Abstract
Background
Since December 8, 2019, an epidemic of coronavirus disease 2019 (COVID‐19) has spread rapidly, but information about children with COVID‐19 is limited.
Methods
This retrospective and the single‐center study were done at the Public Health Clinic Center of Changsha, Hunan, China. We identified all hospitalized children diagnosed with COVID‐19 between January 8, 2019 and February 19, 2020, in Changsha. Epidemiological and clinical data of these children were collected and analyzed. Outcomes were followed until February 26th, 2020.
Results
By February 19, 2020, nine pediatric patients were identified as having 2019‐nCoV infection in Changsha. Six children had a family exposure and could provide the exact dates of close contact with someone who was confirmed to have 2019‐nCoV infection, among whom the median incubation period was 7.5 days. The initial symptoms of the nine children were mild, including fever (3/9), diarrhea (2/9), cough (1/9), and sore throat (1/9), two had no symptoms. Two of the enrolled patients showed small ground‐glass opacity of chest computed tomography scan. As of February 26, six patients had a negative RT‐PCR for 2019‐nCoV and were discharged. The median time from exposure to a negative RT‐PCR was 14 days.
Conclusions
The clinical symptoms of the new coronavirus infection in children were not typical and showed a less aggressive clinical course than teenage and adult patients. Children who have a familial clustering or have a family member with a definite diagnosis should be reported to ensure a timely diagnosis.
Keywords: children, clinical characteristics, coronavirus disease 2019
1. INTRODUCTION
Since December 8, 2019, a cluster of acute respiratory illness, now known as coronavirus disease 2019(COVID‐19), occurred in Wuhan, Hubei Province, China. 1 The disease has rapidly spread from Wuhan to other areas. The World Health Organization (WHO) subsequently named this novel coronavirus as 2019 Novel Coronavirus (2019‐nCoV). The virus is similar to the coronavirus responsible for the severe acute respiratory syndrome (SARS‐CoV), a member of the subgenus Sarbecovirus (Beta‐CoV lineage B). 2 Common clinical manifestations in COVID‐19 patients included fever, fatigue, dry cough, nasal congestion, runny nose, and other respiratory symptoms, as well as diarrhea, nausea, vomiting, abdominal pain, and other gastrointestinal symptoms. A few patients have developed severe pneumonia and they may present with acute respiratory distress syndrome (ARDS), septic shock, metabolic acidosis, and coagulation dysfunction or even with death. Previous studies suggested that the infection of 2019‐nCoV appeared in clusters was more likely to infect older men with comorbidities, and the earlier stage of the COVID‐19 epidemic primarily involved adults older than 15 years. 3 , 4 , 5 Compared with adults, the present 2019‐nCoV infection in children is relatively rare, but there is an increasing trend, which requires great attention.
During the period from January 2020 to February 19, 2020, 246 patients were found infected with 2019‐nCoV and hospitalized in the Public Health Clinic Center of Changsha, the capital city of Hunan province near from Wuhan, where the first COVID‐19 patient was reported on January 21, 2020. Among all hospitalized patients, we identified nine patients under 14 years old. The purpose of this study was to describe the clinical characteristics and outcomes of children with COVID‐19 in Changsha. As COVID‐19 is still spreading in other areas and continents, we believe this information would be helpful to clinicians and researchers.
2. METHODS
This retrospective and single‐center study were done at the Public Health Clinic Center of Changsha, Hunan, China. We identified all hospitalized children diagnosed with COVID‐19 between January 8, 2019 and February 19, 2020, in Changsha. Epidemiological, clinical, laboratory, and radiological characteristics and treatment and outcomes data were obtained with data collection forms from electronic medical records. The data were reviewed by two physicians. The information recorded included demographic information (age, sex, and geographic location), medical history, exposure history, clinical features (signs and symptoms at admission, dates of admission and diagnosis), important laboratory results, chest computed tomographic (CT) scans, treatment (intensive care unit, oxygen therapy, antiviral therapy, antibiotics, corticosteroid therapy), prognosis (any severe complications, including death), time from exposure to reverse transcription polymerase chain reaction (RT‐PCR) negative, and discharge date. Efforts were made to reach families of patients to confirm the information.
All patients with COVID‐19 were classified as follows 6 : (a) Asymptomatic infection: without any clinical symptoms and signs and the chest imaging is normal, while the RT‐PCR is positive. (b) Mild: with symptoms of acute upper respiratory tract infection, including fever, fatigue, myalgia, cough, sore throat, runny nose, and sneezing. Physical examination shows the congestion of the pharynx. Some cases may show no fever, or have only digestive symptoms such as nausea, vomiting, abdominal pain, and diarrhea. (c) Moderate: with pneumonia, frequent fever and cough, mostly dry cough, followed by productive cough, some may have wheezing, but no obvious hypoxemia such as dyspnea, and lungs can hear sputum or dry snoring and/or wet snoring. Some cases have no clinical signs or symptoms with lung lesions in CT images, which are subclinical. (d) Severe: Early respiratory symptoms such as fever and cough, may be accompanied by gastrointestinal symptoms such as diarrhea. The disease usually progresses within 1 week, and dyspnea occurs, with central cyanosis. Oxygen saturation is less than 92%, with other hypoxia manifestations. (e) Critical: Children can quickly progress to ARDS or respiratory failure, and may also have shock, encephalopathy, myocardial injury or heart failure, coagulation dysfunction, and acute kidney injury. Organ dysfunction can be life‐threatening.
This study was approved by the Medical Ethical Committee of the Second Xiangya Hospital of Central South University (Approved Number KL‐2020003), which waived the requirement for patients' informed consent referring to the CIOMS guideline.
3. RESULTS
Nine infected children were identified between January 30, 2019 and February 26, 2020 (Table 1). Six patients were female. The median age was 8 years old, the youngest was 1‐year‐old and the oldest was 12 years old. Two children lived in Wuhan for several days before they came to Changsha and the others were infected locally. Six children could provide the exact dates of close contact with someone who was confirmed to have 2019‐nCoV infection, among whom the median incubation period was 7.5 days, the shortest incubation period was 1 day and the longest incubation period was 16 days. The median time from onset of symptoms to hospital admission was 3 days (range 0‐17 days).
Table 1.
Characteristics of nine hospitalized children infected with 2019‐nCoV outside of Wuhan, China
| Patient | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| Demographics | |||||||||
| Age, y | 1 | 2 | 8 | 8 | 8 | 9 | 9 | 11 | 12 |
| Sex | Female | Female | Male | Female | Male | Female | Female | Female | Male |
| Exposure history in Wuhan | No | Yes | No | No | No | No | No | Yes | No |
| Family source of exposure | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Time between exposure to symptoms, d | None | None | 10 | 1 | 9 | 14 | 3 | 6 | None |
| Time between onset symptoms to hospital admission, d | 10 | 2 | 5 | 15 | 0 | 1 | 17 | 3 | 2 |
| Symptoms at onset | None | Fever | Fever | Fever and Diarrhea | Diarrhea | Sore throat | None | Cough | Fever |
| Highest temperature, °C | 36.8 | 39.1 | 37.8 | 36.7 | 36.9 | 36.8 | 36.5 | 37.2 | 37.5 |
| Laboratory results | |||||||||
| White blood cell count ×109(4‐10) | 16.1 | 7.88 | 5.43 | 4.98 | 5 | 5.45 | 7.06 | 6.06 | 8.34 |
| Lymphocyte count×109 (0.8‐4) | 9.54 | 4.05 | 1.15 | 2.38 | 1.82 | 2.19 | 3.7 | 2.65 | 3.81 |
| C‐creative protein,mg/L (0‐8) | 3.58 | 13.17 | 2.24 | 0.2 | 5.9 | 1.78 | 0.2 | 1.45 | 0.3 |
| Erythrocyte sedimentation rate, mm/h (0‐15) | 3.28 | 27 | 2 | 4 | 23 | 25 | 15 | 40 | 8 |
| Lactate dehydrogenase, U/L (135‐225) | 218 | 230.7 | 153.2 | 159.4 | 152.9 | 177.1 | 175 | 140.9 | 132.1 |
| Alanine aminotransferase, U/L(0‐42) | 15.66 | 18.69 | 19.69 | 17.1 | 15.81 | 14.48 | 14.2 | 13.55 | 10.5 |
| Aspartate aminotransferase, U/L(0‐37) | 31.42 | 40.49 | 28.5 | 27.8 | 23.19 | 31.36 | 55.1 | 22.21 | 14.8 |
| Chest x‐ray and CT findings | Normal | Small ground‐glass opacity | Normal | Normal | Normal | Small ground‐glass opacity | Normal | Normal | Normal |
| Treatment | |||||||||
| Oxygen support | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Intensive unit care | No | No | No | No | No | No | No | No | No |
| Mechanical ventilation | No | No | No | No | No | No | No | No | No |
| Severe complications | No | No | No | No | No | No | No | No | No |
| Antiviral therapy | lopinavir/ritonavir | lopinavir/ritonavir | lopinavir/ritonavir | lopinavir/ritonavir | lopinavir/ritonavir | lopinavir/ritonavir | lopinavir/ritonavir | lopinavir/ritonavir | lopinavir/ritonavir |
| Antibiotic therapy | Azithromycin | Azithromycin | Azithromycin | No | No | No | Azithromycin | No | Azithromycin |
| Meprednisone | No | Yes | No | No | No | No | No | No | No |
| Gamma globulin | No | Yes | No | No | No | No | No | No | No |
| Prognosis | |||||||||
| Time between exposure to RT‐PCR negative, d | 14 | 10 | 20 | Positive | 9 | 14 | Positive | 13 | Positive |
| Time of hospitalization, d | 16 | 12 | 22 | In hospital | 11 | 16 | In hospital | 15 | In hospital |
Abbreviation: CT, computed tomography; RT‐PCR, reverse transcription polymerase chain reaction.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
The initial symptoms of illness of the nine children were fever (3/9; the highest temperature of the three febrile patients ranged from 37.5°C to 39.1°C, diarrhea (2/9), mild upper respiratory tract symptoms including cough (1/9) and sore throat (1/9). Two children had no symptoms but were tested positive for 2019‐nCoV in a designated screening because of exposure to infected family members.
On admission the number of white blood cells and leukocytes of the children was in the normal range except for an elevation in the 1‐year‐old child, lactate dehydrogenase of all children was in the normal range, and the aspartate aminotransferase of two children was above the normal range. One child had an elevation of the level of erythrocyte sedimentation rate and two had an elevation of C‐reactive protein (CRP) positive. Only two of the enrolled patients showed unilateral small unilateral ground‐glass opacities of chest CT scans (Figure 1).
Figure 1.
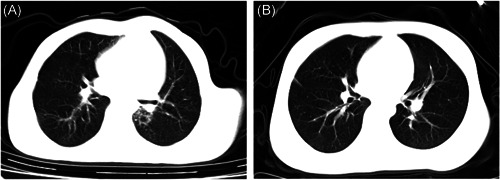
Chest computed tomographies (CTs) on a 9‐year‐old patient Case: (A) CT image showed ground‐glass opacity in the left pulmonary lobe of a 9‐year‐old female with no sign and symptom on the first day of hospitalization, the followed (B) image showed absorption on day 7
Nine patients received oxygen inhalation through a nasal cannula, but none of the nine children required intensive care or mechanical ventilation or had any severe complications. All of them received antiviral therapies (lopinavir/ritonavir tablets or oral liquid, 10/2.5 mg/kg twice daily, orally), four received antibiotics (azithromycin for suspension, 10 mg/kg once daily, orally) and one received meprednisone and immune globulin therapy for febrile convulsion. As of February 26, six patients tested negative for RT‐PCR for 2019‐nCoV and were discharged.
4. DISCUSSION
In this retrospective study, we reported nine pediatric patients of 246 patients with laboratory‐confirmed 2019‐nCOV infection in Changsha. We described and analyzed the clinical characteristics of pediatric patients with COVID‐19. Five of them were mild patients, two were moderate COVID‐19 patients and two were asymptomatic carriers. More than half children were confirmed to have had a family source of exposure. Fever was the most frequent symptoms at the onset of illness. The pediatric patients showed few abnormalities in routine blood examinations. Most demonstrated normal CT images.
The proportion of infected children identified was very low. A case report (72 314) from the Chinese Center for Disease Control and Prevention found the frequency of positive COVID‐19 patients under 19 years was 2% (965 cases). 7 This may be due to a lower risk of exposure or incomplete identification due to a mild or asymptomatic presentation. 8 , 9 Most cases demonstrated familial clustering and the study of COVID‐19 in Chinese infants also showed that infants who were be infected by 2019‐nCoV had come from families that had at least 1 other infected family member with the infant's infection occurring after the family member's infection. 10 In the SARS studies, a majority of young children and teenage patients had a definite contact history with adult SARS patients (usually an immediate family member), which pattern was consistent with our study. 11 , 12 , 13 Six childhood patients were female, consistent with an infant COVID‐19 case report (9), 10 but previous studies in adults found higher percentages of infection in men than women. 4 , 5 A retrospective study of 2143 pediatric patients with COVID‐19 in China showed at all ages appeared susceptible to COVID‐19, and there was no significant gender difference. 14 Whether or not female children and infants may be more susceptible to 2019‐nCoV infection than males requires further study.
The symptoms of childhood patients were mild and two of them were asymptomatic carriers. Most children had normal images. Two children showed pneumonia according to their CT scan, but the lesions were limited to small areas of mottling or ground‐glass opacities. The most common symptoms at the onset of illness were fever, diarrhea, cough, and sore throat, which was different from the adult presentations. Studies across the country had shown that fever, cough, and fatigue were the most frequent symptoms and fever affected nearly 90% and cough more than 50%. 7 , 15 , 16 , 17 Studies of SARS in newborns and children reported that the clinical course and prognosis differed between pediatric and adult SARS patients. Young children (<12 years) experienced in general a less aggressive clinical course than teenage or adult patients. 18
In 2019‐nCoV infected children, most peripheral blood white blood cell counts and an absolute number of lymphocytes were normal, CRP was normal or slightly elevated, and liver enzymes were elevated in some children, 19 which was consistent with our study. In our study, the child with an elevation level of white blood cells and lymphocytes was 1‐year‐old, however, considering other laboratory results of this patient, other possible infection of these patients was excluded. The immature immune system of the infant may as an interpretation of these abnormal results but still need more studies to prove that. No one required intensive care or mechanical ventilation or had any severe complications and most of them did not need to use meprednisone or immune globulin. But in existing studies of patients with COVID‐19. About 20% to 30% of patients infected with 2019‐nCoV need intensive care and more than 20% needed glucocorticoid therapy. 4 , 5 , 20
Most pediatric patients' cases were mild, with much fewer severe and critical cases (5.9%) than adult patients (18.5%). 14 , 21 Why the children and infant patients demonstrated minimal symptoms and less severe compared with adults is not clear. This may be related to the fact that the 2019‐nCoV has been reported to share the same receptor, angiotensin‐converting enzyme 2 (ACE2) with SARS‐CoV and that the ACE2 virus receptor expression is concentrate in a small population of type II alveolar cells (AT2). While the 2019‐nCoV virus is able to use human ACE2 as a receptor, thus having some potential for replication in human cells, 22 , 23 the inadequate expression of ACE2 cells or inadequate expression of ACE2 receptors or the immature function of ACE2 cells could possibly explain the milder symptoms in a child with COVID‐19. Another explanation for this difference may be related to the immature immune system of children. Since the immune function of adults is well developed, the highly pathogenic coronavirus could induce a robust immune response, causing a substantial cytokine storm and producing severe clinical symptoms. 24 As the immune function of children is not mature, serious immune damage would plausibly not be stimulated, and the clinical expression would be milder, just as the minimally pathogenic coronavirus infecting the upper respiratory tract and causing mild, cold‐like respiratory illness. More research is needed to clarify the age‐related changes in the inflammatory responses to these viral agents.
Limitations of this study include the small sample size and the restricted geographic origin of the subjects. A larger, more geographically diverse cohort study population will be needed to characterize more fully the clinical characteristics of childhood COVID‐19 patients.
In conclusion, the clinical symptoms of the new coronavirus infection in children are not typical, which deserves a further and large‐scale study. Children who have family clustering or have family members with a definite diagnosis should be reported to ensure a timely diagnosis.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ACKNOWLEDGMENTS
The authors would like to acknowledge the doctors including Jinhua Li, Wenlong He, Zhiguo Zhou, Hong Peng, and all healthcare professionals who helped and took care of the patients infected with 2019‐nCoV, they have made huge contributions and self‐dedication against the disease, and some of them even gave their precious life. We are like to express our best sincere respect and honorable thanks. This work was supported by Innovative Major Emergent Project against the Outbreak of New Coronavirus Pneumonia in Hunan Province (No. 2020SK3014), the National Natural Science Foundation of China (No. 81370164 and No. 81670062), the National Natural Science Foundation of Hunan Province (No. 2015JJ4087), and the National key clinical specialist construction Programs of China.
Shen Q, Guo W, Guo T, et al. Novel coronavirus infection in children outside of Wuhan, China. Pediatric Pulmonology. 2020;55:1424–1429. 10.1002/ppul.24762
Contributor Information
Zhiguo Zhou, Email: 13807311490@163.com.
Hong Peng, Email: penghong66@csu.edu.cn.
REFERENCES
- 1. Paules CI, Marston HD, Fauci AS. Coronavirus infections—more than just the common cold. JAMA. 2020;323(8):707‐708. [DOI] [PubMed] [Google Scholar]
- 2. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382:1199‐1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA ‐ J Am Med Assoc. 2020;323:1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Society of Pediatrics, Chinese Medical Association . Recommendations for the diagnosis, prevention and control of the 2019 novel coronavirus infection in children. Chinese J Pediatr. 2020;58(3):169‐174. [DOI] [PubMed] [Google Scholar]
- 7. Tian S, Hu N, Lou J, et al. Characteristics of COVID‐19 infection in Beijing. J Infect. 2020;80:401‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Paules CI, Marston HD, Fauci AS. Coronavirus infections‐more than just the common cold. JAMA ‐ J Am Med Assoc. 2020;323(8):707‐708. [DOI] [PubMed] [Google Scholar]
- 9. Del Rio C, Malani PN. 2019 Novel coronavirus—important information for clinicians. JAMA ‐ J Am Med Assoc. 2020;323:1039. [DOI] [PubMed] [Google Scholar]
- 10. Wei M, Yuan J, Liu Y, Fu T, Yu X, Zhang ZJ. Novel coronavirus infection in hospitalized infants under 1 year of age in China. JAMA ‐ J Am Med Assoc. 2020. [published online ahead of print February 14, 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leung TF, Wong GWK, Hon KLE, Fok TF. Severe acute respiratory syndrome (SARS) in children: epidemiology, presentation and management. Paediatr Respir Rev. 2003;4(4):334‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hon K, Leung C, Cheng W, et al. Clinical presentations and outcome of severe acute respiratory syndrome in children. Lancet. 2003;361(9370):1701‐1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bitnun A, Allen U, Heurter H, et al. Children hospitalized with severe acute respiratory syndrome‐related illness in Toronto. Pediatrics. 2003;112(4):e261. [DOI] [PubMed] [Google Scholar]
- 14. Dong Y, Mo X, Hu Y, et al. Epidemiological characteristics of 2143 pediatric patients With 2019 coronavirus disease in China. Pediatrics. 2020:e20200702.32179660 [Google Scholar]
- 15. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang JJ, Dong X, Cao Y, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy Eur J Allergy Clin Immunol. 2020. [published online ahead of print February 19, 2020]. [DOI] [PubMed] [Google Scholar]
- 17. Wu J, Liu J, Zhao X, et al. Clinical characteristics of imported cases of COVID‐19 in Jiangsu province: a multicenter descriptive study. Clin Infect Dis. 2020. [published online ahead of print February 29, 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ng PC, Leung C, Chiu WK, Wong SF, Hon EKL. SARS in newborns and children. Biol Neonate. 2004;85(4):293‐298. [DOI] [PubMed] [Google Scholar]
- 19. Hu T, Fang L, Junling W, Hu T, Hu T. Clinical characteristics of 2019 novel coronavirus (2019‐nCoV) infection in children and family prevention and control. Medical Journal of Wuhan University. 2020. [published online ahead of print February 19, 2020]. [Google Scholar]
- 20. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Novel Coronavirus Pneumonia Emergency Response Epidemiology Team . The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID‐19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145‐151.32064853 [Google Scholar]
- 22. Xu X, Chen P, Wang J, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China: Life Sci. 2020;63(3):457‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single‐cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019‐nCov. bioRxiv. 2020. [published online ahead of print January 26, 2020]. [Google Scholar]
- 24. Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39(5):529‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]


