Abstract
In December 2019, the world started to face a new pandemic situation, the severe acute respiratory syndrome‐coronavirus 2 (SARS‐CoV‐2). Although coronavirus disease (COVID‐19) clinical manifestations are mainly respiratory, major cardiac complications are being reported. Cardiac manifestations etiology seems to be multifactorial, comprising direct viral myocardial damage, hypoxia, hypotension, enhanced inflammatory status, ACE2‐receptors downregulation, drug toxicity, endogenous catecholamine adrenergic status, among others. Studies evaluating patients with COVID‐19 presenting cardiac injury markers show that it is associated with poorer outcomes, and arrhythmic events are not uncommon. Besides, drugs currently used to treat the COVID‐19 are known to prolong the QT interval and can have a proarrhythmic propensity. This review focus on COVID‐19 cardiac and arrhythmic manifestations and, in parallel, makes an appraisal of other virus epidemics as SARS‐CoV, Middle East respiratory syndrome coronavirus, and H1N1 influenza.
Keywords: arrhythmia, COVID‐19, myocardial damage, myocarditis, SARS‐CoV‐2
Abbreviations
- ATP
antitachycardia pacing
- CHF
congestive heart failure
- CO
cardiac output
- COVID‐19
coronavirus disease‐2019
- CRT‐D
cardiac resynchronization therapy defibrillator
- HCQ
hydroxychloroquine
- HF
heart failure
- HR
hazard ratio
- hs‐cTnI
high‐sensitivity cardiac troponin I
- ICD
implantable cardiac defibrillator
- ICU
intensive care unit
- LVEF
left ventricular ejection fraction
- MERS‐CoV
Middle East respiratory syndrome coronavirus
- NHC
National Health Commission of China
- OR
odds ratio
- RBBB
right bundle branch block
- SARS
severe acute respiratory syndrome
- SARS‐CoV
severe acute respiratory syndrome‐coronavirus
- SARS‐CoV‐2
severe acute respiratory syndrome‐coronavirus 2
- SCD
sudden cardiac death
- SVT
supraventricular tachycardia
- TnT
troponin T
- VA
ventricular arrhythmias
- VF
ventricular fibrillation
- VT
ventricular tachycardia
1. INTRODUCTION
Since the last December, the entire world is facing a new pandemic situation, the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). After emerging from Wuhan‐China, the coronavirus disease‐2019 (COVID‐19) quickly spread throughout the world. Due to all its unknown factors, physicians are passing through real‐time learning process. As the disease spreads, a massive wave of information takes over scientific journals and media.
This new virus belongs to the same severe acute respiratory syndrome‐coronavirus (SARS‐CoV) and Middle East respiratory syndrome‐coronavirus (MERS‐CoV) family. Although COVID‐19 clinical manifestations are mainly respiratory, with the growing number of infected patients, major cardiac complications have been reported in a considerable number of patients with COVID‐19. 1 , 2 , 3
SARS‐CoV‐2 infection is associated with a variety of pro‐inflammatory mediators that may play important roles in the pathophysiology of cardiac and arrhythmic complications. In a single center study 1 cardiac injury was observed in 19% of hospitalized patients with COVID‐19, and it was associated with higher risk of in‐hospital mortality. Therefore, it is plausible that these patients have an even higher risk of cardiac arrhythmias.
Aiming to shed some light in this issue, we performed this review focused on COVID‐19 cardiac manifestations not only by analyzing the preliminary available evidence about the virus, but also by making comparative considerations with SARS‐CoV, MERS‐CoV, and H1N1 influenza.
1.1. Lesson from previous epidemics
Much of our present knowledge of SARS‐CoV‐2 comes from previous historical epidemics that preceded the current outbreak, as SARS‐CoV, MERS‐CoV, and H1N1 influenza syndromes. It was observed, during these outbreaks, a significant association between underlying cardiovascular disease, myocardial injury, and worse outcomes. 4
The first human infection by a new strain of coronavirus, the SARS‐CoV, was reported in 2002. At that time it was known that, at least in rabbits, coronavirus infections could induce cardiomyopathy resulting in cardiac chambers dilatation and systolic function impairment, simulating other dilated cardiomyopathies. 5
In humans, hypotension, cardiac arrhythmias, and even sudden cardiac death (SCD) were described as possible SARS‐CoV manifestations. 6 In a cohort of 121 patients, Yu et al demonstrated that sinus tachycardia was the commonest cardiovascular SARS‐CoV finding with an overall incidence of 72%. Persistent tachycardia mean duration was 12.7 days with a mean heart rate of 117 beats/min (range: 102‐150 beats/min) and the tachycardia remained persistent in nearly 40% of patients within 30 days after hospital discharge. The incidence of tachycardia during the third hospitalization week, when most patients were afebrile, could be related to drug treatment, such corticosteroid and ribavirin. However, corticosteroid therapy was not associated with persistent tachycardia during follow‐up. Hence, longstanding tachycardia could eventually be due to autonomic tone changing. Or, alternatively, sinus tachycardia secondary to cardiopulmonary or peripheral deconditioning since this disease resulted in prolonged bed rest. 7
Besides these findings, significant sinus bradycardia was seen in 18 (14.9%) patients. Unlike tachycardia, which was persistent, bradycardia was somewhat transient with a mean heart rate of 43 beats/min (range: 38‐49 beats/min) and a mean duration of 2.6 days. Reversible cardiomegaly was also reported in 13 (10.7%), with no clinical evidence of heart failure (HF). Transient atrial fibrillation was observed in one patient. 7
Lau et al additionally described that palpitation, in the form of tachycardia at rest or mild exertion, was noted amongst patients recovering from SARS. Possible causes, according to them, were deconditioning, impaired pulmonary function, impaired cardiac function, cardiac arrhythmia, thyroid dysfunction, anemia, autonomic dysfunction, and anxiety state. 8
Trying to explain the occurrence of cardiac arrest in 15 patients with SARS, Pan et al suggested some possible mechanisms: (a) lung injury caused by SARS virus leading to hypoxemia and an unsteady state in myocardial electricity; (b) SARS direct causing new myocardial cells and/or conduction system damage; (c) SARS infection aggravating pre‐existing myocardial conditions, or conduction disturbances; and (d) extreme anxiety leading to further endogenous catecholamine release, causing myocardial electrical instability (see Figure 1). 9
Figure 1.
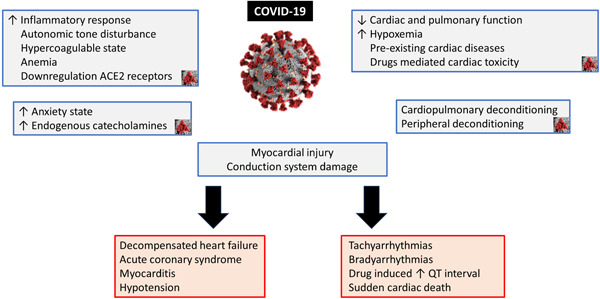
Mechanisms and consequences of COVID‐19 myocardial damage. COVID‐19, coronavirus disease
In the setting of the 2012 MERS‐CoV syndrome, despite some similarities with SARS‐CoV, the early mortality rate for the former achieved 60%, 10 remaining higher than 35% during the overall outbreak period, while for SARS‐CoV the mortality rate was about 10%. 11 A meta‐analysis suggested that MERS‐CoV infection was more likely to occur in patients with underlying cardiovascular diseases. 12 In terms of overall complications, renal failure (40.9%), cardiac arrhythmias (15.7%), hepatic dysfunction (31.4%), 13 besides pericarditis, and hypotension were the most commonly reported. 14 In a case report published by Alhogbani, he describes an acute myocarditis caused by MERS‐CoV; a 60‐year‐old presenting with respiratory symptoms, chest pain, and persistent tachycardia (120 bpm). Echocardiogram demonstrated severe left ventricular function impairment, cardiac magnetic resonance showed typical findings of acute myocarditis, and sputum was positive for MERS‐CoV. The patient was intubated and required hemodialysis. After 6 weeks of intensive care unit (ICU) and 1 month of ward hospitalization, he was discharged in stable condition. 15
Last but not least, influenza virus infection is well‐known to aggravate plenty of cardiovascular disorders, being associated with myocarditis, myocardial infarction, and HF exacerbation. 16
An interesting survey conducted by Madjid et al tested the possible effect of seasonal influenza on the occurrence of ventricular arrhythmias (VA) requiring shock or antitachycardia pacing (ATP) treatment in patients with implantable cardiac defibrillator or cardiac resynchronization therapy defibrillator. The results indicated that more shocks were delivered during influenza season than during other periods of the year, suggesting a correlation between higher arrhythmia burden and influenza season. The multivariate generalized linear model showed that during high influenza activity, patients were more likely to have a VA treated with shock (odds ratio [OR]: 1.06; P < .001) or ATP (OR: 1.06; P < .0001). 17
Multiple mechanisms have been proposed to explain influenza triggering arrhythmias, among them severe systemic, arterial, and myocardial inflammatory reaction seems to be one of the most plausible. Moreover, influenza is known to exacerbate congestive heart failure (CHF) and increase CHF‐related hospital admissions. 18 Decompensated CHF, besides leading to hospitalization, is related to electrical myocardial homeostasis impairment, causing ventricular tachycardias (VTs) treated with shock or ATP therapy. In patients with underlying ischemic cardiomyopathy, the worsening of ischemia by increased oxygen demand and potential acute coronary syndromes led by influenza can also have a role in the increase of arrhythmic events. 17
These concepts were strengthened by a nationwide Denmark studied, which showed a strong relationship between yearly influenza vaccination and mortality in patients with HF. In this study, annual influenza vaccination was associated with 18% reduction in the adjusted risk of all‐cause death and 18% reduction in the adjusted risk of cardiovascular death (P < .001, for both). Remarkably, those who received more than one seasonal vaccination also had a more pronounced reduction in atrial fibrillation incidence (hazard ratio [HR]: 0.94; P = .009). According to this study, influenza infection may result in increased metabolic demand, hypoxia, and adrenergic surges, which may lead to acute decompensation or exacerbation of HF. Additionally, the infection may induce a hypercoagulable state and trigger acute coronary syndromes, resulting in further left ventricular function deterioration, or it could cause direct myocardial depression. Based on these results, the authors advocated that influenza vaccination may be a valuable treatment strategy to improve survival in patients with HF. 19
1.2. Coronavirus disease
Despite not being particularly lethal, SARS‐CoV‐2 is very contagious. In a published clinical cohort of patients with COVID‐19, they observed that acute cardiac injury, shock, and arrhythmias were present in 7.2%, 8.7%, and 16.7% of patients, respectively, with higher prevalence amongst patients requiring intensive care. 2 In this report, myocardial injury biomarkers levels were significantly higher in patients requiring ICU admission than in those not treated in the ICU (median creatine kinase‐MB level 18 U/l vs 14 U/l; P < .001; and high‐sensitivity cardiac troponin I [hs‐cTnI] level 11.0 pg/mL vs 5.1 pg/mL; P = .004), suggesting that patients with severe symptoms often have complications involving acute myocardial injury. 2 Overall, arrhythmia rate was also more frequent in ICU patients (44.4% vs 6.9%; P < .001). Despite the relevance of these initial data, the authors did not provide any arrhythmia classification or definition Table 1.
Table 1.
Cohorts that evaluated cardiac manifestations in SARS‐CoV, MERS‐CoV, H1N1, and SARS‐CoV‐2
| First author, y | Number of patients | Cardiac manifestations | Troponin | In‐hospital mortality | |
|---|---|---|---|---|---|
| SARS‐CoV | Lee et al 39 | 138 | Acute HF (1 pt) | None | 3.6% |
| Booth et al 40 | 144 | Pulse >100 bpm (46%) | ND | 6.5% | |
| Chest pain (10.4%) | |||||
| Li et al 23 | 46 | RBBB 15.2% | ND | 13% | |
| LVEF‐HF 1 pt (EF 30.2%) | |||||
| Initial TTE compare with 30 d control: | |||||
| Lower LVEF | |||||
| Lower doppler‐derived CO | |||||
| Yu et al 7 | 121 | Tachycardia (71.9%) | ND | ND | |
| Hypotension (50.4%) | |||||
| Bradycardia (14.9%) | |||||
| Rev cardiomegaly (10.7%) | |||||
| MERS‐CoV | Saad et al 13 | 70 | Arrhythmias (15.7%) | ND | 60% |
| Al‐Tawfiq et al 41 | 17 | X‐ray cardiomegaly (53%) | ND | 76% | |
| Chest pain (7%) | |||||
| Assiri et al 43 | 47 | Chest pain (15%) | ND | 60% | |
| Al‐Albdallat et al 14 | 9 | Chest pain (44%) | ND | 22% | |
| Pericarditis (1 pt) | |||||
| VT (1 pt) | |||||
| SVT (1 pt) | |||||
| H1N1 Influenza | Schoen et al 42 | 160 | Chest pain (5%) | ND | Zero |
| SARS‐CoV‐2 | Huang et al 20 | 41 | Shock (7%) | Elevated in 12.2% | 15% |
| Wang et al 2 | 118 | Arrhythmia (16.7%) | Mean 6.4 pg/mL | 4.3% | |
| Shock (8.7%) | |||||
| Acute cardiac injury (7.2%) | |||||
| Shi et al 1 | 416 | Chest pain (3.4%) | Elevated in 19.7% | 13.7% | |
| ST‐depression on ECG (0.7%) | |||||
| Zhou et al 22 | 191 | HF (23%) | Elevated in 17% | 28.2% | |
| Hypotension (1%) | |||||
| HR > 125 bpm (1%) | |||||
| Guo et al 21 | 187 | VT/VF (5.9%) | Elevated in 27.8% | 23% |
Abbreviations: CO, cardiac output; ECG, electrocardiogram; EF, ejection fraction; HF, heart failure; HR, heart rate; MERS‐CoV, Middle East respiratory syndrome coronavirus; ND, not disclosed; pt, patient; LVEF, left ventricular ejection fraction; TTE, transthoracic echocardiogram; RBBB, right bundle branch block; Rev, reversible; SARS‐CoV, severe acute respiratory syndrome‐coronavirus; SVT, supra ventricular tachycardia; VF, ventricular fibrillation; VT, ventricular tachycardia.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
A study from Shi et al evaluated a single‐center cohort of 416 patients hospitalized due to COVID‐19. He observed that cardiac lesion, defined by hs‐cTnI >99th percentile of on admission, was present in 19.7%, with median value of 0.19 (0.08‐1.12) μg/L in this group. Compared with those without cardiac injury, patients with cardiac injury required more noninvasive ventilation (46.3% vs 3.9%; P < .001) and invasive mechanical ventilation (22.0% vs 4.2%; P < .001), and also had a higher mortality (51.2% vs 4.5%; P < .001). It is remarkable that the elevated troponin group was older and significantly more ill, but after adjustment for all the possible confounding factors, still the cardiac injury was a predictor of mortality (HR: 4.26; 95% CI: 1.92‐9.49). 1
In another small report, Huang et al demonstrated that SARS‐CoV‐2 associated myocardial injury occurred on 5 out of 41 patients, and was manifested as an increase in hs‐cTnI levels (>28 pg/mL). Among these five patients, ICU management was required in four, indicating the severe nature of the myocardial injury in patients with COVID‐19. 20
In a study by Guo et al, 187 patients with SARS‐CoV‐2 positive were analyzed, stratified by the level of troponin, which was elevated in 27.8%. During hospitalization, patients with elevated troponin T (TnT) levels developed more frequently complications as acute respiratory distress syndrome (57.7% vs 11.9%), malignant VAs (11.5% vs 5.2%), acute coagulopathy (65.8% vs 20.0%), and acute kidney injury (36.8% vs 4.7%), compared with those with normal TnT levels. But the most impressive observation is that mortality was markedly higher in patients with elevated plasma TnT levels than in patients with normal TnT levels (59.6% vs 8.9%). 21
Contrary to the above mentioned studies Zhou et al comparing survivors and non‐survivors in a cohort of 191 patients from two hospitals in Wuhan, found that, despite more frequent in non‐survivors (46% vs 1%; P < .001), hs‐cTnI >28 pg/mL was not associated with mortality in multivariate analysis. Even though, it is remarkable that this study was unpowered to draw conclusions from this analysis due to the excess of variable for only 54 events. 22
Acute myocarditis, as well as VAs might represent the first clinical manifestation of SARS‐CoV‐2 infection. 3 , 44 In the epicenter of the current Italian epidemic, SCD likely occurred in many nonhospitalized patients with mild symptoms who were found dead home while in quarantine. Myocardial biomarkers should be evaluated in all patients with COVID‐19 for risk stratification and prompt intervention. Even after hospital discharge, we should consider that myocardial injury might result in atrial or ventricular fibrosis, the substrate for subsequent cardiac arrhythmias. The extent of myocardial scar, as assessed with cardiac magnetic resonance, might be a powerful tool to better stratify the arrhythmic risk in patients recovered from COVID‐19 who had evidence of myocardial injury at the time of infection.
Another relevant aspect of COVID‐19 infection is that early diagnosis can be confounded in patients with chronic cardiac conditions, once the most frequent symptoms, like fatigue (51%, 95% CI: 34%‐68%), dyspnea (30%, 95% CI: 21%‐40%), and cough (67%, 95% CI: 59%‐76%) 25 can also be manifestations of decompensated HF or arrhythmic syndrome. Corroborating this concern, the National Health Commission of China (NHC) reported that among SARS‐CoV‐2 infection confirmed cases, cardiovascular symptoms were the first presentation in some patients. The problem behind these atypical presentations is that patients suffering from heart palpitations and chest tightness rather than respiratory symptoms, such as fever and cough, had a delayed COVID‐19 diagnosis. 26 Still according to the NHC, among the people who died from COVID‐19, 11.8% had substantial heart damage, with elevated troponin I levels or cardiac arrest during hospitalization. 26
Explanatory theories regarding COVID‐19 cardiovascular affection postulate that chronic cardiovascular diseases may become unstable in the setting of a viral infection as a consequence of the imbalance between the infection‐induced increase in metabolic demand and reduced cardiac reserve. 2 This imbalance, concurrent with an accentuated inflammatory response and myocardial damage, could raise the risk of acute coronary syndromes, HF, and arrhythmias.
The deleterious SARS‐CoV‐2 infection myocardial effects could also be perpetuated by the prompt and severe downregulation of myocardial and pulmonary ACE2 pathways, thereby mediating myocardial inflammation, lung edema, and acute respiratory failure. 27 ACE2 is widely expressed not only in the lungs but also in the cardiovascular system and, therefore, ACE2‐related signaling pathways might even have a role in heart injury. Other proposed mechanisms of myocardial injury include a cytokine storm triggered by an imbalanced response by type 1 and 2 T‐helper cells, 20 , 28 strong interferon‐mediated immunopathological events, 29 and respiratory dysfunction and hypoxemia caused by COVID‐19, resulting in damage to myocardial cells. Therapeutic use of corticosteroids, in this context, would further augment the possibility of adverse cardiovascular events.
Regarding hypoxemia caused by COVID‐19, it is relevant to highlight that this condition can trigger atrial fibrillation, which is the most common arrhythmia among elderly individuals, and that atrial fibrillation can become persistent even before pulmonary improvement. Furthermore, the systemic inflammatory response would make anticoagulation therapy for atrial fibrillation very complex. 30
1.3. Chloroquine and hydroxychloroquine side effects on cardiovascular system
Another essential aspect to be discussed is about chloroquine cardiovascular side effects since this is one of the promising drugs that have been tested in patients with COVID‐19. It is well‐reported that long‐term chloroquine use may increase depolarization length duration and Purkinje fiber refractory period, 31 , 32 , 33 , 34 ultimately leading to atrioventricular nodal and/or His system malfunction. 31
As an antimalarial drug, both chloroquine and hydroxychloroquine (HCQ) are accumulated in lysosomes, directly inhibiting phospholipase activity, inducing cytoplasmic inclusion body formation, increasing lysosomal pH, and causing protein inactivity. 31 , 35 Due to these properties, drug‐induced atrial and VAs have been associated with their use. 31 , 32 , 33 , 34 , 35 The most usual electrocardiographic alteration is fascicular block, which can lead to advanced types of atrioventricular block, generally associated with syncope. 36
HCQ can also induce QT interval prolongation, an extremely rare but potential fatal side effect, due to the risk of induced polymorphic VT and SCD. The proposed mechanism by which HCQ causes QT interval prolongation is not well understood. In 2015, Capel et al demonstrated, in guinea pig sinoatrial node myocytes, an inhibitory effect of the HCQ on the hyperpolarization‐activated current ion channels (also known as “funny current” channels), along with delayed rectifier potassium currents, and L‐type calcium ion currents. 37 Inhibitory effects on pacemaker cells were shown to cause delayed rates in depolarization leading to decreased heart rates. These findings may correlate with a proposed mechanism by which refractory action potentials in cardiac myocytes may lead to prolongation of QT interval due to delayed depolarization and repolarization from abnormal ion currents. 38 QT prolongation in individual medical therapy is not always predictable, dose adjustments and/or additional monitoring with electrocardiograms may be appropriate in some cases. HCQ proarrhythmic risk must be monitored in patients with underlying cardiovascular or renal disorders, and high caution should be posed in the case of electrolyte imbalance, dysrhythmias or concurrent use of QTc‐prolonging drugs. 38
2. CONCLUSION
Acute lung injury is a common problem in patients with COVID‐19 and results in significant morbidity and mortality. However, increasing clinical and epidemiological evidence suggests that COVID‐19 infection is associated with myocardial injury and arrhythmic complications.
Even though the prevalence of COVID‐19 arrhythmogenic effects has yet not been reported, close cardiovascular surveillance is advisable, particularly in patients with more severe presentation and in those with increased baseline risk due to previous cardiac comorbidities. Since many medications are being used empirically to treat the infection and/or symptoms, there is a need to increase awareness to possible drug interactions and close monitoring in atrioventricular conduction and QT interval.
Kochi AN, Tagliari AP, Forleo GB, Fassini GM, Tondo C. Cardiac and arrhythmic complications in patients with COVID‐19. J Cardiovasc Electrophysiol. 2020;31:1003–1008. 10.1111/jce.14479
REFERENCES
- 1. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA. 2020. 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in wuhan, China. JAMA. 2020. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020. 10.1093/eurheartj/ehaa190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sellers SA, Hagan RS, Hayden FG, Fischer WA 2nd. The hidden burden of influenza: a review of the extra‐pulmonary complications of influenza infection. Influenza Other Respir Viruses. 2017;11:372‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alexander LK, Small JD, Edwards S, Baric RS. An experimental model for dilated cardiomyopathy after rabbit coronavirus infection. J Infect Dis. 1992;166:978‐985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xiong TY, Redwood S, Prendergast B, Chen M. Coronaviruses and the cardiovascular system: acute and long‐term implications. Eur Heart J. 2020. 10.1093/eurheartj/ehaa231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yu CM, Wong RS, Wu EB, et al. Cardiovascular complications of severe acute respiratory syndrome. Postgrad Med J. 2006;82:140‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lau ST, Yu WC, Mok NS, Tsui PT, Tong WL, Cheng SW. Tachycardia amongst subjects recovering from severe acute respiratory syndrome (SARS). Int J Cardiol. 2005;100:167‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pan SF, Zhang HY, Li CS, Wang C. Cardiac arrest in severe acute respiratory syndrome: analysis of 15 cases. Zhonghua Jie He He Hu Xi Za Zhi. 2003;26:602‐605. [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention (CDC) . Update: severe respiratory illness associated with Middle East respiratory syndrome coronavirus (MERS‐CoV)‐worldwide, 2012‐2013. MMWR Morb Mortal Wkly Rep. 2013;62:480‐483. [PMC free article] [PubMed] [Google Scholar]
- 11. Maslow JN. Vaccines for emerging infectious diseases: lessons from MERS coronavirus and Zika virus. Hum Vaccin Immunother. 2017;13:2918‐2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Badawi A, Ryoo SG. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS‐CoV): a systematic review and meta‐analysis. Int J Infect Dis. 2016;49:129‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saad M, Omrani AS, Baig K, et al. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single‐center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Al‐Abdallat MM, Payne DC, Alqasrawi S, et al. Hospital‐associated outbreak of Middle East respiratory syndrome coronavirus: a serologic, epidemiologic, and clinical description. Clin Infect Dis. 2014;59:1225‐1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alhogbani T. Acute myocarditis associated with novel Middle East respiratory syndrome coronavirus. Ann Saudi Med. 2016;36:78‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nguyen JL, Yang W, Ito K, Matte TD, Shaman J, Kinney PL. Seasonal influenza infections and cardiovascular disease mortality. AMA Cardiol. 2016;1:274‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Madjid M, Connolly AT, Nabutovsky Y, Safavi‐Naeini P, Razavi M, Miller CC. Effect of high influenza activity on risk of ventricular arrhythmias requiring therapy in patients with implantable cardiac defibrillators and cardiac resynchronization therapy defibrillators. Am J Cardiol. 2019;124:44‐50. [DOI] [PubMed] [Google Scholar]
- 18. Ang LW, Yap J, Lee V, et al. Influenza‐associated hospitalizations for cardiovascular diseases in the tropics. Am J Epidemiol. 2017;186:202‐209. [DOI] [PubMed] [Google Scholar]
- 19. Modin D, Jørgensen ME, Gislason G, et al. Influenza vaccine in heart failure. Circulation. 2019;139:575‐586. [DOI] [PubMed] [Google Scholar]
- 20. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020. 10.1001/jamacardio.2020.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li SS, Cheng C, Fu C, et al. Left ventricular performance in patients with severe acute respiratory syndrome: a 30‐day echocardiographic follow‐up study. Circulation. 2003;108:1798‐1803. [DOI] [PubMed] [Google Scholar]
- 24. Harris JE, Shah PJ, Korimilli V, Win H. Frequency of troponin elevations in patients with influenza infection during the 2017‐2018 influenza season. Int J Cardiol Heart Vasc. 2019;22:145‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID‐19) infection: a systematic review and meta‐analysis. Int J Infect Dis. 2020. 10.1016/j.ijid.2020.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zheng YY, Ma YT, Zhang JY, Xie X. COVID‐19 and the cardiovascular system. Nat Rev Cardiol. 2020. 10.1038/s41569-020-0360-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oudit GY, Kassiri Z, Jiang C, et al. SARS‐coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SAR. Eur J Clin Invest. 2009;39:618‐625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wong CK, Lam CW, Wu AK, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cameron MJ, Bermejo‐Martin JF, Danesh A, Muller MP, Kelvin DJ. Human immunopathogenesis of severe acute respiratory syndrome (SARS). Virus Res. 2008;133:13‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang C, Jin Z. An acute respiratory infection runs into the most common noncommunicable epidemic—COVID‐19 and cardiovascular diseases. JAMA Cardiol. 2020. 10.1001/jamacardio.2020.0934 [DOI] [PubMed] [Google Scholar]
- 31. Ratliff NB, Estes ML, McMahon JT, Myles JL. Chloroquine‐induced cardiomyopathy. Arch Pathol Lab Med. 1988;112:578. [PubMed] [Google Scholar]
- 32. Seshadri MS, John L, Varkey K, Koshy TS. Ventricular tachycardia in a patient on dehydroemetine and chloroquine for amoebic liver abscess. Med J Aust. 1979;1:406‐407. [DOI] [PubMed] [Google Scholar]
- 33. Fauchier JP, Fauchier L, Babuty D, Breuillac JC, Cosnay P, Rouesnel P. Drug‐induced ventricular tachycardia. Arch Mal Coeur Vaiss. 1993;86:757‐767. [PubMed] [Google Scholar]
- 34. Siqueira‐Batista R, Ramos Júnior AN, Pessanha BS, Sforza‐de‐Almeida MP, Potsch DF. Chloroquine and cardiac arrhythmia: case report. East Afr Med J. 1998;75:117‐119. [PubMed] [Google Scholar]
- 35. Harris L, Downar E, Shaikh NA, Chen T. Antiarrhythmic potential of chloroquine: new use for an old drug. Can J Cardiolo. 1988;4:295‐300. [PubMed] [Google Scholar]
- 36. Verny C, de Gennes C, Sebastien P, et al. Heart conduction disorders in long‐term treatment with chloroquine: two new cases. Presse Med. 1992;2:800‐804. [PubMed] [Google Scholar]
- 37. Capel RA, Herring N, Kalla M, et al. Hydroxychloroquine reduces heart rate by modulating the hyperpolarization‐activated current if: novel electrophysiological insights and therapeutic potential. Heart Rhythm. 2015;12:2186‐2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. O'Laughlin JP, Mehta PH, Wong BC. Life threatening severe QTc prolongation in patient with systemic lupus erythematosus due to hydroxychloroquine. Case Rep Cardiol. 2016:4626279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee N, Hui D, Wu A, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986‐1994. [DOI] [PubMed] [Google Scholar]
- 40. Booth CM, Matukas LM, Tomlinson GA, et al. Clinical features and short‐term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289:2801‐2809. [DOI] [PubMed] [Google Scholar]
- 41. Al‐Tawfiq JA, Hinedi K, Ghandour J, et al. Middle East respiratory syndrome coronavirus: a case‐control study of hospitalized patients. Clin Infect Dis. 2014;59:160‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schoen K, Horvat N, Guerreiro NFC, de Castro I, de Giassi KS. Spectrum of clinical and radiographic findings in patients with diagnosis of H1N1 and correlation with clinical severity. BMC Infect Dis. 2019;19:964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Assiri A, Al‐Tawfiq JA, Al‐Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752‐761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Inciardi RM, Lupi L, Zaccone G, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020. 10.1001/jamacardio.2020.1096 [DOI] [PMC free article] [PubMed] [Google Scholar]


