Abstract
Comorbidities are associated with the severity of coronavirus disease 2019 (COVID‐19). This meta‐analysis aimed to explore the risk of severe COVID‐19 in patients with pre‐existing chronic obstructive pulmonary disease (COPD) and ongoing smoking history. A comprehensive systematic literature search was carried out to find studies published from December 2019 to 22 March 2020 from five databases. The languages of literature included English and Chinese. The point prevalence of severe COVID‐19 in patients with pre‐existing COPD and those with ongoing smoking was evaluated with this meta‐analysis. Overall 11 case series, published either in Chinese or English language with a total of 2002 cases, were included in this study. The pooled OR of COPD and the development of severe COVID‐19 was 4.38 (fixed‐effects model; 95% CI: 2.34‐8.20), while the OR of ongoing smoking was 1.98 (fixed‐effects model; 95% CI: 1.29‐3.05). There was no publication bias as examined by the funnel plot and Egger's test (P = not significant). The heterogeneity of included studies was moderate for both COPD and ongoing smoking history on the severity of COVID‐19. COPD and ongoing smoking history attribute to the worse progression and outcome of COVID‐19.
Keywords: COPD, COVID‐2019, smoking
Abbreviations
- COVID‐19
coronavirus disease 2019
- COPD
chronic obstructive pulmonary disease
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- MINORS
methodological index nonrandomized studies
- OR
pooled odds ratio
1. BACKGROUND
Coronavirus disease 2019 (COVID‐19) is now a declared pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). 1 , 2 Due to its extremely high infectivity, COVID‐19 has caused over 292 142 infections and 12 784 deaths worldwide as of 22 March 2020 (World Health Organization coronavirus disease situation reports). 3
SARS‐CoV‐2 primarily invades the pulmonary alveolar epithelial cells. Although most infections with SARS‐CoV‐2 are thought to be subclinical or mildly symptomatic, it may result in acute respiratory distress syndrome and occasionally into multiorgan failure. 4 , 5 The impact of underlying respiratory compromises, such as the presence of chronic obstructive pulmonary disease (COPD) or ongoing smoking history on the clinical manifestation and course of SARS‐CoV‐2 infection, is uncertain. A large case series reporting the clinical characteristics and outcomes of patients with COVID‐19 from China have reported a higher prevalence of COPD in patients with severe presentation and worse outcomes. 6 The same case series also reported a higher prevalence of active smokers in severe COVID‐19. 6 A recent meta‐analysis, however, failed to demonstrate any correlation between ongoing smoking history and the severity of COVID‐19. 7 The results of a meta‐analysis are dependent on the studies included, sample size and frequency of events. A large number of COVID‐19 related studies, case reports, and case series are reported in native Chinese languages. A meta‐analysis that includes published Chinese literature regarding COVID‐19 is likely to improve on the conclusions by increasing the sample size and the number of events. We conducted this meta‐analysis by including both English and Chinese language published literature to explore the impact of underlying respiratory illness and smoking history on the severity of COVID‐19 manifestations.
2. METHODS
2.1. Literature search and selection
A comprehensive systematic literature search of online databases including PubMed, Web of Science, Cochrane, WanFang Database, and CNKI from December 2019 to 22 March 2020 to identify all reported case studies in Chinese and English languages was carried out. The WanFang and CNKI databases are Chinese databases available online which can be used to find full‐text articles. The following terms and their relative variants were used for the literature search: “Covid‐19” or “2019 novel coronavirus infection” or “coronavirus disease 2019” or “2019 novel coronavirus disease” or “coronavirus 2019” or “2019‐nCoV” or “SARS‐CoV‐2” or “COVID19” or “coronavirus disease‐19.”
The titles, abstracts, and full text of all documents identified according to this search strategy were then screened by two investigators (MM and QZ). The reference list of each review and the original article was then reviewed for identifying other eligible reports. The criteria for the studies to be included in the meta‐analysis were as follows: studies presenting data on COPD or smoking history in COVID‐19 cases with or without severe presentation. As the very definition of severity of COVID‐19 was not consistent across included studies, for this meta‐analysis, we defined severe COVID‐19 as COVID‐19 diagnosed according to the guidance issued by the Chinese National Health Committee along with either the requirement of intensive care/mechanical ventilation or resulting in death by bias risk assessment
All the search results were evaluated according to the methodological index nonrandomized studies (MINORS) statement. MINORS statement includes the following items: a clearly stated aim, the inclusion of consecutive patients, prospective collection of data, endpoints appropriate to the aim of the study, unbiased assessment of the study endpoint, follow‐up period appropriate to the aim of the study, loss to follow up less than 5%, and prospective calculation of the study size. The items were scored “0” if it was not reported, “1” if it was reported but inadequate, “2” if reported and adequate.
2.2. Data extraction and quality assessment
Data extraction and the evaluation of literature quality were conducted independently by two investigators (MM and QZ). Microsoft Excel database was created to record the available information including baseline details and the rate of development of severe COVID‐19 in patients with different respiratory conditions. Any disagreement was resolved by another investigator (SL).
2.3. Statistical analysis of data
Microsoft Excel was used to analyze the clinical symptoms and laboratory results. Meta‐analysis was carried out using R‐software (version 3.6.3, available on https://www.r-project.org). Heterogeneity among studies was tested using the Cochran χ 2 test and I 2 . When I 2 was less than 50%, a fixed‐effects model was used, and when I 2 was more than 50%, a random‐effects model was used. Sensitivity analysis was conducted to determine the source of heterogeneity by excluding one study at a time. After excluding significant clinical heterogeneity, the random‐effects model was used for meta‐analysis. Funnel plot and Egger test were used to detect any publication bias. A P value of less than 0.05 was considered statistically significant.
3. RESULTS
3.1. Research selection and quality assessment
On the basis of predefined search strategy, a total of 2472 studies were found in the five online databases as described above. After removing the duplicate records, 1596 articles were obtained. Besides, another 1544 articles were excluded as they were not relevant to current meta‐analysis. Full texts of the remaining 48 articles were then assessed for eligibility, 37 of which were removed for various reasons. Eventually 11 articles (1 in Chinese and 10 in English) 6 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 were included in the analysis (Figure 1). The patient characteristics and demographic data of the included studies are shown in Table 1. All the reported and thus included studies were case series, sample size calculation was not relevant. The follow‐up period was either not or inadequately reported in most studies. The overall quality of available literature was moderate with MINORS scores ranging from 10 to 13. The quality of the included articles is evaluated and shown in Table S1.
Figure 1 .

A flow diagram of literature screening
Table 1.
Characteristics and demographic data of the included studies
| Author | Year | Origin | Language | Outcome | Mean age, y | Male (n) (%) | Severe cases/total cases (%) | Severe cases in smoke/total cases of smoke (%) | Severe cases in COPD/total cases of COPD (%) |
|---|---|---|---|---|---|---|---|---|---|
| Chen 16 | 2020 | Chongqing | Chinese | Severe COVID‐19 a | 45.33 | 76 (54.68%) | 31/139 | 3/14 | Not report |
| (22.30%) | (21.43%) | ||||||||
| Guan 6 | 2020 | 30 provinces | English | ICU, or mechanical ventilation, or death | 46.67 | 459 (58.12%) | 67/1099 | 17/137 | 7/12 (58.33%) |
| (6.10%) | (12.41%) | ||||||||
| Gao 15 | 2020 | Anhui | English | Severe COVID‐19 a | 44.08 | 26 (60.47%) | 15/43 | Not report | 3/8 (37.50%) |
| (34.88%) | |||||||||
| Huang 8 | 2020 | Wuhan | English | ICU | 49.33 | 30 (73.17%) | 13/41 | 0/3 | 1/1 (100%) |
| (31.71%) | (0.00%) | ||||||||
| Liu 17 | 2020 | Wuhan | English | Disease progression | 42.67 | 39 (50.00%) | 11/78 | 3/5 | 1/2 (50.00%) |
| (14.10%) | (60.00%) | ||||||||
| Liu 9 | 2020 | Shenzhen | English | Mechanical ventilation | 53.67 | 8 (66.67%) | 3/12 | Not report | 1/1 (100.00%) |
| (25.00%) | |||||||||
| Wang 10 | 2020 | Wuhan | English | ICU | 55.33 | 75 (54.35%) | 36/138 | Not report | 3/4 (75.00%) |
| (26.09%) | |||||||||
| Wang 14 | 2020 | Wuhan | English | Severe COVID‐19 a | 46.33 | 32 (46.38%) | 14/69 | Not report | 2/4 (50.00%) |
| (20.29%) | |||||||||
| Yang 11 | 2020 | Wuhan | English | Death | 59.7 | 35 (66.67%) | 32/52 | 0/2 | 2/4 (50.00%) |
| (61.54) | (0.00%) | ||||||||
| Zhou 12 | 2020 | Wuhan | English | Death | 56.33 | 119 (62.30%) | 54/191 | 5/11 | 4/6 (66.67%) |
| (28.27%) | (45.45%) | ||||||||
| Zhang 13 | 2020 | Wuhan | English | Severe COVID‐19 a | 56.33 | 71 (50.71%) | 58/14 | 6/9 | 2/2 (100.00%) |
| (41.43%) | (66.67%) |
Abbreviations: COPD, chronic obstructive pulmonary disease; COVID‐19, coronavirus disease 2019; ICU, intensive care unit; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Severe cases are diagnosed according to the defined according to the diagnostic and treatment guideline for SARS‐CoV‐2 issued by Chinese National Health Committee, which are: respiratory distress with respiratory frequency ≥ 30/min; (b) pulse oximeter oxygen saturation ≤ 93% at rest; and (c) oxygenation index (artery partial pressure of oxygen/inspired oxygen fraction, PaO2/FiO2) ≤ 300 mm Hg.
3.2. Clinical data
Pooling the clinical data of the 11 studies included in this meta‐analysis resulted in a total of 2002 patients and 334 meetings the definition of severe COVID‐19. Out of the 11 studies, 1 study reported only on the smoking history, 16 and 4 others reported only on the presence of COPD. 9 , 10 , 14 , 15 The rest of the studies reported both, the smoking history and the presence of COPD. Other respiratory ailments, such as asthma, bronchiectasis, or interstitial lung disease, were not documented in the included literature. The original data were presented as the prevalence of COPD or smoking history in severe or not severe patients. To better visualize the risk, we converted the dataset to present the data as the incidence of severe COVID‐19 in patients with pre‐existing COPD or with a history of smoking.
3.3. Pre‐existing COPD and the severity of COVID‐19
A total of 10 studies were included in the analysis 6 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 17 to determine the effect of pre‐existing COPD on the severity of COVID‐19, as 1 of the studies did not report on the prevalence of COPD in their patient population. The pooled odds ratio (OR) of COPD for the development of severe COVID‐19 is summarized in Figure 2A, which shows that the presence of COPD is associated with a nearly fourfold higher risk of developing severe COVID‐19 (fixed‐effects model; OR = 4.38; 95% confidence interval [CI]: 2.34‐8.20), heterogeneity among the different studies being moderate (I 2 = 41%; P = .08). Sensitivity analysis showed that the results were not affected by any individual study. Publication bias, as accessed by funnel plot (Figure 2B) and Egger's test, showed no publication bias in this analysis (P for Egger's test was 0.5492).
Figure 2.

Forest plot and funnel plot of all outcomes
3.4. Pre‐existing COPD and mortality
Only two of the included studies reported the association between death and pre‐existing COPD. 11 , 12 Death was reported in 6 of 10 (60%) of patients with COPD and 80 of 233 (34.3%) of non‐COPD patients. The pooled OR of COPD for death was 1.93 (95%CI: 0.59‐7.43); however, the heterogeneity in this analysis (I 2 = 61%; P = .11) was quite high.
3.5. Smoking history and the severity of COVID‐19
Seven studies reported the relationship between active smoking and the severity of COVID‐19. 6 , 8 , 11 , 12 , 13 , 16 , 17 The exact duration of smoking was not reported in most studies. The pooled OR is summarized in Figure 2C, which shows that smoking increases the risk of severe COVID‐19 (fixed‐effects model; OR = 1.98; 95% CI: 1.29‐3.05) by around twofolds. The heterogeneity among the different studies was moderate (I 2 = 44%; P = .10). Sensitivity analysis by excluding each study one by one showed that the study from Guan 6 was a major source of heterogeneity. After excluding this study, the effect of smoking on the severity of COVID‐19 became insignificant with the OR of 1.55 (95% CI: 0.83‐ 2.87). Egger's test (P = .3244 > .1) and the funnel plot indicated no publication bias inside this study (Figure 2D).
3.6. Subgroup analysis
As the reported endpoints varied between including literatures, a subgroup analysis was performed to find out the impact of different endpoints on the effect of COPD or smoking on COVID‐19. As shown in Figure 3A, pre‐existing COPD is significantly associated with mechanical ventilation, requirement ICU or death (random‐effects model; OR = 6.44; 95% CI: 1.85‐22.46). However, when the endpoint was defined as severe COVID‐19 or disease progression, the subgroup analysis showed the effect of pre‐existing COPD on COVID‐19 was no longer significant (Figure 3B, fixed‐effects model; OR = 2.63; 95% CI: 0.93‐7.43). The smoking history was not related to the severity of COVID‐19 in either of the subgroups (Figure 3C,D).
Figure 3.
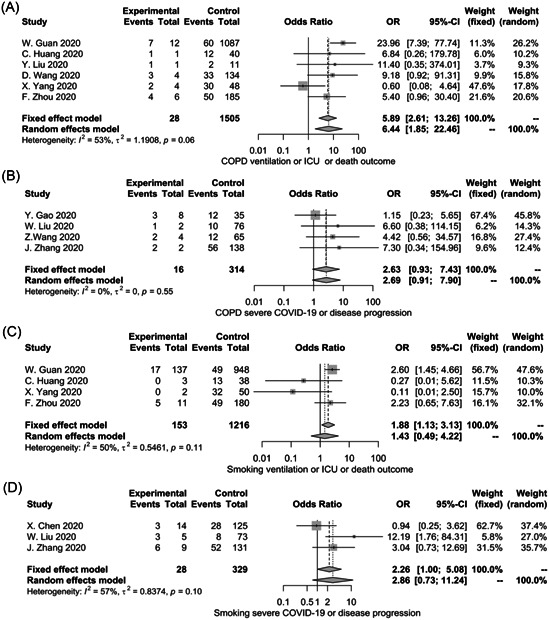
Subgroup analysis for the impact of COPD and smoking histology on the severity of COVID‐19. CI, confidence interval; COPD, chronic obstructive pulmonary disease; COVID‐19, coronavirus disease 2019; ICU, intesive care unit; OR, odds ratio
4. DISCUSSION
This meta‐analysis which includes studies on SARS‐CoV‐2 infection from December 2019 to 22 March 2020, published in either English or Chinese language, shows that the pre‐existing COPD has a fourfold increased risk of developing severe COVID‐19. Active smoking although increased the risk of severe COVID‐19, the result was heavily influenced by one study 6 and after removing the study from analysis association between active smoking and severe COVID‐19 was found to be nonsignificant.
COPD is characterized by chronic inflammation of the large (central) airways, small (peripheral) bronchioles, and destruction of lung parenchyma. The functional consequence of these abnormalities is expiratory airflow limitation. 18 Pathogenic infections are a common cause of acute exacerbation of COPD, which can result in respiratory failure in many patients. 19 The clinical symptomatology of COVID‐19 and acute exacerbation of COPD is difficult to differentiate which may potentially result in delayed or inappropriate medical intervention. It is, thus, not surprising to find a worse prognosis of COVID‐19 in patients with COPD. The result of this meta‐analysis, which was stable on sensitivity analysis, confirms that the risk of severe COVID‐19 in a patient with pre‐existing COPD was four folds higher than in patients without COPD. In subgroup analysis, this effect remained statistically significant in the subgroup of those death or ICU requirements. These findings highlight the importance of strict control of COVID‐19 spread and an urgent need for mitigation strategies in patients with pre‐existing COPD.
The impact of active, ongoing smoking on COVID‐19 progression is controversial. Lippi et al 7 showed that active smoking was not associated with the severity of COVID‐19, which is contrary to our conclusion. The results of our study show that active smoking increases the risk of developing severe COVID‐19 by around two folds.
However, in the subgroup analysis, this effect was no longer significant in either subgroup. The weight of a single study on the overall sample size could be a possible explanation. When the study with the largest sample size was excluded from sensitivity analysis, the effect of smoking on the severity of COVID‐19 was no longer significant. Another possible explanation is that most studies did not differentiate between current and ex‐smokers except for the study from Guan et al. 6 In addition, smoking duration was not reported in all available, included studies. It is well‐known that cessation of smoking improves pulmonary function; however, this benefit is less among older smokers due to the cumulative injury to lung over a prolonged period of time. 20 These results indicate a complicated relationship between smoking history and the severity of COVID‐19.
One of the important limitations of our study is that all the included studies were case series; however, due to the rapid spread of COVID‐19, randomized controlled trial or prospective studies have not been possible so far. Even though all the literature included in this study were case series, the heterogeneity in terms of the impact of COPD in COVID‐19 was relatively low, and the sensitivity analysis showed that the results were stable without any publication bias. The impact of ongoing and ex‐smoking remains an area of further evaluation. Although a majority of the included cases are from Asia and that may be a limitation of this study; however, so far to the best of our knowledge, there is no report of any ethnic differences in the pathogenesis of COVID‐19.
In conclusion, the results of this study indicate that pre‐existing COPD is likely to worsen the progression and prognosis of COVID‐19. Strong efforts should be directed to ovoid infection in patients with underlying COPD.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Guarantor of the article: SL; designed the study, interpreted data, and wrote the manuscript: SL and RK; screened and extracted data: QZ and MM; statistical analyses: SL; reviewed the results and made critical comments on the manuscript: NL, YD, JH, and YW. All authors approved the final version of the manuscript.
Supporting information
Supporting information
ACKNOWLEDGMENT
This project was funded by the Fujian Provincial Health Technology Project (2018‐ZQN‐54), Scientific Research “Pei Yu” Project of Beijing Municipal Hospital Administration (PZ2018011), and the Chinese National 13th Five‐Year Plan's Science and Technology Projects (2017ZX10202201).
Zhao Q, Meng M, Kumar R, et al. The impact of COPD and smoking history on the severity of COVID‐19: A systemic review and meta‐analysis. J Med Virol. 2020;92:1915–1921. 10.1002/jmv.25889
Qianwen Zhao, Meng Meng, and Rahul Kumar contributed equally to this study and are co‐first authors.
DATA AVAILABILITY STATEMENT
All data are fully available online without restriction.
REFERENCES
- 1. El Zowalaty ME, Järhult JD. From SARS to COVID‐19: a previously unknown SARS‐related coronavirus (SARS‐CoV‐2) of pandemic potential infecting humans—call for a one health approach. One Health (Amsterdam, Netherlands). 2020;9:100124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. He F, Deng Y, Li W. Coronavirus disease 2019: what we know? J Med Virol. 2020;1. https://pubmed.ncbi.nlm.nih.gov/32170865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO coronavirus disease situation reports . https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. Accessed 22 March 2020.
- 4. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS‐CoV2 may play a role in the respiratory failure of COVID‐19 patients. J Med Viro. 2020;92(6):552–555. 10.1002/jmv.25728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cao Y, Liu X, Xiong L, Cai K. Imaging and clinical features of patients with 2019 novel coronavirus SAR‐CoV‐2: a systematic review and meta‐analysis. J Med Virol. 2020. 10.1002/jmv.25822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. W‐J Guan, Z‐Y Ni, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lippi G, Henry BM. Active smoking is not associated with severity of coronavirus disease 2019 (COVID‐19). Eur J Intern Med. 2020. 10.1016/j.ejim.2020.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu Y, Yang Y, Zhang C, et al. Clinical and biochemical indexes from 2019‐nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63(3):364‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020. 10.1016/s2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020. [DOI] [PubMed] [Google Scholar]
- 14. Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gao Y, Li T, Han M, et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID‐19. J Med Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen X, Tong J, Xiang J, Hu J. Retrospective study on the epidemiological characteristics of 139 patients with novel coronavirus pneumonia on the effects of severity. Chongqing Med. 2020. [Google Scholar]
- 17. Liu W, Tao ZW, Lei W, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saetta M, Turato G, Maestrelli P, Mapp CE, Fabbri LM. Cellular and structural bases of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163(6):1304‐1309. [DOI] [PubMed] [Google Scholar]
- 19. Leung JM, Tiew PY, Mac Aogáin M, et al. The role of acute and chronic respiratory colonization and infections in the pathogenesis of COPD. Respirology (Carlton, Vic). 2017;22(4):634‐650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Burns DM. Cigarette smoking among the elderly: disease consequences and the benefits of cessation. Am J Health Promot. 2000;14(6):357‐361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Data Availability Statement
All data are fully available online without restriction.


