Abstract
Objective
The ongoing pandemic of coronavirus disease (2019 coronavirus disease [COVID‐19]), caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) virus, is highly contagious with high morbidity and mortality. The role of the nasal and paranasal sinus cavities is increasingly recognized for COVID‐19 symptomatology and transmission. We therefore conducted a systematic review, synthesizing existing scientific evidence about sinonasal pathophysiology in COVID‐19.
Study Design
Systematic review.
Methods
Systematic searches were performed of all indexed studies in PubMed/Medline and Cochrane databases through 28 March 2020 and studies searchable on preprints.com (including ArXiv and Scilit repositories) through 30 March 2020. Data extraction focused on sinonasal pathophysiology in COVID‐19.
Results
A total of 19 studies were identified. The sinonasal cavity may be a major site of infection by SARS‐CoV‐2, where susceptibility genes required for infection are expressed at high levels and may be modulated by environmental and host factors. Viral shedding appears to be highest from the nose, therefore reflecting a major source for transmission. This has been highlighted by multiple reports of health care‐associated infection (HAI) during rhinologic procedures, which are now consequently considered to be high risk for SARS‐CoV‐2 transmission to health care workers. While sinonasal symptomatology, such as rhinorrhea or congestion, appears to be a rarer symptom of COVID‐19, anosmia without nasal obstruction is reported as highly specific predictor of COVID‐19+ patients.
Conclusion
Sinonasal pathophysiology is increasingly important in our understanding of COVID‐19. The sinonasal tract may be an important site of infection while sinonasal viral shedding may be an important transmission mechanism—including HAI. Anosmia without nasal obstruction may be a highly specific indicator of COVID‐19.
Level of Evidence
2a.
Keywords: anosmia, congestion, coronavirus, COVID‐19, nose, olfactory dysfunction, paranasal sinus, respiratory epithelium, rhinorrhea, SARS, SARS‐CoV‐2, SARS‐CoV2, sinonasal, systematic review, transmission
1. INTRODUCTION
The 2019 coronavirus disease (COVID‐19) was first identified in December 2019 in Wuhan, China and subsequently found to be caused by a novel coronavirus, now referred to as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2). 1 , 2 SARS‐CoV‐2 is highly infectious, with an estimated basic reproduction number in the range of 2 to 3—indicating that on average one infected person will infect 2 to 3 others. 3 To date, SARS‐CoV‐2 is confirmed to have infected over 1 million individuals worldwide and killed over 50 000. COVID‐19 consists of upper and lower respiratory tract components of the SARS‐CoV‐2 infection. 1 , 4 Mortality associated with SARS‐CoV‐2 is due to lower respiratory tract manifestations of the disease, such as severe acute respiratory distress syndrome. This is similar to outbreaks of other coronaviruses including SARS‐CoV‐1 in 2003 and the middle east respiratory syndrome coronavirus (MERS‐CoV) in 2012. 5 However, as the COVID‐19 pandemic has spread and our knowledge about it grown, sinonasal pathophysiology has been uniquely brought to the forefront with important roles in infection, transmission, and pathognomonic symptomatology that may identify infected individuals. Given the extreme morbidity that is associated with COVID‐19 and the fact that the world's attention is entrained on this disease, many anecdotal reports are disseminated through conventional or social media without the provision of detailed scientific methodology or the performance of a scientific review. Although our understanding of COVID‐19 continues to rapidly evolve, there are already clinically informative insights with respect to sinonasal pathophysiology that have been uncovered in the scientific literature. The objective of this systematic review was to synthesize existing scientific evidence on the role of sinonasal pathophysiology in COVID‐19.
2. METHODS
2.1. Literature search
A computerized search of the PubMed/Medline and Cochrane databases was performed of all indexed studies in those databases up to 28 March 2020 in order to identify all relevant manuscripts. Similarly, given that the majority of knowledge about COVID‐19 is still emerging, we also performed a computerized search of scientific manuscript preprints up to 30 March 2020 using the search tool on preprints.com, which queries preprints of scientific manuscripts in not only the preprints.com repository but also the MDPI, ArXiv, and Scilit repositories. The preprints.com search tool therefore allows a broad search of preprints across many repositories.
We included only articles that were in English or Chinese. Combined search terms included: “COVID‐19, SARS‐CoV‐2, coronavirus, nose, nasal mucosa, nasal cavity, respiratory epithelium, sinonasal pathophysiology, sinonasal disease, chronic rhinosinusitis, upper respiratory tract, endoscopic sinus surgery, anosmia, hyposmia, olfactory epithelium, olfactory bulb, otolaryngology.” Articles mapping to the exploded medical subject heading “sinonasal pathophysiology and SARS‐CoV‐2” were combined into one group. Medical subject headings “COVID‐19 and otolaryngology” were exploded and the manuscripts were collected into a second group. Medical subject headings “olfaction and coronavirus” were combined into a third group. Medical subject headings “respiratory epithelium and coronavirus” were combined into a fourth group. The four groups were then cross‐referenced. Studies were excluded if they did not have full texts or could not be obtained. Adjunctive searches were performed based on the studies that were identified (and their references). Titles and abstracts were then evaluated according to the inclusion/exclusion criteria described hereafter. Two individual reviewers (I. G. and J. C. W.) performed searches independently, blinded to each other's results, with the search results additionally reviewed by the senior author (A. R. S.). Titles and abstracts for all identified studies were reviewed.
2.2. Inclusion and exclusion criteria
Articles identified by the above search strategy were evaluated to meet these inclusion criteria: (a) studies that included COVID‐19 and (b) evaluation of nasal, sinonasal pathophysiology. In the context of the urgency of the pandemic and the rapid accumulation of new information, we also included two studies, one in review and one in press whose data were obtained via professional otolaryngology society communication and/or direct communication.
Articles were excluded if there was no discussion of the upper respiratory tract, and they were only abstracts indexed in a searched database without an associated and accessible full manuscript.
2.3. Data extraction
Extracted data included study design; epidemiology and population description, including Country in which study/observations were reported; mechanisms of infection in the sinonasal cavity, type of COVID‐19 test conducted if applicable; signs and symptoms of COVID‐19; COVID‐19 risks, and sinonasal anatomy.
3. RESULTS
Our search identified a total of 2013 studies (1830 published articles, 181 prints, and 2 communicated studies). A total of 1988 studies (1816 published studies, 178 preprints) were excluded as off scope or having unobtainable full text (Figure 1 ). A total of 19 studies were included (14 published studies, 4 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 3 preprints, 19 , 20 , 21 and 2 communicated studies 22 , 23 ), which described the sinonasal pathophysiology—as it related to infection by SARS‐CoV‐2, transmission of SARS‐CoV‐2, and symptoms of COVID‐19. Primary literature was sourced and integrated with other available and supporting literature in this review.
FIGURE 1.
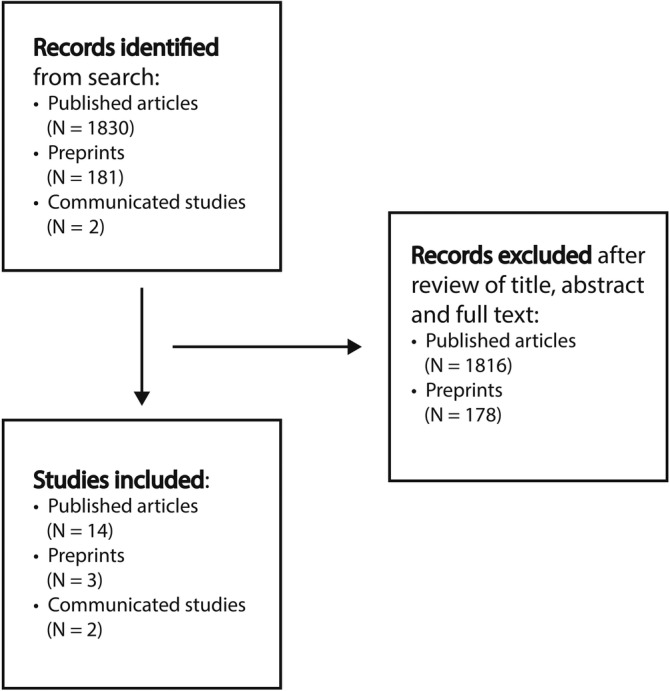
Flow diagram for identifying references
4. DISCUSSION
4.1. Infection by SARS‐CoV‐2
Infection by SARS‐CoV‐2 may occur via inhaled particles as small as aerosol (less than 5 μm in size; capable of staying suspended in the air for long periods of time and easily inhaled into the lungs and distal alveoli) up to droplets (measuring greater than 20 μm in size; quickly pulled to the ground by gravity or, when inhaled, mostly deposited in the nasal cavity), 24 or by direct inoculation of the respiratory epithelium (ie, touching a surface with live virus and then touching one's face). As up to 90% (or more) of inspiration is through the nose, 25 it is reasonable that the sinonasal cavities may be important sites for initial infection by SARS‐CoV‐2. In fact, SARS‐CoV‐2 infection via the ocular route is hypothesized to occur via drainage of virus‐laden tears into the nasal cavity through the nasolacrimal duct. 19
Recent work has shown that SARS‐CoV‐2 utilizes its S1 spike glycoprotein, which resides in the virus' envelope, for attachment to—and infection of—host target cells. 26 Infection by SARS‐CoV‐2 is likewise dependent on two host proteins for cellular entry and infection. 27 Angiotensin‐converting enzyme 2 (ACE2) serves as the cell surface receptor for SAR‐CoV‐2, which binds to the S1 spike glycoprotein and is required for cellular entry of the virus through endocytosis. A second protein, known as transmembrane protease serine 2 (TMPRSS2), is a protease that resides in the endosomal compartment and is required for priming/cleavage of the S1 spike glycoprotein, which then allows fusion of the viral envelope with the endosomal compartment (with introduction of the viral contents, including genetic material, into the cytoplasm of the host target cell).
ACE2, the host cell surface receptor for SARS‐CoV‐2, has been shown to be expressed throughout the aerodigestive tract, including the mucosa of the nasal cavity (as well as the mucosa of the oral cavity, and in the epithelium of the lungs and enterocytes of the gastrointestinal tract). 7 , 28 Recent single‐cell RNA sequencing of cells in the aerodigestive tract shows that expression of ACE2 in the nasal cavity is as high as it is in any other site within the aerodigestive tract. 21 Analysis of gene expression data from the Human Cell Atlas Project has further shown that ACE2 is most highly expressed within the ciliated epithelium and goblet cells in the nose. 20 Moreover, single‐cell RNA sequencing has shown that ACE2 may be expressed in tissues throughout the body (such as in the heart and kidneys), suggesting direct viral pathogenesis as a potential mechanism for extra‐aerodigestive symptoms of COVID‐19. 29
TMPRSS2, which is also required for SARS‐CoV‐2 infection of host target cells, has also been shown to be expressed in nasal epithelial cells 30 and the upper airway. 7 Expression level of TMPRSS2 in the respiratory epithelium—including the nasal mucosa—may be modulated by external factors such as air pollution or inflammatory airway conditions such as atopy or asthma. 30 , 31 Such modulation of expression in susceptibility genes for SARS‐CoV‐2 may, in part, explain geographic and individual‐level variation in SARS‐CoV‐2 infection rates. Although not directly studied with respect to SARS‐CoV‐2, the inflammatory milieu of the sinonasal mucosa has previously been shown to be associated with higher rates of respiratory virus isolation from the sinonasal cavity, 8 which further suggests that modulation of host susceptibility gene expression in the nasal mucosa could potentially impact SARS‐CoV‐2 infection rates. However, whether the modulation of SARS‐CoV‐2 susceptibility gene expression by environmental or host factors translates to a clinically significant impact on susceptibility to SARS‐CoV‐2 infection is yet unknown and represents an area of future research.
4.2. Transmission of COVID‐19
Different modes of transmission for COVID‐19 have been described, and all contribute to the exponential spread of the virus 32 over 196 countries. The major mode of transmission is through the upper respiratory tract, 13 similar to what was observed during the SARS‐CoV‐1 epidemic. 14 Nasal shedding of live virus is usually quite high early in the course of COVID‐19, precedes lower respiratory tract viral shedding and may even continue after the virus is no longer detected from the lower respiratory tract. 10 , 18 Zou et al showed higher viral load in nasal swabs when compared to throat swabs obtained from 17 symptomatic patients. 18 They concluded that the viral shedding pattern in patients with SARS‐CoV‐2 is similar to influenza, and is similar between symptomatic and asymptomatic patients, 18 with suspected prolonged shedding of COVID‐19 after recovery.
The important role for transmission of COVID‐19 due to sinonasal pathophysiology—and the infectiousness of nasal secretions—is further highlighted by a recent report by Patel et al, 22 who shared the experiences of rhinology colleagues from COVID‐19 hot spots around the world, including China, Iran, and Italy. In this report, the risk of health care‐associated infection (HAI) posed to health care workers by nasal transmission of SARS‐CoV‐2 was described during rhinology procedures. One illustrative case described HAI of up to 14 health care workers during a single endoscopic pituitary case procedure in Wuhan, China. The working hypothesis regarding the mechanisms for enhanced HAI during rhinology procedures includes both the higher viral loads in nasal secretions 18 as well as the generation of aerosols by powered rhinologic instrumentation such as the microdebrider or drill. 33 This possibility is also consistent with evidence in the dental literature reporting the generation of 5 μm droplet nuclei, capable of getting through a surgical mask using motorized instrumentation on the oral mucosa. 12 , 34 At present, rhinology and otolaryngology societies from around the world are recommending that endonasal surgeries be approached as high‐risk procedures. 35 , 36
4.3. Sinonasal symptomatology of COVID‐19
Cases series of patients with COVID‐19 from both China 16 , 37 , 38 and Europe 39 have reported a paucity of sinonasal symptomatology. Rhinorrhea has been described as individual cases, for example, being experienced by one child in a cohort of nine children with COVID‐19, 17 one adult in a cohort of eleven, 9 or one adult in another cohort of 18 with COVID‐19. 4 By contrast, lower respiratory tract and constitutional symptoms, such as fever, cough, fatigue, shortness of breath, and myalgias, are much more commonly reported. However, olfactory dysfunction (hyposmia or anosmia) has recently gained attention as an important symptom in COVID‐19.
4.4. Olfactory dysfunction in COVID‐19
The olfactory epithelium resides at the superior aspect of the nasal cavities where olfactory sensory neurons are in direct contact with the environment. The olfactory epithelium comprises approximately 9 cm 2 of the total 150 cm2 mucosal surface area of the nasal cavities. 40 As the adjacent respiratory epithelium is the primary site of SARS‐CoV‐2 attachment and infection, it may not be surprising for COVID‐19 to impact olfactory function. This is especially true as other coronaviruses, such as the related SARS‐CoV‐1, have previously been shown to have neurotropic properties with respect to olfactory neurons. 6 , 11 , 15
ACE2, the necessary cell surface host receptor for SARS‐CoV‐2, has been previously shown to be expressed on neurons and found to play a role in neurodegeneration. 41 The TMPRSS2 gene, also required for SARS‐CoV‐2 infection, has not been well studied in the nervous system although one study in a rat model has described expression of TMPRSS2 in neurons of the central nervous system. 42 Previous studies in mice have shown that after intranasal inoculation, the SARS‐CoV‐1 virus is neuroinvasive with infection directly transmitted through olfactory neurons into the central nervous system. 43 , 44 , 45
Olfactory dysfunction presenting as hyposmia or anosmia is not uncommon as a symptom of active viral upper respiratory tract infections, with up to 30% experiencing some kind of olfactory dysfunction. 40 Described as beginning suddenly in the context of a “common cold,” postviral olfactory dysfunction is characteristically described as occurring in a setting with other sinonasal symptoms. 46 In the long term, however, postviral olfactory dysfunction may persist in some as a lasting sequela without other sinonasal symptomatology. 47
Representing the first systematically collected data on olfactory dysfunction in COVID‐19 released for public knowledge, a recent study lead by Pr Dominique Salmon, MD, PhD (Hôtel Dieu, Paris), and Dr Alain Corré, MD (Hôpital Fondation Adolph de Rothschild, Paris) of Parisian COVID‐19 patients has revealed several novel insights into the olfactory dysfunction as a symptom of COVID‐19 (personal communication shared with permission from Dr Alain Corré; manuscript in review). In a cohort of 55 patients presenting to them with the symptom of anosmia without nasal obstruction within 7 days of the occurrence of this symptom, 94% were found to be COVID‐19+ by nasopharyngeal swabbing and reverse transcription polymerase chain reaction testing. In a press release given to us dated 28 March 2020, 23 their group writes: “Patients with allergic rhinitis seem more affected. It occurs suddenly 2 to 3 days after the beginning of usually rather mild symptoms related to COVID 19 disease such as headaches, low‐grade fever, and diarrhea. In most cases, the signs of cold (such as cough, fever) are absent or have disappeared. The sense of smell usually starts recovering after a few days (5‐10 days) and this recovery seems already complete in some patients around day 10 to 15 but unfortunately this trouble of smell persists longer in others.” Thus, decreased sense of smell in the absence of nasal obstruction may be a highly predictive marker for COVID‐19, which may be particularly helpful in identifying asymptomatic carriers or those with mild symptoms who otherwise would not think that they have the infection. However, it should be noted that while the presence of anosmia without nasal obstruction may be a uniquely described viral phenomenon in COVID‐19, it is unknown how many cases of idiopathic anosmia may have occurred in the setting of a viral upper respiratory tract infection that went unnoticed by the affected patient. 46
5. CONCLUSION
In COVID‐19, there is increasing evidence for the importance of sinonasal pathophysiology. The sinonasal cavity may be an important route for infection and virus shed from the sinonasal cavity may be an important source of transmission. The high viral load in sinonasal secretions may also reflect an especially high risk for HAI from rhinologic procedures. Although sinonasal symptoms do not appear to be a major component of the clinical presentation of COVID‐19, decreased sense of smell may be an underappreciated symptom of COVID‐19. Anosmia without nasal obstruction, in particular, appears to be a highly specific indicator of COVID‐19.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Gengler I, Wang JC, Speth MM, Sedaghat AR. Sinonasal pathophysiology of SARS‐CoV‐2 and COVID‐19: A systematic review of the current evidence. Laryngoscope Investigative Otolaryngology. 2020;5:354–359. 10.1002/lio2.384
REFERENCES
- 1. Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID‐19) outbreak ‐ an update on the status. Mil Med Res. 2020;7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sohrabi C, Alsafi Z, O'Neill N, et al. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID‐19). Int J Surg. 2020;76:71‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu D, Wu T, Liu Q, Yang Z. The SARS‐CoV‐2 outbreak: what we know. Int J Infect Dis. 2020. 10.1016/j.ijid.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS‐CoV‐2 in Singapore. JAMA. 2020. 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14:523‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID‐19 virus targeting the CNS: tissue distribution, host‐virus interaction, and proposed neurotropic mechanisms. ACS Chem Nerosci. 2020;11:995‐998. [DOI] [PubMed] [Google Scholar]
- 7. Bertram S, Heurich A, Lavender H, et al. Influenza and SARS‐coronavirus activating proteases TMPRSS2 and HAT are expressed at multiple sites in human respiratory and gastrointestinal tracts. PLoS One. 2012;7:e35876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cho GS, Moon BJ, Lee BJ, et al. High rates of detection of respiratory viruses in the nasal washes and mucosae of patients with chronic rhinosinusitis. J Clin Microbiol. 2013;51:979‐984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dong X, Cao YY, Lu XX, et al. Eleven faces of coronavirus disease 2019. Allergy. 2020. 10.1111/all.14289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim JY, Ko JH, Kim Y, et al. Viral load kinetics of SARS‐CoV‐2 infection in first two patients in Korea. J Korean Med Sci. 2020;35:e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS‐CoV2 may play a role in the respiratory failure of COVID‐19 patients. J Med Virol. 2020. 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peng X, Xu X, Li Y, Cheng L, Zhou X, Ren B. Transmission routes of 2019‐nCoV and controls in dental practice. Int J Oral Sci. 2020;12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019‐nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970‐971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Teleman MD, Boudville IC, Heng BH, Zhu D, Leo YS. Factors associated with transmission of severe acute respiratory syndrome among health‐care workers in Singapore. Epidemiol Infect. 2004;132:797‐803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wheeler DL, Athmer J, Meyerholz DK, Perlman S. Murine olfactory bulb interneurons survive infection with a neurotropic coronavirus. J Virol. 2017;91:e01099‐17. 10.1128/JVI.01099-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020. 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 17. Zhou Y, Yang GD, Feng K, et al. Clinical features and chest CT findings of coronavirus disease 2019 in infants and young children. Zhongguo Dang Dai Er Ke Za Zhi. 2020;22:215‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zou L, Ruan F, Huang M, et al. SARS‐CoV‐2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177‐1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sun CB, Wang YY, Liu GH, Liu Z. Role of the Eye in Transmitting Human Coronavirus: What We Know and What We Do Not Know. https://www.scilit.net/article/3498497ae4fd4d7954701bba57684bda. Accessed March 30, 2020. [DOI] [PMC free article] [PubMed]
- 20. Sungnak W, Huang N, Bécavin C, Berg M, Network HLB. SARS‐CoV‐2 Entry Genes Are Most Highly Expressed in Nasal Goblet and Ciliated Cells within Human Airways. https://arxiv.org/pdf/2003.06122v1.pdf. Accessed March 30, 2020.
- 21. Wu C, Zheng M. Single‐Cell RNA Expression Profiling Shows that ACE2, the Putative Receptor of Wuhan 2019‐nCoV, Has Significant Expression in the Nasal, Mouth, Lung and Colon Tissues, and Tends to Be Co‐Expressed with HLA‐DRB1 in the Four Tissues. https://www.preprints.org/manuscript/202002.0247/v1. Accessed March 30, 2020.
- 22. Patel ZM, Fernandez‐Miranda J, Hwang PH, et al. Precautions for endoscopic transnasal skull base surgery during the COVID‐19 pandemic. Neurosurgery. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Salmon Ceron D, Hautefort C, Bequignon E, Corre A, Canoui‐Poitrine F, Papon JF. Anosmia without Nasal Obstruction: A Pathognomonic Sign of COVID‐19 Infection. Press Release, March 28, 2020.
- 24. Tellier R, Li Y, Cowling BJ, Tang JW. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect Dis. 2019;19:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Camner P, Bakke B. Nose or mouth breathing? Environ Res. 1980;21:394‐398. [DOI] [PubMed] [Google Scholar]
- 26. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS‐CoV‐2 spike glycoprotein. Cell. 2020. 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020. 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single‐cell RNA‐seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019‐nCoV infection. Front Med. 2020. 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kesic MJ, Meyer M, Bauer R, Jaspers I. Exposure to ozone modulates human airway protease/antiprotease balance contributing to increased influenza A infection. PLoS One. 2012;7:e35108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kesic MJ, Hernandez M, Jaspers I. Airway protease/antiprotease imbalance in atopic asthmatics contributes to increased influenza A virus cleavage and replication. Respir Res. 2012;13:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus‐infected pneumonia. N Engl J Med. 2020;382:1199‐1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vukkadala N, Qian ZJ, Holsinger FC, Patel ZM, Rosenthal E. COVID‐19 and the otolaryngologist ‐ preliminary evidence‐based review. Laryngoscope. 2020. 10.1002/lary.28672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sun H, Lau A, Heo YC, Lin L, Delong R, Fok A. Relationships between tissue properties and operational parameters of a dental handpiece during simulated cavity preparation. J Dent Biomech. 2013;4:1758736013483747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. European Rhinologic Society . Information for Rhinologists on COVID‐19. https://www.europeanrhinologicsociety.org/?page_id=2143. Accessed March 30, 2020.
- 36. American Academy of Otolaryngology‐Head and Neck Surgery . Academy Supports CMS, Offers Specific Nasal Policy. https://www.entnet.org/content/academy‐supports‐cms‐offers‐specific‐nasal‐policy. Updated March 26, 2020. Accessed March 30, 2020.
- 37. Adhikari SP, Meng S, Wu YJ, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID‐19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lescure FX, Bouadma L, Nguyen D, et al. Clinical and virological data of the first cases of COVID‐19 in Europe: a case series. Lancet Infect Dis. 2020. 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dalton P. Olfaction and anosmia in rhinosinusitis. Curr Allergy Asthma Rep. 2004;4:230‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Abiodun OA, Ola MS. Role of brain renin angiotensin system in neurodegeneration: an update. Saudi J Biol Sci. 2020;27:905‐912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ubuka T, Moriya S, Soga T, Parhar I. Identification of transmembrane protease serine 2 and forkhead box A1 as the potential bisphenol a responsive genes in the neonatal male rat brain. Front Endocrinol. 2018;9:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Desforges M, Le Coupanec A, Dubeau P, et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McCray PB Jr, Pewe L, Wohlford‐Lenane C, et al. Lethal infection of K18‐hACE2 mice infected with severe acute respiratory syndrome coronavirus. J Virol. 2007;81:813‐821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008;82:7264‐7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hummel T, Whitcroft KL, Andrews P, et al. Position paper on olfactory dysfunction. Rhinol Suppl. 2017;54:1‐30. [DOI] [PubMed] [Google Scholar]
- 47. Seiden AM. Postviral olfactory loss. Otolaryngol Clin North Am. 2004;37:1159‐1166. [DOI] [PubMed] [Google Scholar]


