Abstract
177Lu-PSMA-617 is a radioligand with high affinity for prostate-specific membrane antigen (PSMA), enabling targeted β-irradiation of prostate cancer. We have previously reported favorable activity with low toxicity in a prospective phase II trial involving 30 men with metastatic castration-resistant prostate cancer. We now report their longer-term outcomes, including a 20-patient extension cohort and outcomes of subsequent systemic treatments after completion of trial therapy. Methods: Fifty patients with PSMA-avid metastatic castration-resistant prostate cancer who had progressed after standard therapies received up to 4 cycles of 177Lu-PSMA every 6 wk. Endpoints included prostate-specific antigen (PSA) response (Prostate Cancer Working Group 2), toxicity (Common Terminology Criteria for Adverse Events, version 4.03), imaging response, patient-reported health-related quality of life, progression-free survival, and overall survival. We also describe, as a novel finding, outcomes of men who subsequently progressed and had further systemic therapies, including 177Lu-PSMA. Results: Seventy-five men were screened to identify 50 patients eligible for treatment. Adverse prognostic features of the cohort included short median PSA doubling time (2.3 mo) and extensive prior treatment, including prior docetaxel (84%), cabazitaxel (48%), and abiraterone or enzalutamide (92%). The mean administered radioactivity was 7.5 GBq/cycle. A PSA decline of at least 50% was achieved in 32 of 50 patients (64%; 95% confidence interval [CI], 50%–77%), including 22 patients (44%; 95% CI, 30%–59%) with at least an 80% decrease. Of 27 patients with measurable soft-tissue disease, 15 (56%) achieved an objective response by RECIST 1.1. The most common toxicities attributed to 177Lu-PSMA were self-limiting G1–G2 dry mouth (66%), transient G1–G2 nausea (48%), G3–G4 thrombocytopenia (10%), and G3 anemia (10%). Brief Pain Inventory severity and interference scores decreased at all time points, including at the 3-mo follow-up, with a decrease of −1.2 (95% CI, −0.5 to −1.9; P = 0.001) and −1.0 (95% CI, −0.2 to −0.18; P = 0.013), respectively. At a median follow-up of 31.4 mo, median overall survival was 13.3 mo (95% CI, 10.5–18.7 mo), with a significantly longer survival of 18.4 mo (95% CI, 13.8–23.8 mo) in patients achieving a PSA decline of at least 50%. At progression after prior response, further 177Lu-PSMA was administered to 15 (30%) patients (median of 2 cycles commencing 359 d from enrollment), with a PSA decline of at least 50% in 11 patients (73%). Four of 21 patients (19%) receiving other systemic therapies on progression experienced a PSA decline of at least 50%. There were no unexpected adverse events with 177Lu-PSMA retreatment. Conclusion: This expanded 50-patient cohort of men with extensive prior therapy confirms our earlier report of high response rates, low toxicity, and improved quality of life with 177Lu-PSMA radioligand therapy. On progression, rechallenge 177Lu-PSMA demonstrated higher response rates than other systemic therapies.
Keywords: prostate-specific membrane antigen, PSMA, theranostics, prostate cancer, 177Lu, radioligand therapy
Most prostate cancers express prostate-specific membrane antigen (PSMA) on their surface, with increased expression in higher-grade and castration-resistant cancers (1–3). PSMA represents an excellent target for both the imaging and the therapy of prostate cancer and is the focus of extensive research (4–6). 177Lu-PSMA-617 (177Lu-PSMA) is a radiolabeled small molecule that binds with high affinity to the enzymatic site of PSMA, enabling highly targeted delivery of β-radiation to prostate cancer cells. Multiple mainly retrospective series of β-radiolabeled small molecules targeting PSMA have demonstrated that treatment is effective in patients with advanced and heavily pretreated prostate cancers (7–13).
In a prospective phase II clinical trial, we demonstrated single-agent activity of 177Lu-PSMA in 30 men with metastatic castration-resistant prostate cancer who had progressed on most systemic therapies (14). Encouraging clinical, biochemical, and imaging responses and limited acute normal-tissue toxicity were observed in this heavily treated cohort of men. Furthermore, we also observed improvement in quality-of-life measures. Radiation dosimetry in these men demonstrated high tumor-absorbed doses yet low exposure of critical normal tissues (15). These dosimetric findings have also been observed by other groups in retrospective analyses (16–21) and suggest that multiple cycles of therapy can safely be administered without a significant risk of either acute or delayed radiation toxicity.
We now present longer-term follow-up in an expanded 50-patient cohort to validate our earlier clinical findings and assess overall survival and any late toxicity. Additionally, we report outcomes of patients treated within the original trial protocol who subsequently received additional cycles of 177Lu-PSMA therapy or other systemic therapies at relapse, documenting their response to treatment and patterns of failure.
MATERIALS AND METHODS
This was an investigator-initiated, single-institution phase II trial that initially recruited 30 patients. Given the high clinical activity observed, the study was expanded to a total of 50 patients. Patients were treated and monitored as previously described (14). All patients gave written informed consent, and the protocol was approved by the institutional ethics board and conducted in accordance with the Declaration of Helsinki and good clinical practice. The study was sponsored by the Peter MacCallum Cancer Centre. Study data were collected and managed using REDCap electronic data capture tools (22). The trial was registered with the Australian New Zealand Clinical Trials Registry, ACTRN12615000912583.
Inclusion criteria mandated that patients have pathologically confirmed metastatic castration-resistant prostate cancer with progressive disease after standard therapies, including taxane-based chemotherapy and second-generation antiandrogen therapy (abiraterone, enzalutamide, or both) unless deemed medically unsuitable or refused by the patient. Patients must have had progressive disease within the prior 12 mo as defined by radiographic progression or new pain in an area of radiographically evident disease and an Eastern Cooperative Oncology Group Performance Status of 2 or less. Patients were excluded if they had an estimated glomerular filtration rate (eGFR) of less than 40 mL/min, a platelet count of less than 75,000 × 109/L, a neutrophil count of less than 1.5 × 109/L, hemoglobin of less than 9.0 g/dL, albumin of no more than 25 g/L, prior radiotherapy (within 6 wk) to sole sites of assessable disease, or uncontrolled intercurrent illness.
All patients underwent imaging with both 68Ga-PSMA-11 PET and 18F-FDG PET/CT. Inclusion mandated that the PSMA intensity at sites of disease had to be significantly greater than that in normal liver, as defined by a tumor SUVmax at least 1.5 times the SUVmean of liver. Patients were excluded if 18F-FDG PET demonstrated major discordant disease, that is, sites of 18F-FDG–positive and PSMA-negative disease, which we anticipated would be less likely to respond to therapy.
Procedures
Assessments
At baseline, all patients underwent 68Ga-PSMA-11 PET/CT and 18F-FDG PET/CT; radionuclide bone scanning; contrast-enhanced CT of the chest, abdomen, and pelvis; 51Cr-ethylenediaminetetraacetic acid eGFR; a full blood count, urea, and electrolytes; liver function tests; lactate dehydrogenase; testosterone; and prostate-specific antigen (PSA). Safety reviews and blood tests (full blood count, urea, and electrolytes; liver function tests; and PSA) were performed at 2 and 4 wk after each treatment cycle (i.e., every 6 wk). Additionally, all patients were reviewed 24 h after 177Lu-PSMA administration. Patient-reported health-related quality-of-life outcomes were assessed using the European Organisation for Research and Treatment of Cancer (EORTC) Quality-of-Life Questionnaire for cancer patients (EORTC-QLQ-C30) (23) and the Brief Pain Index (BPI) questionnaire (24) before each cycle of therapy and at the 12-wk follow-up. Adverse events were graded and causality assigned according to the Common Terminology Criteria for Adverse Events, version 4.03, at each clinical review up to the 12-wk follow-up visit after the last administration of 177Lu-PSMA. Beyond the 12-wk follow-up visit, only adverse events deemed to be related to treatment were reported. At the 12-wk follow-up visit, 51Cr-ethylenediaminetetraacetic acid eGFR, 68Ga-PSMA-11, and 18F-FDG PET/CT; bone scanning; and CT of the chest, abdomen, and pelvis were repeated. The low-dose CT portion of the posttreatment SPECT/CT scan was also used to assess soft-tissue RECIST response. Thereafter, patients were managed at the discretion of the treating physician and followed for biochemical progression, further anticancer treatments, and overall survival. The study protocol mandated formal assessment every 3 mo until 12 mo of follow-up. PSA reassessment, however, was performed more frequently in most patients.
Administration of Therapy
177Lu-PSMA was administered intravenously over a period of 2–10 min. Patients were encouraged to be well hydrated by consuming 1.5 L of oral fluids on the day of 177Lu-PSMA administration. No specific measures to minimize xerostomia were used. The administered radioactivity was adjusted according to tumor burden, patient weight, and renal function, starting from a dose of 6 GBq. This was adapted from our 177Lu-DOTATATE experience (25); activity was increased by 1 GBq if there were more than 20 sites of disease, or it was decreased by 1 GBq if there were fewer than 10 sites. Activity was increased by 0.5 GBq per factor if weight was more than 90 kg or eGFR more than 90 mL/min; activity was decreased by 0.5 GBq if weight was less than 70 kg or eGFR less than 60 mL/min. Patients could receive up to 4 cycles of 177Lu-PSMA—one every 6 wk.
Radiation emission was measured with a handheld γ-counter, and patients were discharged when the reading was below 9 μSv/h at a distance of 2 m from the patient as per local regulations; this level was generally reached within 2–4 h and after the first bladder voiding. Planar and quantitative SPECT/CT scans were acquired at 4, 24, and 96 h after 177Lu-PSMA therapy in the first 30-patient cohort for dosimetry studies. As a result of their findings (15), only 24-h imaging was performed in the expanded cohort to confirm tumor localization.
Cycle Delays and Early Treatment Cessation in Exceptional Responders
In patients with cytopenias (hemoglobin < 9.0 g/dL, platelet count < 75,000 × 109/L, neutrophil count < 1.5 × 109/L), blood tests were repeated weekly and therapy delayed until the patient had recovered to acceptable levels. If posttherapy imaging demonstrated no or minimal uptake of radionuclide at sites of tumor, indicative of an exceptional response to prior cycles, no further cycles were administered. Treatment was also ceased in patients who were deemed to be no longer clinically benefiting from treatment after multidisciplinary discussion.
Retreatment
Patients who initially responded to therapy, as defined by a PSA decline of at least 50% with an imaging response, and who subsequently progressed were considered for further 177Lu-PSMA as part of a compassionate-access program using the Australian Therapeutic Goods Administration Special Access Scheme. The selection criteria and procedures followed the study protocol. Patients underwent repeat 68Ga-PSMA-11 and 18F-FDG imaging to confirm they had sufficiently PSMA-avid disease and no sites of discordant disease.
Outcomes and Statistical Analysis
The initial sample size of 30 was pragmatic and was later expanded to 50 on the basis of department resources and a supply agreement for 177Lu. Primary endpoints included PSA response, defined as an at least 50% PSA decline from baseline; toxicity according to Common Terminology Criteria for Adverse Events version 4.03; imaging responses; and patient-reported quality of life. Secondary endpoints were overall survival and PSA progression-free survival, defined as time to PSA progression according to the criteria of the Prostate Cancer Working Group 2; both endpoints were measured from the date of patient enrollment or consent. A further secondary endpoint was determination of the radiation dosimetry of therapy and has been reported separately (15).
For PSA response, the best percentage change in PSA levels was recorded with a 2-sided exact binomial 95% confidence interval (CI). Time-to-event outcomes, including PSA progression-free survival and overall survival, was analyzed using Kaplan–Meier statistics; to compare groups, the log-rank test was applied. Linear mixed models were used to assess the EORTC-QLQ-C30 and BPI endpoints; no imputation for missing values was used. Mean differences from baseline and 95% CIs were estimated from the linear mixed-model contrasts. Statistical analyses were conducted using R-Statistics, version 3.4.0, with the ggplot2 package.
RESULTS
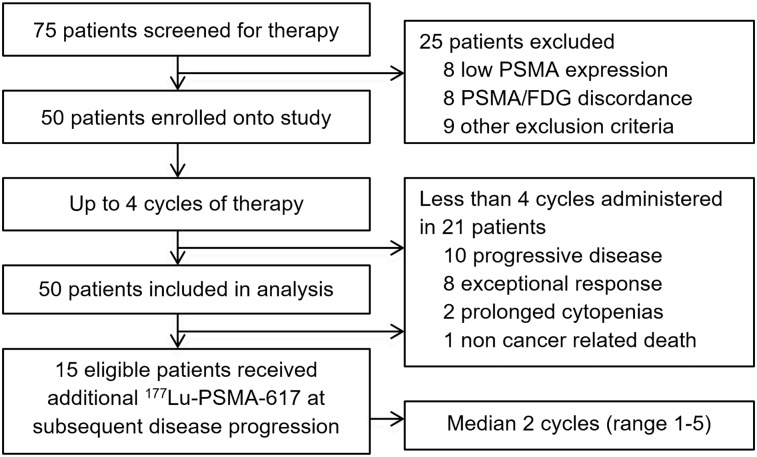
The initial 30-patient cohort was recruited between August 2015 and December 2016, and the 20-patient expanded cohort was enrolled from March 2017 to June 2017. Results are reported after a median follow-up of 31.4 mo (interquartile range, 25.1–36.3 mo; cutoff date, May 29, 2019). Seventy-five patients were screened to identify 50 evaluable patients as outlined in Figure 1. Sixteen patients were excluded because of either low PSMA expression (n = 8) or discordant sites of 18F-FDG–positive PSMA-negative disease (n = 8); the outcomes of these patients have been reported previously (26).
FIGURE 1.
Study schema.
The patient characteristics are outlined in Table 1. The patients were heavily pretreated, with 84% having received docetaxel and 92% having received therapy with a second-generation antiandrogen (abiraterone or enzalutamide). The median PSA doubling time before the first administration of 177Lu-PSMA was 2.3 mo.
TABLE 1.
Patient Characteristics
| Characteristic | Median or number | Range |
| Age (y) | 71 | 50–87 |
| Time since diagnosis of prostate cancer (y) | 8 | 2–17 |
| Gleason score | 8 | 6–10 |
| Alkaline phosphatase (U/L) | 131 | 49−1,896 |
| Hemoglobin (g/L) | 117 | 88–151 |
| Lactate dehydrogenase (U/L) | 268 | 148–1,331 |
| PSA (ng/mL) | 189.8 | 7–4,022 |
| PSA doubling time (mo) | 2.3 | −5.8 to 22.9 |
| Eastern Cooperative Oncology Group performance status | ||
| 0 | 20 (40%) | |
| 1 | 22 (44%) | |
| 2 | 8 (16%) | |
| Prior therapies | ||
| Abiraterone or enzalutamide or both | 46 (92%) | |
| Docetaxel | 42 (84%) | |
| Cabazitaxel | 24 (48%) | |
| Docetaxel + enzalutamide/abiraterone ± cabazitaxel | 39 (78%) | |
| Stage of disease (68Ga-PSMA-11 PET) | ||
| Node only (M1a) | 2 (4%) | |
| Bone (M1b) | 38 (76%) | |
| Visceral (M1c) | 10 (20%) | |
| Pain at baseline (BPI pain severity score) | ||
| None (<1) | 8 (16%) | |
| Mild (1–4) | 29 (58%) | |
| Moderate to severe (5–10) | 13 (26%) |
The median number of cycles received on protocol was 4 (range, 1–4). Twenty-one patients received fewer than 4 cycles because of progressive disease during therapy (n = 10), an exceptional response to therapy (n = 8), prolonged cytopenias (n = 2), or non–cancer-related death (n = 1). The mean injected activity delivered per cycle was 7.5 GBq (range, 4–8.9 GBq), with a mean cumulative activity of 24.7 GBq. The median time to first treatment after enrollment was 5.0 wk, and the time between cycles was 6.0 wk. Whole-body planar imaging at 24 h after treatment demonstrated an average retention of 22.6% (range, 4.6%–45.5%).
Biochemical and Imaging Response
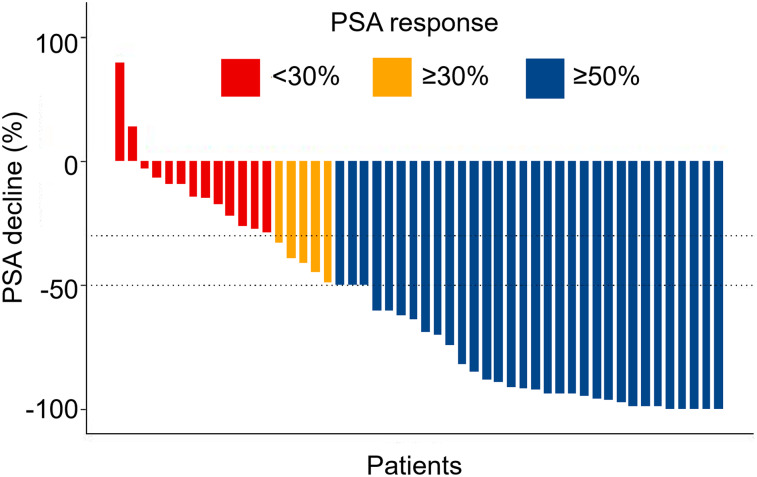
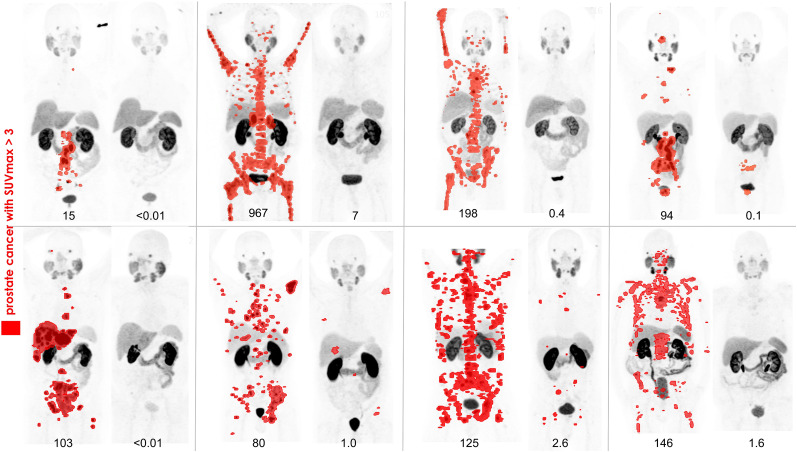
The primary endpoint of PSA decline greater than or equal to 50% from baseline was seen in 64% of patients (95% CI, 50%–77%), with an at least 80% decline seen in 44% of patients (95% CI, 30%–59%) (Fig. 2). Eight patients (16%) achieved an at least 98% PSA decline (Fig. 3).
FIGURE 2.
Waterfall plot of best PSA decline compared with baseline.
FIGURE 3.
68Ga-PSMA-11 PET/CT before and 3 mo after therapy in 8 patients with PSA declines of ≥98% after 177Lu-PSMA therapy. Prostate cancer with SUV > 3 is highlighted in red. (Image of the Year at 2018 Society of Nuclear Medicine and Molecular Imaging annual meeting.)
Imaging response was assessed at 3 mo after the last cycle of therapy and is shown in Table 2. In 27 patients with measurable soft-tissue disease on CT at baseline, 56% had an objective response (complete or partial response) by RECIST 1.1. Complete or partial molecular imaging responses on 68Ga-PSMA-11 and 18F-FDG PET/CT were seen in 42% and 30%, respectively. The most common pattern of progression was in the marrow (56%), typically at new sites compared with baseline evaluation, or in the liver (19%).
TABLE 2.
Imaging Response at 3 Months After Last Cycle of Induction 177Lu-PSMA
| Response | Bone scintigraphy | Soft-tissue lesions on CT (nodal or visceral) (n = 27) | 68Ga-PSMA-11 PET | 18F-FDG PET |
| Complete response | 16 (32%) | 5 (19%) | 6 (12%) | 7 (14%) |
| Partial response | 10 (37%) | 15 (30%) | 8 (16%) | |
| Stable disease | 0 | 0 | 3 (6%) | |
| Progressive disease | 12 (24%) | 9 (33%) | 14 (28%) | 15 (30%) |
| Not performed* | 22 (44%) | 3 (11%) | 15 (30%) | 17 (34%) |
Because of clinical progression or death.
PET responses were assessed visually using Hicks criteria (33) and CT with RECIST 1.1 with Prostate Cancer Working Group 2 caveats; CT component of posttherapy SPECT/CT was also used for soft-tissue measurements.
Treatment-Related Toxicity
Therapy was well tolerated, with no infusion-related complications or treatment-related deaths. Toxicities causally related to therapy are outlined in Table 3. The most common acute toxicity was xerostomia, which was reported in 66% of patients and was grade 2 or less in severity and transient. Grade 1–2 nausea and vomiting were seen in 48% and 26%, respectively; this generally occurred within the first 24 h of therapy, was transient, and was manageable with antiemetics. Grade 3–4 toxicity was primarily hematologic, including lymphopenia (32%), thrombocytopenia (10%), anemia (10%), and neutropenia (6%). No episodes of neutropenic sepsis were observed. Grade 4 toxicity was limited to a single case of thrombocytopenia. Grade 1–2 renal injury occurred in 10% of patients; in 28 patients who had 51Cr-ethylenediaminetetraacetic acid measured before and 3 mo after completion of 177Lu-PSMA, there was a mean decline of 11.7 mL/min (95% CI, −19 to −4 mL/min).
TABLE 3.
Treatment-Related Toxicity Occurring up to 12 Weeks After Treatment Cessation
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Dry mouth | 29 (58%) | 4 (8%) | 0 (0%) | 0 (0%) |
| Lymphocytopenia | 7 (14%) | 13 (26%) | 16 (32%) | 0 (0%) |
| Thrombocytopenia | 11 (22%) | 3 (6%) | 4 (8%) | 1 (2%) |
| Fatigue | 15 (30%) | 3 (6%) | 1 (2%) | 0 (0%) |
| Nausea | 20 (40%) | 4 (8%) | 0 (0%) | 0 (0%) |
| Anemia | 3 (6%) | 6 (12%) | 5 (10%) | 0 (0%) |
| Neutropenia | 6 (12%) | 6 (12%) | 3 (6%) | 0 (0%) |
| Bone pain | 5 (10%) | 4 (8%) | 0 (0%) | 0 (0%) |
| Vomiting | 11 (22%) | 2 (4%) | 0 (0%) | 0 (0%) |
| Anorexia | 8 (16%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Dry eyes | 4 (8%) | 1 (2%) | 0 (0%) | 0 (0%) |
| Renal injury | 4 (8%) | 1 (2%) | 0 (0%) | 0 (0%) |
| Weight loss | 3 (6%) | 1 (2%) | 0 (0%) | 0 (0%) |
Possibly, probably, or definitely related treatment-emergent adverse events were graded with Common Terminology Criteria for Adverse Events, version 4.03.
No patients developed myelodysplasia during the extended follow-up period. Of note, one patient with lymph node–only disease had pancytopenia (grade 2 thrombocytopenia, grade 2 neutropenia, and grade 3 anemia) commencing after cycle 3. The platelet nadir occurred at 28 d, with full recovery. Bone marrow biopsy demonstrated no specific cause. Restaging 68Ga-PSMA-11 PET/CT on progression again demonstrated only nodal disease.
Quality of Life
Health-related quality-of-life assessments were available for 79% of time points, with missing data attributable to death (9%), illness (7%), or not being recorded (4%). Overall, global health status improved significantly on the EORTC QLQ-C30 by cycles 2 and 3, with an increase of 6 and 7, respectively (95% CI, 0–11 and 1–13; P = 0.04 and 0.03, respectively); at the 3-mo follow-up this was stable compared with baseline. Changes in specific functional scales or symptom items are shown in Supplemental Figures 1 and 2 and Supplemental Table 1 (supplemental materials are available at http://jnm.snmjournals.org).
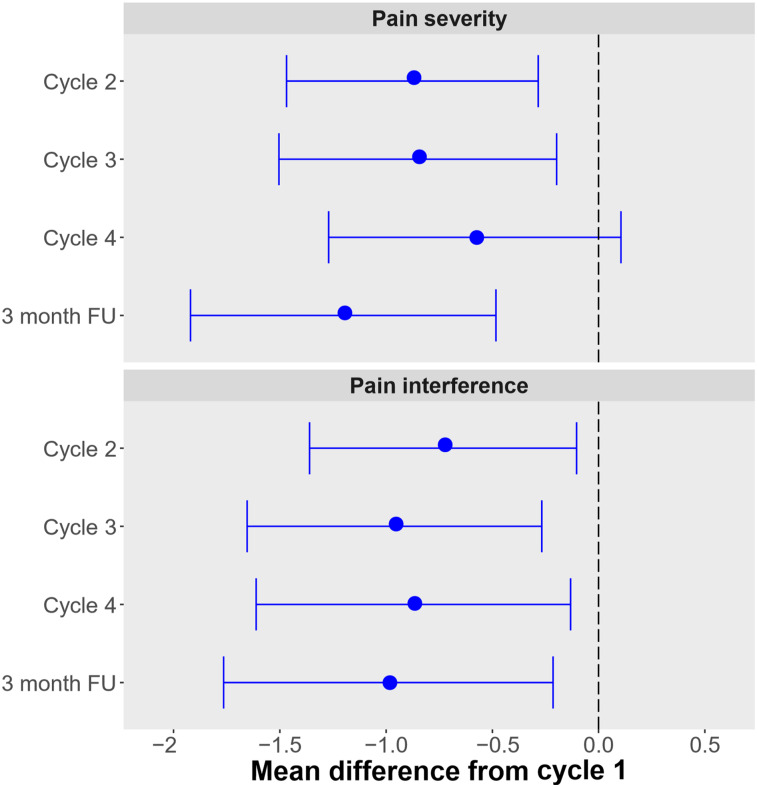
Overall, BPI pain severity and interference scores decreased at all time points, including at the 3-mo follow-up, with a decrease of −1.2 (95% CI, −0.5 to −1.9; P = 0.001) and −1.0 (95% CI, −0.2 to −0.18, P = 0.013), respectively (Fig. 4; Table 4). The pain scale on the EORTC QLQ-C30 also improved (Supplemental Table 1), concordant with the BPI findings.
FIGURE 4.
BPI scores compared with baseline with 95% CI. FU = follow-up.
TABLE 4.
BPI Mean Difference in Scores from Baseline
| Cycle 2 (46 evaluable) |
Cycle 3 (36 evaluable) |
Cycle 4 (29 evaluable) |
3 mo follow-up (26 evaluable) |
|||||
| Dimension | Mean | P | Mean | P | Mean | P | Mean | P |
| Pain severity | −0.9 (−1.5 to −0.3) | 0.004 | −0.9 (−1.5 to −0.2) | 0.011 | −0.6 (−1.3 to 0.1) | 0.096 | −1.2 (−1.9 to −0.5) | 0.001 |
| Pain interference | −0.7 (−1.4 to −0.1) | 0.023 | −1 (−1.7 to −0.3) | 0.007 | −0.9 (−1.6 to −0.1) | 0.021 | −1 (−1.8 to −0.2) | 0.013 |
Data in parentheses are 95% CIs.
PSA Progression-Free and Overall Survival
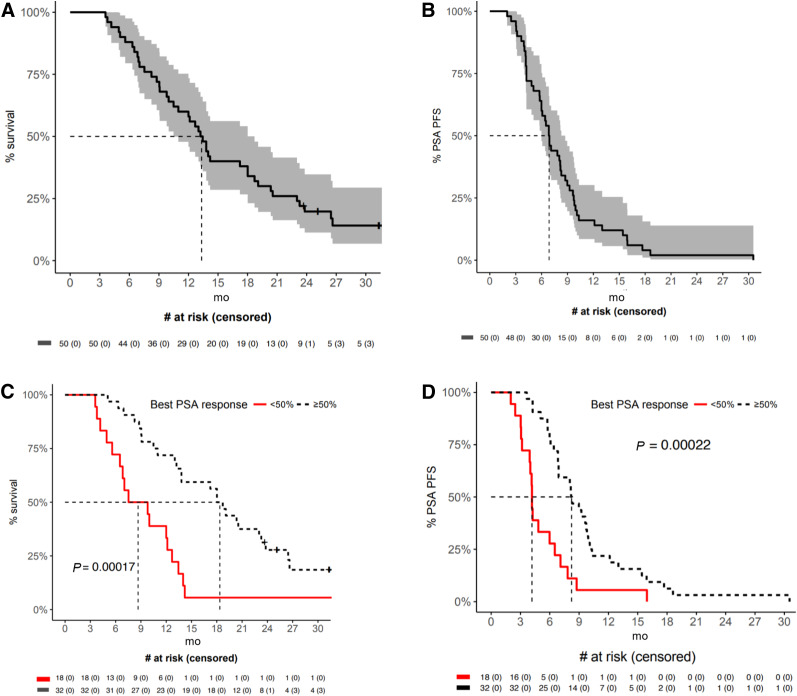
At the time of analysis, 43 of 50 patients were deceased. Median overall survival was 13.3 mo (95% CI, 10.5–18.7 mo) (Fig. 5). Survival was significantly longer in patients who achieved a PSA decline of at least 50%, with a median of 18.4 mo (95% CI, 13.8–23.8 mo) compared with 8.7 mo if the PSA decline was less than 50% (95% CI, 6.5–13.4 mo). PSA response at 12 wk was predictive of survival, with the optimal cutoff defined as 34% (Supplemental Fig. 3).
FIGURE 5.
Kaplan–Meier survival curves: overall survival (A) and PSA progression-free survival (B) for entire cohort; and overall survival (C) and PSA progression-free survival (D) in patients with PSA decline of ≥50% compared with PSA decline of <50%.
All patients eventually had PSA progression, with the longest duration of response being 31 mo in a patient with lymph node–only disease. Median PSA progression-free survival was 6.9 mo (95% CI, 6.0–8.7 mo). PSA progression-free survival was also significantly longer in patients with a PSA decline of at least 50%, at 8.2 mo (95% CI, 6.9–10.3 mo) compared with 4.2 mo for those with a decline of less than 50% (95% CI, 3.9–7.1 mo). In 37 patients with at least 3 PSA values at baseline and time of progression, the mean PSA doubling time was 1.4 mo at time of progression compared with 3.3 mo at baseline (P = 0.002).
Retreatment Cohort
On progression, 30 patients went on to receive further systemic therapy. At first relapse, these included 177Lu-PSMA (n = 14), cabazitaxel (n = 7), docetaxel (n = 6), mitoxantrone (n = 1), and olaparib (n = 1). At subsequent progression, additional lines of therapy included cabazitaxel (n = 7), pembrolizumab (n = 4), docetaxel (n = 2), 177Lu-PSMA (n = 1) (after olaparib), enzalutamide (n = 1), and carboplatin (n = 1). In total, 15 patients received further 177Lu-PSMA and 21 patients had at least 1 line of other systemic therapy.
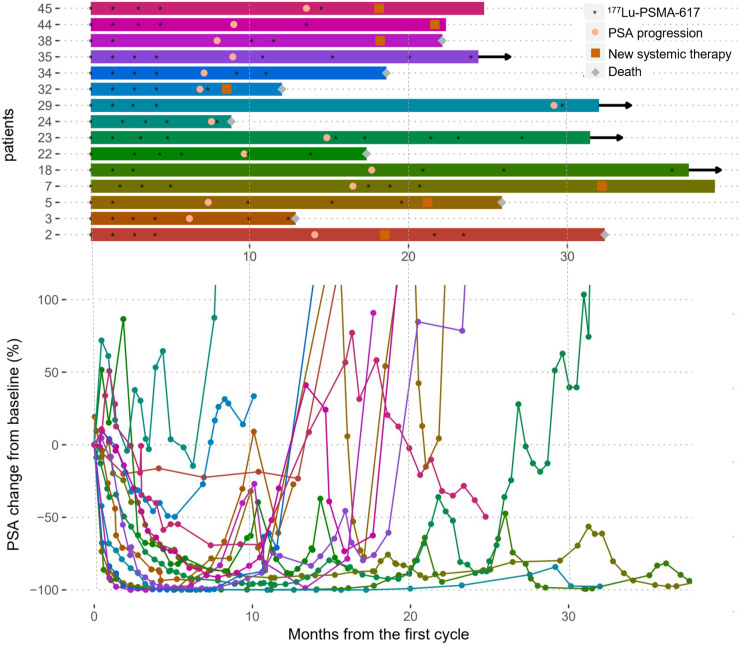
Of 15 patients receiving further 177Lu-PSMA at first or second relapse after initial response to 177Lu-PSMA, 11 (73%) had a PSA decline of at least 50% (Fig. 6). Retreatment commenced a median of 359 d after study enrollment (median, 2 cycles; range, 1–5). The mean best percentage fall in PSA in 177Lu-PSMA responders was 76.7% (range, 60%–98%). However, responses after further 177Lu-PSMA were less durable (Table 5). The median overall survival from time of study enrollment in the 15 patients who received retreatment was 26.6 mo.
FIGURE 6.
Outcomes of 15 patients who received further 177Lu-PSMA. (Top) Swimmer plot of progress over time. Arrows indicate patients who were alive at follow-up. (Bottom) Corresponding spider plot of percentage PSA change over time compared with baseline.
TABLE 5.
Patients Who Received Further 177Lu-PSMA on Progression
| No. of 177Lu-PSMA cycles |
Best % PSA decline |
PSA progression-free survival* (d) |
||||
| Patient no. | Initial | Retreatment | Initial | Retreatment | Initial | Retreatment |
| 2 | 4 | 2 | 39 | 60 | 485 | 106 |
| 3 | 4 | 2 | 94 | 64 | 249 | 75 |
| 5 | 2 | 3 | 99 | 80 | 248 | 259 |
| 7 | 4 | 3 | 92 | 54 | 538 | 132 |
| 18 | 3 | 3 | 100 | 98 | 566 | 432 |
| 22 | 4 | 2 | 89 | 70 | 314 | 90 |
| 23 | 4 | 5 | 97 | 63 | 469 | 291 |
| 24 | 4 | 1 | 15 | No response | 266 | No response |
| 29 | 4 | 1 | 100 | 85 | 929 | In follow-up |
| 32 | 4 | 1 | 50 | No response | 245 | No response |
| 34 | 4 | 2 | 100 | No response | 273 | No response |
| 35 | 4 | 4 | 99 | 98 | 296 | 237 |
| 38 | 2 | 3 | 100 | 98 | 293 | 179 |
| 44 | 4 | 1 | 91 | 73 | 302 | 123 |
| 45 | 4 | 1 | 69 | No response | 484 | No response |
“Initial” measured from date of first treatment to PSA nadir, and “Retreatment” measured from date of treatment to PSA nadir.
Treatment-emergent adverse events were similar to initial therapy. One patient, already described above with node-only disease, experienced pancytopenia, with G4 thrombocytopenia, G4 neutropenia and a platelet nadir at 42 d, G3 lymphopenia, and G2 anemia. This patient went on to receive cabazitaxel chemotherapy without significant cytopenias. One patient experienced grade 3 chronic kidney disease, with eGFR declining progressively from 91 mL/min at baseline to 38 mL/min over the course of 30 mo after receiving 9 177Lu-PSMA treatments. Two patients died within 30 d of 177Lu-PSMA administration from subdural hematomas unrelated to treatment, 7 and 24 d after 177Lu-PSMA administration.
Of 21 patients receiving other systemic therapies at first or second relapse, 4 (19%) had a PSA decline of at least 50%. Twelve of these had undergone 68Ga-PSMA-11 and 18F-FDG PET/CT imaging previously, and in 6 patients disease was PSMA-positive without 18F-FDG discordance, indicating potential suitability for further 177Lu-PSMA therapy. Two patients had PSMA superscans with pancytopenia, and 4 patients had PSMA-positive disease with 18F-FDG discordance and were thus deemed unsuitable for further 177Lu-PSMA therapy.
DISCUSSION
In a prospective phase II clinical trial, we have previously reported high single-agent activity for 177Lu-PSMA in 30 patients with metastatic castration-resistant prostate cancer (14), confirming findings observed from numerous retrospective series (7,8,10,11,27,28). In this expanded cohort of patients, we report a PSA response rate of at least 50% in 64% of patients, compared with 57% in the original cohort. Importantly, no new or delayed treatment-related toxicity was reported except for G1–G2 renal injury. We also report for the first time—to our knowledge—patterns of disease progression after treatment with this novel therapeutic agent and the outcome of patients who were retreated with additional systemic agents, including further doses of 177Lu-PSMA, at disease progression.
Approximately one third of screened patients in this cohort were not offered 177Lu-PSMA therapy, largely because of either low PSMA expression or the presence of PSMA-negative, 18F-FDG-positive disease (18F-FDG–discordant disease) on pretreatment screening (Supplemental Fig. 4). These stringent selection criteria have likely enriched our cohort with patients most likely to benefit from 177Lu-PSMA therapy and explains our relatively high PSA response rates compared with other series that did not perform screening 18F-FDG PET studies. Radiation dosimetry from the original cohort (15) and others (29) shows that the SUVmean of screening 68Ga-PSMA-11 PET correlates with absorbed dose in tumor. We have also reported that whole-body tumor dose correlates with therapeutic response (15), justifying our approach to limiting treatment with single-agent 177Lu-PSMA to patients with relatively high uptake on diagnostic PSMA scanning. Nevertheless, we acknowledge that patients with more heterogeneous PSMA expression may still have derived clinical benefit from 177Lu-PSMA treatment, particularly if this were combined with other effective systemic therapies.
The optimal dose and administration schedule for 177Lu-PSMA are not clearly defined, and the choice of treatment cycles in the design of this study was pragmatic. Therefore, in some patients who relapsed after completion of study therapy and who continued to fulfill study eligibility criteria, namely maintained expression of PSMA, we administered further cycles of therapy via a compassionate-access program. Administration of further therapy in this group of patients was effective, achieving a PSA response rate of at least 50% in 73% of patients. These findings suggest that progression after therapy in responding patients should not be a barrier to further treatment, as long as patients are carefully selected. The findings also provide safety data to support administering more than 4 cycles of therapy. One of our patients has had 9 cycles of therapy between 2017 and 2019 and continued to benefit from 177Lu-PSMA treatment but had declining renal function. Loss of renal cortical mass due to the age of the population and prior obstructive uropathy may render men with advanced prostate cancer at increased risk of progressive renal impairment, because a greater percentage of the residual nephron mass is within the 1- to 3-mm range of β-radiation emitted by 177Lu-PSMA as it is excreted by the kidneys.
Patients who further went on to receive other systemic therapies, primarily salvage systemic chemotherapy, had much worse biochemical responses. In a single patient treated with a poly(adenosine diphosphate ribose) polymerase inhibitor without any response, a subsequent biochemical response was achieved after further 177Lu-PSMA therapy. Nevertheless, the duration of response in patients given additional 177Lu-PSMA was significantly shorter than after de novo treatment. The high rate of eventual treatment failure supports efforts to increase the depth and durability of response, which may require combination therapies to enhance control of micrometastatic disease, increase radiosensitivity of disease sites, or activate an adaptive immune response. Trials are currently under way to test combination therapies with 177Lu-PSMA to assess the safety and efficacy of such approaches (NCT03874884, NCT03658447). First, however, we await the results of 2 key randomized controlled trials currently under way; the ANZUP TheraP trial comparing 177Lu-PSMA to cabazitaxel (NCT03392428) and the Endocyte VISION trial comparing 177Lu-PSMA plus best supportive care/standard of care to best supportive care/standard of care alone (NCT03511664).
After an initial response, patients predominantly progressed, with new focal or diffuse areas of involvement in the bone or new hepatic metastases. Diffuse marrow infiltration was the most common pattern of eventual demise, manifesting as leukoerythroblastic pancytopenia and sufficient to cause cessation of 177Lu-PSMA treatment. Liver metastases were the second most common pattern of progression; these generally had low PSMA expression and high metabolic activity in line with recent data (30).
The high response rates and limited toxicity of 177Lu-PSMA have stimulated interest in the wider application of this therapy earlier in the course of the disease (31,32). Radiation dosimetry suggests that earlier introduction of therapy should be safe, but data on long-term toxicity to verify this suggestion are sparse, given the current application of therapy in end-stage patients who, even with excellent responses to treatment, have a relatively limited prognosis. Renal function requires ongoing monitoring in patients receiving serial treatment.
CONCLUSION
This study confirms the findings of our earlier report, demonstrating high therapeutic efficacy and low toxicity for 177Lu-PSMA in men with metastatic castration-resistant prostate cancer who have progressed after standard therapies. The study also provides evidence of improvement in quality of life in multiple domains. Finally, we demonstrated high response rates but less durable responses in patients rechallenged with 177Lu-PSMA on progression.
DISCLOSURE
177Lu (no carrier added) was supplied by the Australian Nuclear Science and Technology Organization (ANSTO, Sydney, Australia), and PSMA-617 was supplied by Advanced Biochemical Compounds (ABX, Radeberg, Germany) and subsequently Endocyte (now part of Advanced Accelerator Applications, a Novartis company). Interim results of this study were presented at the 2019 ASCO Genitourinary Symposium (San Diego) and the 2018 annual meeting of the Society of Nuclear Medicine and Molecular Imaging (Philadelphia). Michael Hofman is the chair of the ANZUP TheraP Study, which receives research support from the Prostate Cancer Foundation of Australia (PCFA), Endocyte (a Novartis company), and the Australian Nuclear Science and Technology Organisation (ANSTO, Sydney, Australia). Michael Hofman additionally receives research support from Movember Australia, the Prostate Cancer Foundation (PCF), the Prostate Cancer Foundation of Australia (PCFA), and the Victoria Cancer Agency (VCA). Unrelated to this work, he has received honoraria and travel support for educational lectures from Janssen, Ipsen, and Sanofi Genzyme. Rodney Hicks holds shares in Telix on behalf of the Peter MacCallum Cancer Centre and has received travel support from GE Medical Systems and Siemens Healthineers. Ben Tran receives consulting and honoraria from Amgen, Astellas, Bayer, Bristol-Myers Squibb, IQVIA, Janssen-Cilag, Sanofi, Novartis, and Ipsen and research funding from Amgen and Astellas. Michael Hofman and Shahneen Sandhu receive from the Peter MacCallum Foundation a Clinical Fellowship directly supporting this research. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: What is the role of 177Lu-PSMA in men with metastatic castration-resistant prostate cancer who have progressed after standard therapies?
PERTINENT FINDINGS: In this 50-patient phase II single-center clinical trial, we observed high response rates (PSA decline ≥50% in 64%) with low toxicity and improved health-related quality of life. Furthermore, in patients rechallenged with 177Lu-PSMA on progression, the response rate was high, whereas responses to other forms of systemic therapies were lower.
IMPLICATIONS FOR PATIENT CARE: In men with limited therapeutic options and PSMA-avid prostate cancer, 177Lu-PSMA is an effective therapy with low toxicity.
Supplementary Material
Acknowledgments
We thank the nuclear medicine and nursing staff at the Peter MacCallum Cancer Centre and all the patients who participated in the study. We also thank Dr. Mathias Bressel (Biostatistician) for analyzing the quality-of-life data.
REFERENCES
- 1.Smith-Jones PM, Vallabahajosula S, Goldsmith SJ, et al. In vitro characterization of radiolabeled monoclonal antibodies specific for the extracellular domain of prostate-specific membrane antigen. Cancer Res. 2000;60:5237–5243. [PubMed] [Google Scholar]
- 2.Ghosh A, Heston WD. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J Cell Biochem. 2004;91:528–539. [DOI] [PubMed] [Google Scholar]
- 3.Sweat SD, Pacelli A, Murphy GP, Bostwick DG. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology. 1998;52:637–640. [DOI] [PubMed] [Google Scholar]
- 4.Hofman MS, Murphy DG, Williams SG, et al. A prospective randomized multicentre study of the impact of gallium-68 prostate-specific membrane antigen (PSMA) PET/CT imaging for staging high-risk prostate cancer prior to curative-intent surgery or radiotherapy (proPSMA study): clinical trial protocol. BJU Int. 2018;122:783–793. [DOI] [PubMed] [Google Scholar]
- 5.Perera M, Papa N, Roberts M, et al. Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions—a systematic review and meta-analysis. Eur Urol. February 14, 2019 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 6.Farolfi A, Fendler W, Iravani A, et al. Theranostics for advanced prostate cancer: current indications and future developments. Eur Urol Oncol. 2019;2:152–162. [DOI] [PubMed] [Google Scholar]
- 7.Ahmadzadehfar H, Rahbar K, Kurpig S, et al. Early side effects and first results of radioligand therapy with 177Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: a two-centre study. EJNMMI Res. 2015;5:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahbar K, Ahmadzadehfar H, Kratochwil C, et al. German Multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med. 2017;58:85–90. [DOI] [PubMed] [Google Scholar]
- 9.Yadav MP, Ballal S, Tripathi M, et al. 177Lu-DKFZ-PSMA-617 therapy in metastatic castration resistant prostate cancer: safety, efficacy, and quality of life assessment. Eur J Nucl Med Mol Imaging. 2017;44:81–91. [DOI] [PubMed] [Google Scholar]
- 10.Heck MM, Retz M, D’Alessandria C, et al. Systemic radioligand therapy with 177Lu labeled prostate specific membrane antigen ligand for imaging and therapy in patients with metastatic castration resistant prostate cancer. J Urol. 2016;196:382–391. [DOI] [PubMed] [Google Scholar]
- 11.Baum RP, Kulkarni HR, Schuchardt C, et al. 177Lu-labeled prostate-specific membrane antigen radioligand therapy of metastatic castration-resistant prostate cancer: safety and efficacy. J Nucl Med. 2016;57:1006–1013. [DOI] [PubMed] [Google Scholar]
- 12.Kulkarni HR, Singh A, Schuchardt C, et al. PSMA-based radioligand therapy for metastatic castration-resistant prostate cancer: the Bad Berka experience since 2013. J Nucl Med. 2016;57(suppl):97S–104S. [DOI] [PubMed] [Google Scholar]
- 13.Emmett L, Crumbaker M, Ho B, et al. Results of a prospective phase 2 pilot trial of 177Lu-PSMA-617 therapy for metastatic castration-resistant prostate cancer including imaging predictors of treatment response and patterns of progression. Clin Genitourin Cancer. 2019;17:15–22. [DOI] [PubMed] [Google Scholar]
- 14.Hofman MS, Violet J, Hicks RJ, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825–833. [DOI] [PubMed] [Google Scholar]
- 15.Violet J, Jackson P, Ferdinandus J, et al. Dosimetry of 177Lu-PSMA-617 in metastatic castration-resistant prostate cancer: correlations between pretherapeutic imaging and whole-body tumor dosimetry with treatment outcomes. J Nucl Med. 2019;60:517–523. [DOI] [PubMed] [Google Scholar]
- 16.Hohberg M, Eschner W, Schmidt M, et al. Lacrimal glands may represent organs at risk for radionuclide therapy of prostate cancer with [Lu]DKFZ-PSMA-617. Mol Imaging Biol. 2016;18:437–45. [DOI] [PubMed] [Google Scholar]
- 17.Delker A, Fendler WP, Kratochwil C, et al. Dosimetry for 177Lu-DKFZ-PSMA-617: a new radiopharmaceutical for the treatment of metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:42–51. [DOI] [PubMed] [Google Scholar]
- 18.Kabasakal L, AbuQbeitah M, Aygun A, et al. Pre-therapeutic dosimetry of normal organs and tissues of 177Lu-PSMA-617 prostate-specific membrane antigen (PSMA) inhibitor in patients with castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42:1976–1983. [DOI] [PubMed] [Google Scholar]
- 19.Okamoto S, Thieme A, Allmann J, et al. Radiation dosimetry for 177Lu-PSMA-I&T in metastatic castration-resistant prostate cancer: absorbed dose in normal organs and tumor lesions. J Nucl Med. 2017;58:445–450. [DOI] [PubMed] [Google Scholar]
- 20.Yadav MP, Ballal S, Tripathi M, et al. Post-therapeutic dosimetry of 177Lu-DKFZ-PSMA-617 in the treatment of patients with metastatic castration-resistant prostate cancer. Nucl Med Commun. 2017;38:91–98. [DOI] [PubMed] [Google Scholar]
- 21.Fendler WP, Kratochwil C, Ahmadzadehfar H, et al. 177Lu-PSMA-617 therapy, dosimetry and follow-up in patients with metastatic castration-resistant prostate cancer [in German]. Nucl Med (Stuttg). 2016;55:123–128. [PubMed] [Google Scholar]
- 22.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. [DOI] [PubMed] [Google Scholar]
- 24.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- 25.Hofman MS, Hicks RJ. Peptide receptor radionuclide therapy for neuroendocrine tumours: standardized and randomized, or personalized? Eur J Nucl Med Mol Imaging. 2014;41:211–213. [DOI] [PubMed] [Google Scholar]
- 26.Thang SP, Violet J, Sandhu S, et al. Poor outcomes for patients with metastatic castration-resistant prostate cancer with low prostate-specific membrane antigen (PSMA) expression deemed ineligible for 177Lu-labelled PSMA radioligand therapy. Eur Urol Oncol. 2019;2:670–676. [DOI] [PubMed] [Google Scholar]
- 27.Kratochwil C, Giesel FL, Stefanova M, et al. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with 177Lu-labeled PSMA-617. J Nucl Med. 2016;57:1170–1176. [DOI] [PubMed] [Google Scholar]
- 28.Yadav MP, Ballal S, Tripathi M, et al. 177Lu-DKFZ-PSMA-617 therapy in metastatic castration resistant prostate cancer: safety, efficacy, and quality of life assessment. Eur J Nucl Med Mol Imaging. 2017;44:81–91. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Zang J, Wang H, et al. Pretherapeutic 68Ga-PSMA-617 PET may indicate the dosimetry of 177Lu-PSMA-617 and 177Lu-EB-PSMA-617 in main organs and tumor lesions. Clin Nucl Med. 2019;44:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paschalis A, Sheehan B, Riisnaes R, et al. Prostate-specific membrane antigen heterogeneity and DNA repair defects in prostate cancer. Eur Urol. 2019;76:469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy DG, Sathianathen N, Hofman MS, Azad A, Lawrentschuk N. Where to next for theranostics in prostate cancer? Eur Urol Oncol. 2019;2:163–165. [DOI] [PubMed] [Google Scholar]
- 32.Murphy DG, Hofman MS, Azad A, Violet J, Hicks RJ, Lawrentschuk N. Going nuclear: it is time to embed the nuclear medicine physician in the prostate cancer multidisciplinary team. BJU Int. May 24, 2019 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 33.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(suppl 1):122S–150S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.