Abstract
COVID‐19 was first identified in Wuhan, China and is caused by the novel coronavirus SARS‐CoV 2. It has now spread rapidly to over 190 countries and territories around the world and has been declared a global pandemic by the World Health Organization. The virus is spread through droplet transmission and currently has a mortality rate of over 4% globally. The pediatric population has been found to be less susceptible to the disease with the majority of children having milder symptoms and only one pediatric death being reported globally so far. Despite this, strategies need to be put in place to prevent further spread of the virus. We present a summary of the general measures implemented at a large adult and pediatric tertiary hospital in Singapore (National University Hospital) as well as the specific strategies in place for the operating room and pediatric intensive care unit.
Keywords: COVID‐19, general anesthesia, pediatric, PICU, SARS‐CoV 2
1. INTRODUCTION
In December 2019, a cluster of viral pneumonia cases of unknown etiology was reported in Wuhan, China. 1 Sequence analysis identified the virus to be related to the SARS‐CoV clade and was later named COVID‐19. 2 Since the virus subtype was discovered in January 2020, it has spread quickly to over 190 countries and territories around the world. 3 The newest strain of coronavirus has been found to cause a range of respiratory symptoms from a cough or fever, to acute respiratory distress. 1 , 4 The virus is spread through droplet transmission from close contact with infected individuals (symptomatic or asymptomatic) and by touching contaminated surfaces. 5 It has a higher rate of transmission than other members of the coronavirus family such as Middle East Respiratory Syndrome and Severe Acute Respiratory Distress Syndrome, but a significantly lower mortality rate 3 , 6 , 7
On March 11, 2020, COVID‐19 was declared a global pandemic by the World Health Organization and many countries are now in a state of emergency. The mortality rate for COVID‐19 at the time of writing is 4.36% and varies in each country. 3 The pediatric population has been found to be less susceptible to the disease with children having milder symptoms and only one death reported globally so far. 5 , 8 , 9 , 10
The first COVID‐19 case in Singapore was confirmed on January 23, 2020. Initial cases were imported cases from Wuhan followed later by local transmission from 3 distinct clusters. 11 On February 7, 2020, Singapore raised its Disease Outbreak Response System Condition (DORSCON) to Orange prompting the nation to increase temperature screening, implement quarantine measures as well as visitor restrictions in hospitals.
Singapore currently has 631 cases and 2 deaths; both patients were aged between 60 and 75 years and had underlying cardiac disease. To curb the spread of the virus, rigorous contact tracing and quarantine measures have been implemented. 12 At the National University Hospital in Singapore, a large tertiary hospital with 1200 beds, 32 operating rooms (OR), and an 18‐bed pediatric intensive care unit (PICU) with 16 negative pressure rooms, we describe our experience and protocols for the management of COVID‐19 patients in the OR and PICU.
2. GENERAL CONSIDERATIONS
DORSCON orange measures were instituted in both public and healthcare settings. Temperature screening and travel declarations were made compulsory for all schools and businesses. Designated General Practitioner clinics were converted to Public Health Preparedness Clinics to allow for the assessment, investigation, and treatment of patients with a fever or respiratory symptoms in the community. All patients with mild symptoms were given a medical certificate for 5 days and advised to stay at home. Any patient with symptoms of pneumonia or respiratory distress was sent to hospital by a dedicated ambulance. The public were provided with surgical masks (four per household) and encouraged to wear masks only if they felt unwell, and to maintain social distancing. A dedicated team was created and a smartphone app developed to assist with contact tracing. Anyone who had close contact with a confirmed COVID‐19‐positive individual was tested and isolated for 14 days if they were asymptomatic. Any person that tests positive is admitted to hospital.
DORSCON orange measures were instituted early throughout the hospital, to provide perioperative care to known or suspected COVID‐19 patients and to reduce the risk of viral transmission to healthcare workers and other non‐COVID‐19 patients. 13 Restrictions were imposed on all visitors to the hospital including temperature screening and reduced visitors per patient. All elective surgeries were canceled to free up hospital beds and ORs for suspected or confirmed COVID‐19 patients. Isolation wards were created and workflows set‐up for the management of COVID‐19 patients in the PICU and OR. The hospital management sent out daily communications to staff updating them on case definitions or new guidance on the management of suspected/confirmed cases.
Every department in the hospital was required to divide their staff into two teams; each team member was to have minimal to no contact with the other team to ensure social distancing and business continuity in the event of COVID‐19 transmission to a healthcare worker. All healthcare workers submitted their temperatures twice a day via the hospital intranet (prior to starting work and before leaving). Staff were allowed to go home but were told not to return to work if they became unwell (temperature > 37.5°C or if they developed a cough, shortness of breath or other symptoms of flu). Staff with the abovementioned symptoms were given 5 days of medical leave and must stay at home. They were only allowed to return to work if they were afebrile and if their respiratory symptoms were resolving. If the symptoms persisted or worsened, the healthcare worker would be swabbed for COVID‐19 and the medical leave extended. In addition, any healthcare worker returning from overseas had to observe a strict 2‐week leave of absence at home before commencing work.
To prevent the spread of COVID‐19 to healthcare workers, additional infection control measures were implemented. Mask fitting exercises and personal protective equipment (PPE) training was provided to all healthcare personnel. Our PPE includes an N95 mask, eye protection with goggles or an eye shield, gown, and gloves. For healthcare workers who have failed their mask fitting, a powered air‐purifying respirator (PAPR) can be used instead of an N95 mask. A PAPR consists of a head hood, a motor‐driven fan, a filter, and a battery. It can also be donned over an N95 mask for aerosol generating procedures in known COVID‐19 patients. PAPR training was also provided to the healthcare personnel. Specific guidelines for the use of PPE and/or PAPR are outlined in Table 1. In situ simulations were scheduled to enable staff to familiarize themselves with PAPR use and the management of resuscitation of COVID‐19 patients. To date, there have been no nosocomial infections with these infection control measures in our hospital.
Table 1.
Hospital Personal Protective Equipment (PPE) Policy as on Mar 12, 2020
| Clinical Areas | Management | Recommended use of PPE | Frequency of PPE use |
|---|---|---|---|
| Operating Room | Aerosol generating procedures a in confirmed or suspected cases |
Full PPE (N95 mask, gown, gloves, eye protection), and powered air‐purifying respirator (PAPR) should be used. Staff who have failed N95 mask fitting should use PAPR |
Extended use up to 6 h for mask and eye protection. Single use for gown and gloves. |
| All other procedures | Full PPE (N95 mask, gown, gloves, eye protection) | Extended use up to 6 h for mask and eye protection. Single use for gown and gloves. | |
| PICU | Rooms with suspected/confirmed cases | Full PPE (N95 mask, gown, gloves, eye protection) | Extended use up to 6 h for mask and eye protection. Single use for gown, gloves. |
| Rooms without suspected/confirmed cases | Surgical mask | Extended use up to 6 h | |
| All other areas on PICU | Surgical mask | Single use |
Abbreviations: PAPR, powered air‐purifying respirator; PPE, personal protective equipment.
Intubation, extubation, non‐invasive ventilation, tracheostomy, high‐flow nasal oxygen, cardiopulmonary resuscitation prior to intubation.
3. OPERATING ROOM
The OR workflow for a suspected or confirmed COVID‐19 pediatric patient is the same as for an adult patient in our hospital. 14 All surgeries are done in a designated negative pressure OR with a designated access route separate from non‐COVID‐19 patients. Minimal equipment and personnel should be allowed inside the OR to avoid contamination and reduce exposure. A dedicated anesthetic machine with a new breathing circuit, heat and moisture exchanger filter, closed, in‐line tracheal suctioning, and soda lime should be in place. Intubation and drug trolleys should be prepared prior to patient arrival. Disposable equipment should be used as much as possible.
All OR personnel are required to wear full PPE for confirmed or suspected COVID‐19 patients (Table 1). The anesthesiologist and anesthesia nurse must wear full PPE (N95 mask, gown, gloves, and eye protection) and a PAPR to minimize aerosol transmission during intubation, extubation or for other aerosol generating procedures (Figure 1). For all asymptomatic patients without known COVID‐19 disease, an N95 mask and eye protection (goggles) are worn by the anesthesiologist and anesthesia nurse. The PAPR hood with goggles may blur the view and make airway management potentially challenging (Figure 1). Airway management should be done by the most experienced anesthesiologist present using a disposable pediatric video laryngoscope blade to minimize exposure to respiratory secretions. 15 Closed breathing systems should be considered instead of an Ayre's T‐piece. Rapid sequence intubation may be preferred to avoid mask ventilation and possible aerosol generation.
Figure 1.
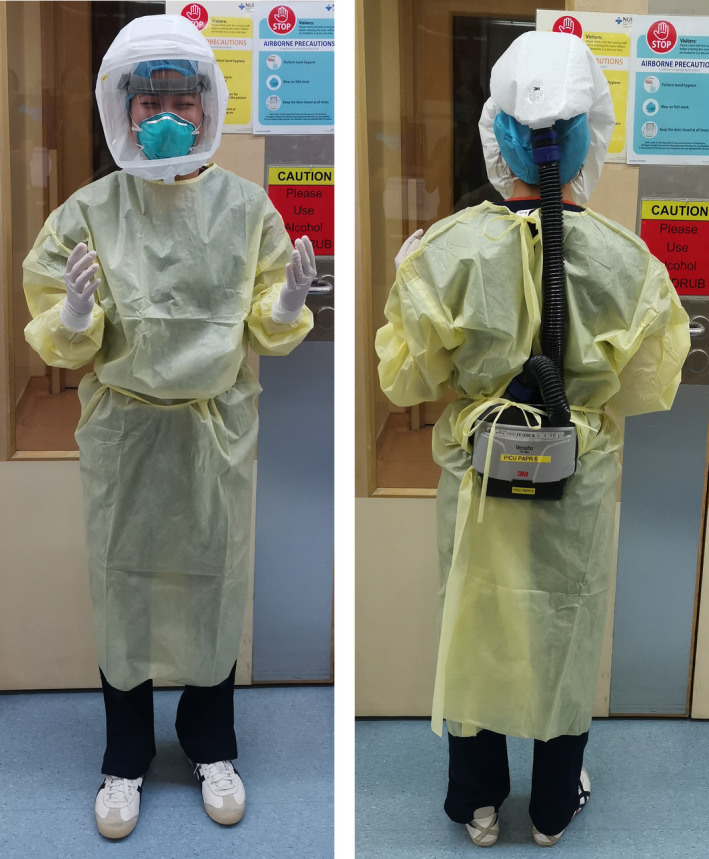
Powered air‐purifying respirator (PAPR) with personal protective equipment (PPE)
The preoperative review, induction, and recovery of the pediatric patient should be done in the negative pressure OR itself. One parent accompanies the child into the OR and leaves the room as soon as the child is asleep. Both the child and the parent are to wear a surgical face mask prior to entering the OR. Recovery of the patient should be undertaken in the operating theater itself before being transferred to the ward via the designated access route. Any unused drugs and consumables should be thrown away at the end of the operation. Disinfection guidelines should be strictly followed for cleaning of the OR and anesthesiology equipment. The need for additional staff (eg, OR coordinator) and increased turnaround times for decontamination and showering for staff should also be considered. A summary of the anesthesia considerations in the OR is provided in Table 2.
Table 2.
Anesthetic and PICU considerations for COVID‐19‐positive pediatric patients
| Anesthesia considerations in the OR |
|
| Pediatric intensive care unit considerations |
|
Abbreviations: BVM, bag valve mask; HEPA, high‐efficiency particulate air; OR, operating room; PAPR, powered air‐purifying respirator; PPE, personal protective equipment.
4. PEDIATRIC INTENSIVE CARE UNIT
All patients presenting with an upper respiratory infection requiring hospital admission, or with a lower respiratory infection, are considered to be a suspected COVID‐19 patient. Therefore, according to this definition, children presenting with common pediatric illnesses, such as bronchiolitis or bronchitis, would be treated as a suspected case. These children would be admitted to a negative pressure room, and as a result, PICU beds are often used for isolation purposes. All suspected cases will undergo 2 nasopharyngeal swabs for COVID‐19 during their admission in PICU, each separated by a 24‐hour interval. They are only de‐isolated when both of their swab results are negative. This is in line with the observation that initial SARS‐CoV‐2 testing by RT‐PCR can be falsely negative in the early phase of illness. 14 While the confirmed COVID‐19 rate is low, the burden of care lies in the management of suspected cases in the initial 48‐hour period prior to the swab results.
All our negative pressure rooms have a dedicated anteroom, and the patient is given one‐to‐one nursing. Each child can only be accompanied by one caregiver who is provided with a surgical facemask and advised to stay in the room with the child at all times. Most of our negative pressure rooms have en suite bathrooms to minimize the movement of both parent and child on the ward.
Healthcare workers are required to don PPE before entering the patient's room for physical examinations and other clinical work. The use of PAPR in addition to PPE is recommended for all trained personnel performing aerosol generating procedures such as suctioning, tracheal intubation, and bronchoscopy. Nebulization should be avoided due to the risk of aerosolization of infected droplets. 15 Instead, metered dose inhalers are preferred, especially because both modes of delivery for beta‐agonist have similar efficacy. 16 , 17
Depending on the severity of the respiratory illness, early intubation is recommended in our center, due to risk of aerosolization of respiratory droplets with other forms of non‐invasive ventilation. This is done to protect healthcare workers, as well as avoiding emergency intubations while having to rapidly don PPE. This practice has raised some concerns in the PICU community as it is difficult to differentiate COVID‐19 infection from other viral illnesses before the nasopharyngeal swab results are confirmed. It would be difficult to justify the morbidity associated with invasive ventilation in this group of patients. Active heated humidification is avoided in children with suspected or confirmed COVID‐19 because there is a concern that respiratory droplets could form and condensate within the ventilatory circuits. These droplets could potentially be aerosolized from the expiratory limb of non‐invasive ventilation circuits, or when open suctioning is performed. In addition, these droplets could also travel backward and accumulate inside the ventilator itself when the circuit limbs are handled, potentially contaminating the ventilators. Disposable mechanical bacterial filters that are hydrophobic and have greater than 99.99% filtration efficacy for bacteria and viruses are attached to both the inspiratory and expiratory limb of the mechanical ventilators. Closed in‐line suction is recommended for all suspected cases.
Special consideration should be given to pediatric patients who are on long‐term home ventilation. They are often admitted for respiratory illnesses such as aspiration pneumonia and would fulfill Singapore's case definition for a suspected case. At present, our practice so far has been to allow these children to remain on non‐invasive ventilation while waiting for swab results if their respiratory illnesses are mild.
Under normal circumstances, the use of extracorporeal membrane oxygenation (ECMO) would be considered for children with severe respiratory disease requiring high ventilatory pressures. In large outbreaks like COVID‐19, the use of ECMO for confirmed patients who are severely ill may offer limited benefit. 18 Cannulation is likely to be challenging given the need to wear PPE, the difficulty of communicating in a large group in PPE, and the technical difficulty the cannulating surgeon may face operating in PPE.
Simulation training has been conducted within the PICU for emergency airway management for COVID‐19 patients. Intubation of these patients is a high‐risk procedure and requires early anticipatory planning as it is difficult to obtain extra equipment and medication while in the patient's room. We have implemented the use of an intubation checklist, prepacked emergency drugs as well as resuscitation algorithms placed both inside and outside the rooms to facilitate the process. We have also found communication to be especially challenging during these simulation training sessions due to the inability to hear through the PAPR and limitation of healthcare worker's movement while wearing PAPR. In order to reduce the amount of movement and to facilitate communication, a whiteboard with markers has since been provided to mitigate this issue. Curtains are also kept open during resuscitation to facilitate communication with colleagues outside of the negative pressure room via a whiteboard.
5. CODE BLUE WORKFLOW
The pediatric code blue workflow has also been revisited in light of COVID‐19. The code blue team consists of 2 doctors, 1 nurse, and 1 pediatric respiratory therapist. A box containing multiple sets of PPEs, a disposable bag valve mask, and airway equipment has been prepacked. Team members are all required to wear full PPE. The most senior physician is responsible for the airway and should don PAPR in addition to PPE. We should ideally avoid bagging the patient. However, if necessary, a high‐efficiency particulate air (HEPA) filter should be attached between the mask and bag, and manual ventilation with small tidal volumes are given. Transport of patients to PICU is accompanied by security personnel, to ensure that the route is clear of other patients, staff, and visitors.
CONFLICTS OF INTEREST
The authors report no conflict of interest.
ACKNOWLEDGMENT
We would like to acknowledge our colleagues in the Department of Anesthesia and Pediatric Intensive Care.
Thampi S, Yap A, Fan L, Ong J. Special considerations for the management of COVID‐19 pediatric patients in the operating room and pediatric intensive care unit in a tertiary hospital in Singapore. Pediatr Anesth. 2020;30:642–646. 10.1111/pan.13863
Section Editor: Britta S. von Ungern‐Sternberg
REFERENCES
- 1. WHO . Novel coronavirus – China. Jan 12, 2020. http://www.who.int/csr/don/12‐january‐2020‐novel‐coronavirus‐china/en/ Date accessed 18 Mar 2020.
- 2. Outbreak of acute respiratory syndrome associated with a novel coronavirus, Wuhan, China first update 22 January 2020. https://www.ecdc.europa.eu/sites/default/files/documents/Risk‐assessment‐pneumonia‐Wuhan‐China‐22‐Jan‐2020.pdf. Date accessed 18 Mar 2020.
- 3. WHO . Coronavirus disease 2019 (COVID‐19) Situation Report –63, World Health Organization. https://www.who.int/docs/default‐source/coronaviruse/situation‐reports/20200323‐sitrep‐63‐covid‐19.pdf?sfvrsn=d97cb6dd_2 Accessed 24 Mar 2020).
- 4. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shen KYY, Wang T, Zhao D, et al. Diagnosis, treatment, and prevention of 2019 novel coronavirus infection in children: experts’ consensus statement. World Journal of Pediatrics. 2020;7:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. WHO . Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. Dec 31, 2003. https://www.who.int/csr/sars/country/table2004_04_21/en/ (accessed Mar 18, 2020).
- 7. WHO . Middle East respiratory syndrome coronavirus (MERS‐CoV). November, 2019. http://www.who.int/emergencies/mers‐cov/en/ (accessed Mar 18, 2020).
- 8. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239. [DOI] [PubMed] [Google Scholar]
- 9. Dong YMX, Hu Y, Hu Y, et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics. 2020;e20200702. 10.1542/peds.2020-0702 [DOI] [PubMed] [Google Scholar]
- 10. Liu W, Zhang Q, Chen J, et al. Detection of COVID‐19 in children in early January 2020 in Wuhan, China. N Engl J Med. 2020;382(14):1370‐1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pung HCC, Young B, Young BE, et al. Investigation of three clusters of COVID‐19 in Singapore: implications for surveillance and response measures. Lancet. 2020;395(10229):1039‐1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Singapore MoH . https://www.moh.gov.sg/covid‐19. Date accessed ( 24 Mar 2020).
- 13. Wong J, Goh QY, Tan Z, et al. Preparing for a COVID‐19 pandemic: a review of operating room outbreak response measures in a large tertiary hospital in Singapore. Can J Anaesth. 2020;1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bai YYL, Wei T, Wei T, et al. Presumed asymptomatic carrier transmission of COVID‐19. JAMA. 2020. 10.1001/jama.2020.2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hui DS, Chow BK, Chu LCY, et al. Exhaled air and aerosolized droplet dispersion during application of a jet nebulizer. Chest. 2009;135(3):648‐654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Batra V, Sethi GR, Sachdev HP. Comparative efficacy of jet nebulizer and metered dose inhaler with spacer device in the treatment of acute asthma. Indian Pediatr. 1997;34(6):497‐503. [PubMed] [Google Scholar]
- 17. Mitselou N, Hedlin G, Hederos CA. Spacers versus nebulizers in treatment of acute asthma ‐ a prospective randomized study in preschool children. J Asthma. 2016;53(10):1059‐1062. [DOI] [PubMed] [Google Scholar]
- 18. MacLaren GFD, Fisher D, Brodie D. Preparing for the most critically Ill patients with COVID‐19: the potential role of extracorporeal membrane oxygenation. JAMA. 2020;323(13):1245. [DOI] [PubMed] [Google Scholar]


