Abstract
The outbreak of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has evolved into an emergent global pandemic. Coronavirus disease 2019 (COVID‐19) can manifest on a spectrum of illness from mild disease to severe respiratory failure requiring intensive care unit admission. As the incidence continues to rise at a rapid pace, critical care teams are faced with challenging treatment decisions. There is currently no widely accepted standard of care in the pharmacologic management of patients with COVID‐19. Urgent identification of potential treatment strategies is a priority. Therapies include novel agents available in clinical trials or through compassionate use, and other drugs, repurposed antiviral and immunomodulating therapies. Many have demonstrated in vitro or in vivo potential against other viruses that are similar to SARS‐CoV‐2. Critically ill patients with COVID‐19 have additional considerations related to adjustments for organ impairment and renal replacement therapies, complex lists of concurrent medications, limitations with drug administration and compatibility, and unique toxicities that should be evaluated when utilizing these therapies. The purpose of this review is to summarize practical considerations for pharmacotherapy in patients with COVID‐19, with the intent of serving as a resource for health care providers at the forefront of clinical care during this pandemic.
Keywords: infectious disease, antivirals, cytochrome P450, dialysis, renal, liver
The recent outbreak of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and subsequent coronavirus disease 2019 (COVID‐19) has created a global health emergency with unparalleled worldwide burden. 1 The full epidemiological impact of this virus remains to be seen, but an understanding of the approximate denominator will be important when considering the clinical characteristics of these cases and the percentage of cases with more severe disease or resultant mortality. 2 In December 2019, the first reports of COVID‐19 included a cluster of patients hospitalized with pneumonia in Wuhan, China. The most common presenting symptoms in these patients were fever (98%), cough (76%), dyspnea (55%), and myalgia or fatigue (44%). 3 Characteristic laboratory and radiologic findings included lymphopenia (63%) and rapid development of multiple patchy consolidations and ground glass opacities bilaterally with a notable peripheral distribution seen on chest computed tomography (CT). 3 , 4 Patients may present with a chest CT suggestive of COVID‐19 even before developing a positive reverse transcription‐polymerase chain reaction assay result. 5
Epidemiology and diagnostics for COVID‐19 are rapidly evolving, giving us greater understanding of those at risk and elucidating more potential pharmacologic targets. 6 SARS‐CoV‐2 is a betacoronavirus, in the same subgroup as severe acute respiratory syndrome‐coronavirus (SARS‐CoV) that caused the SARS outbreak in 2002 and the Middle East respiratory syndrome coronavirus (MERS‐CoV) that caused the MERS outbreak in 2012. 7 As such, a rational approach to initial therapies to target COVID‐19 would be those that were studied during the SARS and MERS outbreaks. Novel therapies and repurposing of old drugs are now being studied as therapeutic strategies against COVID‐19.
Treatment strategies that have been identified thus far include medications uncommonly used in clinical practice. Our understanding of important practical considerations is quite limited. In one study, ~25% of patients with COVID‐19 required admission to an intensive care unit (ICU). These patients were older (66 vs 51 yrs of age) and more likely to have underlying comorbidities (72% vs 37%). 8 A study in Wuhan showed that of 52 critically ill ICU patients infected with SARS‐CoV‐2, mortality was 61.5%; among all ICU patients with COVID‐19, organ dysfunction included acute respiratory distress syndrome (ARDS) (67%), acute kidney injury (29%), and hepatic dysfunction (29%). 9 In the ICU, the level of complexity required in the care of these patients involves the underlying disease state(s), multitudes of comorbidities, and polypharmacy.
The intent of this review is to provide health care providers with foundational knowledge on the therapies that have been proposed for use against COVID‐19. We review critical key concepts for consideration in the use of these therapies in practice, particularly in the ICU setting where the most severe patients will be managed.
1. Pharmacologic Targets for COVID‐19
Pharmacologic therapies against SARS‐CoV‐2 target its viral structure and genome (Figure 1). SARS‐CoV‐2 expresses viral proteins on its outer surface that facilitate attachment on to host cells via angiotensin‐converting enzyme 2 (ACE2). SARS‐CoV‐2 is a single‐stranded ribonucleic acid (RNA) beta coronavirus that replicates by recruiting nonstructural proteins such as 3‐chymotrypsin‐like protease, papain‐like protease, helicase, and RNA‐dependent RNA polymerase. 10 These structural components have some similarities to other known viruses such as hepatitis B, hepatitis C, and human immunodeficiency virus (HIV), prompting repurposing of currently approved antiviral therapies targeted at these viruses. Nucleoside analogs available for HIV and respiratory viruses may have a therapeutic role in blocking RNA synthesis by targeting the RNA‐dependent RNA polymerase found in SARS‐CoV‐2. Furthermore, the currently available HIV protease inhibitors have displayed some in vitro activity against the 3‐chymotryspin‐like protease found in SARS and MERS. 10
Figure 1.
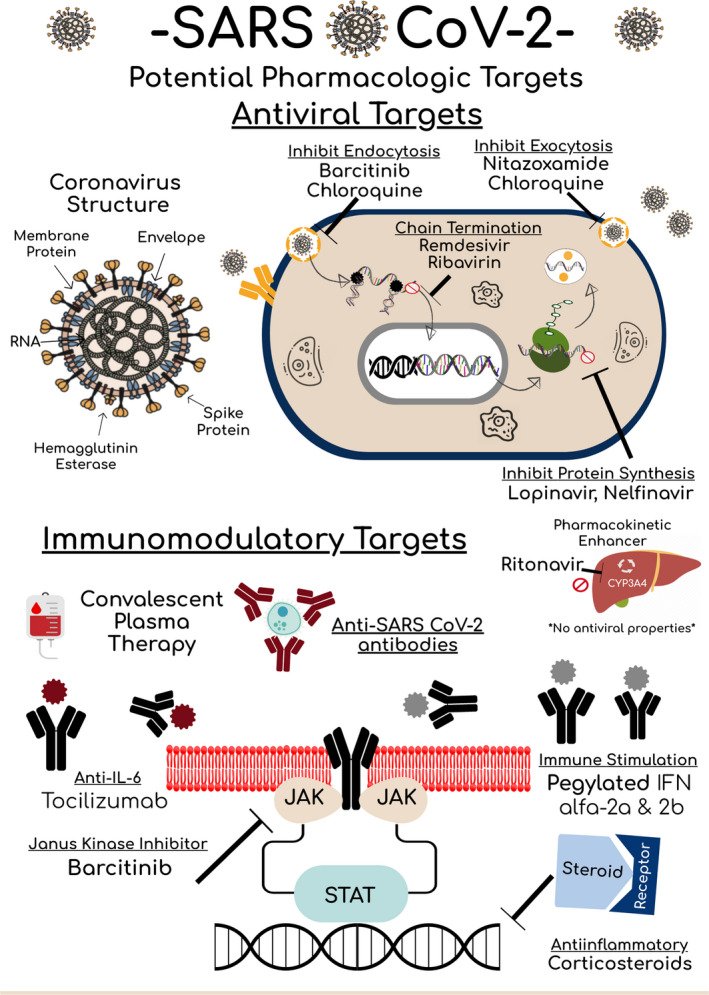
SARS‐CoV‐2 Potential Pharmacologic Targets. COVID = coronavirus; CYP3A4 = cytochrome 3A4; JAK = Janus kinase; IFN = interferon; IL‐6 = interleukin 6; RNA = ribonucleic acid; SARS‐CoV‐2 = severe acute respiratory syndrome coronavirus 2; STAT = signal transducer and activator proteins.
It is critical to be aware that currently available therapies have been specifically designed to target the viral genome of a certain virus, and the SARS‐CoV‐2 physical structure may differ, limiting in vivo efficacy. 10 Other nonstructural or accessory proteins have a role for potential therapeutic targets in development as shown in Figure 1. As opposed to directly targeting viral replication, other therapeutic approaches can be aimed at modulating the innate immune system to attack the virus or inhibit cytokines that are upregulated during viral replication to dampen the physiologic response to disease. 11 , 12 Practical considerations for current therapies available against SARS‐CoV‐2 are summarized in Tables 1 and 2 and discussed at length in the following sections.
Table 1.
Practical Considerations of Antiviral Therapies Proposed against Severe Acute Respiratory Syndrome Coronavirus 2
| Drug | Remdesivir 21 , 22 , 35 | Chloroquine 39 | Hydroxychloroquine 33 , 40 | LPV/r 44 , 55 , 93 | Ribavirin 59 | Nitazoxanide 68 | Nelfinavir 82 |
|---|---|---|---|---|---|---|---|
| Dosing | 200 mg IV on day 1, then 100 mg IV/day for 5–10 days | 500 mg by mouth twice/day for 10 days | 400 mg by mouth twice/day for 1 day, then 200 mg twice/day for 4 days | 400 mg/100 mg by mouth twice/day for 14 days |
Not established In clinical trials, 400 mg by mouth twice/day for 14 days |
500 mg by mouth twice/day Duration not defined |
Not studied in humans for SARS‐CoV‐2 |
| Formulation | IV contains β‐cyclodextrin | Oral tablet, oral solution contains propylene glycol | Oral tablet | Oral tablet, oral solution contains ethanol 42.4% a and propylene glycol | Oral tablet, oral solution contains propylene glycol, inhaled, IV not available in United States |
Controlled‐release tablet, oral suspension Formulations are not bioequivalent |
Oral tablet |
| Administration | IV over 30 min |
Administer with food Can be crushed Consider diluting when used in a feeding tube |
Administer with food Do not crush Film coating must be removed to before compounding |
Administer with or without food Do not crush tablets Do not dilute solution (risk of precipitation), administer solution down a feeding tube with milk (no water) Solution incompatible with polyurethane feeding tubes |
Administer with food Do not crush (hazardous) PPE must be worn during administration with all dosage forms |
Administer with or without food Can be crushed and mixed with food Reconstituted powder stable for 7 days |
Administer with food Can be crushed Avoid mixing with acidic foods |
| Dose adjustments |
Renal: Clcr < 30 ml/min: Not studied RRT: Not studied Hepatic: Not studied |
Renal: N/A RRT: HD = 50% dose reduction CRRT = full dose Hepatic: N/A, use with caution |
Renal: N/A RRT: N/A, use with caution Hepatic: N/A, use with caution |
Renal: N/A RRT: Avoid against once/day dosing Hepatic: Caution in severe liver impairment |
Renal: Clcr 30–50 ml/min: 50% reduction Clcr < 30 ml/min 75% reduction RRT: Not established Hepatic: Child‐Pugh class B and C not recommended |
Renal: N/A RRT: No data Hepatic: N/A |
Renal: N/A RRT: N/A Hepatic: Child‐Pugh class B and C not recommended |
| Drug interactions | None identified; metabolized to active form via intracellular kinase |
Avoid CYP2D6 inhibitors or inducers Avoid use with LPV/r Avoid antacids within 4 hrs of a dose Caution use with medications that increase QTc or cause hypoglycemia |
Avoid CYP3A4, P‐gp, OATP1B1, OATP1B3, and UGT substrates | No Interactions | No interactions | Avoid CYP3A4 and CYP2C19 inducers or inhibitors | |
| Side effects | Data limited, reports of increased LFTs, nausea, vomiting, gastroparesis, rectal bleeding | Nausea, vomiting, abdominal cramping, metallic taste, hemolysis (G6PD deficient), QTc prolongation, skin eruptions | Diarrhea, nausea, vomiting, increased serum amylase and LFTs | Hemolytic anemia, hypocalcemia, hypomagnesemia, nausea, dry cough, rashes, teratogenic | Abdominal pain, nausea, headache, urine discoloration, diarrhea, dizziness, urticaria | Diarrhea, nausea, flatulence, increase LFTs | |
Clcr = creatinine clearance; CRRT = continuous renal replacement therapy; CYP = cytochrome P450; G6PD = glucose‐6‐phosphate‐dehydrogenase deficiency; HD = hemodialysis; IV = intravenous; LFTs = liver function tests; LPV/r = lopinavir/ritonavir; N/A = no adjustment; OATP = organic anion transporting polypeptide; P‐gp = P‐glycoprotein; PPE = personal protective equipment; RRT = renal replacement therapy; SARS‐CoV‐2 = severe acute respiratory syndrome coronavirus 2; UGT = UDP‐glucuronosyltransferase.
Disulfiram‐like reactions may occur with disulfiram or other drugs that produce these reactions (e.g., metronidazole).
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Table 2.
Practical Considerations of Immunomodulatory Therapies Proposed against Severe Acute Respiratory Syndrome Coronavirus 2
| Drug | Interferon α/β 52 , 87 | Baricitinib 98 | Tocilizumab 93 , 102 , 108 |
|---|---|---|---|
| Dosing |
Not established Clinical trial: Interferon‐β‐1b 0.25 mg subcutaneous injection alternate day for 3 days |
Not studied in humans |
4–8 mg/kg IV once (maximum 800 mg) or 400 mg IV once dose Consider additional dose 8–12 hrs later if continued clinical decompensation (maximum total dose of 2 doses) |
| Formulation |
Subcutaneous injection Formulations studied a : alpha‐2b, beta‐1b |
Oral tablet | IV |
| Administration |
Injection can be performed into the abdomen or thigh Rotate injection sites |
Without regard to meals No data for compounding, may be hazardous (carcinogenic) |
IV infusion over 1 hr |
| Dose adjustments |
Renal: Clcr < 30 ml/min b RRT: Not established, may experience increased adverse effects Hepatic: Child‐Pugh class B and C not recommended |
Renal: eGFR 30–60 ml/min/1.73 m2 decrease to 1 mg/day c Clcr < 30 ml/min: Avoid RRT: N/A Hepatic: Caution in severe liver impairment |
Renal: N/A RRT: N/A Hepatic: Hold if LFTs 1‐3 × ULN (may resume when LFTs normalize) |
| Drug interactions | N/A | Avoid strong OAT 3 inhibitors | Moderate induction of CYP1A2, CYP2B6, CYP2C9, CYP2C19, CYP2D6, CYP3A4, may decrease concentration of substrates |
| Side effects | Flulike symptoms (fever, myalgias, and headaches), leukopenia, lymphopenia, depression, injection site pain, autoimmune hepatitis, and thyroiditis |
Boxed warnings: Infection, upper respiratory tract infection, malignancy, thrombosis Other: Neutropenia, elevations in platelets, LFTs, lipids, creatinine, and creatinine phosphokinase |
Infusion reactions, infection, neutropenia, thrombocytopenia, increased liver enzymes, and lipid abnormalities |
Clcr = creatinine clearance; CYP = cytochrome P450; eGFR = estimated glomerular filtration rate; IV = intravenous; LFTs = liver function tests; N/A = no adjustment; OAT = organic anion transporting; RRT = renal replacement therapy; ULN = upper limit of normal.
Inhaled studied; formulation not available in United States.
Pegylated formulation only.
Based on dose adjustments for rheumatoid arthritis.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
2. Antiviral Therapeutics
2.1. Remdesivir
2.1.1. Therapeutic Uses
Remdesivir (GS‐5734) is an investigational antiviral agent undergoing phase 3 clinical trials for the treatment of COVID‐19. Remdesivir was initially developed for the treatment of Ebola hemorrhagic fever with trials still ongoing; however, it has not been approved globally for any indication. 13 In vitro and in vivo animal data suggest activity against paramyxoviridae, filoviridae, and the coronaviridae including MERS‐CoV, SARS‐CoV, and SARS‐CoV‐2. 3 , 14 , 15 , 16
2.1.2. Mechanism of Action
Remdesivir is a 1′‐cyano‐substituted adenosine nucleotide analog. 17 As a prodrug, remdesivir is metabolized in cells and tissues to an active nucleoside triphosphate (GS‐443902) that inhibits viral RNA‐dependent RNA polymerases early in the viral infectious cycle. 18 , 19 Other potential mechanisms of this adenosine nucleotide analog may involve lethal mutagenesis and chain termination. 18 The incorporation of active nucleoside triphosphate via remdesivir at the beginning stages of replication of murine hepatitis virus in vitro had the most profound effect at 2 hours pre‐ and postinfection with a decreasing effect greater than 4 hours postinfection, suggesting a time‐dependent effect for drug activity. 18
2.1.3. Rationale for Proposed Therapy
Nucleotide analogs are used for viral RNA or DNA polymerase inhibition and have demonstrated decreased viral replication with their use. Resistance to mutagens of other medications in vitro has resulted in exo‐ribonuclease proofreading and removal. Remdesivir has shown potential to avoid this proofreading and thus removal via the exo‐ribonuclease. 18 In vitro studies in human airway epithelial cell cultures as a lung model have found activity against coronaviruses. 15 Studies assessing the potency of remdesivir were efficacious among divergent coronaviruses in human airway epithelial cells. 18 Based on the existing evidence for remdesivir activity against SARS‐CoV and similar viruses, remdesivir is being investigated for its use against SARS‐CoV‐2. In the United States, remdesivir has been used for compassionate purposes for a duration of 4–10 days. In these patients, remdesivir was continued until improvement in respiratory symptoms. 20
2.1.4. Dosing and Pharmacokinetics
Ongoing clinical trials are studying remdesivir with a loading dose of 200 mg intravenously followed by 100 mg/day intravenously for 5 to 10 days in adult patients. 21 , 22 Dosing for patients weighing less than 40 kg with a 5 mg/kg loading dose followed by a 2.5 mg/kg once/day maintenance dose has been studied for the treatment of Ebola. 23
The existing remdesivir pharmacokinetic data are extrapolated from in vitro human cell data and in vivo mouse and nonhuman primate data. Remdesivir is converted from the parent metabolite to the active metabolite, a triphosphorylated nucleoside, via anabolic intracellular kinase. 24 , 25 Mice received remdesivir subcutaneously as 50 mg/kg once/day or 25 mg/kg twice/day with a decrease in plasma concentrations of the prodrug and a steady concentration of the activated drug in the lung. 15 In the murine model, twice/day dosing maintained lung concentrations for a longer duration. In the in vitro human lung cell model and the in vivo nonhuman primate model, once/day dosing was found to reach sufficient concentrations with remdesivir demonstrating an activated nucleoside triphosphate half‐life of ~20 hours. 19 , 26
No data are available to date regarding remdesivir dosing in patients with renal or hepatic impairment. Data on clearance of remdesivir in renal replacement therapy are also not yet available. Important pharmacokinetic considerations will need to be elucidated, given that patients receiving remdesivir are likely to be critically ill with end‐organ failure. 9
2.1.5. Adverse Effects
Reported adverse effects of remdesivir after use in three patients in the United States included nausea, vomiting, gastroparesis, or rectal bleeding with no other reported symptoms. These patients also experienced elevated aminotransferase levels occurring 1–5 days after initiating remdesivir. 20 However, it is unclear whether this laboratory change is due to the drug itself or the virus because a third of critically ill patients with COVID‐19 have been observed with liver dysfunction. 9 In a study using remdesivir for Ebola virus in humans, one patient experienced hypotension during infusion of the loading dose, necessitating discontinuation; that patient ultimately died due to cardiac arrest. 27 A patient experienced neurologic complications after receiving remdesivir for Ebola treatment in a phase 1 study; however, it is not clear if this was due exclusively to having received remdesivir. 19 Currently, the data on remdesivir use in humans are too sparse to have a clear adverse effect profile for this agent. Current phase 3 randomized clinical trials will provide more conclusive insight into the adverse effects of remdesivir. 21 , 22 , 28
2.1.6. Administration Considerations
Remdesivir is manufactured as a lyophilized powder that requires reconstitution and is administered intravenously over 30 minutes. The infusion duration has also been extended over longer times to obtain higher intracellular concentrations of the active metabolite. 29 No data are available regarding compatibility of remdesivir with other intravenous medications. To date, drug‐drug and drug‐nutrition interactions have not been reported.
2.2. Chloroquine and Hydroxychloroquine
2.2.1. Therapeutic Uses
Chloroquine analogs, chloroquine phosphate and hydroxychloroquine, have been used for the prevention and treatment of malaria since the early 1900s. 30 Aside from their antimalarial properties, chloroquine analogs possess immunomodulatory effects lending to their utility for the treatment of autoimmune conditions such as systemic lupus erythematosus and rheumatoid arthritis. The multimodal properties of chloroquine analogs and their minimal toxicity profile have led to their investigation in the treatment of virus‐induced illness where the pathologic features are driven by an inflammatory response, such as in HIV‐associated immune reconstitution inflammatory syndrome. 31
2.2.2. Mechanism of Action
Chloroquine analogs are weak bases, that, in their non‐protonated form, penetrate and concentrate within acidic intracellular organelles such as endosomes and lysosomes. Once present intracellularly, chloroquine analogs become protonated and increase intravesicular pH, resulting in prevention of endosomal trafficking, dysfunctional cellular enzymes, and impaired protein synthesis. 30 Chloroquine‐mediated pH alterations have been proposed to result in early inhibition of viral replication through interference with endosome‐mediated viral entry or late transport of the enveloped virus. This mechanism has translated to the potential role of chloroquine analogs in the treatment of COVID‐19 and also appears to interfere with the terminal glycosylation of ACE2 receptor expression that prevents SARS‐CoV‐2 receptor binding and subsequent spread of infection. 32
2.2.3. Rationale for Proposed Therapy
Clinical data supporting the use of chloroquine analogs in the treatment of coronavirus have centered around several in vitro studies from the SARS‐CoV outbreak in the early 2000s with the use of chloroquine. One group tested the cytotoxic potential of chloroquine against SARS‐CoV in an in vitro cell culture. Chloroquine demonstrated a potent cytotoxic response with a 99% inhibition of viral replication at day 3. 33 Further in vitro models supported its cytotoxic response and observed that application of chloroquine before viral exposure resulted in favorable inhibition of viral spread, suggesting its potential role as an effective prophylactic intervention. 32
Hydroxychloroquine, a hydroxy derivative of chloroquine with an improved safety profile, has also been studied in vitro against SARS‐CoV‐2 with a 3‐fold higher cytotoxic potential compared with chloroquine. 34 One study demonstrated potent antiviral effect at both entry and post‐entry states at plasma concentrations of 6.90 μM. This concentration can be safely achieved in humans. 35 Clinical data in humans thus far have been limited, with the best available evidence to date from more than 100 patients from 10 different hospitals in China during the current COVID‐19 outbreak. This pooled analysis demonstrated superiority of chloroquine compared with control in inhibiting exacerbations of pneumonia, improving lung imaging findings, promoting viral negative conversion, and shortening the disease course in the absence of any adverse effects. 36 The dual mechanism of antiviral and antiinflammatory effects of chloroquine were proposed to account for its utility in preventing COVID‐19–related pneumonia. Ongoing trials for hydroxychloroquine are actively recruiting with hopes to further delineate its role in the treatment paradigm for COVID‐19. 37
2.2.4. Dosing and Pharmacokinetics
Chloroquine is formulated as a tablet in a phosphate salt form for oral administration. In the treatment of COVID‐19, expert consensus recommends a chloroquine phosphate tablet at a dose of 500 mg orally twice/day for a duration of 10 days. 38 Bioavailability after oral administration is complete (~60–100%) and rapid within 30 minutes. Chloroquine is widely distributed into nearly every organ system including the eyes, heart, kidney, liver, and lungs where retention is prolonged. 39 Chloroquine is metabolized into an active metabolite, desethyl chloroquine, via hepatic enzymes cytochrome P450 (CYP) 2C8 and CYP3A4. This metabolite accounts for a small portion of its pharmacologic activity. Chloroquine is a major substrate of CYP2D6. This enzyme accounts for ~30% of its inactivation; inhibitors of this enzyme should be avoided due to a heightened risk of accumulation and toxicity. Chloroquine is predominantly eliminated in the urine with ~50% of a dose recovered unchanged. No dose adjustments are needed if creatinine clearance (Clcr) is above 10 ml/minute; however, administration of 50% of the dose is required in patients on intermittent hemodialysis, peritoneal dialysis, or if Clcr is below 10 ml/minute. Patients receiving continuous renal replacement therapy (CRRT) should receive full‐dose chloroquine. 39
Hydroxychloroquine sulfate is available as a film‐coated tablet for oral administration. Dosing recommendations for COVID‐19 are not well‐defined, and clinical trials and in vitro data are ongoing to assist with dose optimization. Current in vitro pharmacokinetic models suggest a loading dose of hydroxychloroquine 400 mg orally twice/day on day 1, followed by 200 mg orally twice/day for 4 days. Hydroxychloroquine has similar or possibly superior cytotoxic effects against SARS‐CoV‐2 as chloroquine 500 mg twice/day. Translation of the cytotoxic effects of hydroxychloroquine in vivo remains unknown. 34 A clinical trial of hydroxychloroquine for COVID‐19 is actively recruiting, with a planned intervention of 400 mg by mouth once/day for a total duration of 5 days. 37 The major difference in dosing strategies is the utility of a loading dose. Given that the terminal half‐life of hydroxychloroquine is ~50 days in blood and remains in the body for up to 3 months, a loading dose is a reasonable approach until the clinical outcomes from ongoing trials are published. Hydroxychloroquine has rapid absorption after oral administration, a wide volume of distribution (Vd), and prolonged half‐life. Metabolism and elimination of hydroxychloroquine differ from chloroquine because reliance on CYP enzymes for elimination is minimal and clearance is not correlated with renal function. No renal or hepatic dose adjustments are recommended; however, cautious use with severe impairment is advised. Given the wide Vd (~15.5 L/hr) and high lipophilicity of the compound, CRRT is likely to have minimal impact on drug removal.
2.2.5. Adverse Effects
Chloroquine and hydroxychloroquine are generally well tolerated. The most common adverse effects are gastrointestinal intolerances such as nausea, vomiting, abdominal cramping, and a metallic taste. Acute toxicity in the setting of supratherapeutic dosing may manifest as cardiomyopathy, neuropathy, or myopathy. Retinopathy, a hallmark toxicity of hydroxychloroquine and chloroquine, is predominantly associated with high doses, long‐term administration (i.e., hydroxychloroquine for systemic lupus erythematosus or rheumatoid arthritis), and are unlikely of concern in the setting of COVID‐19 treatment. Although the manufacturer’s labeling of chloroquine and hydroxychloroquine cautions against their use in patients with glucose‐6‐phosphate dehydrogenase (G6PD) deficiency, only limited data support this risk, and no incidence of hemolytic anemia was seen in patients with G6PD in 30 years of drug exposure. 39 , 40 Both agents are safe in pregnancy with minimal risk of congenital malformations. 41 Albeit rare, use in patients with preexisting cardiac disease or QTc prolongation should be avoided due to the risk of torsades de pointes.
Concurrent administration of QTc prolonging agents and strong 2D6 inhibitors (chloroquine only) should be avoided to minimize cardiac adverse effects. Notable dermatologic adverse effects can include photosensitivity, lichenoid reactions, and drug eruptions with chloroquine, and acute generalized exanthematous pustulosis with hydroxychloroquine. 39 , 40 In postmarketing data, case reports of hypoglycemia progressing to loss of consciousness occurred with these agents in patients with a history of diabetes on concurrent antidiabetic agents. 39 , 40 The proposed mechanism of hypoglycemia involves inhibited insulin degradation. Patients have required up to a 30% reduction in insulin doses with concurrent hydroxychloroquine administration. 42 Baseline and routine monitoring of blood glucose and electrocardiogram for the QTc interval are recommended for patients on chloroquine analog therapy.
2.2.6. Administration Considerations
To mitigate nausea and vomiting, it is recommended to administer chloroquine and hydroxychloroquine with food. Chloroquine can be crushed and mixed with flavored syrups or enclosed in gelatin capsules to mask the bitter taste. 39 In patients who are mechanically ventilated, chloroquine can be extemporaneously compounded into oral suspension. Hydroxychloroquine tablets should not be crushed or split at the bedside for administration but instead require removal of the film coating to be compounded extemporaneously into a solution for administration via enteral tubes. Neither agent is available for intravenous administration and would be an unfavorable option in patients who are not permitted to take anything by mouth or have no enteral access.
Administration of antacids within 4 hours of chloroquine should be avoided due to a potential for chelation and reduced bioavailability. This interaction is not present with hydroxychloroquine. A theoretical drug‐drug interaction exists between proton pump inhibitors (PPIs) and chloroquine analogs because the blockage of H+, K+ −ATPase by PPIs in lysosomes can raise the pH and reduce drug accumulation that could weaken immunomodulatory effects. However, effects of this interaction in vivo are unknown. 39 , 40
2.3. Lopinavir/Ritonavir
2.3.1. Therapeutic Uses
Lopinavir (LPV) is an aspartic acid protease inhibitor developed for the treatment of HIV. Lopinavir is co‐formulated with ritonavir (LPV/r) to boost the pharmacokinetic activity and half‐life of LPV through inhibition of CYP450. LPV/r provides potent and sustained viral load reductions for patients with HIV, which has prompted interest in assessing its efficacy in other viruses that function with similar machinery. 43
2.3.2. Mechanism of Action
Proteases are essential enzymes for the production and maturation of viral genomes. The primary antiviral effect of protease inhibitors is prevention of viral replication, thus limiting spread into host cells. 43 The therapeutic rationale for LPV/r arises from in vitro studies demonstrating inhibition of the 3‐chymotrypsin‐like protease found in novel coronaviruses. 44 , 45 However, LPV was specifically designed to match the structure of the C2 catalytic site within the HIV aspartic acid protease. SARS‐CoV‐2 protease is a cysteine protease family and structurally dissimilar because it lacks a C2 catalytic site. 10
2.3.3. Rationale for Proposed Therapy
The therapeutic value of LPV/r emerged from in vitro studies testing its utility for SARS and MERS. In vitro testing of LPV/r was performed for severe MERS‐CoV in marmosets. Lopinavir/ritonavir improved clinical scores for weight loss and symptom burden, improved radiologic clearance, and decreased viral loads in necroscopied lungs. 45 The benefit of LPV/r has yet to be clearly replicated in human cell cultures, with some studies showing complete lack of in vitro activity. 46 Clinical in vivo studies from SARS and MERS outbreaks demonstrated that the use of LPV/r 400–100 mg by mouth twice/day combined with ribavirin with or without corticosteroids initiated during the early phase of viral acquisition reduced rates of ARDS or death when compared with a matched cohort that received supportive care or ribavirin alone. 44 , 47 , 48 However, these findings did not remain significant if LPV/r was initiated later in the disease course, reiterating the lack of pharmacologic effect against a viral genome that has been integrated into host DNA. 48
Available literature to date for LPV/r in the treatment of COVID‐19 stems from a descriptive case series of five patients in Singapore receiving LPV/r 200–100 mg twice/day for 14 days. Three patients had reductions in ventilation requirements within 3 days of treatment initiation, but two had progressive respiratory failure. Despite the lower dosing regimen compared with previous studies, four of the five patients had premature treatment discontinuation secondary to nausea, vomiting, diarrhea, and increased liver function tests. No deaths occurred in the study, but efficacy outcomes are difficult to interpret based on the lower dosing strategy compared with previous data. This does raise concerns about the potential tolerability of the higher dose regimen. 49
Another retrospective cohort showed that LPV/r has limited influence on the duration of viral shedding that can occur for a median of 20 days after initially contracting the virus. 49 , 50 In a recent randomized controlled open‐label trial including hospitalized patients with SARS‐CoV‐2 infection, patients receiving LPV/r 400−100 mg twice/day for 14 days versus standard of care found no benefit versus standard of care treatment. 51 Multiple ongoing studies have evaluated the clinical utility of LPV/r as monotherapy and in combination with other therapies such as ribavirin and interferon (IFN). 52 , 53 , 54
2.3.4. Dosing and Pharmacokinetics
Lopinavir/ritonavir is available as a single‐tablet formulation (Kaletra) in dosage strengths of 400/100 mg or 200/100 mg. Given the largest available evidence from in vitro studies, if LPV/r is used as an adjunctive agent for COVID‐19, a dose of 400 mg/100 mg by mouth twice/day for 14 days is recommended. The drug is highly protein bound (~98%), and its primary pathway for elimination is through the hepatobiliary system. No dose adjustments are required for renal impairment. In patients receiving intermittent hemodialysis, however, once/day dosing is not recommended when indicated for treatment of HIV. This does not influence use in COVID‐19 because the recommended schedule is twice/day. 43 No dosage adjustments are recommended in hepatic impairment, but caution should be advised if used in patients with advanced liver disease. 55 Lower dosages of 200/100 mg twice/day can be considered where adverse effects limit tolerability, with the understanding that lower doses may not substantially mitigate toxicities. Based on information collected by the Antiretroviral Pregnancy Registry, an increased risk of teratogenic effects has not been observed in humans. The Health and Human Services perinatal HIV guidelines consider LPV/r a therapeutic option for pregnant women living with HIV and can be continued if conception occurs during therapy. 56
The considerable potential for drug‐drug interactions with LPV/r requires a thorough review of concomitant medications. Ritonavir is a strong inhibitor of CYP3A4, a major enzyme responsible for the metabolism and elimination of many medications. In addition, ritonavir is a moderate inhibitor of p‐glycoprotein (P‐gp), organic anion transporter (OATP1B1), OATP1B3, and is an inducer of UDP‐glucuronosyltransferase. 55 Patients requiring ICU admission for COVID‐19 are often elderly with multiple comorbidities and polypharmacy. The potential therapeutic benefit of LPV/r should be weighed against considerable interactions with commonly used medications in the ICU (e.g., midazolam, fentanyl, antiarrhythmics). 3
2.3.5. Adverse Effects
Treatment with LPV/r may result in intolerable gastrointestinal toxicities, although administration with food may ameliorate the severity of these symptoms. Up to 24% of patients experience diarrhea, although it usually improves within 2 weeks. 55 Adverse events particularly relevant to ICU patients include potentially fatal pancreatitis, hepatitis, hepatic decompensation among those with preexisting liver disease, and increased PR intervals, especially in those with congenital QTc prolongation. 55
Previous studies using LPV/r for SARS demonstrated increased serum amylase and liver enzyme elevations compared with a matched cohort. Routine monitoring of liver function tests is critical, and supportive care with antiemetics and antimotility agents should be considered. 44 Cardiovascular and metabolic toxicities such as lipid abnormalities are commonly observed in patients with HIV receiving these therapies for prolonged periods; these are less likely when used for the abbreviated durations for COVID‐19.
2.3.6. Administration Considerations
Lopinavir/ritonavir tablets should not be chewed or crushed for patients unable to swallow. 55 In patients who are intubated or have limited oral access, an oral suspension is available for delivery via a nasogastric tube. 55 Of note, the suspension does contain 42.4% ethanol. Available data suggest administration with enteral nutrition does not adversely impact drug concentrations. 57 Coadministration with food that contains a moderate to high fat content can enhance LPV/r bioavailability. 55
2.4. Ribavirin
2.4.1. Therapeutic Uses
Ribavirin is a purine nucleoside analog that elicits its antiviral affect through inhibition of viral RNA synthesis. Ribavirin is a prodrug that undergoes metabolic conversion by the liver, after which its metabolic structure closely mimics the purine analog guanosine that enhances its incorporation into RNA. The structural elements prohibit the subsequent addition of nucleoside analogs, effectively halting the synthesis of RNA. 58 Ribonucleic acid is ubiquitous in many viruses, which is why ribavirin has been studied in a broad range of viral diseases including hepatitis B, C, and respiratory syncytial virus. 59
2.4.2. Rationale for Proposed Therapy
The nonselective antiviral properties of ribavirin prompted investigators to evaluate its use during the SARS and MERS outbreaks. During the initial SARS outbreak, ribavirin was used throughout Hong Kong in both oral and intravenous formulations. 60 However, the distribution of the intravenous formulation, which is not available in all countries including the United States, was restricted in the midst of the epidemic given the concern for lack of in vitro susceptibility and considerable toxicity. In 126 patients treated with ribavirin, hemolysis and anemia occurred in up to 76% and 49% of cases, respectively. 61 , 62 In vivo data suggest serum concentrations of ribavirin needed to blunt viral replication effectively is higher than what is safely achievable in humans. 61 In vitro data suggest rapid resistance against SARS and MERS when used as a monotherapy but potential activity when combined with other antivirals such as LPV/r or chloroquine analogs. 38 , 63 Outcomes for ribavirin use in combination with interferon (IFN)‐α‐2a for MERS‐CoV is discussed in the IFN section.
2.4.3. Dosing and Pharmacokinetics
Ribavirin is commercially available as an oral capsule, oral solution, and inhaled formulation. 59 The inhaled formulation has not been studied for SARS, MERS, or COVID‐19, and the intravenous form is not available in the United States. Oral ribavirin has been dosed as a 4‐g loading dose followed by 1.2 g every 8 hours in two small studies for SARS. 44 , 64 In the management of COVID‐19, data are limited to ongoing studies using a dosing strategy of 400 mg by mouth twice/day for 14 days as a part of a combination regimen.
Ribavirin has a prolonged half‐life of ~40 days. Loading doses may aid in achieving steady state more rapidly. 65 Ribavirin is metabolized to its active form via extrahepatic enzymes. 59 However, its use in hepatic impairment Child‐Pugh class B and C is not recommended given that it is metabolized by CYP enzyme pathways. Ribavirin is eliminated primarily by renal excretion, requiring strict dose reductions for renal insufficiency that varies based on indication. 59 A review on the use of ribavirin in SARS recommended a 50% dose reduction for Clcr between 30 and 50 ml/minute, and a 75% reduction for Cl of below 30 ml/minute. 65 There is no established safety of ribavirin in patients receiving renal replacement therapy; it should be restricted to those with stable renal function.
2.4.4. Adverse Effects
Ribavirin carries a boxed warning for hemolytic anemia. 59 In a study assessing the adverse effects associated with the use of ribavirin use for SARS, the most common adverse effects were hemolytic anemia (61%), hypocalcemia (58%), and hypomagnesemia (46%). 66 The onset of hemolytic anemia occurs as early as 3–5 days after therapy with high doses (above 1–2 g) that were used as loading doses for coronavirus. 44 , 67 The risk is highest in patients who are elderly or have poor renal function, but consideration should also be taken in patients with preexisting cardiac disease where reduction in hemoglobin increases the risk of decompensation. 59 Ribavirin is a teratogen with a significant potential for embryonic toxicity and contraindicated in women who are pregnant and in male partners of women who are pregnant. If ribavirin is considered in women or men of childbearing potential, use of systemic and barrier contraception must be strictly enforced. Because active metabolites of ribavirin persist in plasma for up to 6 months, contraception is required both during treatment and for at least 6 months after treatment is completed. 59
Ribavirin does not have drug‐drug interactions because its metabolism and elimination does not rely on the CYPP450 system. However, certain therapies should be used cautiously with ribavirin due to the potential for enhanced toxicities. Ribavirin in combination with other immunosuppressive therapies, particularly azathioprine or IFN, can lead to severe pancytopenia. 59
2.4.5. Administration Considerations
Ribavirin administered orally as a solution or capsule has an absolute bioavailability of 40–50% compared with the intravenous or as an aerosol formulation. 59 Oral formulations have enhanced bioavailability when taken with a high‐fat meal, and it is recommended to administer both oral formulations with food. Due to the teratogenic potential of ribavirin, it was identified as a hazardous medication on the National Institute for Occupational Safety and Health (NIOSH) list and requires significant safety precautions with its use. The capsules should not be opened, crushed, or broken. 59 Health care providers handling ribavirin must also follow NIOSH recommendations to wear gloves when packaging, administering, or receiving intact capsules. When handling the solution, double gloving, a protective gown, and eye/face protection is required, especially for administration of the oral liquid through a feeding tube.
2.5. Nitazoxanide
2.5.1. Therapeutic Uses
Nitazoxanide is a 2‐(acetyloxy)‐N‐(5‐nitro‐2‐thiazolyl) benzamide with an approved Food and Drug Administration (FDA) indication for its antiprotozoal activity to treat diarrhea caused by Giardia parvum and Giardia lamblia. 68 Nitazoxanide has since been found to have activity against Cryptosporidium and in vitro activity against anaerobic gram‐positive and gram‐negative bacteria. 69 , 70 Another studied use of nitazoxanide was for the treatment of influenza. 71 When nitazoxanide was studied for severe acute respiratory illness, no benefit was seen in clinical outcomes, specifically length of hospital stays. 72
2.5.2. Mechanism of Action
Nitazoxanide interferes with pyruvate ferredoxin oxidoreductase enzyme‐dependent electron transfer, thus impairing anaerobic energy metabolism of protozoa. 68 Nitazoxanide is metabolized to its active circulating metabolite tizoxanide. 73 Tizoxanide selectively blocks the posttranslational influenza viral hemagglutinin maturation and intracellular movement in addition to blocking protein implantation into the plasma membrane. 74 Nitazoxanide may potentiate the production of type 1 IFNs produced by host cell fibroblasts that may potentiate antiviral activity via hemagglutinin inhibition. 75
2.5.3. Rationale for Proposed Therapy
Nitazoxanide has demonstrated in vitro activity against MERS‐CoV. 71 An anti‐coronavirus effect was observed with nitazoxanide in an in vitro screen of small molecules against a recombinant murine coronavirus. 76 In vitro studies of canine coronavirus found that the use of nitazoxanide inhibited viral replication. 71 Based on this in vitro animal data, it is thought that nitazoxanide may have activity against SARS‐CoV‐2. Human data are limited regarding the efficacy of nitazoxanide against human coronavirus. In a 260‐patient double‐blind placebo‐controlled trial in patients with influenza‐like illness, five participants who received nitazoxanide were coronavirus positive. 72 Subgroup analysis of these five coronavirus‐positive patients found no difference in the primary outcome of days of hospitalization (p=0.61).
2.5.4. Dosing and Pharmacokinetics
The recommended nitazoxanide dosing for Cryptosporidium parvum or G. lamblia is 500 mg oral suspension or tablet every 12 hours for 3 days. 68 The dosing used for acute respiratory illness was twice/day for 5 days, and dose strength was based on age. Patients 12 years of age and older received two 300‐mg tablets, children 4–11 years old received 200 mg of the suspension, and toddlers 1–3 years old received 100 mg of the suspension. 72
It is recommended that nitazoxanide be administered with food because pharmacokinetic studies found increased area under the curve (AUC) and maximum concentration (Cmax) of tizoxanide. 68 Specifically, a doubling of AUC and 50% increase of Cmax was observed when the tablet was administered with food. When the suspension was administered with food, there was a 45–50% increase in AUC and up to a 10% increase in Cmax. An important consideration is that the oral suspension is not bioequivalent to the tablet. The suspension has a bioavailability of 70%, necessitating dose adjustments when transitioning between these dosage forms. 68 In plasma, tizoxanide is more than 99% bound to proteins. Metabolism of nitazoxanide to tizoxanide occurs via hydrolysis and then conjugation via glucuronidation. Nitazoxanide has not been studied in patients with impaired renal and hepatic function, and no dosage adjustment recommendations exist. 68 Data in geriatric and pediatric populations are not available.
2.5.5. Adverse Effects
The most common adverse effects of nitazoxanide are gastrointestinal, mainly abdominal pain, nausea, and headache. Patients may also experience discoloration of eyes and urine, diarrhea, dizziness, gastroesophageal reflux disease, skin rash, or urticaria. Compared with patients receiving standard of care and placebo, patients receiving nitazoxanide did not have additional adverse effects, and both groups of patients experienced gastrointestinal and respiratory effects. 72 Headache and diarrhea were reported most in patients receiving placebo versus low‐dose nitazoxanide versus high‐dose nitazoxanide for treatment of viral respiratory infection with no significant difference among all three groups. 77
2.5.6. Administration Considerations
Nitazoxanide is available in dosage forms administered enterally including a controlled‐release film‐coated tablet and oral suspension. Nitazoxanide tablets can be crushed and mixed with food. There are no existing data regarding the absorption of nitazoxanide when administered via gastric feeding tubes. However, if gastric feeding tube administration is needed, the oral suspension can be used.
Drug‐drug interactions with nitazoxanide are not attributed to cytochrome CYP enzymes because in vitro metabolism data found no significant effect of tizoxanide on these enzyme pathways. 68 Most notably, nitazoxanide may have binding site competition with other highly protein‐bound drugs. A narrow therapeutic index drug that would be of concern in this case is warfarin. However, a pharmacokinetic study in which patients received nitazoxanide twice/day for 6 days with a onetime warfarin 25 mg dose compared with patients receiving warfarin did not result in pharmacokinetic or dynamic changes and was well tolerated. 78
2.6. Nelfinavir
2.6.1. Therapeutic Uses
Nelfinavir is a HIV‐1 protease inhibitor originally developed for use in combination with antivirals that target HIV via alternative mechanisms. With development of newer combination antivirals for HIV, nelfinavir has fallen out of favor for this indication. 79
2.6.2. Mechanism of Action
Nelfinavir binds to the site of HIV‐1 protease activity and inhibits cleavage of viral Gag‐Pol polyprotein precursors into functional proteins that are needed for HIV. What remains are nonviable HIV particles that are no longer infectious.
2.6.3. Rationale for Proposed Therapy
During the 2002 SARS outbreak, nelfinavir was identified as a potential agent with activity against SARS‐CoV based on in vitro studies. 80 Nelfinavir was found to inhibit the replication of SARS‐CoV in Vero E6 cells originating from the African green monkey. Most recently, nelfinavir was identified via homology models based on three‐dimensional SARS structures specifically at the main protease or chymotrypsin‐like protease. 81 These models assessed the ability of small molecule drugs to dock to the SARS structures. The SARS‐CoV was used because its sequence is 96% similar to that of SARS‐CoV‐2. Of 30 drugs assessed, nelfinavir was found to have the most promising activity against SARS‐CoV‐2.
2.6.4. Dosing and Pharmacokinetics
Nelfinavir has not been studied in humans for its efficacy against SARS‐CoV or SARS‐CoV‐2. The recommended dosing of nelfinavir for the treatment of HIV is 750 mg by mouth 3 times/day or 1250 mg by mouth twice/day. The half‐life of nelfinavir is 3.5–5 hours. The effective dose for treatment of COVID‐19 is unknown. Nelfinavir dosage adjustments do not need to be made with renal impairment because less than 2% of nelfinavir is excreted in the urine. 82 For patients with hepatic impairment, nelfinavir is not recommended in patients with Child‐Pugh class B or C. 79 , 83 , 84 Pharmacokinetics in patients using nelfinavir with chronic liver disease may be unpredictable. 85 No dosage adjustments are recommended for use in patients with Child‐Pugh class A impairment.
Nelfinavir is metabolized by CYP3A4 and CYP2C19 and could potentially interact with medications that are inhibitors or inducers of these enzymes. In vitro data found that only CYP3A enzymes were inhibited by nelfinavir at therapeutic concentrations. 82
2.6.5. Adverse Effects
The major adverse effect of nelfinavir is gastrointestinal intolerance, primarily diarrhea, nausea, and flatulence. A nonspecific rash may also occur. The incidence of other side effects are minimal, occurring less than 2% of the time in phase 2 and 3 trials. 82
2.6.6. Administration Considerations
Nelfinavir is available in a tablet dosage form. The 250‐mg tablets can be dissolved in water and readily dissolve. For patients with a gastric tube as enteral access, nelfinavir can be crushed, dissolved in water, and administered via gastric tube. After dissolving, nelfinavir should be taken or administered immediately. Notably, nelfinavir may have a bitter taste if mixed with acidic food or juice. 82
Absorption of nelfinavir was found to result in less pharmacokinetic variability and higher nelfinavir levels when taken with food. Nelfinavir has a volume of distribution of 2–7 L/kg and is highly protein bound (more than 98%). 82
3. Immunomodulating Therapeutics
3.1. Interferon‐α
3.1.1. Therapeutic Uses
Interferons are endogenous signaling proteins released by host cells during response to infections or inflammation. Upregulation of IFNs stimulates the immune system to blunt viral replication and eradicate offending pathogens. 86 Two IFNs mediate host immune responses: α and β. Interferon‐α elicits a potent host‐mediated immune cell response that has generated interest in the treatment of viral diseases such as hepatitis B and C and various cancers such as melanoma and chronic lymphocytic leukemia. Interferon‐β has been primarily used in the treatment of the autoimmune condition multiple sclerosis. 87 These nonspecific immunomodulatory effects have gained attraction by investigators for other viral illnesses including COVID‐19.
3.1.2. Mechanism of Action
During the 2002 SARS outbreak, it was observed that after viral inoculation, SARS‐CoV was able to effectively evade upregulation of IFNs in human macrophages. This allowed for continued viral replication due to suppression of the innate immune response. 87 Two IFN products are available, α and β. It is currently unknown whether both products would influence coronavirus disease expression. Interferon‐α has the most studies related to its use.
3.1.3. Rationale for Proposed Therapy
Thus far, human data for the use of IFN‐α for novel coronaviruses are sparse. During the SARS outbreak, pegylated IFN‐α was assessed in an in vitro analysis of macaque cells at different periods of SARs exposure. 88 When used before SARS inoculation, IFN‐α reduced viral replication and excretion. However, these results were not observed post‐SARS exposure. 88 An in vitro analysis assessing IFN‐α as monotherapy for MERS demonstrated the serum concentrations required to effectively inhibit viral replication were higher than what can be safely achieved in humans. 63 Nevertheless, in vitro isolates of IFN‐α or ‐β as a multimodal regimen with ribavirin for SARS displayed potent synergy against the virus. 89 , 90 In vivo studies have yet to be able to replica the same benefits, with some studies showing no influence on the disease course for MERS, whereas others suggest a small improvement in survival at 14 days but not 28 days when used in combination with ribavirin. 91 , 92 These viruses may display different in vitro and in vivo susceptibilities than the current SARS‐CoV‐2; extrapolation to the current COVID‐19 pandemic is difficult. This has not dampened the potential application of this therapy, and investigators await the results of ongoing studies assessing the efficacy of IFN‐α‐2b as a part of combination therapy with ribavirin for COVID‐19 to further elucidate any benefit from IFN treatment. 93 , 94
3.1.4. Dosing and Pharmacokinetics
Interferon‐α‐2b also comes as a pegylated form to prolong its half‐life from 4.6 hours to 22–60 hours, permitting a lower dosing frequency. 87 Due to the lack of established human data with IFNs for COVID‐19, this therapy should only be considered for COVID‐19 as a part of a clinical trial. There is no established dosing regimen for IFN in the treatment of COVID‐19. The only in vivo data are with IFN‐α‐2a for the treatment of MERS using a dose of 180 µg per week for 2 weeks, similar to hepatitis B virus. 92 There is no established dosing regimen for IFN in the treatment of COVID‐19. The only study currently recruiting at the time of this publication is using IFN‐β‐1b at a dose of 0.25 mg (8 million IU) on day 1, 3, and 5 in combination with other therapies (ribavirin, LPV/r). 93 Additional studies evaluating IFN‐α‐1b are evaluating its utility as an inhaled therapy. 94
If used as a part of a trial, there are key clinical considerations to consider for safe use. The drug labeling provides a cautionary statement for use in Child‐Pugh class B or C. 87 Renal elimination accounts for 30% of total clearance dose reductions required for patients with a Clcr below 50 ml/minute when IFN‐α is used in combination with ribavirin for hepatitis C. 87 Given that these agents are both under investigation for COVID‐19, if used as a part of combination therapy, the same precaution should be considered. Interferon therapies have an increased risk of adverse effects when used during renal replacement therapy; thus caution is recommended in this population. 95 , 96
Interferon does not pose a risk of drug‐drug interactions through CYP enzyme pathways. Concomitant medications that enhance the myelosuppressive or hepatotoxic effects of IFN should be used sparingly. 87
3.1.5. Adverse Effects
Interferons are associated with significant adverse reactions, even when used as a short‐term therapy. Injection site reactions resulting in flulike symptoms (fever, myalgias, and headaches) occur within the first 2–8 hours after administration. Rotation of injection sites, application of ice before and after application, and premedication with acetaminophen can mitigate these reactions. Additional adverse reactions include leukopenia, lymphopenia, autoimmune hepatitis, and thyroid disease. Patients exposed to IFNs for currently approved indications can develop neutralizing antibodies that reduce the therapeutic efficacy of this agent. The development of neuropsychiatric adverse effects is not expected to occur with short‐term use. 97 The safety of IFN in pregnancy has not been established. 87
3.1.6. Administration Considerations
Interferon is available in injectable and inhaled formulations. The current study is enrolling patients using β‐1b injected subcutaneously in the abdomen or thigh. 93
3.2. Baricitinib
3.2.1. Therapeutic Uses
Baricitinib is a Janus kinase (JAK) inhibitor originally developed for the treatment of rheumatoid arthritis. It is approved by the FDA for moderate or severe active rheumatoid arthritis in patients who have not responded to one or more tumor necrosis factor antagonists.
3.2.2. Mechanism of Action
Baricitinib reversibly and selectively inhibits JAK1 and JAK2 with less inhibition of JAK3 and tyrosine kinase. Inhibition of JAK results in inability of signal transmission from cytokine or growth receptors resulting in decreased hematopoiesis and immune cell function. 98 This inhibition of signal transmission prevents phosphorylation and thus activation of signal transducers and activators of transcription. Baricitinib has a high affinity for associated protein kinase 1 (AAK1) binding and could prevent the intracellular passage of viral cells and development of viral particles. 99
3.2.3. Rationale for Proposed Therapy
COVID‐19 may result in secondary hemophagocytic lymphohistiocytosis (sHLH) as a result of viral infection. 100 Patients with COVID‐19 have exhibited ferritin and inflammatory marker elevations similar to sHLH including elevated interleukin (IL)‐2, IL‐7, granulocyte stimulating factor, and tumor necrosis factor (TNF)‐α. Evaluation of risk factors for mortality of COVID‐19 cases in Wuhan found ferritin and IL‐6 levels to be higher in those who ultimately died versus survivors (p<0.001). 101 Considering the potential for increased lung injury with corticosteroid use in patients with COVID‐19 and reported use of tocilizumab for COVID‐19, JAK‐STAT inhibitors were theorized to have an effect on the hyperinflammatory state that occurs in COVID‐19. Baricitinib may also prevent endocytosis and viral infection by inhibiting AAK1 activity. Baricitinib has not been studied in humans for COVID‐19.
3.2.4. Dosing and Pharmacokinetics
Baricitinib is typically dosed as 2 mg/day by mouth for rheumatoid arthritis. 98 No data exist for treatment of COVID‐19; however, it is proposed that a 7‐ to 14‐day course would be needed. 102 Dose adjustments are recommended for patients with lymphopenia, neutropenia, and anemia. 98 It is recommended to hold baricitinib in patients with an absolute lymphocyte count less than 500 cells/mm2, an absolute neutrophil count less than 1000 cells/mm2, and hemoglobin less than 8 g/dl. In patients with hepatic impairment, baricitinib is not recommended. Dose adjustments are recommended with renal impairment. For patients with an estimated glomerular filtration rate (eGFR) less than 30 ml/minute/1.73 m2, it is recommended to hold baricitinib. For patients with an eGFR 30–60 ml/minute/1.73 m2, it is recommended to decrease dose to 1 mg once/day. There are no dosing recommendations for patients requiring CRRT.
Baricitinib reaches peak concentrations within 1 hour of oral administration with steady state achieved at 2–3 days. 98 Baricitinib has an oral bioavailability of 80% and is not significantly impacted by taking with or without food. The baricitinib volume of distribution is 76 L; it is 50% bound to plasma proteins and 45% bound to serum proteins. It is a substrate of P‐gp, breast cancer resistance protein OAT, and multidrug and toxin extrusion protein transporters. Metabolism of baricitinib occurs mainly by CYP3A4, and elimination is ~75% renal.
Relevant drug‐drug interactions with baricitinib include strong OAT 3 inhibitors that may increase baricitinib exposure. 98 For patients concomitantly taking strong OAT 3 inhibitors, the recommended dose of baricitinib is 1 mg once/day.
3.2.5. Adverse Effects
Several boxed warnings exist for baricitinib including risk of infection, particularly upper respiratory tract infection, malignancy, and thrombosis. 98 Of note, these side effects were higher in patients receiving the 4‐mg dose of baricitinib that is not a recommended dose due to these safety concerns. Patients in clinical trials also experienced increased incidence of neutropenia, platelet elevations, aminotransferase elevations, lipid elevations, creatinine elevations, creatine phosphokinase elevations, and nausea. Monitoring for long‐term treatment of rheumatoid arthritis is recommended with baseline complete blood counts including neutrophils, liver function tests, and lipids. 98 Monitoring for signs and symptoms of infection while treating with baricitinib is recommended in addition to skin examinations in patients at an increased risk for skin cancer.
3.2.6. Administration Considerations
Baricitinib is available as a film‐coated immediate‐release tablet for enteral administration. 98 It can be given with or without consideration of food. No data are available regarding crushing baricitinib and administration via gastric feeding tubes. Although baricitinib is not on the NIOSH list of medications, it is carcinogenic by virtue of the adverse effect profile, and it may be treated as a hazardous drug.
3.3. Tocilizumab
3.3.1. Therapeutic Uses
Tocilizumab is a monoclonal antibody with FDA approval for chimeric antigen receptor (CAR)‐T cell‐induced cytokine release syndrome (CRS), giant cell arteritis, rheumatoid arthritis, and polyarticular or systemic juvenile idiopathic arthritis. 103
3.3.2. Mechanism of Action
Evolving data suggest patients with severe COVID‐19 experience significant lung injury secondary to a surge of inflammatory cytokines, resulting in a cytokine storm. Viral replication activates the innate immune system to secrete various signaling proteins such as ILs that results in hyperinflammation and further lung damage. Interleukin‐6 is a key inflammatory protein involved in this pathway. 100 Tocilizumab binds to IL‐6 receptors, thereby blunting cell signaling and effectively downregulating the excess inflammatory response. 103
3.3.3. Rationale for Proposed Therapy
The rationale for use of tocilizumab in COVID‐19 stems from data discovered with SARS and MERS. The SARS patients were noted to have CRS as a result of proinflammatory marker release including IL‐6 and TNF‐α. 104 Patients with MERS were discovered to have elevations of IL‐6, IL‐8, and IL‐1β. 105 The data from Wuhan in critically ill patients with COVID‐19 have also found increased levels of cytokines including IL‐6 and granulocyte‐colony stimulating factor. 8 , 106 Lung tissue obtained postmortem from a patient with COVID‐19 showed elevated inflammatory factors suggestive of a cytokine storm. 107 Interleukin‐6 may be a key driver of the robust inflammatory response within the lungs of ICU patients with COVID‐19. Recently published data from Wuhan indicate that tocilizumab added to lopinavir, methylprednisolone, and oxygen therapy in 20 patients with severe COVID‐19 resulted in rapid reductions in fever in all patients, improvement in oxygenation for 75%, and facilitated discharged from the hospital in 95% of patients. 108 Additional studies are underway with tocilizumab combined with other antivirals or compared against renal replacement therapy for management of CRS associated with COVID‐19. 109 , 110 Sarilumab, another anti‐IL‐6 antibody, is currently under evaluation for the treatment of COVID‐19. It will be studied against supportive care measures and provide enhanced insight into the utility of targeting this pathway. 111
3.3.4. Dosing and Pharmacokinetics
Tocilizumab for most indications is weight based with a maximum dose of 800 mg. The dosing of tocilizumab for COVID‐19 is not well established. When used in a case series of patients with COVID‐19, a onetime dose of intravenous tocilizumab 400 mg was administered. 108 , 112 For CRS secondary to chimeric antigen receptor CAR T‐cell therapy, three additional doses of tocilizumab can be given with at least 8 hours between each dose. In the COVID‐19 reports, three patients received an additional dose of tocilizumab 12 hours later as a result of continued fevers. 108
Tocilizumab AUC varies based on body weight, and the Vd of intravenous tocilizumab at steady state is 6.4 L. 103 There are no published data regarding the use of tocilizumab 400 mg in obese patients with COVID‐19 and whether the 400‐mg dose is adequate. The half‐life of tocilizumab is concentration dependent, at 11 days when dosed 4 mg/kg and 13 days when dosed 8 mg/kg. Tocilizumab should be held when the absolute neutrophil count is 1000 cells/mm2 or below, platelet count is 100,000 cells/mm2 or below, or liver enzymes are more than 3–5 times the upper limit of normal. This is particularly relevant for the treatment of COVID‐19 that was demonstrated to elevate liver enzymes in severe cases. 113 Tocilizumab elimination is not influenced by renal dysfunction. No dose adjustments are required. In patients receiving tocilizumab for COVID‐19, only one patient had a history of chronic kidney disease, and no patients had reported hepatic impairment. 108
3.3.5. Adverse Effects
Limited efficacy data with tocilizumab should be weighed against its significant cost and potential for toxicities. Secondary to interference with the host immune response, infections ranging from bacterial, viral, and fungal sources may develop. 103 Patients should be screened for active or latent tuberculosis infection before administering tocilizumab. Gastrointestinal perforation after the use of tocilizumab was reported in patients with a history of diverticulitis or receiving high‐dose corticosteroids. 103 Infusion reactions causing hypertension, headache, and skin reactions within 24 hours of administration were reported after administration of tocilizumab. 103
3.3.6. Administration Considerations
Tocilizumab is recommended to be administered intravenously over 1 hour. 103 , 112 Tocilizumab should not be administered in the same line with other medications because it has not been studied for compatibility. 103
Pertinent drug‐drug interactions with tocilizumab may occur as a result of increased CYP450 enzyme activity. In vitro studies reported that tocilizumab may increase CYP1A2, CYP2B6, CYP2C9, CYP2C19, CYP2D6, and CYP3A4 activity. In vivo studies reported that tocilizumab may interact with simvastatin via increased activity of CYP3A4, resulting in decreased simvastatin exposure. Findings were also similar when omeprazole and tocilizumab were combined, resulting in decreased omeprazole AUC through increased CYP19 activity.
3.4. Convalescent Plasma Therapy
Passive immunity delivered as anti‐coronavirus antibodies from convalescent human plasma may offer a novel therapeutic approach for COVID‐19. The proposed mechanism of benefit from convalescent human plasma derived from survivors of the coronavirus is the transfer of passive immunity in an effort to restore the immune system during critical illness and neutralize the virus to suppress viremia. 114 The main driver for the investigation of convalescent human blood products in the treatment of COVID‐19 originated from its use in the H1N1 pandemic, during which patients derived a clinically significant mortality benefit and improved viral clearance from convalescent blood products. 115
In a retrospective review of 40 patients with SARS who failed treatment with methylprednisolone and ribavirin at 3 days, 74% (19) of patients who received convalescent plasma were discharged by day 22 compared with only 19% (21) of patients who received high‐dose corticosteroids (p<0.001). Five deaths were reported in the steroid group compared with no deaths with convalescent plasma. To obtain the greatest benefit from convalescent plasma, treatment should be used early in the course of disease (before day 16) because viremia from SARS was shown to peak in the first week of treatment followed by a primary immune response by days 10 to 14. 116 Preliminary data from ongoing convalescent plasma therapy data in the COVID‐19 outbreak suggest improvement in clinical symptoms with no signal of adverse effects. 117
Targeted anti‐SARS‐CoV‐2 antibodies that allow for a more selective therapy against coronavirus are in development, and some companies have begun active recruitment for investigation into its clinical efficacy and safety treatment of COVID‐19. 118 Takeda Pharmaceutical has announced investigation into a new plasma‐derived therapy coined TAK‐888. This plasma‐derived therapy involves removing plasma from COVID‐19 survivors, extracting coronavirus‐specific antibodies to administer to infected patients to stimulate a potent immune response against SARS‐CoV‐2. Convalescent therapy remains in the experimental phase, but appears it may favorably influence the treatment course, and enrollment of patients into a clinical trial will aid in defining its role in therapy.
3.5. Corticosteroids
Given lack of efficacy and risk of delayed viral clearance, the World Health Organization consensus document on the management of COVID‐19 strongly recommends against the use of corticosteroids unless alternative indications are present. Acute lung injury and respiratory distress syndrome secondary to systemic inflammation are well‐characterized sequelae of SARS and MERS outbreaks with pulmonary histology demonstrating evidence of inflammation and diffuse alveolar damage. 119 Corticosteroids were proposed to be of benefit in suppression of lung inflammation in MERS and SARS due to their immunomodulatory properties. However, clinical data are now clear that patients derive no benefit from corticosteroids in the management of SARS, MERS, or COVID‐19 but have instead demonstrated evidence of increased risk of harm including prolonged mechanical ventilation, avascular necrosis, delayed viral clearance, and secondary infections. 120 , 121
A retrospective observational study of critically ill patients with MERS who received hydrocortisone 300 mg per day did not find a 90‐day mortality benefit (adjusted odds ratio 0.8, 95% confidence interval [CI] 0.5–1.1, p<0.12) but did find delayed viral clearance (adjusted HR 0.4, 95% CI 0.2–0.7, p<0.0005). 122 The lack of survival benefit was further supported by a systematic review of corticosteroids in patients with SARS but showed an increased risk of psychosis, avascular necrosis, prolonged viremia, and hyperglycemia with corticosteroid treatment. 120 Although severe, potentially life‐threatening ARDS can occur, generalizing the evidence for corticosteroids to the COVID‐19–associated ARDS is problematic and fails to account for the differing pathogenesis of viral‐induced lung injury. 121
Corticosteroids should only be considered if required for an alternative indication (i.e., septic shock, bronchoconstriction). Despite the lack of benefit defined in previous trials, a clinical trial is actively recruiting to assess the efficacy and safety of methylprednisolone 1 mg/kg/day for a duration of 7 days in noncritically ill patients to further identify the risks and benefits of therapy. 123
4. Other Emerging Therapies for Treatment of COVID‐19
In addition to the drug therapies previously discussed, several additional drugs are being evaluated as potential therapies for COVID‐19 in clinical trials. Arbidol, a drug used in Russia and China for prophylaxis and treatment of influenza and respiratory viral infections, works by blocking viral fusion to the target cell membrane and has demonstrated activity against several viruses including SARS. 124 Arbidol is currently being evaluated for the treatment of COVID‐19 in several studies in China and is currently a recommended treatment option for COVID‐19 there. Arbidol is not available in the United States. Favipiravir, a drug licensed in Japan for the treatment of influenza, is another potential agent due to its activity against a wide spectrum of RNA viruses including coronaviruses. 125 Several studies are underway evaluating favipiravir for the treatment of COVID‐19. Other antiviral agents being evaluated in China for the treatment of COVID‐19 include antiretroviral agents darunavir/cobicistat and emtricitabine/tenofovir alafenamide, as well as the influenza drug baloxavir. Finally, oseltamivir, a neuraminidase inhibitor used for treatment of influenza, is also being studied for treatment of COVID‐19. However, oseltamivir is unlikely to be active against SARS‐CoV‐2 based on previous studies with SARS‐CoV. 61
Drugs to decrease the inflammation associated with COVID‐19 are also being evaluated. One of these drugs is thalidomide that may decrease inflammation through its antiinflammatory and immunomodulatory effects. 126
5. Conclusions
The COVID‐19 pandemic has presented an enormous global health crisis. The pharmaceutical industry is working to develop novel treatments targeted at SARS‐CoV‐2. In the meantime, the medical community is trialing a variety of therapies that target different antiviral and immunomodulating mechanisms to combat this virus. Some of the drugs are investigational, and some are old drugs being repurposed for a new pathogen. As we gain more information both about the effectiveness of the drugs against SARS‐CoV‐2 and their pharmacokinetics, especially in the complex setting of critical illness, we will be better equipped to optimize therapeutic strategies.
Conflict of interest: The authors have declared no conflicts of interest for this article.
References
- 1. Velavan TP, Meyer CG. The COVID‐19 epidemic. Trop Med Int Health 2020;25(3):278–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lipsitch M, Swerdlow DL, Finelli L. Defining the epidemiology of Covid‐19—studies needed. N Engl J Med 2020;382:1194–6. [DOI] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wei J, Xu H, Xiong J, et al. 2019 Novel coronavirus (COVID‐19) pneumonia: serial computed tomography findings. Korean J Radiol 2020;21(4):501– 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT‐PCR testing in coronavirus disease 2019 (COVID‐19) in China: a report of 1014 cases [published online ahead of print February 26, 2020]. Radiology 2020;200642 10.1148/radiol.2020200642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID‐19). Drug Discov Ther 2020;14(1):58–60. [DOI] [PubMed] [Google Scholar]
- 7. Ashour HM, Elkhatib WF, Rahman MM, Elshabrawy HA. Insights into the recent 2019 novel coronavirus (SARS‐CoV‐2) in light of past human coronavirus outbreaks. Pathogens 2020;9(3):E186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 2020;323:1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study [published online ahead of print February 24, 2020]. Lancet Respir Med 2020. 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li G, Clercq ED. Therapeutic options for the 2019 novel coronavirus (2019‐nCoV). Nat Rev Drug Discov 2020;19(3):149–50. [DOI] [PubMed] [Google Scholar]
- 11. Paragas J, Blatt LM, Hartmann C, Huggins JW, Endy TP. Interferon alfacon1 is an inhibitor of SARS‐corona virus in cell‐based models. Antiviral Res 2005;66(2–3):99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang W, Ye L, Ye L, et al. Up‐regulation of IL‐6 and TNF‐alpha induced by SARS‐coronavirus spike protein in murine macrophages via NF‐kappaB pathway. Virus Res 2007;128(1–2):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoenen T, Groseth A, Feldmann H. Therapeutic strategies to target the Ebola virus life cycle. Nat Rev Microbiol 2019;17(10):593–606. [DOI] [PubMed] [Google Scholar]
- 14. Lo MK, Feldmann F, Gary JM, et al. Remdesivir (GS‐5734) protects African green monkeys from Nipah virus challenge. Sci Transl Med 2019;11(494):eaau9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sheahan TP, Sims AC, Graham RL, et al. Broad‐spectrum antiviral GS‐5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med 2017;9(396):eaal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gordon CJ, Tchesnokov EP, Feng JY, Porter DP, Gotte M. The antiviral compound remdesivir potently inhibits RNA‐dependent RNA polymerase from Middle East respiratory syndrome coronavirus [published online ahead of print February 24, 2020]. J Biol Chem 2020;295(15):4773–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tchesnokov EP, Feng JY, Porter DP, Götte M. Mechanism of inhibition of Ebola virus RNA‐dependent RNA polymerase by Remdesivir. Viruses 2019;11(4):326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Agostini ML, Andres EL, Sims AC, et al. Coronavirus susceptibility to the antiviral remdesivir (GS‐5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio 2018;9(2): pii: e00221‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. European Medicines Agency Committee for Medicinal Products for Human Use (CHMP) . CHMP assessment report. Available from http://ema.europa.eu/en/documents/referral/assessment-report-article-53-procedure-medicinal-products-under-development-treatment-ebola_en.pdf. Accessed March 16, 2020.
- 20. The COVID‐19 Investigation Team . First 12 patients with coronavirus disease 2019 (COVID‐19) in the United States [published online ahead of print and peer review March 12, 2020]. medRxiv 2020. 10.1101/2020.03.09.20032896 [DOI] [Google Scholar]
- 21. ClinicalTrials.gov [Internet]. Bethesda, MD: National Library of Medicine (US) 2020 Feb 5‐. Identifier NCT04252664, Mild/Moderate 2019‐nCoV; 2020 Feb 24 [cited 2020 Mar 18]; [about 4 screens]. Available from https://clinicaltrials.gov/ct2/show/NCT04252664 . [Google Scholar]
- 22. ClinicalTrials.gov [Internet]. Bethesda, MD: National Library of Medicine (US) 2020 Feb 6‐. Identifier NCT04257656, Severe 2019‐nCoV Remdesivir RCT; 2020 Feb 24 [cited 2020 Mar 18]; [about 4 screens]. Available from https://clinicaltrials.gov/ct2/show/NCT04257656 . [Google Scholar]
- 23. ClinicalTrials.gov [Internet]. Bethesda, MD: National Library of Medicine (US) 2018 Oct 25‐. Identifier NCT03719586, Investigational Therapeutics for the Treatment of People With Ebola Virus Disease. 2019 Oct 21 [cited 2020 Mar 18]; [about 4 screens]. Available from https://clinicaltrials.gov/ct2/show/NCT03719586 . [Google Scholar]
- 24. Warren TK, Jordan R, Lo MK, et al. Therapeutic efficacy of the small molecule GS‐5734 against Ebola virus in rhesus monkeys. Nature 2016;531(7594):381–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cho A, Saunders OL, Butler T, et al. Synthesis and antiviral activity of a series of 1′‐substituted 4‐aza‐7,9‐dideazaadenosine C‐nucleosides. Bioorg Med Chem Lett 2012;22(8):2705–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Madelain V, Nguyen THT, Olivo A, et al. Ebola virus infection: review of the pharmacokinetic and pharmacodynamic properties of drugs considered for testing in human efficacy trials. Clin Pharmacokinet 2016;55(8):907–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mulangu S, Dodd LE, Davey RT, et al. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med 2019;381(24):2293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. ClinicalTrials.gov [Internet]. Bethesda, MD: National Library of Medicine (US) 2020 Feb 21‐. Identifier NCT04280705, Adaptive COVID‐19 Treatment Trial. 2020 Mar 19 [cited 2020 Mar 18]; [about 4 screens]. Available from https://clinicaltrials.gov/ct2/show/NCT04280705 . [Google Scholar]
- 29. World Health Organization . Deliberations on design options for randomized controlled clinical trials to assess the safety and efficacy of investigational therapeutics for the treatment of patients with Ebola virus disease Appendix 4. Summaries of evidence from selected experimental therapeutics. Available at https://www.who.int/ebola/drc-2018/summaries-of-evidence-experimental-therapeutics.pdf?ua=1. Accessed March 18, 2020.
- 30. Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today’s diseases? Lancet Infect Dis 2003;3(11):722–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Savarino A, Gennero L, Sperber K, Boelaert JR. The anti‐HIV‐1 activity of chloroquine. J Clin Virol 2001;20(3):131–5. [DOI] [PubMed] [Google Scholar]
- 32. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J 2005;2(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Keyaerts E, Vijgen L, Maes P, Neyts J, Van Ranst M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun 2004;323(1):264–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yao X, Ye F, Zhang M, et al. Vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) [published online ahead of print February 26, 2020]. Clin Infect Dis 2020. 10.1093/cid/ciaa237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res 2020;30(3):269–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID‐19 associated pneumonia in clinical studies. Biosci Trends 2020;14(1):72–3. [DOI] [PubMed] [Google Scholar]
- 37. ClinicalTrials.gov [Internet]. Bethesda, MD: National Library of Medicine (US) 2020 Feb 7‐. Identifier NCT04261517, Efficacy and Safety of Hydroxychloroquine for Treatment of Pneumonia Caused by 2019‐nCoV (HC‐nCoV); 2020 Mar 4 [cited 2020 Mar 18]; [about 4 screens]. Available from https://clinicaltrials.gov/ct2/show/NCT04261517 . [Google Scholar]
- 38. Zhonghua J, He HH, Xi ZZ, Zhonghua JHHZCJ. Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia. Tuberc Respir Dis 2020;43(3):185–8. [DOI] [PubMed] [Google Scholar]
- 39. Sanofi‐Aventis USLLC . Aralen (Chloroquine phosphate) package insert. Bridgewater, NJ: Sanofi‐Aventis USLLC; 2017. [Google Scholar]
- 40. Concordia Pharmaceuticals Inc . Plaquenil (hydroxychloroquine) package insert. St. Michael: Concordia Pharmaceuticals Inc; 2017. [Google Scholar]
- 41. Briggs G, Freeman R, Towers C, Forinash A. Drugs in pregnancy and lactation, 11th ed Philadelphia, PA: Wolters Kluwer; 2017. [Google Scholar]
- 42. Pareek A, Chandurkar N, Thomas N, et al. Efficacy and safety of hydroxychloroquine in the treatment of type 2 diabetes mellitus: a double blind, randomized comparison with pioglitazone. Curr Med Res Opin 2014;30(7):1257–66. [DOI] [PubMed] [Google Scholar]
- 43. Cvetkovic RS, Goa KL. Lopinavir/Ritonavir. Drugs 2003;63(8):769–802. [DOI] [PubMed] [Google Scholar]
- 44. Chu CM, Cheng VCC, Hung IFN, et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax 2004;59(3):252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chan JF‐W, Yao Y, Yeung M‐L, et al. Treatment with lopinavir/ritonavir or interferon‐β1b improves outcome of MERS‐CoV infection in a nonhuman primate model of common marmoset. J Infect Dis 2015;212(12):1904–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sheahan TP, Sims AC, Leist SR, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS‐CoV. Nat Commun 2020;11(1):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vastag B. Old drugs for a new bug: influenza, HIV drugs enlisted to fight SARS. JAMA 2003;290(13):1695–6. [DOI] [PubMed] [Google Scholar]
- 48. Chan KS, Lai ST, Chu CM, et al. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong Med J 2003;9(6):399–406. [PubMed] [Google Scholar]
- 49. Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS‐CoV‐2 in Singapore [published online ahead of print March 3, 2020]. JAMA 2020. 10.1001/jama.2020.3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study [published online ahead of print March 11, 2020]. Lancet 2020;395:1054–62. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cao B, Wang Y, Wen D, et al. A trial of lopinavir‐ritonavir in adults hospitalized with severe Covid‐19 [published online ahead of print March 18, 2020]. N Engl J Med 2020. 10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. ClinicalTrials.gov. Bethesda, MD: National Library of Medicine (US) 2016 July 27. Identifier NCT02845843, MERS‐CoV Infection treated With A Combination of Lopinavir /Ritonavir and Interferon Beta‐1b (MIRACLE). 2010 Mar 7 [cited 2020 Mar 18]; [about 4 screens]. Available from https://clinicaltrials.gov/ct2/show/NCT02845843 . [Google Scholar]
- 53. ClinicalTrials.gov. Bethesda, MD: National Library of Medicine (US) 2020 Feb 5. Identifier NCT04252885, The Efficacy of Lopinavir Plus Ritonavir and Arbidol Against Novel Coronavirus Infection. 2020 Feb 5 [cited 2020 Mar 18]; [about 4 screens]. Available from https://clinicaltrials.gov/ct2/show/NCT04252885 . [Google Scholar]
- 54. Chinese Clinical Trial Register . Chengdu (Sichuan): Ministry of Health (China).2020 Jan 23. Identifier ChiCTR2000029308, A randomized, controlled open‐label trial to evaluate the efficacy and safety of lopinavir‐ritonavir in hospitalized patients with 2019‐nCoV infection; 2020 Mar 9. 2020 [cited 2020 Mar 18]; [about 4 screens]. Available from http://www.chictr.org.cn/showprojen.aspx?proj=48684 .
- 55. AbVie Inc . Kaletra (lopinavir and ritonavir) package insert. North Chicago, IL: AbVie Inc; 2016. [Google Scholar]
- 56. Panel on Treatment of Pregnant Women with HIV Infection and Prevention of Perinatal Transmission. Recommendations for the Use of Antiretroviral Drugs in Pregnant Women with HIV Infection and Interventions to Reduce Perinatal HIV Transmission in the United States. Available from https://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. Accessed March 16, 2020.
- 57. Prohaska ES, King AR. Administration of antiretroviral medication via enteral tubes. Am J Health Syst Pharm 2012;69(24):2140–6. [DOI] [PubMed] [Google Scholar]
- 58. Crotty S, Cameron C, Andino R. Ribavirin’s antiviral mechanism of action: lethal mutagenesis? J Mol Med 2002;80(2):86–95. [DOI] [PubMed] [Google Scholar]
- 59. Merck & Co., Inc . Rebetol (ribavirin) package insert. Whitehouse Station, NJ: Merck & Co., Inc; 2013. [Google Scholar]
- 60. Wenzel RP, Edmond MB. Managing SARS amidst uncertainty. N Engl J Med 2003;348(20):1947–8. [DOI] [PubMed] [Google Scholar]
- 61. Tan ELC, Ooi EE, Lin C‐Y, et al. Inhibition of SARS coronavirus infection in vitro with clinically approved antiviral drugs. Emerg Infect Dis 2004;10(4):581–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Booth CM, Matukas LM, Tomlinson GA, et al. Clinical features and short‐term outcomes of 144 patients with SARS in the Greater Toronto Area. JAMA 2003;289(21):2801–9. [DOI] [PubMed] [Google Scholar]
- 63. Falzarano D, de Wit E, Martellaro C, Callison J, Munster VJ, Feldmann H. Inhibition of novel β coronavirus replication by a combination of interferon‐α2b and ribavirin. Sci Rep 2013;3:1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tsang KW, Ho PL, Ooi GC, et al. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med 2003;348(20):1977–85. [DOI] [PubMed] [Google Scholar]
- 65. Koren G, King S, Knowles S, Phillips E. Ribavirin in the treatment of SARS: a new trick for an old drug? CMAJ 2003;168(10):1289–92. [PMC free article] [PubMed] [Google Scholar]
- 66. Knowles SR, Phillips EJ, Dresser L, Matukas L. Common adverse events associated with the use of ribavirin for severe acute respiratory syndrome in Canada. Clin Infect Dis 2003;37(8):1139–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chang CH, Chen KY, Lai MY, Chan KA. Meta‐analysis: ribavirin‐induced haemolytic anaemia in patients with chronic hepatitis C. Aliment Pharmacol Ther 2002;16(9):1623–32. [DOI] [PubMed] [Google Scholar]
- 68. Romark Pharmaceuticals . Alinia (nitazoxanide) package insert. Tampa, FL: Romark Pharmaceuticals; 2005. [Google Scholar]
- 69. Amadi B, Mwiya M, Musuku J, et al. Effect of nitazoxanide on morbidity and mortality in Zambian children with cryptosporidiosis: a randomised controlled trial. Lancet 2002;360(9343):1375–80. [DOI] [PubMed] [Google Scholar]
- 70. Finegold SM, Molitoris D, Väisänen M‐L. Study of the in vitro activities of rifaximin and comparator agents against 536 anaerobic intestinal bacteria from the perspective of potential utility in pathology involving bowel flora. Antimicrob Agents Chemother 2009;53(1):281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rossignol J‐F. Nitazoxanide: a first‐in‐class broad‐spectrum antiviral agent. Antiviral Res 2014;110:94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gamiño‐Arroyo AE, Guerrero ML, McCarthy S, et al. Efficacy and safety of nitazoxanide in addition to standard of care for the treatment of severe acute respiratory illness. Clin Infect Dis 2019;69(11):1903–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rossignol J‐F, Stachulski AV. Syntheses and antibacterial activities of tizoxanide, an N‐(nitrothiazolyl)salicylamide, and its O‐aryl glucuronide. J Chem Res Synop 1999;1:44–5. [Google Scholar]
- 74. Rossignol JF, La Frazia S, Chiappa L, Ciucci A, Santoro MG. Thiazolides, a new class of anti‐influenza molecules targeting viral hemagglutinin at the post‐translational level. J Biol Chem 2009;284(43):29798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Clerici M, Trabattoni D, Pacei M, Biasin M, Rossignol JF. The anti‐infective nitazoxanide shows strong immunomodulating effects (155.21). J Immunol 2011;186(1):155.21. [Google Scholar]
- 76. Cao J, Forrest JC, Zhang X. A screen of the NIH Clinical Collection small molecule library identifies potential anti‐coronavirus drugs. Antiviral Res 2015;114:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Haffizulla J, Hartman A, Hoppers M, et al. Effect of nitazoxanide in adults and adolescents with acute uncomplicated influenza: a double‐blind, randomised, placebo‐controlled, phase 2b/3 trial. Lancet Infect Dis 2014;14(7):609–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Vets E, Rossignol J‐F, Jackson AS. Effects of nitazoxanide on pharmacokinetics and pharmacodynamics of a single dose of warfarin. Am J Health Syst Pharm 2009;66(9):838–42. [DOI] [PubMed] [Google Scholar]
- 79. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV‐1‐infected adults and adolescents. Department of Health and Human Services. Guidelines for the Use of Antiretroviral Agents in HIV‐1‐Infected Adults and Adolescents. Available from http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed March 16, 2020.
- 80. Yamamoto N, Yang R, Yoshinaka Y, et al. HIV protease inhibitor nelfinavir inhibits replication of SARS‐associated coronavirus. Biochem Biophys Res Commun 2004;318(3):719–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nelfinavir was predicted to be a potential inhibitor of 2019‐nCov main protease by an integrative approach combining homology modelling, molecular docking and binding free energy calculation [published online ahead of print January 28, 2020]. bioRxiv. 10.1101/2020.01.27.921627 [DOI]
- 82. Agouron Pharmaceuticals Inc . Viracept (nelfinavir mesylate) package insert. La Jolla, CA: Agouron Pharmaceuticals Inc; 2005. [Google Scholar]
- 83. Trapé M, Barnosky S. Nelfinavir in expanded postexposure prophylaxis causing acute hepatitis with cholestatic features: two case reports. Infect Control Hosp Epidemiol 2001;22(6):333–4. [DOI] [PubMed] [Google Scholar]
- 84. Nelfinavir LiverTox: Clinical and Research Information on Drug‐Induced Liver Injury. Available from http://www.ncbi.nlm.nih.gov/books/NBK548311/. Accessed March 14, 2020. [PubMed]
- 85. Khaliq Y, Gallicano K, Seguin I, et al. Single and multiple dose pharmacokinetics of nelfinavir and CYP2C19 activity in human immunodeficiency virus‐infected patients with chronic liver disease. Br J Clin Pharmacol 2000;50(2):108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Billiau A. The interferons: a brief introduction. Med Clin North Am 1986;70(Suppl):7–11. [DOI] [PubMed] [Google Scholar]
- 87. Merck & Co. Inc . Intron A (interferon alfa‐2b) package insert. Whitehouse Station, NJ: Merck & Co. Inc; 2014. [Google Scholar]
- 88. Haagmans BL, Kuiken T, Martina BE, et al. Pegylated interferon‐alpha protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat Med 2004;10(3):290–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Morgenstern B, Michaelis M, Baer PC, Doerr HW, Cinatl J. Ribavirin and interferon‐beta synergistically inhibit SARS‐associated coronavirus replication in animal and human cell lines. Biochem Biophys Res Commun 2005;326(4):905–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chen F, Chan KH, Jiang Y, et al. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol 2004;31(1):69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Arabi YM, Balkhy H, Al‐Omari A, et al. Ribavirin and interferon for critically ill patients with Middle East respiratory coronavirus (MERS‐CoV) infection. Clin Infect Dis 2019;70(9):1837–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Omrani AS, Saad MM, Baig K, et al. Ribavirin and interferon alfa‐2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis 2014;14(11):1090–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Clinicaltrials.gov [Internet]. Bethesda, MD: National Library of Medicine (US) 2020 Feb 19‐. Identifier NCT04276688, Lopinavir/Ritonavir, Ribavirin and IFN‐beta Combination for nCoV Treatment; 2020 Feb 28. [cited 2020 Mar 18]; [about 4 screens]. Available from https://clinicaltrials.gov/ct2/show/NCT04276688 . [Google Scholar]
- 94. ClinicalTrials.gov [Internet]. Bethesda, MD: National Library of Medicine (US) 2020 Mar 3‐. Identifer NCT04293887, Efficacy and Safety of IFN‐α2β in the Treatment of Novel Coronavirus Patients; 2020 Mar 3 [cited 2020 Mar 18]; [about 4 screens]. Available from https://clinicaltrials.gov/ct2/show/NCT04293887 . [Google Scholar]
- 95. Russo MW, Fried MW. Side effects of therapy for chronic hepatitis C. Gastroenterology 2003;124(6):1711–9. [DOI] [PubMed] [Google Scholar]
- 96. Huraib S, Tanimu D, Romeh SA, et al. Interferon‐α in chronic hepatitis C infection in dialysis patients. Am J Kidney Dis 1999;34(1):55–60. [DOI] [PubMed] [Google Scholar]
- 97. Sulkowski MS, Cooper C, Hunyady B, et al. Management of adverse effects of Peg‐IFN and ribavirin therapy for hepatitis C. Nat Rev Gastroenterol Hepatol 2011;8(4):212–23. [DOI] [PubMed] [Google Scholar]
- 98. Elli Lilly and Company . Olumiant (baricitinib) package insert. Indianapolis, IN: Elli Lilly and Company; 2018. [Google Scholar]
- 99. Lu R, Zhao X, Li J, et al. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020;395(10224):P565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395(10229):1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China [published online ahead of print March 3, 2020]. Intensive Care Med 2020. 10.1007/s00134-020-05991-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Stebbing J, Phelan A, Griffin I, et al. COVID‐19: combining antiviral and anti‐inflammatory treatments. Lancet Infect Dis 2020;20(4):400–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Genentech Inc . Actemra (tocilizumab) injection package insert. South San Francisco, CA: Genentech Inc; 2018. [Google Scholar]
- 104. Cameron MJ, Bermejo‐Martin JF, Danesh A, Muller MP, Kelvin DJ. Human immunopathogenesis of severe acute respiratory syndrome (SARS). Virus Res 2008;133(1):13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lau SKP, Lau CCY, Chan K‐H, et al. Delayed induction of proinflammatory cytokines and suppression of innate antiviral response by the novel Middle East respiratory syndrome coronavirus: implications for pathogenesis and treatment. J Gen Virol 2013;94(Pt 12):2679–90. [DOI] [PubMed] [Google Scholar]
- 106. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395(10223):507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8(4):420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Xu X, Han M, Li T, et al. Effective treatment of severe COVID‐19 patients with Tocilizumab. ChinaXiv 2020; 202003(00026): v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. ClinicalTrials.gov [Internet]. Bethesda, MD: National Library of Medicine (US) 2020 Mar 17‐. Identifier NCT04310228, Favipiravir Combined With Tocilizumab in the Treatment of Corona Virus Disease 2019. 2020 Mar 17 [cited 2020 Mar 18]; [about 4 screens]. Available from https://clinicaltrials.gov/ct2/show/NCT04310228 . [Google Scholar]
- 110. ClinicalTrials.gov [Internet]. Bethesda, MD: National Library of Medicine (US) 2020 Mar 13‐. Identifier NCT04306705, Tocilizumab vs CRRT in Management of Cytokine Release Syndrome (CRS) in COVID‐19. 2020 Mar 17 [cited 2020 Mar 18]; [about 4 screens]. Available from https://clinicaltrials.gov/ct2/show/NCT04306705 . [Google Scholar]
- 111. ClinicalTrials.gov [Internet]. Bethesda, MD: National Library of Medicine (US) 2020 Mar 19‐. Identifier NCT04315298, Evaluation of the Efficacy and Safety of Sarilumab in Hospitalized Patients with COVID‐19. 2020 Mar 19 [cited 2020 Mar 18]; [about 4 screens]. Available from https://clinicaltrials.gov/ct2/show/NCT04315298 . [Google Scholar]
- 112. Guidelines for the diagnosis and treatment of coronavirus disease 2019 (trial version 7) . Beijing: National Health Commission of the People’s Republic of China and National Health Commission of the People’s Republic of China, 2020. Available from http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml. Accessed March 20, 2020.
- 113. Mao R, Liang J, Shen J, et al. Implications of COVID‐19 for patients with pre‐existing digestive diseases [published online ahead of print March 11, 2020]. Lancet Gastroenterol Hepatol 2020;5(5):426–8. 10.1016/S2468-1235(20)30076-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID‐19. Lancet Infect Dis 2020;20(4):398–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta‐analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med 2006;145(8):599–609. [DOI] [PubMed] [Google Scholar]
- 116. Soo YOY, Cheng Y, Wong R, et al. Retrospective comparison of convalescent plasma with continuing high‐dose methylprednisolone treatment in SARS patients. Clin Microbiol Infect 2004;10(7):676–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Liu G, Li S. Convalescent plasma: a valid option in the treatment of COVID‐19? Insights Clin Cell Immunol 2020;4:001–002. [Google Scholar]
- 118. ClinicalTrials.gov [Internet]. Bethesda, MD: National Library of Medicine (US) 2020 Mar 3‐. Identifier NCT04292340, Anti‐SARS‐CoV‐2 Inactivated Convalescent Plasma in the Treatment of COVID‐19. 2020 Mar 3 [cited 2020 Mar 18];[about 4 screens]. Available from https://clinicaltrials.gov/ct2/show/NCT04292340 . [Google Scholar]
- 119. Arabi YM, Balkhy HH, Hayden FG, et al. Middle east respiratory syndrome. N Engl J Med 2017;376(6):584–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med 2006;3(9):e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019‐nCoV lung injury. Lancet 2020;395(10223):473–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Arabi YM, Mandourah Y, Al‐Hameed F, et al. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med 2018;197(6):757–67. [DOI] [PubMed] [Google Scholar]
- 123. ClinicalTrials.gov [Internet]. Bethesda, MD: National Library of Medicine (US) 2020 Feb 18‐. Identifier NCT04273321, Efficacy and Safety of Corticosteroids in COVID‐19. 2020 Feb 24 [cited 2020 Mar 18]; [about 4 screens]. Available from https://clinicaltrials.gov/ct2/show/NCT04273321 . [Google Scholar]
- 124. Blaising J, Polyak SJ, Pécheur E‐I. Arbidol as a broad‐spectrum antiviral: an update. Antiviral Res 2014;107:84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Jordan PC, Stevens SK, Deval J. Nucleosides for the treatment of respiratory RNA virus infections. Antivir Chem Chemother 2018;26:204020661876448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Chen C, Qi F, Shi K, et al. Thalidomide Combined with Low‐dose Glucocorticoid in the Treatment of COVID‐19. Available from http://www.preprints.org/manuscript/202002.0395/v1. Accessed March 15, 2020.


