Summary
In December 2019, a cluster of atypical pneumonia cases were reported in Wuhan, China, and a novel coronavirus elucidated as the aetiologic agent. Although most initial cases occurred in China, the disease, termed coronavirus disease 2019, has become a pandemic and continues to spread rapidly with human‐to‐human transmission in many countries. This is the third novel coronavirus outbreak in the last two decades and presents an ensuing healthcare resource burden that threatens to overwhelm available healthcare resources. A study of the initial Chinese response has shown that there is a significant positive association between coronavirus disease 2019 mortality and healthcare resource burden. Based on the Chinese experience, some 19% of coronavirus disease 2019 cases develop severe or critical disease. This results in a need for adequate preparation and mobilisation of critical care resources to anticipate and adapt to a surge in coronavirus disease 2019 case‐load in order to mitigate morbidity and mortality. In this article, we discuss some of the peri‐operative and critical care resource planning considerations and management strategies employed in a tertiary academic medical centre in Singapore in response to the coronavirus disease 2019 outbreak.
Keywords: COVID‐19, critical care, healthcare, peri‐operative, resource management
Introduction
The responsible pathogen for coronavirus disease 2019 (COVID‐19), severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is an enveloped, RNA betacoronavirus 1, 2, 3, that is rapidly spreading globally. An estimated 14–19% of patients develop severe or critical disease with acute respiratory distress syndrome, septic shock and multi‐organ dysfunction or failure 4, 5, 6. In Italy, 16% of hospitalised COVID‐19 patients required intensive care 7. SARS‐CoV‐2 has a basic reproduction number of 2.2 8 and, in the Chinese experience, 6% of patients required non‐invasive or invasive mechanical ventilation following a median duration of 9 days between symptom onset and critical care admission 5, 9. The number of COVID‐19 patients requiring ventilatory support quickly overwhelmed available intensive care unit (ICU) beds, a problem compounded by a lack of trained personnel and necessary equipment. As a result, just 25% of patients who eventually succumbed to COVID‐19 received tracheal intubation and ventilation 9. At the outbreak epicentre, the case fatality rate showed a seven‐fold increase compared with other regions of China 4, 10, presumably related to healthcare resource shortfall. In their response to the tsunami of infected cases, general ward beds were converted to ICU beds, and general hospitals to critical care hospitals 9. Clearly, critical care will play an important role in the mitigation of COVID‐19, and Table 1 compares the critical care facilities and current COVID‐19 incidence in Singapore, China and some of the hardest hit countries in Europe. The high mortality rates in Italy compared with Germany may reflect a relationship between healthcare resource availability and outcomes. Northern Italy healthcare resources were overwhelmed by the sudden surge of patients 7, 11, compared with a more widespread distribution of cases all over Germany, allowing for better resource utilisation. Germany also has a higher number of critical care beds per 100,000 population compared with Italy 12, again suggesting the role of adequate healthcare resources in determining outcomes.
Table 1.
| Country | Critical care unit beds | Critical care beds per 100,000 population | Total cases | Total deaths | Case‐fatality rate |
|---|---|---|---|---|---|
| Singapore | 671 | 11.4 | 683 | 2 | 0.3% |
| China | 49,453 | 3.6 | 82,078 | 3,298 | 4.0% |
| France | 7,540 | 11.6 | 28,786 | 1,695 | 5.9% |
| Germany | 23,890 | 29.2 | 42,288 | 253 | 0.6% |
| Italy | 7,550 | 12.5 | 80,539 | 8,165 | 10.1% |
| Spain | 4,479 | 9.7 | 56,188 | 4,089 | 7.3% |
| UK | 4,114 | 6.6 | 11,662 | 578 | 5.0% |
Singapore is a city‐state with a land area of 724.2 square km, and a total population of 5.7 million 13, with a doctor‐to‐population and nurse‐to‐population ratio of 1:410 and 1:134, respectively 14. The first locally reported case was diagnosed on 23 January 2020 15, 16, with community transmission reported. Within a month, Singapore had more COVID‐19 cases than any single country outside mainland China 16, 17. Building on lessons from the Chinese experience and response to the outbreak, early strategic planning and preparation is required to ensure adequate health‐system resource availability and capacity for continued care provision, particularly in the context of a small, densely populated island nation. We describe the experience of a 1250‐bed tertiary academic medical centre in Singapore, and the planning considerations and strategies of our ongoing care continuity planning in response to the COVID‐19 outbreak.
Containment strategies
A skilled, healthy and well‐equipped workforce constitutes the backbone of any healthcare system. Apart from community spread, healthcare workers are exposed to the additional risk of nosocomial transmission of COVID‐19, with infected healthcare workers comprising 2.7–3.8% of cases 4, 19. During the severe acute respiratory syndrome (SARS) outbreak in 2003, healthcare workers accounted for 21.1% of cases worldwide 20, with a higher mortality in hospital personnel 21; thus, appropriate measures are required to minimise out‐of‐hospital and in‐hospital exposure.
As part of broader public health responses to the outbreak, overseas travel was restricted, and a mandatory 14‐day stay‐home notice imposed on returning healthcare workers before resuming clinical duties. Additionally, in accordance with government measures to control the spread of COVID‐19, all individuals with acute upper respiratory tract symptoms were issued 5 days of medical leave, during which leaving their accommodation was prohibited except to seek medical attention. Such measures further aggravated the manpower situation, with hospital departments facing sudden staffing shortages. Local and overseas conference leave was suspended due to the transmission risk during large‐group events. All overseas travel plans required prior approval, and as the global pandemic evolved, travel bans were implemented.
As COVID‐19 case numbers escalated with human‐to‐human transmission within the community, staff‐segregation and social distancing measures were implemented (Table 2). All staff monitored and reported their temperature twice a day into a monitored electronic health record, and staff members who developed fever or symptoms of acute respiratory illness were screened at the staff and occupational health clinic or emergency department. Throat swabs were taken if symptomatic or with a history of unprotected exposure. Unwell personnel were discouraged from coming to work.
Table 2.
Segregation and social distancing measures
| Considerations | Practical measures |
|---|---|
| Inter‐hospital segregation |
Personnel with duties at > 1 hospital restricted to one hospital. 14‐day quarantine period for personnel redeployed from one hospital to another where possible, allows time for symptom manifestation to reduce the risk of nosocomial transmission to the receiving hospital from asymptomatic individuals incubating the virus. Suspension of visiting specialists and locums who are employed at > 1 hospital. Restrict unnecessary medical devices or drug representative entry. |
| Intra‐hospital segregation |
Departmental segregation into teams with an equivalent number of personnel and skill mix. Separate ward and operating theatre teams. Separate personnel caring for suspect or diagnosed COVID‐19 patients from those providing routine care. Shift or rotation system. |
| Reduced face‐to‐face interaction |
Reduce unnecessary at‐work and after‐work social interaction. Creation of additional, separate rest areas with adequate distancing between individuals of 1 metre. Staggered meal times and avoidance of food sharing. Suspension of face‐to‐face group gatherings including department teaching events and meetings. Use of web‐based conferencing applications for discussions. |
A level of personal protective equipment (PPE) appropriate to the outbreak situation is required for protection of staff. All staff underwent an N95 respirator fit test and received small‐group, ward‐based training in safe donning and doffing of PPE. Our institutional practice for PPE in the context of the pandemic is summarised in Table 3.
Table 3.
Institutional guidelines on use of personal protective equipment for healthcare workers
| Patient category | Risk activity | Recommendation |
|---|---|---|
| Suspected or diagnosed COVID‐19 patients | Non‐AGP (including regional anaesthesia and monitored anaesthetic care). |
Negative‐pressure environment with a high rate of air exchanges (20 per hour) preferred. Full PPE (i.e. N95 respirator, eye‐protection, cap, gloves, fluid‐resistant gown and boot covers). |
| AGP (including intubation, extubation, supraglottic airway insertion and removal, bronchoscopy and airway surgery). |
Negative‐pressure environment with a high rate of air exchanges (20 per hour) preferred. Full PPE and PAPR are provided for all personnel within 2 m. Keep number of personnel in the room minimum during AGPs. Surgeons, scrub nurses and operating room assistants to wait outside, with PPE donned during AGPs. Only two staff to manage the airway. The most experienced anaesthetist or intensivist to instrument the airway. |
|
| Asymptomatic and non‐suspect patients | Non‐AGP (including regional anaesthesia and monitored anaesthetic care). | Surgical masks. |
| AGP (including intubation, extubation, supraglottic airway insertion and removal, bronchoscopy and airway surgery). | N95 respirators and goggles or face‐shield. |
AGP, aerosol generating procedure; PPE, personal protective equipment; PAPR, powered air‐purifying respirator.
While the effects of COVID‐19 infection and its transmissibility are unclear at this moment 22, 23, 24, any pregnant or immunocompromised staff were exempt from the care of suspected or confirmed cases 24, as were staff aged > 55 years.
Avoid overburdening health resources
With community transmission, a tiered, pandemic response plan is required to accommodate an anticipated surge in suspected and diagnosed COVID‐19 patients. This is achieved by resource conservation, further demand reduction by deferment of non‐urgent services, and contingency planning for additional space creation.
The COVID‐19 crisis couples dysfunctional logistic supply chains with a surge in demand for consumables and disposable single‐use equipment such as PPE, oxygen delivery systems and laryngoscopy blades, in addition to physiological monitors, invasive monitoring systems, closed‐circuit ventilators, extracorporeal support systems, mechanical circulatory support and infusion devices. We recommend several measures to maintain stockpiles and optimise usage (Table 4).
Table 4.
Recommendations to optimise institutional stockpiles and inventory
| Preparation |
Early inventory assessment and identification of alternative logistic channels for procurement once a potential pandemic threat is identified. Inventory transparency is required to ensure equal distribution and identification of deficiencies. |
| Ration |
Conduct rationing or controlled distribution for scarce commodities (e.g. facemasks, face shields, N95 respirators and PPE). Eliminate non‐essential staff for tasks or procedures where possible to reduce PPE required. |
| Conserve |
Avoid unnecessary use of equipment, drugs and disposables. Avoid unnecessary blood and blood component transfusions, consider recombinant blood product therapy and other blood conservation techniques. Cancel non‐essential, non‐emergency procedures and services. Protocols for care, stick to evidence‐based recommendations. |
| Prioritise use |
Reserve disposable single‐use equipment (e.g. videolaryngoscopy blades, breathing circuits) for suspected and confirmed COVID‐19 patients. ICU ventilators should be used for patients with ARDS requiring high ventilatory settings or advanced ventilatory strategies. Portable ventilators or anaesthetic machines can be used for other patients with lower requirements. |
| Triage | Triage and prioritisation of COVID‐19 patients based on severity and prognosis may be required for resource allocation when levels become critical. |
| Extend supply | With appropriate precautions, such as keeping masks and respirators in containers between uses during a shift, the shelf‐life of certain disposables can be prolonged for limited re‐use. |
| Mobilise reserves | Activation of institutional emergency‐preparedness stockpiles reserved for mass casualty incidents. |
| Consider innovation | Accept novel use and re‐purposing of limited available resources with attention to healthcare worker and patient safety. |
| National effort |
Utilise healthcare cluster‐level or national‐level reserves and solutions for procurement. Consider mobilisation of reserves from other organisations such as military disaster‐relief and humanitarian aid stockpiles. |
ARDS, acute respiratory distress syndrome; PPE, personal protective equipment.
Surgical procedures utilise significant manpower, consumables, bed space and critical care facilities – resources which can be reallocated for COVID‐19 patients. Additionally, general anaesthesia may involve aerosol‐generating procedures, is a potential source of in‐hospital transmission, and requires appropriate PPE – a precious commodity during a pandemic. We rescheduled elective surgical procedures that require postoperative inpatient beds, particularly those which require postoperative ICU or high dependency care. Regional anaesthesia is preferred to avoid aerosol generation associated with airway instrumentation. Patients who require critical care for postoperative monitoring underwent extended recovery in the post‐anaesthetic care unit. As the outbreak progressed, more operating theatres were closed to increase capacity. Only urgent, emergency and ambulatory surgeries (Fig. 1) were allowed to proceed; capacity thresholds below which further reductions in ambulatory procedures, and urgent or emergency surgery such as oncological and cardiac surgery, need to be identified. These measures allow for bed space redundancy in anticipation of a surge in COVID‐19 case‐load. Decreased elective case‐load also allows increased operating theatre turnaround time for cleaning, decontamination, isolated recovery of infected patients and allows smaller segregated teams to continue service provision.
Figure 1.
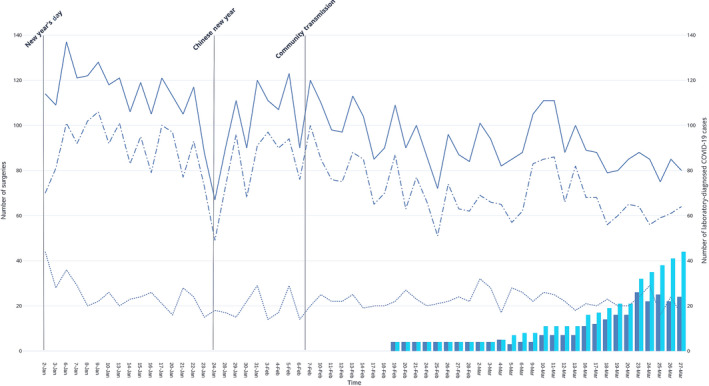
Reduction of daily total (solid line), elective (dots and dashes) and emergency (dotted line) surgical case‐load in relation to number of admitted (blue bars) and cumulative total (teal bars) coronavirus disease 2019 patients. Elective surgeries are not performed on weekends and national holidays, which have been omitted from the x‐axis. ( ) Total surgeries; (
) Total surgeries; ( )Elective surgeries; (
)Elective surgeries; ( )Emergency surgeries; (
)Emergency surgeries; ( ) Admitted COVID‐19 patients; (
) Admitted COVID‐19 patients; ( )Cumulative total COVID‐19 patients.
)Cumulative total COVID‐19 patients.
The COVID‐19 pandemic can rapidly overwhelm available critical care and isolation units, necessitating a tiered response plan for space generation. High dependency units (HDUs) are typically equipped with the necessary infrastructure (oxygen and air outlets, wall‐mounted suction, sufficient electrical outlets) and advanced physiological monitoring for up‐scaling into ICU facilities. With a drop in surgical demand, facilities such as post‐anaesthetic recovery areas and operating theatres are also attractive for repurposing into HDUs and ICUs due to high staffing‐ratios, infrastructure and access to airway equipment, ventilators and advanced physiological monitoring. Additionally, operating theatres are single rooms with high efficiency particulate air (HEPA) filters, a high air exchange rate which can potentially reduce viral particles. They also have the physical space for extracorporeal life support systems. Where possible, we utilise single rooms, with negative pressure capabilities for COVID‐19 patients. Once full, and nursing in an open‐floor plan is required, separation of 2 m between patients is recommended 25.
In our model, we converted one of three operating theatre complexes into an expanded ICU (Fig. 2) to create an additional 42% critical care bed space (Figs. 3 and 4) to transfer HDU cases and facilitate conversion of HDUs into cohort ICUs. This utilised a phased implementation plan where an 80% occupancy rate of existing critical care capacity would trigger activation of the next tier. This trigger level is adjustable to be appropriate for outbreak tempo, with completion of escalation into the next tier within a 24‐h time frame as a planning norm.
Figure 2.
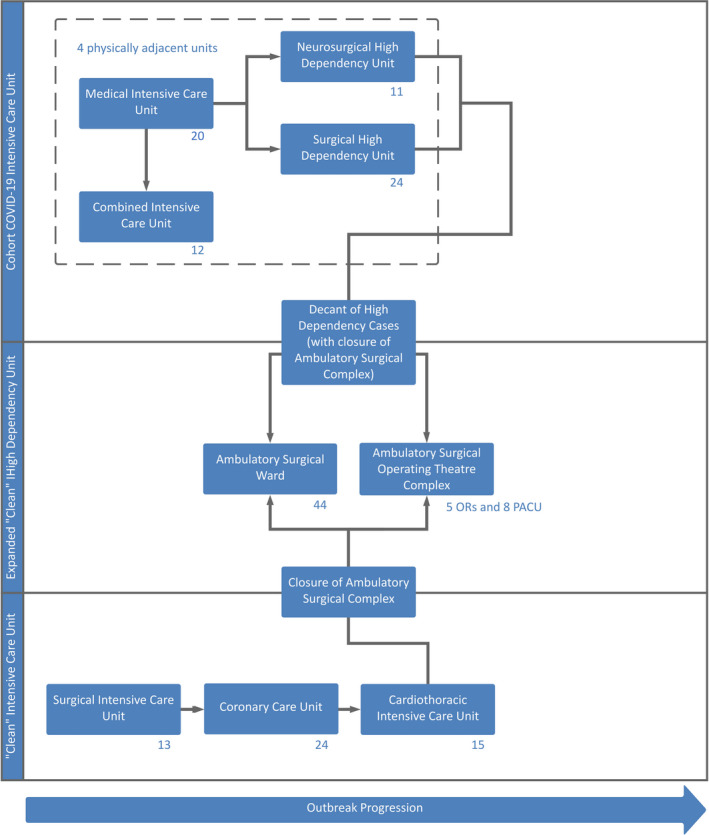
Pandemic expansion schematic for generation of critical care bed space, involving expansion of the cohort intensive care unit into three other adjacent units, with decanting of the high dependency units into the repurposed ambulatory surgical complex. Bed numbers are indicated at the lower right of each unit, representing a total of 176 critical care beds. Each arrow represents activation of the next tier, triggered at 80% capacity of the previous tier. OR, operating room; PACU, post‐anaesthesia care unit.
Figure 3.
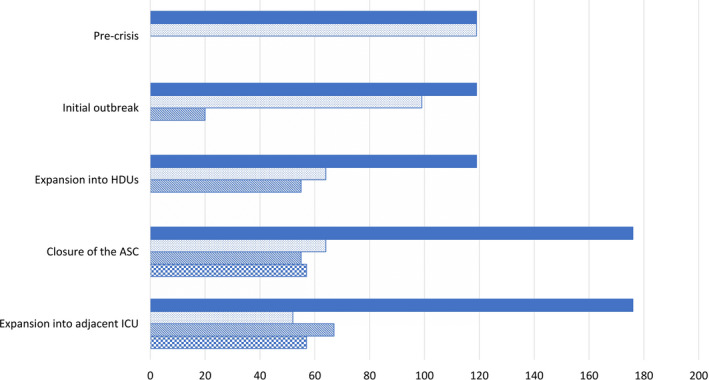
Tiered generation of additional total critical care unit bed space (shaded bars) compared to pre‐crisis levels. Changes in the proportion of clean intensive care unit (ICU) (dotted bars), cohort ICU (striped bars) and expanded clean High dependency units (checked bars) beds are demonstrated according to according to implementation phase. Abbreviations: ambulatory surgical complex (ASC). ( ) total critical care unit bed space; (
) total critical care unit bed space; ( )clean ICU bed space; (
)clean ICU bed space; ( ) cohort ICU bed space; (
) cohort ICU bed space; ( ) expanded clean HDU bed space.
) expanded clean HDU bed space.
Figure 4.
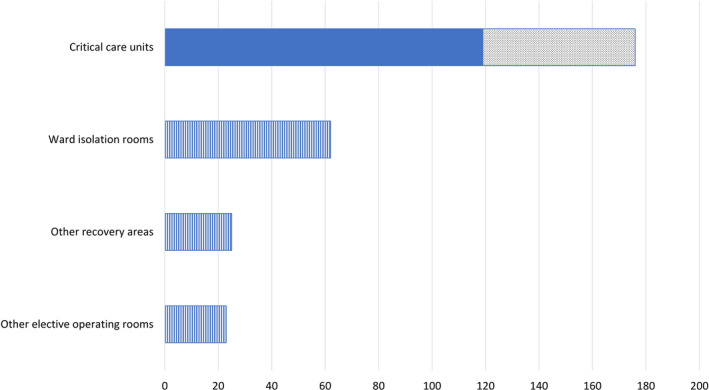
Predicted generation of additional critical care unit bed space (dotted area), compared to pre‐crisis baseline (shaded areas), based on the conversion of the ambulatory surgical complex into a high dependency unit for decanted patients. Additional facilities (striped areas) that may be utilised include re‐purposing suitable ward isolation rooms, elective operating rooms and post‐anaesthetic recovery areas as the pandemic progresses. ( ) pre‐crisis baseline; (
) pre‐crisis baseline; ( )additional critical care unit bed space; (
)additional critical care unit bed space; ( ) additional facilities that may be utilised.
) additional facilities that may be utilised.
A robust system for horizontal and vertical transferring of patients is required as ICUs are converted to cohort ICUs, HDUs to ICUs, and ward and surgical facilities to HDUs. Suitable isolation wards and general wards (which typically have open‐floor plans) with appropriate infrastructure can be converted into HDUs for non‐COVID patients to allow transfer of patients from repurposed HDUs.
Optimisation of healthcare resources
Adequately trained and experienced physicians and nurses supported by an allied health team comprising physiotherapists, pharmacists, respiratory therapists and perfusionists will be required to provide high‐quality peri‐operative and critical care. However, high acuity patient care demands high staffing ratios and a high relief factor to allow for redundancy. We propose some measures adopted in our model to redeploy staff to address the manpower surge as additional ICU beds are created to cater for an increased number of critically ill patients. Based on pre‐crisis institutional planning norms, we use a ratio of approximately one intensivist and one anaesthetist or medical registrar per unit, supported by three resident grade junior physicians for every 10–15 patients in an ICU setting. In our model, we sourced medical manpower from several areas (Table 5) to ensure an adequately staffed critical care workforce at all seniority levels for uninterrupted care (Fig. 5).
Table 5.
Manpower strategies to maintain critical care staffing requirements
| Staffing | Potential manpower sources and measures |
|---|---|
| Physician | Redeploy specialists with formal ICU training and accreditation, but not currently working in critical care units such as anaesthetists and pulmonologists into ICUs. |
| Anaesthetists without formal critical care accreditation but recent, substantial critical care experience may take certain supervisory and leadership roles in the ICU under supervision by an accredited intensivist. | |
| Anaesthetists without formal critical care accreditation and no recent critical care experience may assist in ICU care at registrar level. | |
| Redeploy anaesthesia and medical registrars and trainees with critical care experience and training to ICUs and HDUs, especially with a reduction in surgical load. | |
| Junior physicians from other disciplines with critical care or ICU exposure as part of their training programme such as general surgical trainees who can assist in administrative duties and basic ICU‐related care under direct supervision of an intensivist. | |
| Step‐down of specialist physicians of other disciplines and surgeons to assist in ICU* and HDU duties in appropriate capacities. | |
| Nursing | Redeploy nursing staff with prior critical care training and experience, but not currently working in critical care areas. |
| Redeploy nursing staff currently working in HDUs into ICUs. | |
| Nurses from surgical units such as anaesthetic and recovery nurses who are familiar with a critical care environment and advanced physiological monitoring, may be deployed into ICUs and HDUs. | |
| Nurses from other backgrounds such as outpatient clinics, which will see an anticipated drop in case load can be redeployed into general wards or HDUs. | |
| Agency nurses not part of the hospital permanent nursing staff can assist in general wards. | |
| General | Redeployment of manpower from wards and clinics. |
| Reduce during office hours manpower with a reduction in non‐essential services, and conversion to a shift‐work system. | |
| Conversion to longer shifts (e.g. 8–12‐h shifts), and accept a reduced staffing relief factor. | |
| Recall of medical staff on overseas training programmes. | |
| Recall of staff on pre‐approved annual leave. | |
| Utilisation of retired staff with valid certifications, and preferably recent experience. | |
| Utilisation of personnel in administrative roles such as research or education. | |
| Consider redeploying personnel currently in private practice or other agencies into public health institutions. |
ICU, intensive care unit; HDU, high dependency unit.
Figure 5.
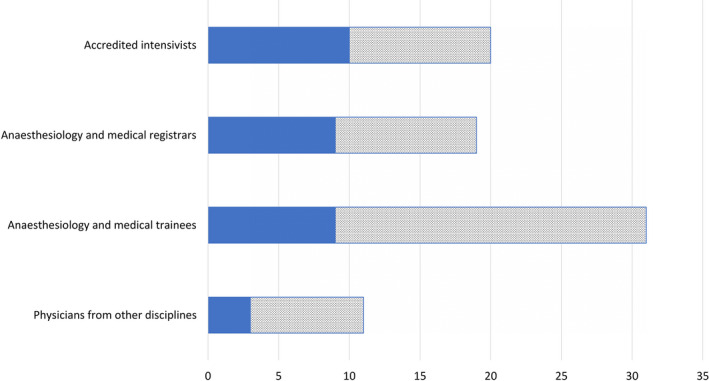
Current pre‐crisis (solid fill) and projected increase (dotted fill) physician manpower for additional critical care areas, assuming maintenance of patient care. ( ) pre‐crisis physician manpower; (
) pre‐crisis physician manpower; ( )projected physician manpower.
)projected physician manpower.
The ideal nurse‐to‐patient ratio of 1:1 for critical care is impossible to maintain in an outbreak (Fig. 6). Additionally, critical care nurses with specialised skills and experience, such as the care of patients on extracorporeal membrane oxygenation (ECMO) 26, 27, are required for managing COVID‐19 and its complications. We propose several measures (Table 5) to mitigate this shortfall to maintain a staffing ratio between 1:1 and 1:1.3. Critical care nurses may be up‐skilled to manage ICU‐specific interventions such as renal replacement therapy and ECMO. Additionally, they may supervise patient care and tasks for multiple patients performed by auxiliary, non‐critical care nursing staff, rather than managing all care aspects for a single patient. Nursing staff who have critical care training or experience but not in current critical care practice are provided refresher training to ensure competency. Nursing staff from other disciplines are trained and up‐skilled for deployment to general wards and HDUs to fill the gaps created from manpower shifts, or into ICUs to perform non‐ICU‐related nursing duties such as administrative tasks and routine nursing care.
Figure 6.
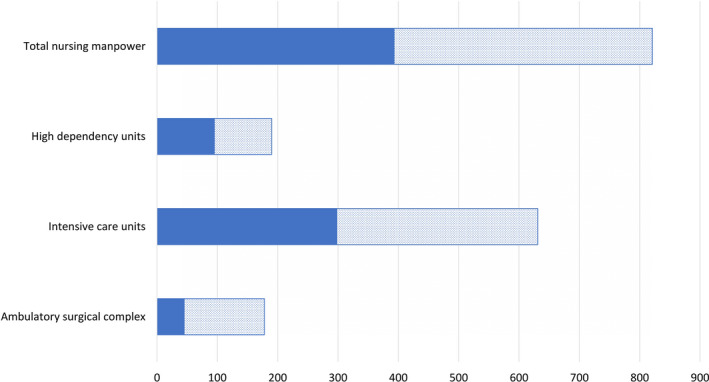
Current pre‐crisis (solid fill) and projected increase (dotted fill) nursing manpower for additional critical care areas, assuming maintenance of patient care. ( ) pre‐crisis nursing manpower; (
) pre‐crisis nursing manpower; ( ) projected nursing manpower.
) projected nursing manpower.
Allied health services have varying staffing levels and surge capacity. Suitably trained physiotherapists, dieticians and pharmacists may be diverted from ward duties. However, respiratory therapists and perfusionists may be employed in smaller numbers and will be spread thin across expanded critical care units. Whereas similar measures such as those listed above can be used to ramp‐up allied health manpower, allied health services will not have the benefit of such staffing numbers where simple redeployment, shift changes and personnel recall alone will suffice. Surge capacity to care for patients on ECMO and emergencies was created by reducing elective cardiac surgery and staggering start times between separate operating theatres in the morning and afternoon such that only one perfusion team supported pre‐planned surgery at any time (as opposed to having multiple teams run concurrently).
Healthcare workers are at risk of in‐hospital transmission due to contact with COVID‐19 patients, and involvement in aerosol‐generating procedures (e.g. intubation and bronchoscopy), sometimes under emergent conditions. Thus, adequate protection and training for COVID‐19 specific scenarios is required. In addition to PPE, training in powered air‐purifying respirators was required for all healthcare workers expected to attend to infected patients. SARS‐CoV‐2 may remain viable in aerosols for up to 3 h, and up to 72 h on plastic 28. Hence, strict compliance to infection control and hand hygiene is important, and surfaces have to be thoroughly wiped‐down after use with appropriate disinfectants.
We propose a differential training programme with early rotation of anaesthetists without critical care accreditation for re‐acclimatisation, refresher training and up‐skilling on advanced ICU‐related care such as bed‐side sonography and advanced ventilatory strategies. For trainees and physicians of disciplines other than critical care and anaesthesia, refresher training before redeployment into critical care units focuses on basic ICU‐related care. The syllabus for this refresher training is summarised and appended in the Supporting Information (Appendix S1).
Similarly, an intensive 4‐week training programme was instituted for nurses from non‐critical care backgrounds deployed into ICUs and HDUs. This covered relevant ICU topics such as care bundles, invasive physiological monitoring, ventilatory support, critical care medications and care and monitoring of chest drains. We also propose multidisciplinary, simulation‐based training to prepare staff for attending to emergencies in COVID‐19 29, 30. This builds familiarity with the nuances of performing resuscitation in smaller teams, wearing unfamiliar equipment and identifying mistakes in infection control precautions and clinical management.
During a pandemic, critical care encompasses both ‘routine’ ICU care, and care for COVID‐19 patients. Our pandemic expansion model involves two physically discrete ICU systems – a ‘clean’ ICU providing critical care for non‐COVID‐19 patients, and a cohort ICU for laboratory‐diagnosed cases, to reduce the risk of nosocomial transmission. Cohort ICUs optimise resource utilisation by concentrating necessary manpower, expertise and equipment (such as powered air‐purifying respirators, PPE, ventilators and extracorporeal support devices). Units with single‐patient rooms and appropriate infrastructure are utilised for the expansion of the cohort ICU (Fig. 2), which would also manage COVID‐19 infected obstetric patients, including labour and delivery. The ‘clean’ ICU providing routine care to non‐COVID‐19 cases is physically distant from the cohort ICU, with provision for tier‐based expansion into other units, including an ambulatory surgical complex.
Similar segregation is required in the operating theatre. An operating theatre capable of generating a negative‐pressure environment and equipped with a HEPA filter was designated to handle all suspected and confirmed COVID‐19 patients during the pandemic in order to reduce virus dissemination. The negative‐pressure function of this operating theatre was kept constantly activated during the outbreak. This operating theatre was located in a corner of the operating theatre complex, and could be isolated from other operating theatres with a separate access route for patient ingress and egress, thereby minimising contact with other patients and staff. The same anaesthetic workstation was used for all suspected and diagnosed COVID‐19 patients, with only the breathing circuit and carbon dioxide absorber changed between cases. Whereas fully equipped drug and airway carts are kept in the induction room, only medications and equipment anticipated necessary for the case are brought into the operating theatre to minimise handling and contamination. During surgery, the operating theatre doors are locked with clearly displayed visual alerts to minimise traffic and airflow contamination.
Task‐based teams may be created to improve efficiency by streamlining manpower. As manpower dwindles, we propose creation of small teams responsible for performing the same task across the unit, such as turning patients, checking lines, performing cannulation and tracheal intubation.
Welfare and administration
Healthcare workers face pressure from the risk of infection, isolation, burnout and public discrimination which adversely impact their overall well‐being and potentially their decision‐making ability 31. In one Chinese study, stress‐related symptoms and depression were prevalent in approximately 73.4% and 50.7% of healthcare workers, respectively 32. Psychological intervention teams and telephone hotlines to support healthcare workers were implemented in China 31, 33. Top‐down outbreak response measures with regular government and institutional engagement with encouragement, updates and free‐flow of information have helped healthcare workers to adjust. Gifts, welfare packages and provisions of food, beverages, special privileges and discounts to healthcare workers by commercial entities have helped to boost morale. Adequate rest periods are required for staff, particularly those working in pandemic wards or critical care facilities to mitigate the effects of fatigue. While direct care of COVID‐19 patients itself does not preclude staff from returning home, provision of accommodation was made for overseas staff who require such support, such as those who make border crossings between Singapore and Malaysia during regular commutes, as well as personnel caring for COVID‐19 patients and living with vulnerable populations, such as the immunocompromised or elderly, and are unable to return home. Assistance can also be provided to healthcare workers requiring child care arrangements.
Clear workflows and protocols are essential to streamline processes and reduce ambiguity, particularly with changing dynamics of the COVID‐19 outbreak, and redeployment of unfamiliar staff from other disciplines. COVID‐19‐specific protocols and workflows for handling, testing, transport and management of COVID‐19 patients are required, including early involvement of an airway team for patients with a difficult airway at risk of deterioration, recommendations for specific populations (e.g. gravid patients and children), considerations for ECMO and for inter‐hospital transfer. Additionally, checklists, references and guidance on various ICU‐related topics were made available online to all personnel within critical care departments.
Advanced preparation and planning is only effective if supported by an efficient administrative system to disseminate regular updates to relevant stakeholders. Such systems should also monitor resource utilisation, facilitate resupply and assist the execution of the expansion plan by monitoring occupancy rates and expediting patient movement across facilities. Transfer of a suspected or diagnosed COVID‐19 patient can be facilitated utilising hospital security personnel in full PPE to clear a route from the patient's origin to the destination, ensuring a smooth, unobstructed transfer route with minimal contact with other patients, healthcare workers and visitors in lifts and corridors.
Supporting information
Appendix S1 Proposed training recommendations and syllabi for physicians of other disciplines and anaesthetists not accredited in critical care who are deployed to intensive care units.
Acknowledgements
No external funding or competing interests declared.
Contributor Information
C. C. M. Lee, Email: melvin_cc_lee@nuhs.edu.sg, @MelvinLee_C2.
S. Thampi, @swapna_thampi.
B. Lewin, @blewin.
T. J. D. Lim, @DannyLimTJ.
References
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. New England Journal of Medicine 2020; 382: 727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gorbalenya AE, Baker SC, Baric RS, et al. The species severe acute respiratory syndrome‐related coronavirus: classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nature Microbiology 2020; 5: 536–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chan JFW, Kok KH, Zhu Z, et al. Genomic characterization of the 2019 novel human‐pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerging Microbes and Infections 2020; 9: 221–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in china: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. Journal of the American Medical Association 2020. Epub 24 February. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 5. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respiratory Medicine 2020. Epub 24 February. 10.1016/s2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. New England Journal of Medicine 2020. Epub ahead of print 28 February. 10.1056/nejmoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID‐19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. Journal of the American Medical Association 2020. Epub 13 March. 10.1001/jama.2020.4031 [DOI] [PubMed] [Google Scholar]
- 8. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. New England Journal of Medicine 2020; 382: 1199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xie J, Tong Z, Guan X, Du B, Qiu H, Slutsky AS. Critical care crisis and some recommendations during the COVID‐19 epidemic in China. Intensive Care Medicine 2020; 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ji Y, Ma Z, Peppelenbosch MP, Pan Q. Potential association between COVID‐19 mortality and health‐care resource availability. Lancet Global Health 2020; 8: e480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Armocida B, Formenti B, Ussai S, et al. The Italian health system and the COVID‐19 challenge. Lancet Public Health 2020. Epub ahead of print 25 March. 10.1016/s2468-2667(20)30074-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rhodes A, Ferdinande P, Flaatten H, Guidet B, Metnitz PG, Moreno RP. The variability of critical care bed numbers in Europe. Intensive Care Medicine 2012; 38: 1647–53. [DOI] [PubMed] [Google Scholar]
- 13. Government of Singapore . Population and population structure. 2019. https://www.singstat.gov.sg/find-data/search-by-theme/population/population-and-population-structure/latest-data (accessed 15/03/2020).
- 14. Ministry of Health, Singapore . Health manpower. 2019. https://www.moh.gov.sg/resources-statistics/singapore-health-facts/health-manpower (accessed 15/03/2020).
- 15. Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS‐CoV‐2 in Singapore. Journal of the American Medical Association 2020. Epub 3 March. 10.1001/jama.2020.3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Government of Singapore . Coronavirus disease 2019: cases in Singapore. 2020. https://www.gov.sg/article/covid-19-cases-in-singapore (accessed 17/03/2020).
- 17. World Health Organization . Coronavirus disease (COVID‐2019) situation reports. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. (accessed 27/03/2020).
- 18. Phua J, Faruq M, Kulkarni A, et al. Critical care bed capacity in Asian countries and regions. Critical Care Medicine 2020. Epub 9 January. 10.1097/ccm.0000000000004222 [DOI] [PubMed] [Google Scholar]
- 19. Wang J, Zhou M, Liu F. Reasons for healthcare workers becoming infected with novel coronavirus disease 2019 (COVID‐19) in China. Journal of Hospital Infection 2020. Epub 5 March. 10.1016/j.jhin.2020.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. World Health Organization . Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. 2003. https://www.who.int/csr/sars/country/table 2004‐04_21/en (accessed 17/03/2020).
- 21. Nuttall I, Dye C. The SARS Wake‐Up Call. Science 2013; 339: 1287. [DOI] [PubMed] [Google Scholar]
- 22. Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID‐19 infection in nine pregnant women: a retrospective review of medical records. Lancet 2020; 395: 809–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schmid MB, Fontijn J, Ochsenbein‐Kölble N, Berger C, Bassler D. COVID‐19 in pregnant women. Lancet Infectious Diseases 2020. Epub 17 March. 10.1016/s1473-3099(20)30175-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Royal College of Obstetricians and Gynaecologists . Professional bodies’ response to government coronavirus advice for pregnant women to reduce social contact. 2020. https://www.rcog.org.uk/en/news/professional-bodies-response-to-government-advice-for-pregnant-women-to-self-isolate (accessed 30/03/2020).
- 25. Centers for Disease Control and Prevention . Interim infection prevention and control recommendations for patients with suspected or confirmed coronavirus disease 2019 (COVID‐19) in healthcare settings. 2020. https://www.cdc.gov/coronavirus/2019-ncov/infection-control/control-recommendations.html (accessed 29/03/2020).
- 26. MacLaren G, Fisher D, Brodie D. Preparing for the Most Critically Ill Patients With COVID‐19: the Potential Role of Extracorporeal Membrane Oxygenation. Journal of the American Medical Association 2020. Epub 19 February. 10.1001/jama.2020.2342 [DOI] [PubMed] [Google Scholar]
- 27. Ramanathan K, Antognini D, Combes A, et al. Planning and provision of ECMO services for severe ARDS during the COVID‐19 pandemic and other outbreaks of emerging infectious diseases. Lancet Respiratory Medicine 2020. Epub 20 March. 10.1016/s2213-2600(20)30121-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and Surface Stability of SARS‐CoV‐2 as Compared with SARS‐CoV‐1. New England Journal of Medicine 2020. Epub 17 March. 10.1056/nejmc2004973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abrahamson SD, Canzian S, Brunet F. Using simulation for training and to change protocol during the outbreak of severe acute respiratory syndrome. Critical Care 2005; 10: R3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fregene TE, Nadarajah P, Buckley JF, et al. Use of in situ simulation to evaluate the operational readiness of a high‐consequence infectious disease intensive care unit. Anaesthesia 2020; 75: 733–8. [DOI] [PubMed] [Google Scholar]
- 31. Kang L, Li Y, Hu S, et al. The mental health of medical workers in Wuhan, China dealing with the 2019 novel coronavirus. Lancet Psychiatry 2020; 7: e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu S, Yang L, Zhang C, et al. Online mental health services in China during the COVID‐19 outbreak. Lancet Psychiatry 2020; 7: e17–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen Q, Liang M, Li Y, et al. Mental health care for medical staff in China during the COVID‐19 outbreak. Lancet Psychiatry 2020; 7: e15–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Proposed training recommendations and syllabi for physicians of other disciplines and anaesthetists not accredited in critical care who are deployed to intensive care units.


