Abstract
Background and Aims
Coronavirus disease 2019 (COVID‐19), the illness caused by the SARS‐CoV‐2 virus, is rapidly spreading throughout the world. Hospitals and healthcare providers are preparing for the anticipated surge in critically ill patients, but few are wholly equipped to manage this new disease. The goals of this document are to provide data on what is currently known about COVID‐19, and how it may impact hepatologists and liver transplant providers and their patients. Our aim is to provide a template for the development of clinical recommendations and policies to mitigate the impact of the COVID‐19 pandemic on liver patients and healthcare providers.
Approach and Results
This article discusses what is known about COVID‐19 with a focus on its impact on hepatologists, liver transplant providers, patients with liver disease, and liver transplant recipients. We provide clinicians with guidance for how to minimize the impact of the COVID‐19 pandemic on their patients’ care.
Conclusions
The situation is evolving rapidly, and these recommendations will need to evolve as well. As we learn more about how the COVID‐19 pandemic impacts the care of patients with liver disease, we will update the online document available at https://www.aasld.org/about-aasld/covid-19-and-liver.
Abbreviations
- ACE
angiotensin‐converting enzyme
- ACEIs
ACE inhibitors
- AIH
autoimmune hepatitis
- ALT
alanine aminotrasnferase
- ARB
angiotensin receptor blocker
- AST
aspartate aminotransferase
- CDC
Centers for Disease Control and Prevention
- CLD
chronic liver disease
- CMS
Centers for Medicare and Medicaid Services
- COVID‐19
coronavirus disease 2019
- CYP3A4
cytochrome P450 3A4
- FDA
U.S. Food and Drug Administration
- HCC
hepatocellular carcinoma
- ICU
intensive care unit
- IL
interleukin
- LT
liver transplantation
- MELD
Model for End‐Stage Liver Disease
- MERS
Middle East Respiratory Syndrome
- PPE
personal protective equipment
- SARS
Severe Acute Respiratory Syndrome
- ULN
upper limit of normal
Overview and Rationale
Coronavirus disease 2019 (COVID‐19), the illness caused by the SARS‐CoV‐2 virus, is rapidly spreading throughout the world.( 1 ) Hospitals and health care providers across the United States are preparing for the anticipated surge in critically ill patients, but few are wholly equipped to manage this new disease. Nonetheless, we all must do our part to prepare our patients, clinics, and hospitals for the drastic changes necessary to mitigate the spread of SARS‐CoV‐2 or we risk overwhelming the capacity of our health care system.( 2 ) In addition, we must continue to manage the care of our patients with liver disease and our liver transplant recipients where unique logistical and pharmacological issues will arise. According to the Centers for Disease Control and Prevention (CDC), patients aged >65 years and those with cardiovascular disease, diabetes mellitus, morbid obesity, chronic obstructive pulmonary disease, or liver disease are at higher risk for severe COVID‐19.( 3 ) However, although the CDC considers those with liver disease to be at increased risk, it is unclear whether patients with cirrhosis, those with autoimmune hepatitis on immunosuppressive medications, and pretransplant and posttransplant patients on immunosuppressant therapy are at increased risk for severe COVID‐19. Given the extraordinary amount of rapidly emerging data on COVID‐19, it is difficult for any single clinician to stay abreast of the latest information. The goals of this document are to provide data on what is currently known about COVID‐19, and how it may impact hepatologists and liver transplant providers and their patients. Our aim is to provide a template for the development of clinical recommendations and policies to mitigate the impact of the COVID‐19 pandemic on liver patients and health care providers. Considering that SARS‐CoV‐2 can be transmitted from asymptomatic individuals, including children, and it can be detected in stool after viral clearance from pharyngeal samples,( 4 , 5 , 6 ) these recommendations have been created to protect our patients, communities, and health care workers. Data from China, Italy, and Spain are staggering, with reports from Italy indicating that up to 20% of health care workers who are taking care of patients with COVID‐19 may become infected.( 7 ) If we do not contain the spread of SARS‐CoV‐2 quickly, our health care system’s capacity will be overwhelmed, including insufficient availability of intensive care unit (ICU) beds, ventilators, and health care workers.
Effects of SARS‐CoV‐2 on the Liver and Evaluation of COVID‐19 Patients With Elevated Liver Biochemistries
The novel coronavirus SARS‐CoV‐2 is most similar to the beta‐coronaviruses, SARS‐CoV and MERS‐CoV, the causative agents of the Severe Acute Respiratory Syndrome (SARS) outbreak in 2002‐2003 and the Middle East Respiratory Syndrome (MERS) outbreak beginning in 2012, respectively. SARS‐CoV‐2 is a single, positive‐stranded RNA virus that replicates using a virally encoded RNA‐dependent RNA polymerase. SARS‐CoV‐2 binds to, and is internalized into, target cells through angiotensin‐converting enzyme (ACE) 2, which acts as a functional receptor.( 8 , 9 ) ACE2 is present in biliary and liver epithelial cells; therefore, the liver is a potential target for infection.( 10 ) Incidence of elevated serum liver biochemistries in hospitalized patients with COVID‐19 ranges from 14% to 53%.( 1 , 11 , 12 , 13 , 14 , 15 ) Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are primarily elevated in COVID‐19, generally 1‐2 times the upper limit of normal (ULN), with normal to modestly elevated total bilirubin levels early in the disease process. Liver injury occurs more commonly in severe COVID‐19 cases than in mild cases, and rare cases of severe acute hepatitis have been described.( 11 , 15 , 16 ) Liver injury in mild COVID‐19 cases is usually transient and does not require specific treatment.( 15 ) In addition to other markers of disease severity, such as lymphopenia and D‐dimer, low serum albumin on hospital admission is a marker of COVID‐19 severity.( 14 , 17 , 18 )
Severe COVID‐19 is uncommon in children.( 19 , 20 ) In the rare cases of severe pediatric COVID‐19, increases in ALT or AST, when present, are usually mild (<2× ULN).( 19 ) Data from Bergamo, a major site of COVID‐19 in Italy, showed no increase in hospitalization among >300 children followed for liver transplantation (LT), autoimmune hepatitis (AIH), or hepatoblastoma (HB).( 20 ) In this report, 3 of 13 hospitalized children following LT or receiving chemotherapy for HB tested positive for SARS‐CoV‐2, and none developed clinical pulmonary disease.( 20 )
Liver histological assessment has been limited, but thus far is nonspecific, and ranges from moderate microvesicular steatosis with mild, mixed lobular and portal activity to focal necrosis.( 21 , 22 ) Elevated liver biochemistries may reflect a direct virus‐induced cytopathic effect and/or immune damage from the provoked inflammatory response.( 12 , 23 ) Therapeutic agents used to manage symptomatic COVID‐19 may be hepatotoxic, including remdesivir and tocilizumab. Less common causes of elevated liver biochemistries include chloroquine, hydroxychloroquine, and azithromycin.
It is unknown whether patients with chronic liver disease (CLD), especially viral hepatitis B and/or C, are more susceptible to liver damage from SARS‐CoV‐2, as was the case with SARS‐CoV.( 14 ) It is also unknown whether SARS‐CoV‐2 infection exacerbates cholestasis in those with underlying cholestatic liver disease such as primary biliary cholangitis (PBC) or primary sclerosing cholangitis (PSC) or with underlying cirrhosis.( 15 ) Emerging data suggest that patients with nonalcoholic fatty liver disease (NAFLD) may be at higher risk for severe COVID‐19.( 24 ) It is unclear whether the risk is specific to NAFLD or to coexisting metabolic risk factors, such as cardiovascular disease, diabetes mellitus, and obesity, which are known to be associated with COVID‐19 severity.( 25 )
It will be difficult to differentiate whether increases in liver biochemistries are attributable to SARS‐CoV‐2 infection itself; its complications, including myositis (particularly with AST>ALT), cytokine release syndrome, ischemia/hypotension; or a drug‐induced liver injury.( 15 , 21 ) An approach to evaluating the patient with COVID‐19 and elevated liver biochemistries is shown in Fig. 1.
FIG. 1.
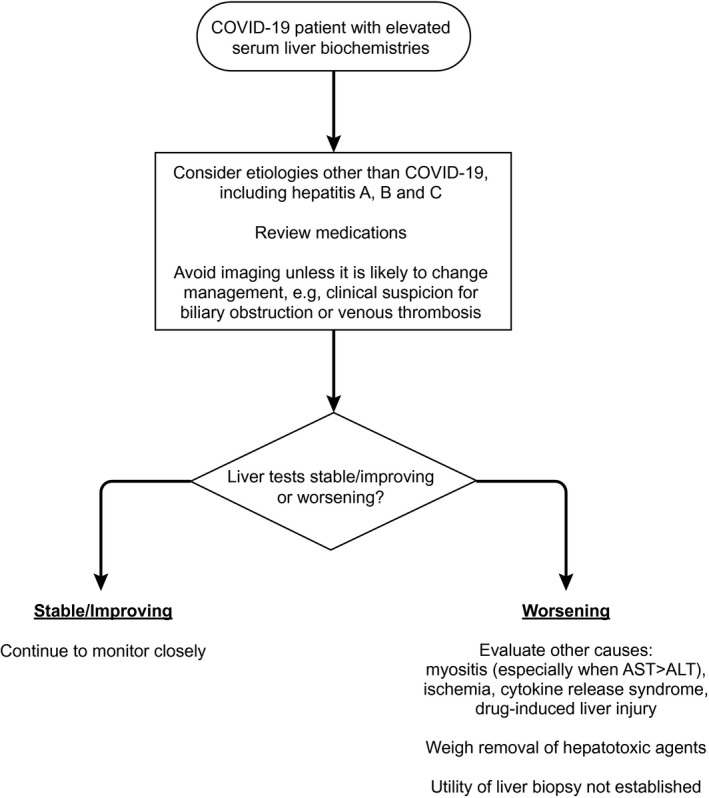
Approach to the patient with COVID‐19 and elevated serum liver biochemistries.
Recommendations
Patients with cirrhosis, those with AIH on immunosuppressive medications, and posttransplant patients on immunosuppressant therapy should be considered to be at increased risk for severe COVID‐19 and should be prioritized for testing until further data become available.
Consider etiologies unrelated to COVID‐19, including other viruses such as hepatitis A, B and C, when assessing patients with COVID‐19 and elevated liver biochemistries.
To limit unnecessary transport of patients with COVID‐19, ultrasound or other advanced imaging (e.g., magnetic resonance imaging/magnetic resonance cholangiopancreatography) should be avoided unless it is likely to change management, for example, clinical suspicion for biliary obstruction or venous thrombosis.
Consider other causes of elevated liver biochemistries, including myositis (particularly when AST>ALT), cardiac injury, ischemia, and cytokine release syndrome.
The presence of abnormal liver biochemistries should not be a contraindication to using investigational or off‐label therapeutics for COVID‐19 (e.g., remdesivir, tocilizumab, chloroquine, and hydroxychloroquine), although AST or ALT levels >5× ULN may exclude patients from consideration of some investigational agents.
Regular monitoring of liver biochemistries should be performed in all hospitalized COVID‐19 patients, particularly those treated with remdesivir or tocilizumab, regardless of baseline values.
In patients with AIH or LT recipients with active COVID‐19 and elevated liver biochemistries, do not presume disease flare or acute cellular rejection without biopsy confirmation.
Evaluate all children with elevated AST or ALT for underlying liver diseases and coexisting infections given that COVID‐19 is not commonly associated with abnormal liver biochemistries in children.( 19 )
Follow guidance in your clinical study protocol and/or by the U.S. Food and Drug Administration (FDA) for monitoring of liver biochemistries and discontinuation of study drug used to treat COVID‐19.
Stable Outpatients With Liver Disease and/or Hepatocellular Carcinoma
Information is limited regarding the effects of SARS‐CoV‐2 in patients with CLD. Data from the CDC on 122,653 COVID‐19 cases, including 7,162 (5.8%) with data on underlying health conditions, showed that approximately one‐third of these patients (37.6%) had at least one underlying condition or risk factor for severe outcomes.( 26 ) Among these patients with underlying conditions, only 41 patients (0.6%) had CLD, including 7 who required ICU admission.( 26 )
Both immunocompetent and immunosuppressed patients can contribute to SARS‐CoV‐2 spread even if they are asymptomatic.( 27 ) Children are less likely to become ill from SARS‐CoV‐2 infection, but can still contribute to spread of the virus.( 19 ) There is no evidence that patients with stable CLD attributed to hepatitis B and/or C, or cholestatic syndromes, such as PBC or PSC, have increased susceptibility to SARS‐CoV‐2 infection.( 15 )
The effect of COVID‐19 in patients with hepatocellular carcinoma (HCC) is not known. A case series reported an association between worse COVID‐19 outcomes and a history of nonhepatic types of cancer.( 28 ) Those who underwent recent chemotherapy had an even higher risk of severe COVID‐19, but the series also included those without recent chemotherapy.( 28 ) It is unknown whether patients with HCC are at increased risk for severe COVID‐19 by virtue of their malignancy. The slow median doubling time of HCC supports a rationale of a short delay in radiological surveillance given the challenges many centers are currently facing with COVID‐19.( 29 )
Recommendations
-
The CDC has provided broad and comprehensive recommendations to limit face‐to‐face visits, optimize supply of personal protective equipment (PPE), clean and disinfect rooms or areas visited by individuals with suspected or confirmed COVID‐19, and monitor health care workers for signs of illness.( 3 , 30 )
-
◦
Consider seeing in person only new adult and pediatric patients with urgent issues and clinically significant liver disease (e.g., jaundice, elevated ALT or AST >500 U/L, or recent onset of hepatic decompensation).
-
◦
Continue treatment for hepatitis B and hepatitis C if already on treatment.
Proceed with treatment of hepatitis B and C in patients without COVID‐19 as clinically warranted. The logistics of monitoring patients during the pandemic should be weighed against the urgency of treatment.
Initiating treatment of hepatitis B in a patient with COVID‐19 is not routinely warranted, but should be considered if there is clinical suspicion of a hepatitis B flare.
Initiating treatment of hepatitis C in a patient with COVID‐19 is not routinely warranted.
-
Continue monitoring in those on or off therapy for HCC and continue surveillance in those at risk for HCC (cirrhosis, chronic hepatitis B) as close to schedule as circumstances allow, although an arbitrary delay of 2 months is reasonable.
-
◦
Discuss the risks and benefits of delaying surveillance with the patient and document the discussion.
-
◦
Review images of new referrals for patients with liver masses in tumor board or with expert radiologists in virtual multidisciplinary conference before scheduling an in‐person visit.
Consider virtual patient visits to discuss diagnosis and management of HCC and other liver tumors.
Proceed with HCC treatments when able rather than delaying them because of the pandemic.
Patients With Decompensated Cirrhosis, LT Evaluations, and Patients on the LT Waiting List
Information is limited regarding the effects of SARS‐CoV‐2 infection in patients with decompensated cirrhosis or those awaiting LT. The complex decision making involved in whether or not to proceed with transplantation is now significantly more challenging because of the COVID‐19 pandemic. It is essential that transplant centers continuously assess their local situation and its impact on patients awaiting transplantation. Some transplant centers may decide that individual candidates should not receive organ offers at this time. Special consideration could be given to wait‐listed patients with high Model for End‐Stage Liver Disease (MELD) scores or HCC based on their risk of dropout and disease progression. A reduction in organ recovery is expected because of COVID‐19‐related limitations on institutional resources and our evolving understanding of the risk of donor‐derived disease transmission. These factors will have a significant impact on the transplant waiting list resulting in increased waiting times. Risk stratification is important to identify patients who need to be evaluated for transplantation or complete their evaluation during the COVID‐19 pandemic, including patients with high MELD scores, risk of decompensation, or tumor progression.
Recommendations
-
Limit the number of patients coming to the clinic for transplant evaluations.
-
◦
Consider evaluating only patients with HCC or those patients with severe disease and high MELD scores who are likely to benefit from immediate LT listing.
-
◦
Develop a policy to decide which listed patients need to be seen in person.
Consider telemedicine alternatives in place of outreach clinics.
-
Obtain labs and imaging only as clinically necessary.
-
◦
Patients should not be asked to update labs simply to update their MELD score. Recent Organ Procurement and Transplantation Network (OPTN) policy changes provide guidance on how to maintain candidate MELD when updated lab results are not obtained.( 31 )
-
◦
Ensure that patients have refills available for essential medications. Provide prescriptions for 90‐day supplies instead of 30‐day supplies. Many insurance companies are waiving early medication refill limits. Consider using medication delivery services.
Consider instructing patients to avoid attending in‐person community recovery support meetings, such as Alcoholics Anonymous, and provide alternative telephone or online resources.
Advise patients not to travel during the COVID‐19 pandemic.
Consider providing documentation to patients, providers, and organ procurement teams to ease essential travel where travel restriction policies are in place.
Have a low threshold for admitting patients on the transplant waiting list who are diagnosed with COVID‐19.
Consider using specific screening facilities and a “COVID‐19‐free” path through the hospital for transplantation candidates.
Conduct patient transplant education and social work, dietitian, and financial consultations by video conference, telemedicine, or telephone whenever possible.
-
Avoid multiple patients in one room for patient education.
-
◦
Consider developing Internet‐based education sessions for patients and family members that can be deployed either by in‐room computers or at home before patient evaluation.
-
◦
LT, Resource Utilization, and Ethical Considerations
Resource utilization and ethical considerations are inherently tied to LT. This is a critical and challenging area for which protocols and policies need to be carefully considered and developed. There is no overarching policy that can or should be applied to every transplant center; these issues will need to be discussed and developed locally (Table 1). Although the Centers for Medicare and Medicaid Services (CMS) recommends limiting all nonessential planned surgeries and procedures until further notice, they specifically exclude transplant surgery from this recommendation and categorize transplant surgery as Tier 3b (“do not postpone”).( 32 )
TABLE 1.
Challenging Issues in LT During the COVID‐19 Pandemic
|
Insufficient data regarding viral transmission of SARS‐CoV‐2 from organ donors are available at this time, but currently most organ procurement organizations are testing donors for SARS‐CoV‐2 RNA, and those who test positive are medically ineligible for organ donation.( 33 ) Based on center capability, testing for SARS‐CoV‐2 should be considered in all recipients before transplant; however, the capacity to test recipients shortly before proceeding with transplant may be limited and can add additional logistical hurdles. There is a significant false‐negative rate and transplant programs should consider symptoms of COVID‐19 to be strongly suggestive of infection despite negative testing. Transplantation in SARS‐CoV‐2–positive recipients is currently not recommended.
Recommendations
-
Develop a hospital‐specific policy for organ acceptance.
-
◦
Ensure hospital administrators are aware of the CMS Tier 3b designation for transplant surgery (“do not postpone”).( 32 )
-
◦
Consider recipient age and comorbidities before organ acceptance.
-
◦
Consider resource utilization, including ICU beds, ventilators, PPE, and supply of blood products (especially platelets and type‐specific packed red cells) in the decision to proceed with LT.
-
◦
Account for local COVID‐19 prevalence data when considering suspension of transplantation.
-
◦
Consider notifying patients that the COVID‐19 pandemic may impact their waiting time on the transplant list.
Notify patients that family and visitor access may be limited or prohibited during their hospital stay.
-
Screen potential donors for exposure and clinical symptoms/fever compatible with COVID‐19 (regardless of test results or availability).( 34 )
-
◦
Alternatives to PCR‐based testing, such as chest radiography, may also be considered.
-
◦
Screen potential recipients with an acceptable organ offer for COVID‐19 symptoms/fever before they are called in from home for transplantation.
When an organ offer becomes available, call in to the hospital potential transplant recipients at the latest possible time to minimize exposure to the hospital environment.
Consider accepting only grafts with a low risk of delayed graft function to minimize complications and postoperative lengths of stay.
-
Consider testing recipients and donors for SARS‐CoV‐2 before transplantation, if testing is available.
-
◦
Consider the risk of false negatives, disease prevalence, and testing turnaround time in your area.
-
◦
Review as much donor history as possible for fever, respiratory symptoms, and radiographical findings.
-
◦
Consider having back‐up transplant recipients wait at home or away from the transplant center.
Consider suspending living donor LT programs during the pandemic, except for pediatric patients with acute liver failure.( 34 )
See the latest updates regarding COVID‐19 related OPTN policy changes.( 31 )
An approach to LT organ offers is shown in Fig. 2.
FIG. 2.
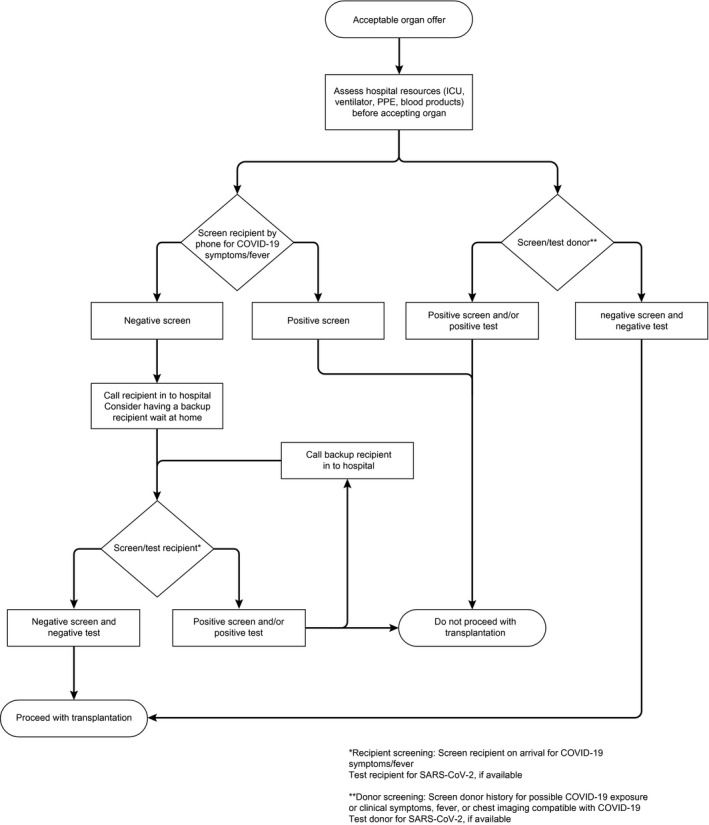
Approach to LT organ offers.
Post‐LT Patients
Data suggest that the immune response may be the main driver for pulmonary injury because of COVID‐19 and that immunosuppression may be protective.( 13 , 20 ) Posttransplant immunosuppression was not a risk factor for mortality associated with SARS (2002‐2003) or MERS (2012‐present).( 20 ) It is too early to know whether posttransplant patients are at greater risk for more‐severe COVID‐19; however, immunosuppressed patients are considered to be at higher risk for severe illness from COVID‐19.( 25 ) Immunosuppression may prolong viral shedding in posttransplant patients with COVID‐19.( 34 , 35 )
Recommendations
Do not reduce immunosuppression or stop mycophenolate for asymptomatic posttransplant patients without known COVID‐19.
Emphasize prevention measures posttransplant patients already know well: frequent hand washing, cleaning frequently touched surfaces, staying away from large crowds, staying away from individuals who are ill, etc.
Advise patients not to travel during the COVID‐19 pandemic.( 34 )
Minimize in‐person visits for posttransplant patients by more‐frequent telephone communication and telemedicine.
Consider advocating for telework options, appropriate excuses from work, or leaves of absence for posttransplant patients and their primary caregivers.
Management of Patients on Immunosuppressive Agents
Effects of immunosuppression on COVID‐19 are not well established. Rapid pulmonary deterioration in COVID‐19 is attributed to a systemic/pulmonary inflammatory response associated with increased serum interleukin (IL)‐6, IL‐8, and tumor necrosis factor alpha levels.( 36 ) The potential role of corticosteroids for the prevention of progression of mild COVID‐19 to severe pneumonia is unknown. The World Health Organization recommends avoiding corticosteroids for treatment of COVID‐19 unless indicated for another therapeutic purpose.( 37 ) Reducing the dosage or stopping immunosuppressants may cause a flare in a patient with AIH or precipitate acute rejection in an LT recipient.
Recommendations
-
In immunosuppressed liver disease patients without COVID‐19:
-
◦
Do not make anticipatory adjustments to current immunosuppressive drugs or dosages.
-
◦
-
In immunosuppressed liver disease patients with COVID‐19:
-
◦
Consider minimizing the dosage of high‐dose prednisone, but maintain a sufficient dosage to avoid adrenal insufficiency.
-
◦
Consider reducing azathioprine or mycophenolate dosages, especially in the setting of lymphopenia, fever, or worsening pneumonia attributed to COVID‐19.
-
◦
Consider reducing, but not stopping, daily calcineurin inhibitor dosage, especially in the setting of lymphopenia, fever, or worsening pulmonary status attributed to COVID‐19.
-
◦
An approach to managing LT recipients with COVID‐19 is shown in Fig. 3.
-
◦
Initiate immunosuppressive therapy in patients with liver disease with or without COVID‐19 who have strong indications for treatment (e.g., AIH, graft rejection).
In patients with COVID‐19, use caution in initiating prednisone, prednisolone, or other immunosuppressive therapy where the potential benefit might be outweighed by the risks (e.g., alcohol‐associated hepatitis).
FIG. 3.
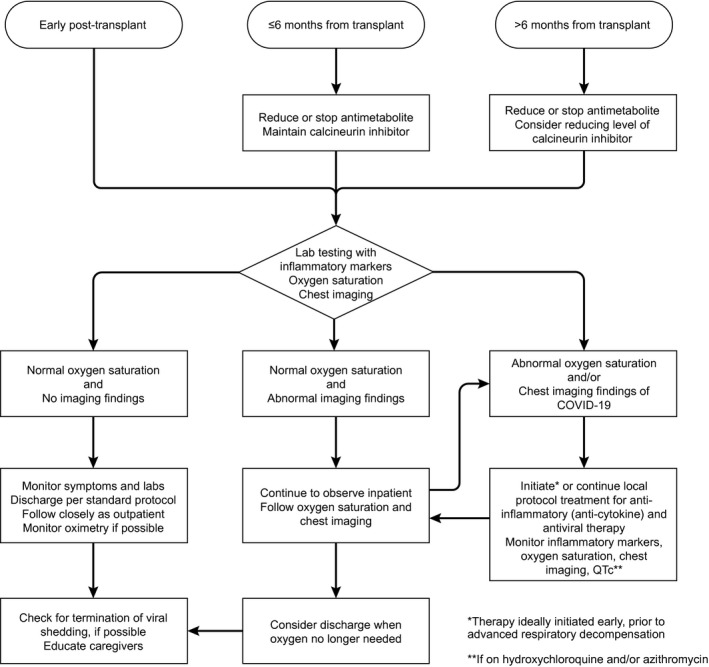
Approach to the LT recipient with COVID‐19.
Inpatients
Health care workers and other hospital staff are at risk for COVID‐19.( 7 ) Health care workers with SARS‐CoV‐2 may spread the virus to patients and to each other, and should remain away from in‐person work until approved to return by local health authorities. Minimizing interactions among health care workers and between health care workers and patients is critical to reducing the spread of SARS‐CoV‐2. Minimizing the transport of patients within and between health care facilities could reduce the spread of SARS‐CoV‐2.
Recommendations
Conduct medical and surgical transplant rounds with the minimum number of personnel needed to provide care at a given time.
Limit the number of team members who enter a patient’s room for patient examinations and encounters.
Limit the personnel permitted to enter patient rooms to the minimum needed for the performance of consultative care.
Discourage in‐person multidisciplinary rounds with dietary, pharmacy, social work, and care coordination staff.
-
Consider developing a policy for review and triage of hospital inpatient transfers. For example, consider accepting for transfer only patients with acute liver failure or those in need of urgent LT evaluation during their hospital stay.
-
◦
Consider accepting for transfer only other liver patients with a unique need for inpatient interventions at the transplant center.
-
◦
Consider evaluating patients with liver disease for COVID‐19 if they develop new‐onset encephalopathy or other acute decompensation.
Have a low threshold for aggressive airway management in COVID‐19 patients with underlying pulmonary diseases, such as hepatic hydrothorax, portopulmonary hypertension, or hepatopulmonary syndrome.
-
Perform a needs assessment before patient discharge to determine whether the patient can have follow‐up encounters by phone or telemedicine and encourage early monitoring by these means to reduce early postdischarge, in‐person visits.
-
◦
Consider home health or visiting nurse services for frequent blood draws needed after posttransplant hospital discharge.
-
◦
Medication Management of Patients With COVID‐19 and Potential Drug‐Drug Interactions
There currently are no FDA‐approved therapies to prevent or treat COVID‐19 infection. Many investigational or off‐label therapeutics for COVID‐19 may be hepatotoxic (Table 2). An open‐label, randomized, controlled trial of lopinavir‐ritonavir versus standard of care in adults hospitalized with severe COVID‐19 showed no clinical benefit.( 38 ) Treatment was stopped early in some patients taking lopinavir‐ritonavir because of adverse events. Ritonavir is a potent inhibitor of cytochrome P450 3A4 (CYP3A4), which is involved in the metabolism of calcineurin inhibitors, sirolimus, and everolimus. The use of ritonavir requires a reduction in tacrolimus dosage to 1/20‐1/50 of baseline because of this drug‐drug interaction.
TABLE 2.
Investigational Treatments for COVID‐19
| Agent (Route/Mechanism) | Target Population | Safety Issues | Efficacy Issues | |
|---|---|---|---|---|
| Antiviral Agents | Remdesivir (IV/nucleotide analogue) | Moderate‐severe | Nausea/vomiting | Investigational |
| Grade 1‐2 ALT elevations | RCT vs. placebo and compassionate‐use protocols | |||
| Drug vehicle accumulation in acute kidney injury | Previously tested in Ebola | |||
| Exclusions: | Few DDIs anticipated | |||
| GFR <30‐50 mL/min | ||||
| AST or ALT >5× ULN | ||||
| Favipiravir (oral/RNA polymerase inhibitor) | Early to mild disease | Investigational | ||
| Approved for influenza in Asia | ||||
| Tested with interferon‐α aerosol × 14 days | ||||
| Lopinavir‐ritonavir (oral/HIV protease inhibitor) | Severe | CYP3A4 substrate | FDA approved for HIV | |
| Severe DDI with CNI | No survival benefit in RCT vs. standard of care × 14 days | |||
| 13% early discontinuation because of side effects | ||||
| Nitazoxanide (oral/host proteins) | Moderate‐severe | Similar to placebo in influenza trials | FDA approved for Cryptosporidium/Giardia | |
| In vitro activity against coronaviruses | ||||
| Hydroxychloroquine (oral/host proteins) | Moderate‐severe | QTc prolongation | FDA approved for lupus/rheumatoid arthritis/malaria | |
| Nausea and vomiting | ||||
| Exclusions: | Available as emergency use | |||
| QTc >415 ms | ||||
| Cardiomyopathy | May work by reducing ACE2 receptor‐mediated endocytosis or inhibiting endosomal acidification | |||
| G6PD deficiency | ||||
| Chloroquine (oral/host proteins) | Moderate‐severe | QTc prolongation | FDA approved for malaria | |
| Nausea and vomiting | ||||
| Exclusions: | May work by reducing ACE2 receptor‐mediated endocytosis or inhibiting endosomal acidification | |||
| QTc >415 ms | ||||
| Cardiomyopathy | Reduced progression of disease and symptom duration in China | |||
| G6PD deficiency | ||||
| Azithromycin (oral/host proteins) | Moderate‐severe | CYP3A4 substrate | FDA approved for bacterial infections | |
| Moderate DDI with CNI | Combined with hydroxychloroquine in a limited number of patients | |||
| Rare cholestatic hepatitis | ||||
| Exclusion: | ||||
| QTc >415 ms | ||||
| Immunomodulatory agents | Tocilizumab (IV/monoclonal IL‐6 receptor antagonist) | Severe (high IL‐6 levels) | Grade 1‐2 ALT 20%‐40% | FDA approved for RA |
| Grade 3+ ALT 1%‐2%. | 8‐mg/kg dose | |||
| Acute liver failure <1% | ||||
| Neutropenia 3% | ||||
| Thrombocytopenia 2% | ||||
| Opportunistic infections | ||||
| Exclusions: | ||||
| ANC <2,000/m3 | ||||
| Platelets <100,000/m3 | ||||
| ALT >5× ULN | ||||
| Sarilumab (SC/monoclonal antibody) | Severe (high IL‐6 levels) | Grade 1‐2 ALT 15%‐25% | FDA approved in RA | |
| Neutropenia 5% | Being tested as IV formulation | |||
| Thrombocytopenia 1% | ||||
| Exclusions: | ||||
| ANC <2,000/mm3 | ||||
| Platelets <150,000/m3 | ||||
| ALT >5× ULN | ||||
| Siltuximab (IV/monoclonal antibody) | Severe (high IL‐6) | Grade 1‐2 ALT | FDA approved in Castleman’s disease | |
| Rash 30% | ||||
| Thrombocytopenia 9% | ||||
| Exclusions: | ||||
| ALT >5× ULN | ||||
| Convalescent plasma (IV/neutralizing antibodies) | Severe or life‐threatening pneumonia | Potential TRALI/anaphylaxis ICU monitoring needed | Investigational | |
| Must screen donor for other transmissible pathogens | Open‐label 400‐mL plasma infusion in 5 patients and 200‐mL plasma infusion in 10 patients | |||
| Finding donors with neutralizing IgG activity not well established | ||||
| Reserved for severe/life‐threatening cases |
Abbreviations: ANC, absolute neutrophil count; CNI, calcineurin inhibitor; DDI, drug‐drug interaction; G6PD, glucose‐6‐phosphate dehydrogenase; GFR, glomerular filtration rate; HIV, human immunodeficiency virus; IgG, immunoglobulin G; IV, intravenous; RA, rheumatoid arthritis; RCT, randomized controlled trial; SC, subcutaneous; TRALI, transfusion‐related acute lung injury.
Remdesivir is a nucleotide analogue with demonstrated activity against SARS‐CoV and MERS‐CoV in cultured cells, mice, and nonhuman primates and, more recently, against SARS‐CoV‐2 in human cell lines.( 39 , 40 ) Remdesivir is being tested in hospitalized patients with moderate‐to‐severe COVID‐19 in randomized controlled trials, and data from compassionate use have reported promising results.( 41 , 42 , 43 ) Drugs that target the IL‐6 receptor are being tested only in hospitalized patients with moderate‐to‐severe COVID‐19.
Hydroxychloroquine (an analogue of chloroquine with a better safety profile) has been shown to have anti–SARS‐CoV‐2 activity in vitro.( 44 ) A single‐arm study from France of 20 patients with COVID‐19 who were treated with hydroxychloroquine with or without azithromycin, compared to 16 nonrandomized controls, reported negative nasopharyngeal swabs for SARS‐CoV‐2 PCR in 70% of the treated group compared to 12.5% of the controls.( 45 ) A separate study from France reported that the combination of hydroxychloroquine and azithromycin was not associated with clinical recovery or viral clearance, and 4 of the 11 patients discontinued therapy because of prolonged QT interval.( 46 )
The CDC recently issued a warning about the danger of using nonpharmaceutical chloroquine phosphate, a commercially available chemical for aquarium use, to treat or prevent COVID‐19.( 47 ) One individual died after using nonpharmaceutical chloroquine, and another became critically ill with gastrointestinal symptoms and cardiac conduction abnormalities.
Convalescent plasma transfusion holds promise for treating critically ill patients with COVID‐19.( 48 , 49 ) The FDA recently announced that it is facilitating access to convalescent plasma for patients with serious COVID‐19 through its emergency Investigational New Drug application process.( 50 )
Treatment with ACE inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) results in up‐regulation of ACE2, the target for SARS‐CoV‐2 entry into cells.( 51 ) Increased ACE2 expression theoretically facilitates infection with SARS‐CoV‐2. Animal studies suggest that ACEIs and ARBs may protect against serious lung complications from SARS‐CoV, but to date there are no data in SARS‐CoV‐2 or in humans.( 52 ) The Council on Hypertension of the European Society of Cardiology highlighted the lack of evidence demonstrating harmful effects of ACEIs and ARBs in the setting of COVID‐19 and recommends that patients should continue with their usual antihypertensive therapy, including ACEIs and ARBs.( 53 )
Recommendations
Monitor studies of antiviral and immunomodulatory approaches to COVID‐19 at the National Institutes of Health’s (NIH) clinicaltrials.gov.
The available evidence does not currently support the use of lopinavir‐ritonavir for the treatment of COVID‐19.
Hydroxychloroquine with or without azithromycin is not routinely recommended and may be associated with serious adverse events, such as prolongation of the QT interval.
Patients receiving ACEIs and ARBs should remain on them even in the setting of COVID‐19.
Acetaminophen at a daily dosage of ≤2 g/d is the preferred analgesic and antipyretic for patients with known or suspected COVID‐19.
Nonsteroidal anti‐inflammatory drugs may also be used or continued as needed.( 54 )
Consult the University of Liverpool Drug Interactions Group document on Interactions with Experimental COVID‐19 Therapies.( 55 )
Procedures
There is potential for fecal‐oral SARS‐CoV‐2 transmission,( 1 , 4 , 6 , 23 , 43 ) and the virus is detected in saliva.( 1 , 23 , 56 ) Multiple societies have strongly recommended rescheduling nonurgent procedures. The Joint Gastroenterology Societies recommend to “strongly consider rescheduling non‐urgent endoscopic procedures”; and the CMS, U.S. Surgeon General, and the American College of Surgeons recommend postponing elective surgeries.( 32 , 57 , 58 , 59 )
Endoscopic procedures should be considered aerosol generating.( 60 ) Nonurgent endoscopic procedures should be rescheduled to reduce the risk of disease transmission from asymptomatic patients, reduce the use of PPE, and reduce hospital admissions.( 60 ) To further limit disease transmission, the Joint Gastroenterology Societies and the American Gastroenterological Association recommend that health care workers involved with endoscopy wear a full set of PPE, including N95 masks and double gloves.( 61 , 62 )
Recommendations
Cancel all elective/nonurgent procedures (e.g., endoscopy, liver biopsy, and transient elastography).( 60 )
Consider, in the interim, primary prophylaxis with beta‐blocker therapy instead of screening endoscopy in patients with clinically significant portal hypertension or high risk of decompensation.
Some procedures may need to be performed (e.g., liver biopsy) to rule out rejection or diagnose AIH, therapeutic paracentesis, transjugular intrahepatic portosystemic shunt, and/or endoscopy for variceal bleeding, follow‐up band ligation in those with recent variceal bleeding, and urgent biliary procedures for symptomatic disease such as cholangitis and sepsis (interventional radiology or endoscopic).
Research
During this unprecedented time, much of clinical and basic science research not directly related to COVID‐19 has come to a standstill. Because of quarantine‐related travel restrictions and potential supply‐chain interruptions, the FDA and NIH have posted guidance documents to advise on the conduct of clinical trials during the COVID‐19 pandemic.( 63 , 64 )
Recommendations
-
Limit clinical trial activity to essential clinical trials, defined as those that enroll or follow patients with life‐threatening or serious conditions for which participation in the clinical trial holds out the clear prospect of directly benefiting the patient. Patients already enrolled in clinical trials who are undergoing safety and efficacy assessments fall within this definition.
-
◦
Continue in‐person research visits for participants already enrolled in essential clinical trials if required for patient safety and/or participation in the clinical trial is an integral part of the patient’s treatment plan.
-
◦
The study physician—in consultation with the study team, patient’s physician, patient, and patient’s family—should carefully assess the necessity and risks of an in‐person visit.
-
◦
Do not initiate new clinical trials at this time unless meeting the definition of an essential clinical trial.
-
Strongly consider not enrolling new research participants into existing clinical trials unless meeting the definition of an essential clinical trial.
-
◦
Postpone all other in‐person clinical research visits.
-
◦
Research staff should make efforts to use alternative methods to conduct research visits or perform testing, such as check‐ins with participants by phone, and/or performing research‐related laboratory testing at laboratory testing centers, if feasible.
Research staff should work remotely, unless their presence is required for the safe conduct of the trial.
Discuss options for conducting telehealth study visits with clinical research organizations and study sponsors.
Principal investigators should notify commercial sponsors that opening new nonessential clinical trials and enrolling subjects into ongoing “nonessential” clinical trials should be temporarily postponed.
Arrange for research medications to be sent to subjects by the study sponsor if the research pharmacy is unavailable.
Institutional policies on clinical research may be more restrictive and should supersede the recommendations contained here.
Laboratory/basic science research may also need to be restricted based on local policies.
Trainees
Although residents and fellows have much to learn from the diagnosis and management of COVID‐19, there is widespread concern that the risks of exposing trainees to SARS‐CoV‐2 may outweigh the benefits. There is also concern about further reducing the already significant PPE shortages by involving trainees in direct patient care. The Accreditation Council for Graduate Medical Education has suspended some activities during the COVID‐19 pandemic; however, requirements for adequate resources and training, adequate supervision, and work‐hour limitations have not changed.( 65 , 66 )
Recommendations
Ensure adequate resources, including PPE, appropriate to the clinical setting for all trainees.
Assign fellows only to participating sites that ensure the safety of patients and fellows.
Ensure appropriate supervision of trainees working remotely if they are conducting patient care activities (telephone/telemedicine visits).
Change all educational conferences to virtual conferences.
Consider assigning fellows and other trainees to indirect patient care activities and/or telemedicine visits.
Consider remote supervision of trainees by concurrently monitoring patient care through appropriate telecommunication technology.
Conclusions
The COVID‐19 pandemic has profoundly strained health care resources around the world. Minimizing the risk of patient and health care worker exposure has been central to our efforts to mitigate the impact of the disease on our ability to continue to provide adequate care to our patients. Growing clinical experience with COVID‐19 evaluation and management has identified subsets of patients who may be at higher risk for a more‐severe disease course. The pandemic has affected patients awaiting LT because of limited transplant center institutional resources and the possibility of donor‐derived infection, which has resulted in longer waiting times. This article provides clinicians caring for patients with CLD with guidance for how to minimize the impact of the COVID‐19 pandemic on their patients’ care. The situation is evolving rapidly, and these recommendations will need to evolve as well. As we learn more about how the COVID‐19 pandemic impacts the care of patients with liver disease, we will update the online document available at: https://www.aasld.org/about‐aasld/covid‐19‐and‐liver.
Disclaimer
This document represents the collective opinion of its authors and approval of the American Association for the Study of Liver Diseases (AASLD) Governing Board as of the date of publication. Its use is voluntary, and it is presented primarily for the purpose of providing information to hepatology and LT care providers. This document is not a practice guideline and has not been subject to the methodical rigor of a practice guideline. There has not been a systematic evidence review as defined by the Health and Medicine Division of the National Academies of Sciences, Engineering, and Medicine (formerly the Institute of Medicine), nor is the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach utilized. This document does not define a standard of practice or a standard of care. It should not be considered as inclusive of all proper treatments or methods of care, nor is it intended to substitute for the independent professional judgment of the treating provider. Hospitals, clinics, and private practices should take into account local standards, practices, and environment.
Author Contributions
Conceptualization: O.K.F., B.H., R.J.F., R.M.K., J.A.B., K.R.R., R.T.C.; Project Administration: O.K.F.; Visualization: O.K.F.; Writing (original draft): O.K.F., B.H., R.J.F., R.M.K., B.M.M., D.C.M., D.S.P., M.W.R., M.L.S., J.A.B., K.R.R., R.T.C.; Writing (review and editing): O.K.F., B.H., R.J.F., R.M.K., B.M.M., D.C.M., D.S.P., M.W.R., M.L.S., E.C.V., R.L., D.E.C., J.A.B., K.R.R., R.T.C.; Supervision: J.A.B., K.R.R., R.T.C.
Acknowledgment
The authors acknowledge the AASLD Governing Board for their contributions to and support of this article: Jorge A. Bezerra, Cincinnati Children’s Hospital; Raymond T. Chung, M.D., F.A.A.S.L.D., Massachusetts General Hospital; Michael W. Fried, M.D., F.A.A.S.L.D., University of North Carolina at Chapel Hill; Meena G. Bansal, M.D., F.A.A.S.L.D., Icahn School of Medicine at Mount Sinai; Vijay H. Shah, M.D., F.A.A.S.L.D., Mayo Clinic Rochester; Laurie D. DeLeve, M.D., Ph.D., F.A.A.S.L.D., University of Southern California; Norah Terrault, M.D., M.P.H., F.A.A.S.L.D., Keck Medical Center of University of Southern California; W. Ray Kim, M.D,. F.A.A.S.L.D., Stanford University Medical Center; John R. Lake, M.D., F.A.A.S.L.D., University of Minnesota; Mary E. McCarthy Rinella, M.D., F.A.A.S.L.D., Northwestern University Feinberg School of Medicine; David C. Mulligan, M.D., F.A.A.S.L.D., Yale University.
An earlier version of this document was first posted online on March 23, 2020 at https://www.aasld.org/about-aasld/covid-19-resources and will continue to be updated.
Potential conflict of interest: Dr. Chung received grants from Gilead, AbbVie, Bristol‐Myers Squibb, Boehringer Ingelheim, Merck, Roche, and Janssen. Dr. Hameed advises for and received grants from Gilead. He advises for Surrozen and received grants from Intercept, Genfit, and Salix. Dr. Fontana consults for Sanofi and received grants from Gilead and AbbVie. Dr. Verna advises for Gilead and received grants from Salix. Dr. Loomba consults for, advises for, and received grants from Boehringer Ingelheim, Bristol‐Myer Squibb, Galmed, Gilead, Intercept, NGM, and Pfizer. He consults for and advises for 89bio, Alnylam, Arrowhead Pharmaceuticals, AstraZeneca, Celgene, Cirius, CohBar, DiCerna, Gemphire, Glympse bio, Ionis, Merck, Metacrine, Novo Nordisk, Sagimet, and Viking Therapeutics. He received grants from Allergan, Eli Lilly and Company, Genfit, Janssen, Madrigal Pharmaceuticals, Novartis, pH Pharma, and Siemens. Dr. Cohen consults for Intercept, advises for Relburn, Intercept, and Surrozen, and received grants from Sanofi. Dr. Reddy consults for, advises for, and received grants from Mallinckrodt, Merck, and Gilead. He received grants from Exact Sciences, Bristol‐Myers Squibb, Intercept, and Grifols.
Contributor Information
Oren K. Fix, Email: oren.fix@swedish.org.
K. Rajender Reddy, Email: reddyr@pennmedicine.upenn.edu.
Raymond T. Chung, Email: chung.raymond@mgh.harvard.edu.
References
- 1. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chopra V, Toner E, Waldhorn R, Washer L. How should U.S. hospitals prepare for coronavirus disease 2019 (COVID‐19)? Ann Intern Med 2020;172:621‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention . Coronavirus disease 2019 (COVID‐19): cleaning and disinfection for community facilities. Published February 11, 2020. https://www.cdc.gov/coronavirus/2019‐ncov/community/organizations/cleaning‐disinfection.html. Accessed April 2020. [Google Scholar]
- 4. Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS‐CoV‐2. Gastroenterology 2020;158:1831‐1833.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu Y, Guo C, Tang L, Hong Z, Zhou J, Dong X, et al. Prolonged presence of SARS‐CoV‐2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol 2020;5:434‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen C, Gao G, Xu Y, Pu L, Wang Q, Wang L, et al. SARS‐CoV‐2‐positive sputum and feces after conversion of pharyngeal samples in patients with COVID‐19. Ann Intern Med 2020. Mar 30. 10.7326/M20-0991. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Remuzzi A, Remuzzi G. COVID‐19 and Italy: what next? Lancet 2020;395:1225‐1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, et al. Structure of the SARS‐CoV‐2 spike receptor‐binding domain bound to the ACE2 receptor. Nature 2020;581:215‐220. [DOI] [PubMed] [Google Scholar]
- 9. Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, et al. Angiotensin‐converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003;27:450‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019‐nCoV infection. BioRxiv 2020. Feb 4. 10.1101/2020.02.03.931766. [Epub ahead of print] [DOI] [Google Scholar]
- 11. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;15:507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fan Z, Chen L, Li J, Tian C, Zhang Y, Huang S, et al. Clinical features of COVID‐19 related liver damage. MedRxiv 2020;18:1561‐1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;15:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int 2020;40:998‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang C, Shi L, Wang FS. Liver injury in COVID‐19: management and challenges. Lancet Gastroenterol Hepatol 2020;5:428‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wander P, Epstein M, Bernstein D. COVID‐19 presenting as acute hepatitis. Am J Gastroenterol. Published April 2020. https://journals.lww.com/ajg/Documents/COVID19_Bernstein_et_al_AJG_Preproof.pdf. Accessed April 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu W, Tao ZW, Lei W, Ming‐Li Y, Kui L, Ling Z, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J 2020;133:1032‐1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA 2020. Feb 7. 10.1001/jama.2020.1585. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, et al. SARS‐CoV‐2 infection in children. N Engl J Med 2020;382:1663‐1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. D’Antiga L. Coronaviruses and immunosuppressed patients. The facts during the third epidemic. Liver Transpl 2020;26:832‐834. [DOI] [PubMed] [Google Scholar]
- 21. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yao XH, Li TY, He ZC, Ping YF, Liu HW, Yu SC, et al. [A pathological report of three COVID‐19 cases by minimally invasive autopsies]. [Article in Chinese; Abstract available in Chinese from the publisher]. Zhonghua Bing Li Xue Za Zhi 2020;49:411‐417. [DOI] [PubMed] [Google Scholar]
- 23. Gu J, Han B, Wang J. COVID‐19: gastrointestinal manifestations and potential fecal‐oral transmission. Gastroenterology 2020;158:1518‐1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, et al. Non‐alcoholic fatty liver diseases in patients with COVID‐19: a retrospective study. J Hepatol 2020. Apr 8. 10.1016/j.jhep.2020.03.044. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Centers for Disease Control and Prevention . Coronavirus disease 2019 (COVID‐19): groups at higher risk for severe illness. Published February 11, 2020. https://www.cdc.gov/coronavirus/2019‐ncov/need‐extra‐precautions/groups‐at‐higher‐risk.html. Accessed April 2020. [Google Scholar]
- 26. Centers for Disease Control and Prevention . Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019—United States, February 12–March 28, 2020. MMWR Morb Mortal Wkly Rep 2020;3:382‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bai Y, Yao L, Wei T, Tian F, Jin DY, Chen L, et al. Presumed asymptomatic carrier transmission of COVID‐19. JAMA 2020. Feb 21. 10.1001/jama.2020.2565. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS‐CoV‐2 infection: a nationwide analysis in China. Lancet Oncol 2020;323:1406‐1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rich NE, John BV, Parikh ND, Rowe I, Mehta N, Khatri G, et al. Hepatocellular carcinoma demonstrates heterogeneous growth patterns in a multi‐center cohort of patients with cirrhosis. Hepatology 2020. Feb 4. 10.1002/hep.31159. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Centers for Disease Control and Prevention . Interim guidance for healthcare facilities: preparing for community transmission of COVID‐19 in the United States. Published February 11, 2020. https://www.cdc.gov/coronavirus/2019‐ncov/healthcare‐facilities/guidance‐hcf.html. Accessed April 2020. [Google Scholar]
- 31. United Network for Organ Sharing . COVID‐19 and solid organ transplant. https://unos.org/covid. Published 2020. Accessed April 2020. [Google Scholar]
- 32. Centers for Medicare and Medicaid Services . Non‐emergent, elective medical services, and treatment recommendations. https://www.cms.gov/files/document/31820‐cms‐adult‐elective‐surgery‐and‐procedures‐recommendations.pdf. Published April 7, 2020. Accessed April 2020. [Google Scholar]
- 33. Association of Organ Procurement Organizations . COVID‐19 (coronavirus) bulletin. Published March 26, 2020. https://www.aopo.org/information‐about‐covid‐19‐coronavirus‐is‐being‐released‐rapidly‐we‐will‐post‐updates‐as‐we‐receive‐them. Accessed April 2020. [Google Scholar]
- 34. American Society of Transplantation . 2019‐nCoV (Coronavirus): FAQs for organ donation and transplantation. Published March 20, 2020. https://www.myast.org/sites/default/files/COVID19%20FAQ%20Tx%20Centers%2003.20.2020‐FINAL.pdf. Accessed April 2020. [Google Scholar]
- 35. Qin J, Wang H, Qin X, Zhang P, Zhu L, Cai J, et al. Perioperative presentation of COVID‐19 disease in a liver transplant recipient. Hepatology 2020. Mar 27. 10.1002/hep.31257. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36. Gong J, Dong H, Xia Q, Huang Z, Wang D, Zhao Y, et al. Correlation analysis between disease severity and inflammation‐related parameters in patients with COVID‐19 pneumonia. MedRxiv 2020. Feb 27. 10.1101/2020.02.25.20025643. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. World Health Organization . Clinical management of severe acute respiratory infection (SARI) when COVID‐19 disease is suspected: interim guidance. Published March 13, 2020. https://www.who.int/publications‐detail/clinical‐management‐of‐severe‐acute‐respiratory‐infection‐when‐novel‐coronavirus‐(ncov)‐infection‐is‐suspected. Accessed April 2020. [Google Scholar]
- 38. Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir‐ritonavir in adults hospitalized with severe Covid‐19. N Engl J Med 2020;382:1787‐1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, et al. Broad‐spectrum antiviral GS‐5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med 2017;9:eaal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res 2020;30:269‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019‐nCoV). Nat Rev Drug Discov 2020;19:149‐150. [DOI] [PubMed] [Google Scholar]
- 42. Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, et al. Compassionate use of remdesivir for patients with severe COVID‐19. N Engl J Med 2020. Apr 10. 10.1056/NEJMoa2007016. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med 2020;382:929‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2). Clin Infect Dis 2020. Mar 9. 10.1093/cid/ciaa237. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. Int J Antimicrob Agents 2020. Mar 20. 10.1016/j.ijantimicag.2020.105949. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46. Molina J, Delaugerre C, Goff J, Mela‐Lima B, Ponscarme D, Goldwirt L, et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID‐19 infection. Med Mal Infect 2020;50:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Federal Laboratory Consortium . CDC warns against using nonpharmaceutical chloroquine phosphate. Published March 30, 2020. https://federallabs.org/news/cdc‐warns‐against‐using‐nonpharmaceutical‐chloroquine‐phosphate. Accessed April 2020. [Google Scholar]
- 48. Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. Treatment of 5 critically ill patients with COVID‐19 with convalescent plasma. JAMA 2020;323:1582‐1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. Effectiveness of convalescent plasma therapy in severe COVID‐19 patients. Proc Natl Acad Sci U S A 2020;117:9490‐9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. US Food and Drug Administration . Revised information for investigational COVID‐19 convalescent plasma. Published April 3, 2020. https://www.fda.gov/vaccines‐blood‐biologics/investigational‐new‐drug‐ind‐or‐device‐exemption‐ide‐process‐cber/revised‐information‐investigational‐covid‐19‐convalescent‐plasma. Accessed April 2020. [Google Scholar]
- 51. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID‐19 infection? Lancet Respir Med 2020;8:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus‐induced lung injury. Nat Med 2005;11:875‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. de Simone G. Position statement of the ESC Council on Hypertension on ACE‐inhibitors and angiotensin receptor blockers. Published March 13, 2020. https://www.escardio.org/Councils/Council‐on‐Hypertension‐(CHT)/News/position‐statement‐of‐the‐esc‐council‐on‐hypertension‐on‐ace‐inhibitors‐and‐ang. Accessed April 2020. [Google Scholar]
- 54. AFP . Updated: WHO now doesn’t recommend avoiding ibuprofen for COVID‐19 symptoms. Published March 17, 2020. https://www.sciencealert.com/who‐recommends‐to‐avoid‐taking‐ibuprofen‐for‐covid‐19‐symptoms/amp. Accessed April 2020. [Google Scholar]
- 55. Liverpool Drug Interaction Group . Liverpool COVID‐19 interactions. Published April 3, 2020. https://www.covid19‐druginteractions.org. Accessed April 2020. [Google Scholar]
- 56. To KKW, Tsang OTY, Yip CCY, Chan KH, Wu TC, Chan JMC, et al. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis 2020. Feb 12. 10.1093/cid/ciaa149. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Joint Gastroenterology Societies . Joint GI Society message: COVID‐19 clinical insights for our community of gastroenterologists and gastroenterology care providers. Published March 15, 2020. https://www.aasld.org/about‐aasld/media/joint‐gi‐society‐message‐covid‐19‐clinical‐insights‐our‐community. Accessed April 2020. [Google Scholar]
- 58. Luthi S. Surgeon General advises hospitals to cancel elective surgeries. Politico. Published March 14, 2020. https://www.politico.com/news/2020/03/14/surgeon‐general‐elective‐surgeries‐coronavirus‐129405. Accessed April 2020. [Google Scholar]
- 59. American College of Surgeons . COVID‐19: recommendations for management of elective surgical procedures. Published March 13, 2020. https://www.facs.org/covid‐19/clinical‐guidance/elective‐surgery. Accessed April 2020. [Google Scholar]
- 60. Soetikno R, Teoh AYB, Kaltenbach T, Lau JYW, Asokkumar R, Cabral‐Prodigalidad P, et al. Considerations in performing endoscopy during the COVID‐19 pandemic. Gastrointest Endosc 2020. Mar 27. 10.1016/j.gie.2020.03.3758. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sultan S, Lim JK, Altayar O, Davitkov P, Feuerstein JD, Siddique SM, et al. Institute rapid recommendations for gastrointestinal procedures during the COVID‐19 pandemic. Gastroenterology 2020. Mar 31. 10.1053/j.gastro.2020.03.072. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Joint Gastroenterology Societies . Joint Gastroenterology Society message: COVID‐19 use of personal protective equipment in GI endoscopy. https://www.aasld.org/sites/default/files/2020‐04/JointSocietyMessage‐PersonalProtectiveEquipmentInGIEndoscopy.pdf. Accessed April 2020. [Google Scholar]
- 63. Food and Drug Administration . FDA guidance on conduct of clinical trials of medical products during COVID‐19 pandemic: guidance for industry, investigators, and institutional review boards. Published April 2, 2020. https://www.fda.gov/media/136238/download. Accessed April 2020. [Google Scholar]
- 64. National Institutes of Health . Guidance for NIH‐funded clinical trials and human subjects studies affected by COVID‐19. Published March 16, 2020. https://grants.nih.gov/grants/guide/notice‐files/NOT‐OD‐20‐087.html. Accessed April 2020. [Google Scholar]
- 65. Nasca TJ . ACGME response to the coronavirus (COVID‐19). Published March 18, 2020. https://acgme.org/Newsroom/Newsroom-Details/ArticleID/10111/ACGME-Response-to-the-Coronavirus-COVID-19. Accessed April 2020. [Google Scholar]
- 66. Accreditation Council for Graduate Medical Education . ACGME response to pandemic crisis. 2020. https://acgme.org/COVID‐19. Accessed April 2020. [Google Scholar]


