Abstract
COVID-19 is a novel, rapidly changing pandemic: consequently, evidence-based recommendations in solid organ transplantation (SOT) remain challenging and unclear. To understand the impact on transplant activity across the United States, and center-level variation in testing, clinical practice, and policies, we conducted a national survey between March 24, 2020 and March 31, 2020 and linked responses to the COVID-19 incidence map. Response rate was a very high 79.3%, reflecting a strong national priority to better understand COVID-19. Complete suspension of live donor kidney transplantation was reported by 71.8% and live donor liver by 67.7%. While complete suspension of deceased donor transplantation was less frequent, some restrictions to deceased donor kidney transplantation were reported by 84.0% and deceased donor liver by 73.3%; more stringent restrictions were associated with higher regional incidence of COVID-19. Shortage of COVID-19 tests was reported by 42.5%. Respondents reported a total of 148 COVID-19 recipients from <1 to >10 years posttransplant: 69.6% were kidney recipients, and 25.0% were critically ill. Hydroxychloroquine (HCQ) was used by 78.1% of respondents; azithromycin by 46.9%; tocilizumab by 31.3%, and remdesivir by 25.0%. There is wide heterogeneity in center-level response across the United States; ongoing national data collection, expert discussion, and clinical studies are critical to informing evidence-based practices.
KEYWORDS: clinical decision-making, epidemiology, guidelines, infectious agents—viral
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BAL, bronchoalveolar lavage; CDC, centers for disease control; CNI, calcineurin inhibitor; COVID-19, coronavirus disease 2019; DDKT, deceased donor kidney transplantation; DDLT, deceased donor liver transplantation; ECMO, extracorporeal membranous oxygenation; HCQ, hydroxychloroquine; IS, immunosuppression; LAS, lung allocation score; LDKT, live donor kidney transplantation; LDLT, live donor liver transplantation; NAT, nucleic acid test; NP, nasopharyngeal; PMP, per million population; PPE, personal protective equipment; RSV, respiratory syncytial virus; RVP, respiratory viral panel; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SOT, solid organ transplant; WHO, World Health Organization
1. INTRODUCTION
COVID-19 has quickly and dramatically impacted the world.1, 2, 3, 4, 5 Given the early nature of the pandemic, knowledge about COVID-19 and its impact on solid organ transplantation (SOT) patients is limited to case reports and expert discussion.6, 7, 8 There is insufficient knowledge about the natural history of COVID-19,9 , 10 including lack of understanding about the potential for donor-derived infection given imperfections in currently available diagnostic tests.2 , 11 There is ongoing nosocomial and community spread,12 and more severe illness has been observed for patients with underlying conditions.3 , 13, 14, 15, 16, 17 Previous experience with related viruses, SARS-CoV in 2003,18 and MERS-CoV in 2015,19 demonstrated that SOT recipients may be anticipated to have prolonged viral shedding, potentially increasing transmissibility, morbidity, and mortality.6 , 20
There are several ways transplant centers can approach the COVID-19 pandemic to mitigate risk for SOT candidates and recipients. Specifically, centers can restrict access to transplantation based on urgency and limit use of donors based on exposure risk. Transplant centers can modify evaluation and monitoring practices of non–COVID-19-SOT patients, develop screening and testing algorithms for suspected cases and treatment protocols for confirmed cases. Furthermore, centers can risk-stratify COVID-19–SOT patients based on disease severity to help allocate appropriate resources to the sickest and most vulnerable patients. However, there are currently no evidence based-guidelines to inform these practices.
To better understand the early impact of COVID-19 on transplant activity across the United States, and to explore center-level variation in testing, clinical practice, and policies, we conducted a national survey of US transplant centers between March 24, 2020 and March 31, 2020. We gathered data in 4 domains: (a) current transplant activity, (b) COVID-19 impact on practices, (c) testing algorithms, and (d) treatment practices. We purposefully conducted our survey at a relatively early stage of US COVID-19 activity in the hopes that rapid dissemination of center-level practices, policies, and perceptions could inform decision-making in other centers in the United States and around the world.
2. METHODS
2.1. Study population
We studied transplant centers in the United States with an annual volume of ≥100 transplants per year (calculated by the average total number of SOTs in 2018 and 2019). These 111 centers perform 87.6% of the adult transplant volume in the United States.
2.2. Survey design
The survey instrument was developed using an iterative process, based on a thorough review of the literature surrounding COVID-19,21, 22, 23, 24, 25 and discussions with transplant surgeons and transplant ID physicians. The final survey was approved by two transplant surgeons and two transplant ID physicians with input from members of the transplant team (Supplement).
2.3. Survey conduct
The survey was conducted between March 24, 2020 and March 31, 2020. At each center, we identified one clinical transplant leader who we anticipated would have knowledge about their center’s COVID-19 practices and policies. Participants were e-mailed links to the survey, and encouraged to either fill out the survey themselves if they felt comfortable, gather data from among colleagues and provide a center-wide response, or pass the survey along to someone who they felt was more appropriate to answer the questions. The online survey was hosted by Qualtrics. Respondents were not compensated.
2.4. Survey domains
We asked questions in 4 domains: (a) current transplant activity, (b) COVID-19 impact on practices, (c) testing algorithms, and (d) treatment practices. For each domain, we asked practice and perception questions. For domain (a), we asked current level of transplant activity (no restrictions, minor restrictions, major restrictions, suspension), and perceptions on what transplant activities should continue. For domain (b), we asked about overall level of concern about risk of COVID-19 for SOT recipients (extremely concerned, highly concerned, neutral, somewhat concerned, not concerned) and perception of shortages of critical supplies, equipment and care areas (anticipated or current). We also asked about changes in outpatient monitoring of SOT recipients. For domain (c), we asked about COVID-19 testing availability. We asked about ease of testing within several time periods (extremely easy, easy, neutral, difficult, extremely difficult), and testing algorithms for recipients and donors. For domain (d), we asked numbers of cases of COVID-19 by organ and perceived severity of illness (mild/no pneumonia, moderate/pneumonia, critically ill), as well as time since transplant (in years). We investigated pharmacologic therapies used by respondents, and changes in immunosuppressive regimens for COVID-19–SOT patients.
2.5. Cumulative incidence by geographic area
Using data from the Johns Hopkins COVID-19 incidence map and the CDC, we linked the cumulative incidence of COVID-19 in each state on March 24 (the day the survey was administered) to each respondent’s survey answers.26 , 27 We divided the total number of cases in each state by state population (from the US Census) to derive cumulative incidence per million population (PMP).28 Twenty-two centers in 8 states whose COVID-19 cumulative incidence were above national average (163 PMP) were defined as centers in high-impact areas.
2.6. Statistical analysis
For statistical purposes, each question was treated as a complete case analysis. We tested the association between transplant activity and level of COVID-19 impact by Fischer’s exact test and reported significance level using P < .05. All analyses were performed using Stata 16.0/MP for Linux.
3. RESULTS
3.1. Study population
Among the 111 transplant centers surveyed, 88 responded (79.3%) ( Table 1). The majority of respondents, 53/88 (60.2%), were transplant surgeons, and 22/88 (25.0%) were transplant infectious diseases (ID) physicians; 82/86 (95.3%) reported expertise in DDKT, and 63/86 (73.3%) in DDLT; 71/88 (80.7%) were academic centers, and 22/88 (25.0%) came from areas of high COVID-19 cumulative incidence.
TABLE 1.
Characteristics of survey respondents and transplant centers
| n = 88 n (%) |
|
|---|---|
| Transplant role | |
| Surgeon | 53 (60.2) |
| Infectious diseases | 22 (25.0) |
| Nephrologist | 8 (9.2) |
| Hepatologist | 1 (1.1) |
| Administrator | 4 (4.6) |
| Expertise with center-level policyb | |
| Live donor KT | 80 (93.0) |
| Deceased donor KT | 82 (95.3) |
| Live donor LT | 34 (39.5) |
| Deceased donor LT | 63 (73.3) |
| Pancreas | 58 (67.4) |
| Heart | 33 (38.4) |
| Lung | 28 (32.6) |
| Center designation | |
| Academic | 71 (80.7) |
| Community | 17 (19.3) |
| COVID-19 prevalence | |
| High-impacta | 22 (25.0) |
| COVID-19 testing availabilityc | |
| Nasopharyngeal NAT | 81 (98.8) |
| Bronchoalveolar lavage NAT | 57 (69.5) |
| Blood NAT | 14 (17.1) |
State cumulative incidence greater than national average (>163 per-million-population) on March 24, 2020.
n = 86.
n = 82 could choose more than one option.
3.2. Current transplant activity
3.2.1. Practices
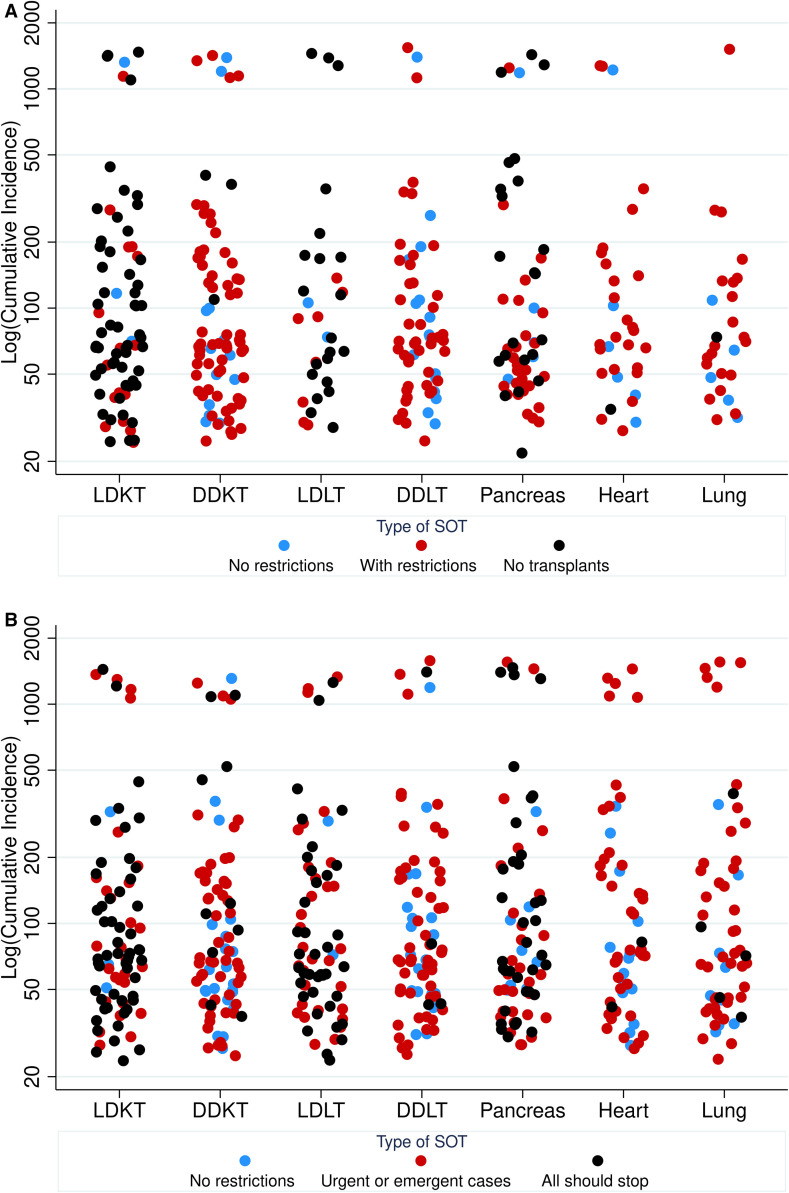
Among LDKT programs, 56/78 (71.8%) reported full suspension of transplantation ( Figure 1A). Operational restrictions (some or major) were reported in 19/78 (24.4%) of LDKT programs. Among DDKT programs, 65/81 (80.2%) were operating with some or major restrictions. Among LDLT programs, 21/31 (67.7%) were suspended. LDLT programs were more likely to be suspended in higher impact areas (P = .03). Among DDLT programs, 16/60 (26.7%) were operating without restriction. Pancreas transplantation was suspended at 22/56 (39.3%). Among heart programs, 6/32 (18.8%) were continuing without restriction. For lung programs, 5/27 (18.5%) were operating without restriction.
FIGURE 1.
A, Transplant center SOT activity by state-level cumulative incidence of COVID-19. B, Perceptions of SOT activity that should continue by organ and state-level cumulative incidence of COVID-19. Cumulative incidence PMP on March 24, 2020 [Color figure can be viewed at wileyonlinelibrary.com]
Examples of LDKT restrictions included: transplanting only preemptive KTs, well-recipients, and those with lack of dialysis access. Some restrictions were driven by limited operating room (OR) staffing. Common DDKT restrictions included: transplanting only highly sensitized patients, those with negative cross-match, higher acuity patients, and those without dialysis access. Others reported transplanting only healthier recipients with the best quality organs and lowest risk of delayed graft function (DGF). Among those LDLT operating with restrictions, respondents noted transplanting patients only with high probability of mortality in 1-3 months; others reported that the LDLT evaluation process has stopped or slowed down. Common restrictions on DDLT included: transplanting higher acuity (MELD > 25 or acute liver failure) patients, inpatients only, those who were not anticipated to require blood products intraoperatively, first-time transplants, and those with tumors without other options. Some respondents reported being limited by supplies and capacity. Reasons for suspension of pancreas transplantation included: avoiding occupying ICU beds, and because of risk of prolonged hospitalization with increased risk of readmission. Some heart programs were restricting to the most severe cases (status 1-3, inpatients). Some lung programs were restricting by lung allocation score (LAS) >45, though some reported inactivating the majority of patients.
3.2.2. Perceptions
Regarding respondents’ perceptions about continuation of transplant practices, 54/85 (63.5%) of respondents thought LDKT should be suspended; 10/85 (11.8%) DDKT; 38/73 (52.1%) LDLT; 4/79 (5.1%) DDLT; 36/75 (48.0%) pancreas; 2/60 (3.3%) heart; and 5/58 (8.6%) lung (Figure 1B).
3.3. COVID-19 impact and transplant center needs
3.3.1. Practices
In-person outpatient visits for SOT recipients were reported to be limited by 62/63 (98.4%) of respondents; 13/63 (20.6%) of respondents reported stopping or limiting lab draws at the hospital; and 61/63 (96.8%) reported using telemedicine ( Table 2). For KT recipients, 53/77 (68.8%) respondents reported developing COVID-19 testing protocols; 43/62 (69.4%) for LT; 28/55 (50.9%) for pancreas; 25/46 (54.4%) for heart, and 24/37 (64.9%) for lung. Fewer respondents reported developing treatment protocols: 46/77 (59.7%) for KT; 34/62 (54.8%) for LT; 30/55 (54.6%) for pancreas recipients; 23/46 (50.0%) for heart recipients; and 18/37 (48.7%) for lung recipients. Donor testing protocols were developed for 67/77 (87.0%) for KT; 53/62 (85.5%) for LT; 47/55 (85.5%) for pancreas; 40/46 (86.9%) for heart; and 31/37 (83.8%) for lung.
TABLE 2.
Respondents who reported changes in outpatient practices and developed COVID-19 recipient/donor testing and treatment protocols
| Changes in OP monitoring | n = 63 n (%) |
|---|---|
| Stopped visits | 5 (7.9) |
| Limited visits | 62 (98.4) |
| Limited laboratory draws | 13 (20.6) |
| Telemedicine | 61 (96.8) |
| Kidney n (%) n = 77 |
Liver n (%) n = 62 |
Pancreas n (%) n = 55 |
Heart n (%) n = 46 |
Lung n (%) n = 37 |
|
|---|---|---|---|---|---|
| SOT testing protocol | 53 (68.8) | 43 (69.4) | 28 (50.9) | 25 (54.4) | 24 (64.9) |
| SOT treatment protocol | 46 (59.7) | 34 (54.8) | 30 (54.6) | 23 (50.0) | 18 (48.7) |
| Donor testing protocol | 67 (87.0) | 53 (85.4) | 47 (85.5) | 40 (87.0) | 31 (83.8) |
Note: Respondents could choose more than one option.
3.3.2. Perceptions
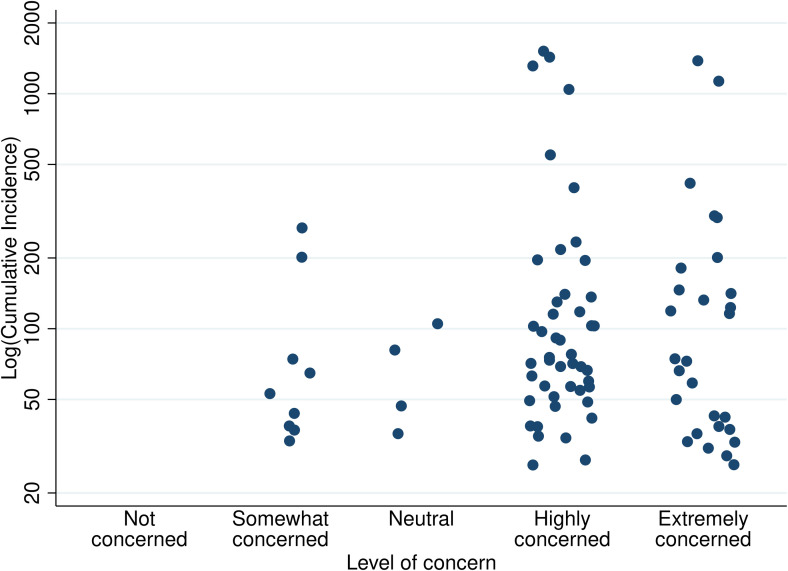
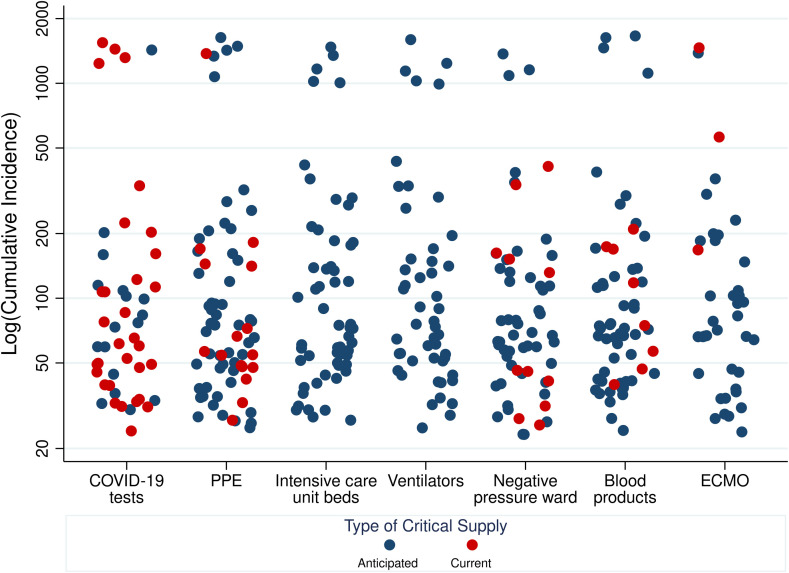
Regarding level of concern about COVID-19 risk for current SOT recipients, 74/87 (85.1%) reported feeling extremely or highly concerned ( Figure 2), which did not change based on state-level cumulative incidence of COVID-19 (P = .8). Regarding COVID-19 testing supplies, 31/73 (42.5%) respondents reported current shortage of COVID-19 tests, and an additional 17/73 (23.3%) anticipated shortage of tests ( Figure 3). Regarding perceptions of shortages of critical supplies, equipment and capacity to care for critically ill patients, 53/75 (70.7%) reported anticipated shortage of personal protective equipment (PPE); 56/73 (76.7%) intensive care unit (ICU) beds; 49/70 (70.0%) ventilators and negative pressure rooms; 51/71 (71.8%) blood products; and 37/64 (57.8%) anticipated shortage of extracorporeal membranous oxygenation (ECMO). These differences in availability of critical supplies were not statistically significantly different by level of COVID-19 impact.
FIGURE 2.
Distribution of respondent concern by state-level cumulative incidence. Cumulative incidence PMP on March 24, 2020
FIGURE 3.
Respondent perception of anticipated and current shortages of supplies by state-level cumulative incidence. Cumulative incidence PMP on March 24, 2020 [Color figure can be viewed at wileyonlinelibrary.com]
3.4. COVID-19–SOT patient testing
3.4.1. Practices
Nasopharyngeal (NP) nucleic acid test (NAT) for COVID-19 was available for 81/82 (98.8%) of respondents; bronchoalveolar lavage (BAL) NAT was available for 57/82 (69.5%); and blood NAT for 14/82 (17.1%) (Table 1).
Regarding testing practices, the most common test for suspected cases of COVID-19 in SOT recipients was simultaneous full respiratory viral panel (RVP) with COVID-19, 35/74 (47.3%) ( Table 3). Other common algorithms were isolated COVID-19 testing 21/74 (28.4%); and reflex COVID-19 testing if influenza, respiratory syncytial virus (RSV), or full RVP were negative.
TABLE 3.
Testing algorithm for suspected COVID-19–SOT recipients and potential deceased donors
| Algorithmc | n (%) |
|---|---|
| Reflex COVID-19 if Influenza/RSVa negative | 13 (17.6) |
| Reflex COVID-19 if full RVPb negative | 8 (10.8) |
| Simultaneous full RVP, COVID-19 | 35 (47.3) |
| Up-front COVID-19 | 21 (28.4) |
| Proposed deceased donor testing | Low-riskd | High-riske |
|---|---|---|
| Nasopharyngeal NAT | 57 (73.1) | 51 (63.8) |
| Bronchoalveolar lavage NAT | 20 (25.6) | 44 (55.0) |
| Blood NAT | 9 (11.3) | 19 (23.8) |
| No NAT | 2 (2.5) | 1 (1.3) |
Note: Respondents could choose more than one algorithm or test.
Respiratory syncytial virus.
Respiratory viral panel.
n = 74.
n = 80.
n = 80.
For low-risk potential deceased donors, 57/80 (71.3%) would test the donor for COVID-19 with NP NAT; 20/78 (25.0%) with BAL NAT. For high-risk donors, 44/80 (55.0%) reported that they would test the donor with BAL NAT.
3.4.2. Perceptions
It was difficult or extremely difficult for 28/83 (32.7%) of respondents to get inpatient COVID-19 testing within 12 hours; 12/82 (14.6%) within 3 days; 4/81 (4.9%) within 7 days ( Table 4). Respondents reported more difficulty in obtaining outpatient COVID-19 tests. It was difficult or extremely difficult for 51/81 (63.0%) of respondents to get outpatient COVID-19 testing within 12 hours; 27/81 (33.3%) within 3 days; 14/78 (17.9%) within 7 days.
TABLE 4.
Perception of ease of arranging for COVID-19 testing at respondents’ hospitals
| Inpatient n (%) | Outpatient n (%) | |||||
|---|---|---|---|---|---|---|
| <12 h n = 83 |
<3 d n = 82 |
<7 d n = 81 |
<12 h n = 81 |
<3 d n = 81 |
<7 d n = 78 |
|
| Extremely easy | 11 (13.3) | 29 (35.4) | 34 (42.0) | 2 (2.5) | 13 (16.0) | 27 (34.6) |
| Easy | 28 (33.7) | 30 (36.6) | 31 (38.3) | 16 (19.8) | 29 (35.8) | 25 (32.1) |
| Neutral | 16 (19.3) | 11 (13.4) | 12 (14.8) | 12 (14.8) | 12 (14.8) | 12 (15.4) |
| Difficult | 19 (22.9) | 10 (12.2) | 2 (2.5) | 28 (34.6) | 18 (22.2) | 9 (11.5) |
| Extremely difficult | 9 (10.8) | 2 (2.4) | 2 (2.5) | 23 (28.4) | 9 (11.1) | 5 (6.4) |
3.5. COVID-19–SOT patient treatment
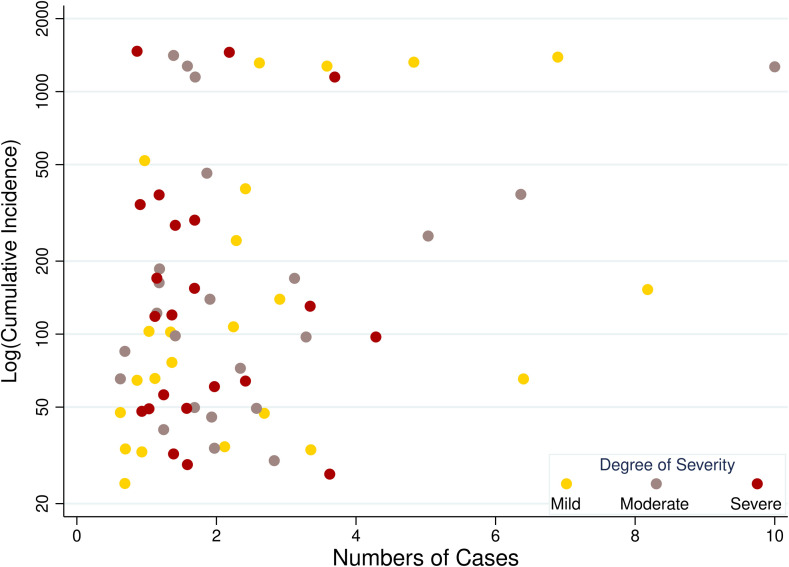
Overall, 31/88 (35.2%) respondents reported caring for at least 1 COVID-19–SOT patients. These respondents reported caring for 148 COVID-19–SOT patients at the time this survey was administered ( Table 5; Figure 4). Participants were asked to classify level of severity of illness: mild (no pneumonia) 80/148 (54.1%); moderate (pneumonia) 31/148 (20.9%); and critically ill 37/148 (25.0%). The majority of COVID-19–SOT patients were KT recipients 103/148 (69.6%). Among the KT recipients, there was a substantial range in the distribution of time since transplant reported by respondents; 10/28 (35.7%) reported caring for KT patients with COVID-19 within 1 year post-KT; 14/28 (50.0%) 1-5 years; 10/28 (35.7%) 6-10 years; and 8/28 (28.6%) >10 years. This was demonstrated by respondents who cared for LT and lung recipients as well.
TABLE 5.
Number of reported cases of COVID-19 in SOT recipients categorized by severity of illness
| Kidney n = 103 n (%) |
Liver n = 23 n (%) |
Heart n = 13 n (%) |
Lung n = 9 n (%) |
Total n = 148 n (%) |
|
|---|---|---|---|---|---|
| Mildlya ill | 58 (56.3) | 11 (47.8) | 8 (61.5) | 3 (33.3) | 80 (54.1) |
| Moderatelyb ill | 18 (17.5) | 6 (26.1) | 5 (38.5) | 2 (22.2) | 31 (20.9) |
| Critically ill | 27 (26.2) | 6 (26.1) | 0 | 4 (44.2) | 37 (25.0) |
No pneumonia.
Pneumonia.
FIGURE 4.
Respondent-reported COVID-19 cases by state-level cumulative incidence. Cumulative incidence PMP on March 24, 2020 [Color figure can be viewed at wileyonlinelibrary.com]
Irrespective of severity of illness, 25/32 (78.1%) of respondents reported having used hydroxychloroquine (HCQ) or chloroquine as off-label therapy for COVID-19; 15/32 (46.9%) reported having used azithromycin; 10/32 (31.3%) reported having used tocilizumab; 8/32 (25.0%) reported having used remdesivir ( Table 6). Pulse steroids were used by 2/32 (6.3%) of respondents, and ACEi/ARB were stopped by 2/32 (6.3%) of respondents. 36/60 (60%) of respondents reported being part of a remdesivir clinical trial; 12/19 (63.2%) respondents who were not participating in a remdesivir clinical trial reported having a compassionate use protocol. Other clinical trials were being participated in by 21/45 (46.7%) respondents; those included HCQ, tocilizumab, interferon lambda, clazakizumab, sarilumab, and convalescent plasma. Adverse effects of HCQ (arrhythmia) was reported by 2/27 (7.4%) of respondents. These therapies varied by type of SOT and severity of illness.
TABLE 6.
Number of respondents who reported using various pharmacologic therapies for COVID-19–SOT patients categorized by degree of illness
| Mild n = 19 n (%) |
Moderate n = 19 n (%) |
Critical n = 18 n (%) |
Overalla n = 32 n (%) |
|
|---|---|---|---|---|
| Anti-inflammatory | ||||
| Hydroxychloroquineb | 10 (52.6) | 16 (84.2) | 15 (83.3) | 25 (78.1) |
| Interferon alpha | 0 | 0 | 0 | 0 |
| Tocilizumabc | 0 | 1 (5.3) | 9 (50.0) | 10 (31.3) |
| Pulse steroids | 0 | 0 | 2 (11.1) | 2 (6.3) |
| Antiviral | ||||
| Remdesivir | 2 (10.5) | 4 (21.1) | 4 (22.2) | 8 (25.0) |
| Oseltamivir/baloxavir | 0 | 0 | 0 | 0 |
| Lopinavir/ritonavir | 1 (5.3) | 0 | 3 (16.7) | 4 (12.5) |
| Ribavirin | 0 | 0 | 0 | 0 |
| Other agents | ||||
| Azithromycin | 6 (31.6) | 9 (47.4) | 11 (61.1) | 15 (46.9) |
| Nitazoxanide | 0 | 0 | 0 | 0 |
| Start ACEi/ARB | 0 | 0 | 0 | 0 |
| Stop ACEi/ARB | 0 | 2 (10.5) | 1 (5.6) | 2 (6.3) |
Note: Respondents could contribute to more than one column (mild, moderate, critical).
This represents the number of individual respondents who reported treating COVID-19–SOT patients; if a respondent contributed to the mild and moderate columns, that respondent would only be counted once in the overall column.
Includes chloroquine.
Anti-IL-6.
Immunosuppression modification varied by SOT type ( Table 7), and severity of illness. For KT recipients, antimetabolites were reported to have been stopped by 24/26 (92.3%) of respondents; calcineurin inhibitor (CNI) were reduced by 7/26 (26.9%) of respondents.
TABLE 7.
Number of respondents who reported changes in immunosuppression for COVID-19–SOT patients
| Kidney n = 26 n (%) |
Liver n = 12 n (%) |
Heart n = 2 n (%) |
Lung n = 4 n (%) |
|
|---|---|---|---|---|
| Antimetabolitea | ||||
| Reduce | 5 (19.2) | 2 (16.7) | 0 | 1 (25.0) |
| Stop | 24 (92.3) | 8 (66.7) | 1 (50.0) | 1 (25.0) |
| Calcineurin inhibitorsb | ||||
| Reduce | 7 (26.9) | 2 (16.7) | 1 (50.0) | 0 |
| Stop | 4 (15.4) | 1 (8.3) | 0 | 1 (25.0) |
| Steroids | ||||
| Reduce | 2 (7.7) | 0 | 0 | 0 |
Note: Respondents could respond to more than one answer.
Includes mycophenolate mofetil (MMF).
Includes mTOR inhibitors.
4. DISCUSSION
In this national survey of transplant centers during the COVID-19 pandemic, we found substantial reduction in transplant activity, wide variation in COVID-19 testing practices, and use of off-label or investigational therapies in the treatment of 148 COVID-19–SOT recipients. Our survey results highlight the heterogeneity in practice patterns for COVID-19 and the lack of treatment protocols.
Given the lack of uniform protocols, the variation in center-specific practices is not surprising and likely driven by the anecdotal and reported experiences of transplant centers in other countries or hotspots within the United States, who have experience exponential increases in COVID-19 hospital admissions and deaths.29 High rates of transplant program cessation are likely reflective of uncertainty regarding the impact of COVID-19 on SOT candidates and recipients and the potential for exponential case growth to overwhelm our current healthcare system.30 This is supported by the large number of respondents who cited concern for inadequate PPE and hospital level resources. Additionally, without successful evidence-based treatment paradigms or even consistent recipient and donor testing availability, uncertainty will likely continue to drive variation and reduction in transplant volume.
This is the first reported national survey of transplant center responses to COVID-19. Strengths include a very high response rate of 79.3%, reflecting a strong national priority to better understand COVID-19. Another strength is the capture of practice, policy, and perceptions early in the pandemic to identify where the needs are and where there was heterogeneity of practice.
Some limitations merit consideration. This survey mostly reflects KT and LT practice given the expertise of transplant leaders with these policies. Also, we were unable to obtain patient-level data on COVID-19 treatments or changes in IS regimens, thus any patient-specific information was relayed through the survey respondent rather than through medical record evaluation.
In conclusion, this national survey of SOT programs suggests that COVID-19 is widely recognized in the United States as a major threat to the field of SOT. However, there were no consistent policies, testing practices, or treatment mechanisms. Given the wide heterogeneity in center-level response across the United States, ongoing national data collection, expert discussion, and clinical studies are critical to informing evidence-based practices.
ACKNOWLEDGMENTS
We acknowledge all respondents for their participation in this time-sensitive survey. This work was supported by grant number T32DK007713-22 (Boyarsky), T32AI007291 (Werbel), F32DK113719 (Jackson), F32DK117563 (Kernodle), K01DK101677 (Massie), K24DK101828 (Segev), and K23DK115908 (Garonzik-Wang) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding information National Institute of Diabetes and Digestive and Kidney Diseases, Grant/Award Number: T32DK007713-22, T32AI007291, F32DK113719, F32DK117563, K01DK101677, K24DK101828 and K23DK115908
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
Supplementary Material
REFERENCES
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu NA, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gandolfini I, Delsante M, Fiaccadori E, et al. COVID-19 in kidney transplant recipients [published online ahead of print March 31, 2020]. Am J Transplant. 10.1111/ajt.15891 [DOI] [PMC free article] [PubMed]
- 7.Zhu L, Xu X, Ma KE, et al. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression [published online ahead of print March 17, 2020]. Am J Transplant. 10.1111/ajt.15869 [DOI] [PMC free article] [PubMed]
- 8.Michaels MG, La Hoz RM, Danziger-Isakov L, et al. Coronavirus disease 2019: implications of emerging infections for transplantation [published online ahead of print 2020]. Am J Transplant. 10.1111/ajt.15869 [DOI] [PMC free article] [PubMed]
- 9.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the seattle region — case series [published online ahead of print 2020]. N Engl J Med. 10.1056/NEJMoa2004500 [DOI] [PMC free article] [PubMed]
- 10.Yazdany J, Kim AHJ. Use of hydroxychloroquine and chloroquine during the COVID-19 pandemic: what every clinician should know [published online ahead of print 2020]. Ann Intern Med. 10.7326/M20-1334 [DOI] [PMC free article] [PubMed]
- 11.Rosenthal PJ. The importance of diagnostic testing during a viral pandemic: early lessons from novel coronavirus disease (COVID-19) [published online ahead of print 2020]. Am J Trop Med Hyg. 10.4269/ajtmh.20-0216 [DOI] [PMC free article] [PubMed]
- 12.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China [published online ahead of print 2020]. JAMA. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed]
- 13.Korean Society of Infectious Diseases and Korea Centers for Disease Control and Prevention Analysis on 54 mortality cases of coronavirus disease 2019 in the Republic of Korea from January 19 to March 10, 2020. J Korean Med Sci. 2020;35(12):e132. doi: 10.3346/jkms.2020.35.e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maddaloni E, Buzzetti R. Covid-19 and diabetes mellitus: unveiling the interaction of two pandemics. Diabetes Metab Res Rev. 2020:e33213321. 10.1002/dmrr.3321 [DOI] [PMC free article] [PubMed]
- 15.D’Adamo H, Yoshikawa T, Ouslander JG. Coronavirus disease 2019 in geriatrics and long-term care: the ABCDs of COVID-19 [published online ahead of print 2020]. J Am Geriatr Soc. 10.1111/jgs.16445 [DOI] [PubMed]
- 16.Guo W, Li M, Dong Y, et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020:e3319. 10.1002/dmrr.3319 [DOI] [PMC free article] [PubMed]
- 17.Emami A, Javanmardi F, Pirbonyeh N, Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch Acad Emerg Med. 2020;8(1):e35. [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar D, Tellier R, Draker R, Levy G, Humar A. Severe Acute Respiratory Syndrome (SARS) in a liver transplant recipient and guidelines for donor SARS screening. Am J Transplant. 2003;3(8):977–981. doi: 10.1034/j.1600-6143.2003.00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.AlGhamdi M, Mushtaq F, Awn N, Shalhoub S. MERS CoV infection in two renal transplant recipients: case report. Am J Transplant. 2015;15(4):1101–1104. doi: 10.1111/ajt.13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fishman JA, Grossi PA. Novel coronavirus-19 (COVID-19) in the immunocompromised transplant recipient: #Flatteningthecurve [published online ahead of print March 31, 2020]. Am J Transplant. 10.1111/ajt.15890 [DOI] [PMC free article] [PubMed]
- 21.Rodriguez-Morales AJ, Cardona-Ospina JA, Gutierrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020:101623. 10.1016/j.tmaid.2020.101623 [DOI] [PMC free article] [PubMed]
- 22.Yang S, Cao P, Du P, et al. Early estimation of the case fatality rate of COVID-19 in mainland China: a data-driven analysis. Ann Transl Med. 2020;8(4):128. doi: 10.21037/atm.2020.02.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. StatPearls. StatPearls Publishing LLC; Treasure Island, FL: 2020. Features evaluation and treatment coronavirus (COVID-19) [PubMed] [Google Scholar]
- 24.Ashour HM, Elkhatib WF, Rahman MM, Elshabrawy HA. Insights into the recent 2019 novel coronavirus (SARS-CoV-2) in light of past human coronavirus outbreaks. Pathogens. 2020;9(3) doi: 10.3390/pathogens9030186. pii:E186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sohrabi C, Alsafi Z, O’Neill N, et al. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int J Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coronavirus (Covid-19) Data in the United States. https://www.aei.org/covid-2019-action-tracker/. Accessed April 2, 2020.
- 27.Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. https://coronavirus.jhu.edu/map.html. Accessed April 2, 2020.
- 28.Bureau USC. Census 2010 and 2018 estimated population by state. http://factinder.census.gov. Accessed April 2, 2020.
- 29.Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3):105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halazun KJ, Rosenblatt R. Lest we forget [published online ahead of print 2020]. Am J Transplant. 10.1111/ajt.15888 [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.