Abstract
Our purpose was to evaluate the performance of 11C-choline PET/CT in detecting biochemically recurrent prostate cancer (PCa) in a large non-European cohort (in the context of emerging evidence for prostate-specific membrane antigen PET in this setting) and to map patterns of PCa recurrence. Methods: We retrospectively analyzed 11C-choline PET/CT scans from 287 patients who were enrolled in an imaging protocol based on rising prostate-specific antigen (PSA) levels (mean, 3.43 ng/mL; median, 0.94 ng/mL; range, 0.15–89.91 ng/mL) and suspected recurrent PCa. A total of 187 patients had undergone primary radical prostatectomy (RP) (79/187 had secondary radiotherapy), 30 had undergone primary radiotherapy, and 70 had a persistent PSA elevation after receiving initial treatment (69 after RP, 1 after radiotherapy). The level of suspicion for recurrence on 11C-choline PET/CT was scored (0, negative; 1, equivocal; 2, positive) by 2 readers. The correlation between 11C-choline PET/CT positivity and initial treatment, Gleason score, National Comprehensive Cancer Network stage, PSA level, PSA doubling time, PSA velocity, and time between initial treatment and PET imaging was evaluated. Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE) criteria were used to map 11C-choline recurrence patterns. Results: Considering scores 1 and 2 as positives, consensus between the 2 readers deemed 66% of the 11C-choline PET/CT scans as positive. When sorted by PSA level, 45% of patients with a PSA of less than 0.5 ng/mL, 56% of patients with a PSA of 0.5–0.99 ng/mL, 70% of patients with a PSA of 1.0–1.99 ng/mL, and 90% of patients with a PSA of at least 2.0 ng/mL scored either 1 or 2 on 11C-choline PET/CT scans. When considering scores of 2 only, 11C-choline PET/CT positivity was 54% (28%, 46%, 62%, and 81%, respectively, for patients with PSA < 0.5 ng/mL, 0.5–0.99 ng/mL, 1.0–1.99 ng/mL, and ≥ 2.0 ng/mL). In multivariate analysis, only PSA level was significantly associated with scan positivity. Pattern analysis showed that pelvic lymph nodes were the most common site of recurrence, and 28% of patients had 11C-choline–positive suspected recurrences outside the initial treatment field. Conclusion: 11C-choline PET/CT can detect PCa recurrence even among patients with low PSA levels when interpretation accounts for the clinical context, providing a certain pretest probability. Until prostate-specific membrane antigen agents are fully approved for PCa, choline PET/CT may provide clinical utility.
Keywords: 11C-choline PET/CT, recurrence, prostate cancer, prostate-specific antigen, PSA relapse
Recurrent prostate cancer (PCa) after primary therapy is common (1,2). The choice of local versus systemic treatment in this setting depends on the location of the recurrence (3–5). Hence, early detection of recurrence and differentiation between local and systemic recurrence are important.
Current conventional imaging studies are suboptimal to detect recurrent PCa, especially when prostate-specific antigen (PSA) levels are low (2–4,6,7). In recent years, PET/CT with 11C-choline or 18F-choline has changed the management of recurrent PCa and has been commonly used to detect early recurrence, particularly in Europe (8,9). However, choline PET has a low detection rate for PSA levels of less than 1.0 ng/mL (10–12). More recently, PET/CT with 68Ga- or 18F-prostate-specific membrane antigen (PSMA) agents is gaining traction for detecting PCa recurrence early (13–17) and has replaced choline PET/CT at some institutions. Although PSMA PET is now routinely used in several countries, it is not currently approved for commercial use in the United States and many other countries. In contrast, 11C-choline was approved by the U.S. Food and Drug Administration in 2012 under an investigational-new-drug application (18). Until PSMA agents are fully approved for PCa, choline PET/CT may provide clinical utility in PCa. We therefore conducted a large retrospective analysis of 11C-choline PET/CT data for patients with recurrent PCa.
MATERIALS AND METHODS
Patients
Patients with biochemical recurrence after biopsy-proven PCa initially treated with curative intent were enrolled in an institutional review board–approved 11C-choline PET/CT expanded-access study (protocol 15-117), with a primary objective of localizing sites of recurrence. From our database, we retrieved patients scanned between March 2016 (opening of protocol) and December 2017 and retrospectively analyzed those who were treated initially by radical prostatectomy (RP), radiotherapy, or RP plus radiotherapy, with biochemical recurrence or persistently elevated PSA and prior negative or equivocal results on other imaging studies (prior tests were performed at the discretion of the treating physicians). Biochemical recurrence was defined as a PSA level of more than 0.2 ng/mL over 2 sequential tests 2 wk apart (for patients after RP) or a PSA level of at least 2.0 ng/mL above the posttherapy nadir (for patients after radiotherapy). Patients initially treated by other local therapies or treated for metastatic disease were excluded. All patients provided written informed consent.
11C-Choline PET/CT
11C-choline was synthesized by our Radiochemistry and Molecular Imaging Probe Core Facility. Patients fasted for at least 4 h before the scan. A low-dose CT scan (120 kVp, 80 mAs, and 3.8-mm slice thickness) was acquired from the skull base to the upper thighs. Starting simultaneously with the 11C-choline injection (370–740 MBq), dynamic PET emission images of the lower pelvis were obtained for 5 min. Afterward, static PET images were acquired from the floor of the pelvis to the lower neck, followed by a final single–field-of-view PET image of the lower pelvis. All images were obtained on a Discovery PET/CT (GE Healthcare). PET emission data were corrected for attenuation, scatter, and random events and then were iteratively reconstructed into a 128 × 128 × 47 matrix (voxel dimensions, 5.46 × 5.46 × 3.27 mm) using the ordered-subset expectation maximization algorithm provided by the manufacturer.
Image Analysis
All scans were analyzed using the GE Healthcare PET VCAR software. Two experienced nuclear medicine physicians interpreted the scans. The first independent reader initially interpreted all scans without access to the patients’ clinical data and then reread the scans with access to the clinical data. This reading was followed by a consensus interpretation, with the second reader aware of the patients’ clinical history and PSA level. The consensus reading by these 2 nuclear medicine physicians was used for the final analysis.
For each patient, the location and level of suspicion for recurrence were recorded on a 3-point scale: 0, no uptake or uptake considered benign or unrelated to PCa; 1, uptake equivocal (not clearly negative but not suggestive enough to clearly be called recurrence); or 2, uptake consistent with recurrence. PET images were assessed visually. Abnormal uptake was defined as nonphysiologic uptake of intensity greater than local background and corresponding to clearly defined anatomic structures on CT. For semiquantitative analysis, SUVmax was recorded, but no specific SUV threshold was applied; rather, visual interpretation was the main criterion to classify findings. Inguinal, axillary, or mediastinal lymph nodes with symmetrically mild to moderate uptake were considered reactive. A rising PSA level after initial curative therapy implies recurrence, whether detectable or not with current imaging studies. In our primary analysis, we therefore aggregated both scores 1 and 2 as positive. In the secondary analysis, we were more stringent and considered only score 2 as positive. To map the patterns of PCa recurrence, we used the Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE) classification recently proposed for PSMA-ligand PET imaging (19,20).
Statistical Analysis
The different readings (masked and unmasked ratings by reader 1, as well as the consensus rating) were compared by pairs. The overall reader agreement was measured by both a Cohen κ-coefficient and a weighted κ-coefficient using squared weights.
Logistic regression was used to assess the association between the risk of having a positive scan and the studied factors (Gleason score 6–7, 8, 9–10; National Comprehensive Cancer Network grade I–II, III, IV; initial treatment type [RP, radiotherapy, persistent elevated PSA after initial treatment]; time between scan and initial treatment; PSA level; PSA doubling time; and PSA velocity). A log transformation was used for continuous variables to ensure a less sparse distribution. Variables with P values of less than 0.20 were introduced in the multivariable model. P values of less than 0.05 were considered significant. Assessing the patient-based and lesion-based sensitivity, specificity, accuracy, negative predictive value, and positive predictive value analysis was not possible since not all choline-positive lesions were biopsied for histopathologic confirmation.
RESULTS
Patient Characteristics
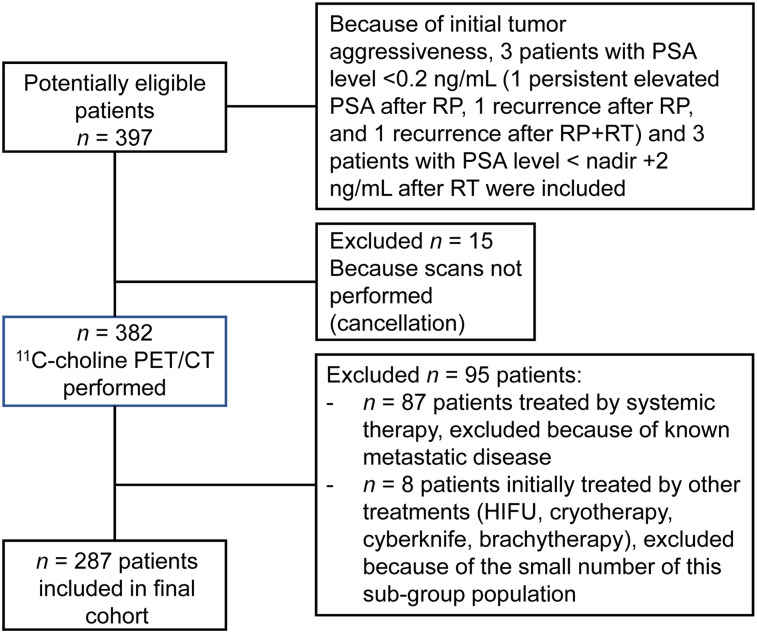
Of the 397 consecutive patients who were planned to undergo 11C-choline PET/CT for biochemically recurrent PCa between March 2016 and December 2017 according to the institutional database, 110 patients were excluded (Fig. 1). Therefore, 287 patients were included in this analysis (Table 1).
FIGURE 1.
Consort diagram. HIFU = high-intensity focused ultrasound; RT = radiotherapy.
TABLE 1.
Patient Characteristics
| Characteristic | Data |
| Mean age (y) | 67 (range, 42–89) |
| Initial Gleason score | |
| 6 | 12 (4%) |
| 7 | 169 (59%) |
| 3 + 4 | 91 |
| 4 + 3 | 77 |
| Unknown | 1 |
| 8 | 45 (16%) |
| 4 + 4 | 43 |
| 3 + 5 | 2 |
| 9 | 58 (20%) |
| 4 + 5 | 53 |
| 5 + 4 | 5 |
| 10 | 1 (0.4%) |
| Unknown | 2 |
| National Comprehensive Cancer Network stage | |
| I | 2 (1%) |
| IIA | 49 (18%) |
| IIB | 36 (13%) |
| III | 98 (36%) |
| IV | 87 (32%) |
| Unknown | 15 |
| Initial treatment | |
| Persistent elevated PSA after RP or radiotherapy | 70 (24%) |
| Rising PSA after initially successful surgery or radiotherapy: | |
| RP | 108 (38%) |
| RP plus radiotherapy | 79 (28%) |
| Radiotherapy | 30 (10%) |
| Time between initial treatment and 11C-choline PET/CT (y) | 4.6 (range, 0.2–23.3) |
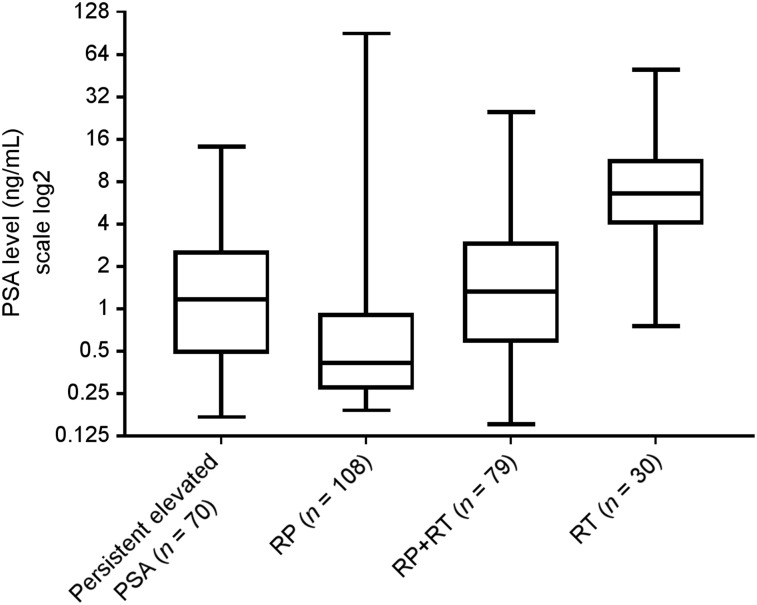
Initial treatments included RP with or without pelvic lymph node dissection (n = 108), RP followed by secondary radiotherapy with or without concomitant hormone therapy (RP plus radiotherapy) (n = 79), and radiotherapy with or without concomitant hormone therapy (n = 30). Another 70 patients had persistently elevated PSA after initial treatment (69 after RP and 1 after radiotherapy). None of these 287 patients was undergoing hormone therapy at the time of the scan. The mean PSA level at the time of imaging was 3.43 ng/mL (median, 0.94 ng/mL; range, 0.15–89.9 ng/mL) (Fig. 2).
FIGURE 2.
PSA level box plots. Boxes contain data between 25th and 75th percentiles (interquartile range), whiskers extend from minimum to maximum, and horizontal line within box indicates median. RT = radiotherapy.
Concordance Between Readers
The κ-coefficient between masked and unmasked readings by reader 1 was 0.86—that is, excellent agreement—and the weighted κ was 0.93. The κ-coefficient between unmasked reading by reader 1 and the consensus reading was 0.76, indicating good agreement, and the weighted κ was 0.87 (Supplemental Table 1; supplemental materials are available at http://jnm.snmjournals.org). Modifications from score 2 to 0 between the unmasked and consensus readings were related mainly to diffuse prostatic uptake after radiotherapy. Modifications from score 0 to 1 or 2 between the masked reading and the unmasked/consensus readings are shown in Supplemental Table 2.
11C-Choline PET/CT Positivity and Proof of Recurrence
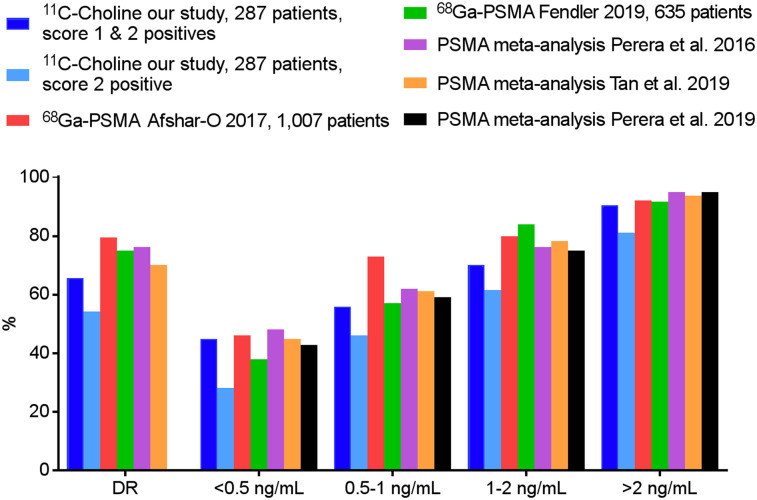
When scores 1 and 2 were considered positive, the overall detection rate of choline PET/CT was 66% (189/287). When sorted by PSA level, for patients with a PSA level of less than 0.5 ng/mL, 45% (43/96) were positive. For patients with higher PSA values of 0.5–0.99 ng/mL, 1.0–1.99 ng/mL, and at least 2.0 ng/mL, choline PET/CT positivity was 56% (28/50), 70% (33/47), and 90% (85/94), respectively. In univariate analysis, PSA, PSA velocity, and type of treatment were associated with the probability of a positive scan. In multivariate analysis, only PSA level was an independent predictor of scan positivity (P < 0.001) (Supplemental Table 3). When only score 2 was considered positive, the overall detection rate was 54% (155/287). The detection rate was 28%, 46%, 62%, and 81% for patients with a PSA level of less than 0.5 ng/mL, 0.5–0.99 ng/mL, 1.0–1.99 ng/mL, and at least 2.0 ng/mL, respectively. Detection rates are shown in Figure 3, case examples in Figure 4, and examples of patients in whom choline PET affected management in Supplemental Table 4.
FIGURE 3.
Comparison of our detection rates and published PSMA data. DR = overall detection rate.
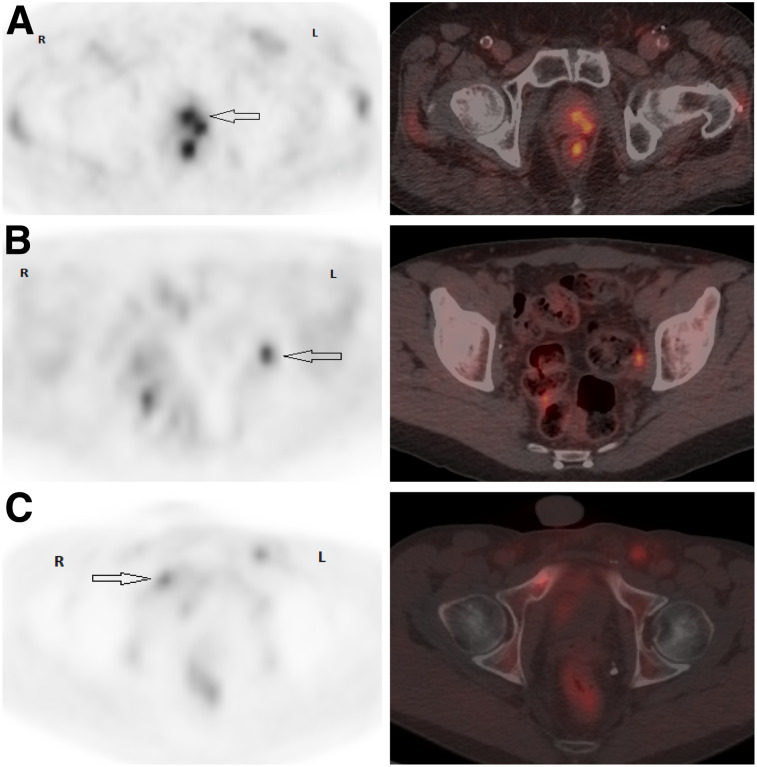
FIGURE 4.
Patient examples of pelvic axial PET and PET/CT images. (A) PCa 4.3 y after intensity-modulated radiotherapy (Gleason 7 [4 + 3]; T2cN0M0; PSA, 2.28 ng/mL). PET/CT shows suspected left posterior prostatic uptake (SUVmax, 3.3), scored 2 (arrow). Local biopsy was positive for PCa. (B) PCa 6.4 y after RP plus pelvic lymph node dissection (Gleason 8 [4 + 4]; pT2bN0M0; PSA, 0.48 ng/mL). PET/CT shows suspected focal uptake (SUVmax, 4.5) in nonenlarged left obturator lymph node, scored 2 (arrow). Histology was positive for PCa. (C) PCa 10 mo after RP plus pelvic lymph node dissection (Gleason 8 [4 + 4]; pT4N1M0; PSA, 0.46 ng/mL; PSA doubling time, 2.1 mo). PET/CT shows 2 suspected bone foci, one in right pubic ramus (SUVmax, 3) with sclerotic lesion on CT (arrow) and the other in posterior eight rib (not shown), scored 2. Biopsy of right pubic ramus was positive for PCa.
Suspected PCa recurrence was confirmed by positive biopsies within 9 mo in 49 patients (Table 2), by other imaging studies in 72 patients, and by decreasing PSA levels after therapy in 103 patients (76 salvage pelvic radiotherapy plus hormone therapy, 1 salvage radiotherapy, 1 brachytherapy, and 25 systemic therapy). For the other patients, 45 continued to be monitored and their PSA levels continued to increase, and 17 were lost to follow-up after imaging. One patient presented with a false-positive PSA measurement caused by human antimouse antibody.
TABLE 2.
11C-Choline PET/CT Findings Compared with Histology Results
| Site | TP | FP | TN | FN |
| Local | 13 | 4 (2 MRI suggesting local recurrence, PSA < 0.05 after salvage radiotherapy [biopsy FN?], 1 with new biopsy planned but not performed for medical reasons and 1 FP without doubt) | 3 | 2 |
| Pelvic lymph node | 24 | 2 (1 perirectal lymph node [possibly FN biopsy; remained on later PSMA PET after salvage pelvic LND and PSA still elevated], 1 FP PSA measurement) | 7 | 1 |
| Bone | 9 | 3 (1 myeloma, 1 benign acetabular lesion, 1 with later imaging positive for bone metastases [possibly FN biopsy]) | 2 | 0 |
| Extrapelvic lymph node | 1 | 0 | 1 | 0 |
| Lung | 1 | 0 | 3 (correctly recognized as not due to PCa: 1 lung primary, 2 inflammatory nodules) | 0 |
| Neural | 1 (neural foramen) | 0 | 0 | 0 |
TP = true-positive; FP = false-positive; TN = true-negative; FN = false-negative.
67 patients and 77 sites were biopsied. Of 67 patients, 49 had at least 1 PCa-positive biopsy site.
In 6 patients, choline uptake was deemed false-positive on the basis of negative biopsies (n = 2), decline of PSA to an undetectable level despite lack of treatment to that site (n = 1), histopathologic confirmation of another malignancy (n = 2), or histopathologic evidence of inflammation (n = 1).
Patterns of Choline-Positive Recurrence
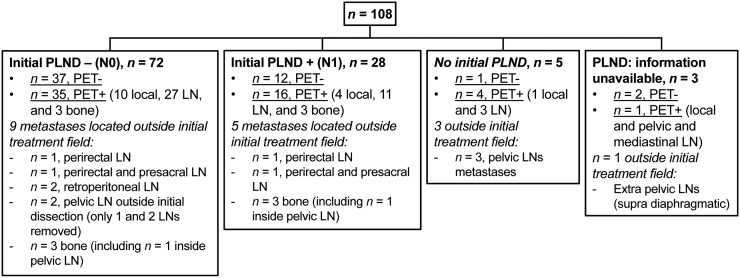
False-positive lesions and the patient with a false-positive PSA measurement were not considered in this analysis. Among the other patients (Table 3), choline PET/CT revealed pelvic lymph node metastases in 48%. Pelvic nodes were the most common site of recurrence after RP, after RP plus radiotherapy, and for patients with a persistently elevated PSA level after local therapy. Local recurrence in the prostate bed, identified in 20.3% of patients, was the most common site of recurrence in patients after radiotherapy. In 27.6% of patients, PET/CT showed recurrence outside the initial treatment field, mainly in perirectal, presacral, and retroperitoneal lymph nodes or in bone (Fig. 5; Supplemental Figs. 1–3).
TABLE 3.
11C-Choline PET/CT Patterns of Recurrence
| Parameter | Persistently elevated PSA (n = 69) | PSA relapse after RP (n = 108) | PSA relapse after RP plus radiotherapy (n = 79) | PSA relapse after radiotherapy (n = 30) |
| PSA level (ng/mL) | 2.1 (0.17–14.13) | 2.6 (0.19–89.91) | 2.8 (0.15–24.98) | 11.3 (0.75–50.15) |
| PET/CT positivity | 50 (72.5%) | 56 (52%) | 53 (67%) | 27 (90%) |
| T+ | 11 (16%) | 16 (15%) | 12 (15%) | 19 (63.5%) |
| N1 | 47 (68%) | 42 (39%) | 34 (43%) | 15 (50%) |
| M1a | 3 (4.5%) | 4 (3.5%) | 7 (9%) | 3 (10%) |
| M1b | 8 (11.5%) | 6 (5%) | 11 (14%) | 5 (17%) |
| M1c | 0 | 0 | 3 (4%) | 1 (3.5%) |
| T+N0M0 | 1 (1.5%) | 10 (9.5%) | 6 (7.5%) | 10 (33.5%) |
| T0N1M0 | 32 (46.5%) | 32 (29.5%) | 22 (28%) | 5 (16.5%) |
| T+N1M0 | 6 (8.5%) | 4 (3.5%) | 5 (6.5%) | 3 (10%) |
| T+N0M1 | 2 (3%) | 0 | 1 (1.5%) | 1 (3.5%) |
| T0N0M1 | 0 | 4 (4%) | 12 (15%) | 1 (3.5%) |
| T0N1M1 | 7 (10%) | 4 (3.5%) | 7 (9%) | 2 (6.5%) |
| T+N1M1 | 2 (3%) | 2 (2%) | 0 | 5 (16.5%) |
| Recurrence outside initial treatment field | 23 (33.5%) | 18 (17%) | 25 (32%) | 13 (43.5%) |
FIGURE 5.
Patients after initial RP with PET/CT-positive recurrence outside initial treatment field. LN = lymph node; PLND = pelvic lymph node dissection.
DISCUSSION
This study showed an overall positivity rate of 66% for localizing recurrent PCa when both definitive and equivocal scans were considered positive, with the rationale that all patients were known to have biochemical recurrence. This approach yielded a detection rate similar to or slightly higher than rates reported in previous publications (10,11,21,22). For instance, in a metaanalysis of 2,126 patients across 18 studies, the pooled detection rate of 11C-choline for PCa recurrence was 62% (21). When considering equivocal scans as negative, we observed a lower detection rate (54%). This is an unlikely low detection rate, as it implies that all equivocal scans would have been false-positives. It is conceivable that the actual detection rate is between 54% and 66%, considering that at least some of the equivocal findings (e.g., mild choline uptake in small pelvic nodes) represented early recurrences. It is important to establish a standardized terminology for reporting choline PET/CT because otherwise some referring physicians may simply dismiss equivocal scan findings as negative instead of considering them suggestive enough to require attention on follow-up. Publications describing choline PET/CT for suspected PCa recurrence remain heterogeneous, largely because of variable inclusion criteria, reading methods, and cohort sizes (11,21,23,24).
Our multivariate analysis showed that the PSA level at the time of PET imaging was the most crucial parameter to predict scan positivity, in line with other studies (11). In univariate analysis, higher Gleason score, higher PSA velocity, persistently elevated PSA after initial treatment, and initial treatment with radiotherapy increased the probability for positive choline scans (10,25,26). Higher scan positivity rates among patients after initial radiotherapy are probably explained by the higher PSA levels in this cohort (Table 3). This was similarly observed by others (24,26) and, at least in part, may be related to the current definition of recurrence after radiotherapy.
The interpretation of choline PET/CT scans poses some unique challenges, particularly compared with PSMA PET/CT, because of the relatively high background activity, low target-to-background activity ratios, and relatively high image noise levels (13,15–17). The challenge to interpretation is reflected in our lower interobserver agreement for sites with equivocal focal choline uptake. Depending on whether readers emphasize sensitivity or specificity, and likely also based on their prior experience, assigning probability scores to sites of focal choline uptake will vary. Thus, in a realistic clinical setting, readers must be aware of all clinical and laboratory data, providing a certain pretest probability, as well as the numerous reasons for choline uptake unrelated to PCa (27–29). For these reasons, in most other studies evaluating the utility of PET/CT in recurrent PCa, readers were aware of clinical data or at least PSA level at the time of imaging, because it is well known (and reproduced in our multivariate analysis) that the probability for a positive scan increases when PSA levels rise.
Analyzing patterns of recurrence may help to understand how choline PET/CT could affect the choice of treatment for recurrent PCa (Supplemental Table 4) (20,30). The patterns observed in our study were generally concordant with those reported previously (31). For instance, pelvic lymph nodes were the most common site of recurrence after initial RP. For these patients, the detection of all involved nodes is particularly important and may lead to extending the standard radiotherapy field or escalating the dose to PET-positive pelvic disease (20). Pattern analysis also suggested that standard pelvic dissection may miss nodal metastases, emphasizing the need for better presurgical or intraoperative staging with modern imaging tests. Unfortunately, both choline (8,32) and more recently also PSMA (33–35) remain suboptimal for this purpose.
PSMA PET imaging is gaining increasing recognition for its ability to detect recurrent PCa. Therefore, we also compared our results with data from some of the largest PSMA studies and metaanalyses in patients with biochemical recurrence (31,36–39). Similar to the literature on choline PET, studies on the utility of PSMA PET in recurrent PCa are heterogeneous, applying variable methods: in some studies, multiple independent readers were aware only of the initial treatment and the PSA level at the time of scan, and a majority vote was used in cases of interobserver disagreement (37); in other studies, multiple physicians classified scan findings during an interdisciplinary conference, with readers aware of clinical data (and possibly prior imaging studies) (36). Recent metaanalyses of PSMA in recurrent PCa (31,39) reported slightly lower detection rates than those published before (38), reflecting a general trend after the introduction of novel radiotracers (as well as other tests): as experience grows and verification becomes more rigorous, initially high sensitivity and specificity tend to decline to more realistic levels. For instance, similar to choline, there are many potential reasons for nonspecific and false-positive PSMA uptake (40). Regardless, our results are in line with those from other studies on recurrent PCa, showing that scan positivity with choline is generally lower than that reported for PSMA (ranging from 70.2% to 79.5% (31,36–39)). However, differences were not as striking among patients with low PSA levels, as had previously been suggested (Fig. 3) (31,37–39).
Like almost all previous studies on recurrent PCa, we focused on scan positivity rates. Since most positive findings cannot be confirmed by biopsies because of practical limitations (e.g., multiple suggestive foci, difficulty accessing sites of suspected disease, and insufficient tissue samples), we used the same composite standard of reference generally accepted in this field.
CONCLUSION
11C-choline PET/CT can detect PCa recurrence even among patients with a low PSA level when the examination is interpreted by readers aware of the clinical context, providing a pretest probability.
DISCLOSURE
This research was funded in part through an NIH/NCI Cancer Center support grant (P30 CA008748). Heiko Schöder served as a consultant to Aileron Therapeutics until June 2018 (outside the submitted work). Wolfgang Weber serves on advisory boards for and receives compensation from Bayer, Blue Earth Diagnostics, Endocyte, and PentixaPharm. He has received research support from Bristol-Myers Squibb, ImaginAb, Ipsen, and Piramal. Michael Morris is an uncompensated consultant for Bayer and Endocyte and a compensated consultant for Advanced Accelerator Applications, Blue Earth Diagnostics, Tokai, Tolmar, and Oric. His research is supported by institutional funds from Bayer, Sanofi, Endocyte, Progenics, Corcept, and Roche, and he has received travel support from Bayer and Endocyte. Jeremy Durack has a patent for biopsy quality assessment technology. He serves as the chair of the Society of Interventional Radiology Foundation. Memorial Sloan Kettering has received funding from Progenics Pharmaceuticals for an imaging–biopsy correlation study. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Can choline PET/CT still provide clinical utility in PCa recurrence?
PERTINENT FINDINGS: In a retrospective analysis of 287 11C-choline PET/CT scans performed to detect recurrent PCa after initial surgery or radiotherapy, the overall positivity rate was 66% when both definitive and equivocal scans were considered positive. When sorted by PSA level, for patients with PSA values of less than 0.5 ng/mL, 0.5–0.99 ng/mL, 1.0–1.99 ng/mL, and at least 2.0 ng/mL, choline PET/CT positivity was 45%, 56%, 70%, and 90.5%, respectively. This detection rate is lower than data reported for PSMA PET, but among patients with low PSA, differences were not as striking as previously suggested.
IMPLICATIONS FOR PATIENT CARE: Until PSMA agents are fully approved for PCa, choline PET/CT maintains utility for detecting PCa recurrence even among patients with low PSA levels and may affect the choice of treatment.
Supplementary Material
REFERENCES
- 1.Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–439. [DOI] [PubMed] [Google Scholar]
- 2.May EJ, Viers LD, Viers BR, et al. Prostate cancer post-treatment follow-up and recurrence evaluation. Abdom Radiol (NY). 2016;41:862–876. [DOI] [PubMed] [Google Scholar]
- 3.Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65:467–479. [DOI] [PubMed] [Google Scholar]
- 4.Vargas HA, Martin-Malburet AG, Takeda T, et al. Localizing sites of disease in patients with rising serum prostate-specific antigen up to 1ng/ml following prostatectomy: how much information can conventional imaging provide? Urol Oncol. 2016;34:482.e485–482.e410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goenka A, Magsanoc JM, Pei X, et al. Long-term outcomes after high-dose postprostatectomy salvage radiation treatment. Int J Radiat Oncol Biol Phys. 2012;84:112–118. [DOI] [PubMed] [Google Scholar]
- 6.Kane CJ, Amling CL, Johnstone PA, et al. Limited value of bone scintigraphy and computed tomography in assessing biochemical failure after radical prostatectomy. Urology. 2003;61:607–611. [DOI] [PubMed] [Google Scholar]
- 7.Hövels AM, Heesakkers RA, Adang EM, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol. 2008;63:387–395. [DOI] [PubMed] [Google Scholar]
- 8.Umbehr MH, Muntener M, Hany T, Sulser T, Bachmann LM. The role of 11C-choline and 18F-fluorocholine positron emission tomography (PET) and PET/CT in prostate cancer: a systematic review and meta-analysis. Eur Urol. 2013;64:106–117. [DOI] [PubMed] [Google Scholar]
- 9.Evangelista L, Zattoni F, Guttilla A, et al. Choline PET or PET/CT and biochemical relapse of prostate cancer: a systematic review and meta-analysis. Clin Nucl Med. 2013;38:305–314. [DOI] [PubMed] [Google Scholar]
- 10.Chiaravalloti A, Di Biagio D, Tavolozza M, Calabria F, Schillaci O. PET/CT with 18F-choline after radical prostatectomy in patients with PSA ≤2 ng/ml: can PSA velocity and PSA doubling time help in patient selection? Eur J Nucl Med Mol Imaging. 2016;43:1418–1424. [DOI] [PubMed] [Google Scholar]
- 11.Graziani T, Ceci F, Castellucci P, et al. 11C-choline PET/CT for restaging prostate cancer: results from 4,426 scans in a single-centre patient series. Eur J Nucl Med Mol Imaging. 2016;43:1971–1979. [DOI] [PubMed] [Google Scholar]
- 12.Beheshti M, Haim S, Zakavi R, et al. Impact of 18F-choline PET/CT in prostate cancer patients with biochemical recurrence: influence of androgen deprivation therapy and correlation with PSA kinetics. J Nucl Med. 2013;54:833–840. [DOI] [PubMed] [Google Scholar]
- 13.Afshar-Oromieh A, Zechmann CM, Malcher A, et al. Comparison of PET imaging with a 68Ga-labelled PSMA ligand and 18F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2014;41:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evangelista L, Cuppari L, Zattoni F, Mansi L, Bombardieri E. The future of choline PET in the era of PMSA. Q J Nucl Med Mol Imaging. 2019;63:19–28. [DOI] [PubMed] [Google Scholar]
- 15.Morigi JJ, Stricker PD, van Leeuwen PJ, et al. Prospective comparison of 18F-fluoromethylcholine versus 68Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J Nucl Med. 2015;56:1185–1190. [DOI] [PubMed] [Google Scholar]
- 16.Bluemel C, Krebs M, Polat B, et al. 68Ga-PSMA-PET/CT in patients with biochemical prostate cancer recurrence and negative 18F-choline-PET/CT. Clin Nucl Med. 2016;41:515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwenck J, Rempp H, Reischl G, et al. Comparison of 68Ga-labelled PSMA-11 and 11C-choline in the detection of prostate cancer metastases by PET/CT. Eur J Nucl Med Mol Imaging. 2017;44:92–101. [DOI] [PubMed] [Google Scholar]
- 18.FDA approves 11C-choline for PET in prostate cancer. J Nucl Med. 2012;53(12):11N. [PubMed] [Google Scholar]
- 19.Eiber M, Herrmann K, Calais J, et al. Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE): proposed miTNM classification for the interpretation of PSMA-ligand PET/CT. J Nucl Med. 2018;59:469–478. [DOI] [PubMed] [Google Scholar]
- 20.Calais J, Czernin J, Cao M, et al. 68Ga-PSMA-11 PET/CT mapping of prostate cancer biochemical recurrence after radical prostatectomy in 270 patients with a PSA level of less than 1.0 ng/mL: impact on salvage radiotherapy planning. J Nucl Med. 2018;59:230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fanti S, Minozzi S, Castellucci P, et al. PET/CT with 11C-choline for evaluation of prostate cancer patients with biochemical recurrence: meta-analysis and critical review of available data. Eur J Nucl Med Mol Imaging. 2016;43:55–69. [DOI] [PubMed] [Google Scholar]
- 22.Castellucci P, Fuccio C, Nanni C, et al. Influence of trigger PSA and PSA kinetics on 11C-choline PET/CT detection rate in patients with biochemical relapse after radical prostatectomy. J Nucl Med. 2009;50:1394–1400. [DOI] [PubMed] [Google Scholar]
- 23.Wei J, Zhu H, Liao X. Trigger pSA predicting recurrence from positive choline PET/CT with prostate cancer after initial treatment. Oncotarget. 2018;9:14630–14641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parker WP, Davis BJ, Park SS, et al. Identification of site-specific recurrence following primary radiation therapy for prostate cancer using C-11 choline positron emission tomography/computed tomography: a nomogram for predicting extrapelvic disease. Eur Urol. 2017;71:340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cimitan M, Evangelista L, Hodolic M, et al. Gleason score at diagnosis predicts the rate of detection of 18F-choline PET/CT performed when biochemical evidence indicates recurrence of prostate cancer: experience with 1,000 patients. J Nucl Med. 2015;56:209–215. [DOI] [PubMed] [Google Scholar]
- 26.von Eyben FE, Kairemo K. Meta-analysis of 11C-choline and 18F-choline PET/CT for management of patients with prostate cancer. Nucl Med Commun. 2014;35:221–230. [DOI] [PubMed] [Google Scholar]
- 27.Corrigan AJ, Schleyer PJ, Cook GJ. Pitfalls and artifacts in the use of PET/CT in oncology imaging. Semin Nucl Med. 2015;45:481–499. [DOI] [PubMed] [Google Scholar]
- 28.Michaud L, Burgess A, Huchet V, et al. Is 18F-fluorocholine-positron emission tomography/computerized tomography a new imaging tool for detecting hyperfunctioning parathyroid glands in primary or secondary hyperparathyroidism? J Clin Endocrinol Metab. 2014;99:4531–4536. [DOI] [PubMed] [Google Scholar]
- 29.Cassou-Mounat T, Balogova S, Nataf V, et al. 18F-fluorocholine versus 18F-fluorodeoxyglucose for PET/CT imaging in patients with suspected relapsing or progressive multiple myeloma: a pilot study. Eur J Nucl Med Mol Imaging. 2016;43:1995–2004. [DOI] [PubMed] [Google Scholar]
- 30.Thompson IM, Valicenti RK, Albertsen P, et al. Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO guideline. J Urol. 2013;190:441–449. [DOI] [PubMed] [Google Scholar]
- 31.Perera M, Papa N, Roberts M, et al. Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions—a systematic review and meta-analysis. Eur Urol. February 14, 2019 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 32.Evangelista L, Guttilla A, Zattoni F, Muzzio PC, Zattoni F. Utility of choline positron emission tomography/computed tomography for lymph node involvement identification in intermediate- to high-risk prostate cancer: a systematic literature review and meta-analysis. Eur Urol. 2013;63:1040–1048. [DOI] [PubMed] [Google Scholar]
- 33.Muteganya R, Goldman S, Aoun F, Roumeguere T, Albisinni S. Current imaging techniques for lymph node staging in prostate cancer: a review. Front Surg. 2018;5:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hope TA, Goodman JZ, Allen IE, Calais J, Fendler WP, Carroll PR. Meta-analysis of 68Ga-PSMA-11 PET accuracy for the detection of prostate cancer validated by histopathology. J Nucl Med. 2019;60:786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yaxley JW, Raveenthiran S, Nouhaud FX, et al. Outcomes of primary lymph node staging of intermediate and high risk prostate cancer with 68Ga-PSMA positron emission tomography/computerized tomography compared to histological correlation of pelvic lymph node pathology. J Urol. 2019;201:815–820. [DOI] [PubMed] [Google Scholar]
- 36.Afshar-Oromieh A, Holland-Letz T, Giesel FL, et al. Diagnostic performance of 68Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: evaluation in 1007 patients. Eur J Nucl Med Mol Imaging. 2017;44:1258–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fendler WP, Calais J, Eiber M, et al. Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: a prospective single-arm clinical trial. JAMA Oncol. 2019;5:856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perera M, Papa N, Christidis D, et al. Sensitivity, specificity, and predictors of positive 68Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70:926–937. [DOI] [PubMed] [Google Scholar]
- 39.Tan N, Bavadian N, Calais J, et al. Imaging of PSMA-targeted radiotracers for the detection of prostate cancer biochemical recurrence after definitive therapy: a systematic review and meta-analysis. J Urol. 2019;202:231–240. [DOI] [PubMed] [Google Scholar]
- 40.Hofman MS, Hicks RJ, Maurer T, Eiber M. Prostate-specific membrane antigen PET: clinical utility in prostate cancer, normal patterns, pearls, and pitfalls. Radiographics. 2018;38:200–217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.