Abstract
An 80‐year‐old man with multiple comorbidities presented to the emergency department with tachypnea, tachycardia, fever, and critically low O2 saturation and definitive chest computerized tomography scan findings in favor of COVID‐19 and positive PCR results in 48 hours. He received antiviral treatment plus recombinant human erythropoietin (rhEPO) due to his severe anemia. After 7 days of treatment, he was discharged with miraculous improvement in his symptoms and hemoglobin level. We concluded that rhEPO could attenuate respiratory distress syndrome and confront the severe acute respiratory syndrome coronavirus 2 virus through multiple mechanisms including cytokine modulation, antiapoptotic effects, leukocyte release from bone marrow, and iron redistribution away from the intracellular virus.
Keywords: acute respiratory distress syndrome, COVID‐19, cytokine, erythropoietin
Highlights
-
–
Recombinant human erythropoietin can rapidly correct anemia and symptoms associated with COVID‐19
-
–
Through cytokine modulation, rhEPO exerts its cytoprotective and anti‐apoptotic effects in COVID pneumonia.
-
–
rhEPO takes iron away from intracellular virus into the bone marrow, undermining the viral enzymatic requirements.
1. INTRODUCTION
By the time of this writing COVID‐19 pandemic has caused over 462 000 confirmed infected cases and 20 000 deaths in more than 200 countries and territories all over the world. 1 Due to its capability of rapid contamination, this virus is very infectious and has caused significant mortality especially in the elderly and populations with comorbidities. The risk factors for developing acute respiratory distress syndrome (ARDS) and subsequent death in COVID‐19, have been reported to be age ≥65, neutrophilia, and development of organ failure and coagulation dysfunction. 2 Many interventions have been used to prevent the virus from progressing further in the host but in vulnerable patients, it finally progresses towards ARDS and cardiopulmonary arrest.
In the present study, we report a patient with COVID‐19 who after treating with recombinant human erythropoietin (rhEPO) due to his severe anemia, exhibited primarily unexplainable rapid symptoms relief and viral regression.
2. CASE PRESENTATION
An 80‐year‐old man with a past medical history of Alzheimer's disease and depression was brought to the emergency department because of a 20‐day history of lightheadedness, fainting, progressive weakness, confusion, loss of appetite, fever, cough, and dyspnea.
The initial physical examination showed a body temperature of 38.9°C, blood pressure of 96/60 mm Hg, pulse rate of 120 beats/minute, respiratory rate of 32 breaths/minute, and oxygen saturation of 80% while the patient was breathing without supplemental oxygen.
He also had generalized pallor, bilateral crackles especially in the right lung, no sign of bleeding in digital rectal examination. Other physical exam findings were unremarkable.
A chest computerized tomography (CT) scan demonstrated peripheral diffuse patchy ground‐glass opacities in both lungs.
An oropharyngeal swab sample was obtained and referred to the lab for the detection of viral respiratory pathogens by reverse transcription polymerase chain reaction and was reported positive for SARS‐CoV‐2 within 48 hours.
laboratory results on the first day of admission showed: WBC 6.4 K/μL, Hgb 5.2 g/dL, PLT count 142 000 cell/mm3, MCV 90.6 fL, RDW 34.9%, neutrophil 86.9%, lymphocyte 5.2%, CRP 142.7 mg/L, Urea 46 mg/dL, Cr 1.03 mg/dL, Iron 24 mcg/dL, TIBC 147 mcg/dL, LDH 639 U/L, Retic RPI 1.2%, ferritin 554.86 ng/mL, direct and indirect Coombs negative.
One week before his admission, the patient's CBC findings were: WBC 6.2 K/μL, Hgb 7 g/dL, PLT count 140 000 cell/mm3, MCV 89 fL.
Transfusion of one unit of packed red blood cell (RBC) and adequate hydration was prescribed for the patient.
According to COVID‐19 national committee treatment protocols, the following medications were started:
-
1)
Hydroxychloroquine Tab 400 mg stat
-
2)
Oseltamivir Cap 75 mg twice daily for 5 days
-
3)
Lopinavir/Ritonavir Tab 400/100 mg twice daily for 5 days
rhEPO was administered at a dose of 300 IU/kg divided into 5 doses of 4000 IU subcutaneous injections every other day during a 9‐day treatment course.
Furthermore, Ceftriaxone 1 g twice daily IV infusion was initiated for the treatment of pneumococcal superinfection because of the observed consolidation and air bronchogram pattern in the CT scan (Figure 1).
Figure 1.
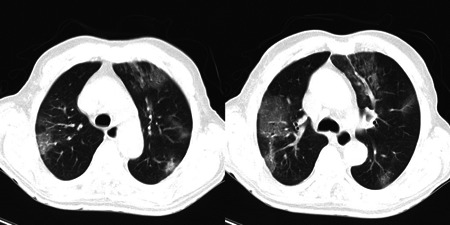
Spiral chest CT scan indicating multilobular patchy and ground‐glass opacities in favor of COVID‐19. CT scan, computed tomography scan
Within 8 days, both anemia severity and patients’ symptoms attenuated significantly.
On discharge day, physical exam revealed a temperature of 37.5°C, blood pressure of 110/70 mm Hg, pulse rate of 90 beats/minute, respiratory rate of 20 breaths/minute, and oxygen saturation of 90%.
Lab data results also showed improvements in lymphocyte count which increased from 333 to 933 cell/µL, and Hgb concentration raised from 5.2 to 6.7 g/dL after one unit of packed RBC transfusion and then up to 9 g/dL after rhEPO administration (Table 1).
Table 1.
Hemoglobin and lymphocyte count progression in 8 days
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | |
|---|---|---|---|---|---|---|---|---|
| Hgb, g/dL | 5.2 | 6.7 | 7.9 | 8.2 | 7.6 | 8.6 | 8.5 | 9 |
| Neutrophil, /µL | 5562 | 5776 | 7331 | 9718 | 6020 | 6288 | 4482 | 4590 |
| Lymph, /µL | 333 | 933 | 958 | 893 | 809 | 748 | 588 | 648 |
| Neut/lymph | 16.7 | 6.19 | 7.65 | 10.88 | 7.44 | 8.41 | 7.62 | 7.08 |
Note: On day 1, one unit of packed RBC was transfused. On days 1, 3, 5, and 7, 4000 IU rhEPO was administered subcutaneously. One week after discharge in follow‐up, he did not have any of the initial symptoms and the oxygen saturation was 94% in the room air.
Abbreviation: rhEPO, recombinant human erythropoietin.
3. DISCUSSION
As mentioned above rhEPO was prescribed for an 80‐year‐old confirmed case of COVID‐19 due to his initial severe anemia in addition to antivirals.
Subsequently, a very fast response considering his age and past medical history both in anemia correction (from Hb:6.7 to Hb:9) and COVID‐19 symptom relief was observed that could not be elaborated simply as a result of anemia correction.
The patient had Iron deficiency anemia and perhaps a mixed component of chronic disease anemia according to the lab results, however a thorough assessment of his anemia was reserved for after his discharge from the hospital.
Erythropoietin (EPO) is a hormone/cytokine produced mainly by the kidneys via hypoxia‐inducible factor‐2 as its primary transcription factor, and through inhibition of RBC precursors’ apoptosis, increases the red cell mass. However, EPO has other beneficial cytoprotective effects including anti‐ischemic, regenerative and antiapoptotic effects in a variety of tissues including lung, kidney, cardiac muscle, nervous system, retina, pancreas, and endothelial cells. 3 Through a special receptor; EPOR‐βcR, it conducts its protective effects following trauma and in critically ill patients. 4
Few animal studies have been conducted to explain the molecular pathways underlying the nonhematopoietic effects of EPO.
In 2019, Zhang et al 5 conducted an animal study on rats and grouped them into three groups of Sham, sepsis‐caused acute lung injury, and an intervention group with sepsis‐caused acute lung injury receiving EPO. The intervention group showed less severe pulmonary interstitial, and alveolar edema, hemorrhage, or lung collapse compared to Sham and sepsis‐caused lung injury groups. The protective effects of EPO towards lung tissue were attributed to its effects in inhibiting expression of nuclear factor‐κB (NF‐κB) in lung tissues, inhibition of interleukin‐6 (IL‐6) and tumour necrosis factor alpha as proinflammatory cytokines, and improvement of anti‐inflammatory cytokine IL‐10 levels. A similar study was also conducted to assess rhEPO effect on human respiratory epithelial cell apoptosis and detected cytoprotective effects of rhEPO through induction of an antiapoptotic Bcl‐xL/Bax phenotype 6 (Figure 2).
Figure 2.
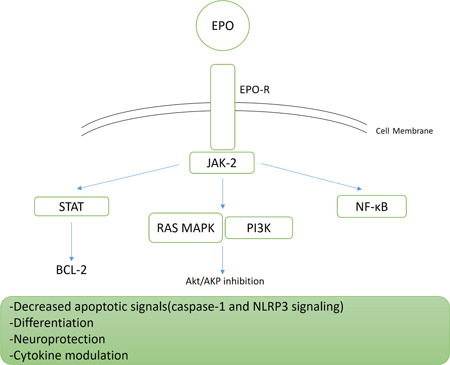
Molecular pathways associated with EPO signaling within the cell. EPO, erythropoietin; EPO‐R, erythropoietin receptor
In another animal study of lipopolysaccharide (LPS) induced sepsis model, 7 EPO effect on hepatic mitochondrial damage was assessed and it was shown that EPO suppressed the LPS effect on the increase of IL‐1β and reactive oxygen species levels, mitochondrial DNA copy number, and also decreased protein expressions of caspase‐1, and NLRP3 (NLR Family Pyrin Domain Containing 3) gene. EPO alleviated LPS‐induced cellular edema in hepatic lobules, lymphocytic infiltration, and hepatocellular necrosis.
Renoprotective effects of EPO in mice with septic acute kidney injury has been observed and has been linked to attenuation of microvascular damage, reducing renal inflammatory response and improvement of renal tissue oxygenation through the decrease of hypoxia‐inducible factor‐1 alpha, inducible nitric oxide synthase, and NF‐κB and also enhancement of erythropoietin receptor (EPO‐R), PeCAM‐1, vascular endothelial growth factor, and VEGFR‐2 expression. 8
Another study conducted earlier by Heitrich et al 9 on a murine model of sepsis‐caused acute lung injury and acute kidney injury demonstrated beneficial protective EPO effects on pulmonary and renal outcomes through EPO‐R and VEGF/VEGFR‐2 expression. Moreover, it has been shown that EPO has cardioprotective effects by reducing the myocardial inflammatory response in septic rats and attenuates the reduction in mitochondrial membrane potential and inhibits myocardial cell apoptosis through mitochondrial pathway and by reducing NF‐κB p65 expression. 10
NF‐κB is a principle factor of multiple inflammatory pathways, and according to the above‐mentioned studies, it can be considered an important target for treatment. Blocking the activation of NF‐κB by EPO may prevent further deterioration caused by the COVID‐19 disease through cytokine modulation and its regenerative and antiapoptotic effects
Another mechanism for an explanation of EPO effect on the improvement of the clinical condition of the presented case could be rooted to the findings of Ito et al 11 on an animal study that revealed that 24 hours after EPO administration, the number of IgDlow immature B cells and mature B cells, as well as CD4+ and CD8+ T cells in the bone marrow, decreased significantly due to their egress into the peripheral blood. This backup leukocyte release into the peripheral bloodstream might be a reason for the optimized viral confrontation of the immune system. Thus in the presented case, after the first dose of rhEPO and packed RBC transfusion, absolute lymphocyte count increased from 333 to 933/μL of blood; a rise quite larger than to be elucidated only by 250 mL of packed RBCs transfusion.
During inflammation, serum Hepcidin levels increase the following stimulation by IL‐6, downregulating cellular ferroportin and this leads to decreased iron absorption and its detainment in liver and spleen macrophages 12 which could promote the survival of intracellular microorganisms. EPO by downregulating IL‐6 and Hepcidin levels could lead to an increased release of iron from macrophages and increased absorption of iron by the bone marrow, thus decreasing iron availability for intracellular organisms like Coronavirus for their required enzymatic activities. This antiviral strategy of keeping iron out of infected cells has previously been explored and considered to be potentially effective in human infections by hepatitis C virus, human immunodeficiency virus‐1, hepatitis B virus, and cytomegalovirus viral infections. 13
Although the above‐mentioned novel mechanisms of EPO effect in septic states could elaborate the rapid clinical improvement of the presented COVID‐19 case, we should not underestimate the definitive effects of rising hemoglobin from 6.7 to 9 g/dL in the improvement of pulmonary oxygenation and thus relieving the existing respiratory symptoms. However, the 2.3 g/dL rise in hemoglobin level in only 7 days with the mentioned dose of rhEPO is both profound and questionable considering the reported peak effects of rhEPO in 2 to 6 weeks after the starting dose. 14 , 15
In spite of the aforementioned benefits for EPO, it can aggravate the formation of microthrombosis and subsequent septic multiorgan failure and coagulopathy. 16 Besides, in patients with chronic kidney disease‐associated anemia, EPO increases thrombotic events and risk of death when administered for Hb more than 12 g/dL. 17 Other side effects of rhEPO in patients receiving large doses have been reported to be hypertension, hyperviscosity, enhancement of tumor progression, and in rare instances pure red blood cell aplasia. 18
Regarding the probable benefits of rhEPO in reversing ARDS and its side effects, it seems to be a reasonable choice to use this agent in critically ill COVID‐19 patients to save their lives from imminent death, however, to determine the optimal dose with maximum cytoprotective and antiapoptotic effects and minimum potential toxicity of rhEPO, more clinical studies are required.
Therefore, we recommend the designation of well‐organized clinical trials with careful consideration of rhEPO administration in anemic COVID‐19 patients to further evaluate its clinical benefits in this critical patient population group without imposing further adverse effects associated with this drug.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Hadadi A, Mortezazadeh M, Kolahdouzan K, Alavian G. Does recombinant human erythropoietin administration in critically ill COVID‐19 patients have miraculous therapeutic effects? J Med Virol. 2020;92:915–918. 10.1002/jmv.25839
REFERENCES
- 1. World Health Organization Available from: www.who.int
- 2. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China [published online ahead of print March 13, 2020]. JAMA Intern Med. 2020:E1–E10. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nekoui A, Blaise G. Erythropoietin and nonhematopoietic Effects. Am J Med Sci. 2017;353(1):76‐81. [DOI] [PubMed] [Google Scholar]
- 4. French C. Erythropoietin in critical illness and trauma. Crit Care Clin. 2019;35(2):277‐287. [DOI] [PubMed] [Google Scholar]
- 5. Zhang X, Dong S. Protective effects of erythropoietin towards acute lung injuries in rats with sepsis and its related mechanisms. Ann Clin Lab Sci. 2019;49(2):257‐264. [PubMed] [Google Scholar]
- 6. MacRedmond R, Singhera GK, Dorscheid DR. Erythropoietin inhibits respiratory epithelial cell apoptosis in a model of acute lung injury. Eur Respir J. 2009;33(6):1403‐1414. [DOI] [PubMed] [Google Scholar]
- 7. Zhang GX, Du YJ, Li XH, et al. Protective effect of erythropoietin against lipopolysaccharide induced inflammation and mitochondrial damage in liver. J Biol Regul Homeost Agents. 2018;32(2):199‐206. [PubMed] [Google Scholar]
- 8. Stoyanoff TR, Rodriguez JP, Todaro JS, Colavita JPM, Torres AM, Aguirre MV. Erythropoietin attenuates LPS‐induced microvascular damage in a murine model of septic acute kidney injury. Biomed Pharmacother. 2018;107:1046‐1055. [DOI] [PubMed] [Google Scholar]
- 9. Heitrich M, Garcia DM, Stoyanoff TR, Rodriguez JP, Todaro JS, Aguirre MV. Erythropoietin attenuates renal and pulmonary injury in polymicrobial induced‐sepsis through EPO‐R, VEGF and VEGF‐R2 modulation. Biomed Pharmacother. 2016;82:606‐613. [DOI] [PubMed] [Google Scholar]
- 10. Zhang X, Dong S, Qin Y, Bian X. Protective effect of erythropoietin against myocardial injury in rats with sepsis and its underlying mechanisms. Mol Med Rep. 2015;11(5):3317‐3329. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11. Ito T, Hamazaki Y, Takaori‐Kondo A, Minato N. Bone marrow endothelial cells induce immature and mature B cell egress in response to erythropoietin. Cell Struct Funct. 2017;42(2):149‐157. [DOI] [PubMed] [Google Scholar]
- 12. Ganz T. Iron and infection. Int J Hematol. 2018;107(1):7‐15. [DOI] [PubMed] [Google Scholar]
- 13. Drakesmith H, Prentice A. Viral infection and iron metabolism. Nat Rev Microbiol. 2008;6(7):541‐552. [DOI] [PubMed] [Google Scholar]
- 14. Bohlius J, Weingart O, Trelle S, Engert A. Cancer‐related anemia and recombinant human erythropoietin—an updated overview. Nat Clin Pract Oncol. 2006;3(3):152‐164. [DOI] [PubMed] [Google Scholar]
- 15. Jurado Garcia JM, Torres Sanchez E, Olmos Hidalgo D, Alba Conejo E. Erythropoietin pharmacology. Clin Transl Oncol. 2007;9(11):715‐722. [DOI] [PubMed] [Google Scholar]
- 16. Shimaoka M, Park EJ. Advances in understanding sepsis. Eur J Anaesthesiol Suppl. 2008;42:146‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Phrommintikul A, Haas SJ, Elsik M, Krum H. Mortality and target haemoglobin concentrations in anaemic patients with chronic kidney disease treated with erythropoietin: a meta‐analysis. Lancet. 2007;369(9559):381‐388. [DOI] [PubMed] [Google Scholar]
- 18. Kakavas S, Demestiha T, Vasileiou P, Xanthos T. Erythropoetin as a novel agent with pleiotropic effects against acute lung injury. Eur J Clin Pharmacol. 2011;67(1):1‐9. [DOI] [PubMed] [Google Scholar]


