Abstract
COVID‐19 is a systemic infection with a significant impact on the hematopoietic system and hemostasis. Lymphopenia may be considered as a cardinal laboratory finding, with prognostic potential. Neutrophil/lymphocyte ratio and peak platelet/lymphocyte ratio may also have prognostic value in determining severe cases. During the disease course, longitudinal evaluation of lymphocyte count dynamics and inflammatory indices, including LDH, CRP and IL‐6 may help to identify cases with dismal prognosis and prompt intervention in order to improve outcomes. Biomarkers, such high serum procalcitonin and ferritin have also emerged as poor prognostic factors. Furthermore, blood hypercoagulability is common among hospitalized COVID‐19 patients. Elevated D‐Dimer levels are consistently reported, whereas their gradual increase during disease course is particularly associated with disease worsening. Other coagulation abnormalities such as PT and aPTT prolongation, fibrin degradation products increase, with severe thrombocytopenia lead to life‐threatening disseminated intravascular coagulation (DIC), which necessitates continuous vigilance and prompt intervention. So, COVID‐19 infected patients, whether hospitalized or ambulatory, are at high risk for venous thromboembolism, and an early and prolonged pharmacological thromboprophylaxis with low molecular weight heparin is highly recommended. Last but not least, the need for assuring blood donations during the pandemic is also highlighted.
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) causing coronavirus disease 2019 (COVID‐19) has rapidly evolved from an epidemic outbreak in Wuhan, China 1 into a pandemic infecting more than 1 million individuals all over the world. Billions of citizens are affected by measures of social distancing and the socioeconomic impact of the pandemic. Note, SARS‐CoV‐2 is approximately 80% similar to SARS‐CoV, and invades host human cells by binding to the angiotensin‐converting enzyme 2 (ACE2) receptor. 1 Although it is well documented that COVID‐19 is primarily manifested as a respiratory tract infection, emerging data indicate that it should be regarded as a systemic disease involving multiple systems, including cardiovascular, respiratory, gastrointestinal, neurological, hematopoietic and immune system. 2 , 3 , 4 Mortality rates of COVID‐19 are lower than SARS and Middle East Respiratory Syndrome (MERS) 5 ; however, COVID‐19 is more lethal than seasonal flu. Older people and those with comorbidities are at increased risk of death from COVID‐19, but younger people without major underlying diseases may also present with potentially lethal complications such as fulminant myocarditis and disseminated intravascular coagulopathy (DIC). 6 , 7 Herein, we summarize the numerous hematologic findings and complications of COVID‐19 and we provide guidance for early prevention and management of the latter.
2. RESULTS
2.1. Full blood count and biochemistry findings: correlation with prognosis
During the incubation period, usually ranging from 1 to 14 days, and during the early phase of the disease, when non‐specific symptoms are present, peripheral blood leukocyte and lymphocyte counts are normal or slightly reduced. Following viremia, SARS‐CoV‐2 primarily affects the tissues expressing high levels of ACE2 including the lungs, heart and gastrointestinal tract. Approximately 7 to 14 days from the onset of the initial symptoms, there is a surge in the clinical manifestations of the disease. This is with a pronounced systemic increase of inflammatory mediators and cytokines, which may even be characterized as a “cytokine storm.” 8 At this point, significant lymphopenia becomes evident. Although more in‐depth research on the underlying etiology is necessary, several factors may contribute to COVID‐19 associated lymphopenia. It has been shown that lymphocytes express the ACE2 receptor on their surface 9 ; thus SARS‐CoV‐2 may directly infect those cells and ultimately lead to their lysis. Furthermore, the cytokine storm is characterized by markedly increased levels of interleukins (mostly IL‐6, IL‐2, IL‐7, granulocyte colony stimulating factor, interferon‐γ inducible protein 10, MCP‐1, MIP1‐a) and tumor necrosis factor (TNF)‐alpha, which may promote lymphocyte apoptosis. 10 , 11 , 12 Substantial cytokine activation may be also associated with atrophy of lymphoid organs, including the spleen, and further impairs lymphocyte turnover. 13 Coexisting lactic acid acidosis, which may be more prominent among cancer patients who are at increased risk for complications from COVID‐19, 14 may also inhibit lymphocyte proliferation. 15 Table 1 presents the results of main studies regarding lymphopenia in COVID‐19.
TABLE 1.
Studies and main findings for lymphocyte count in Covid‐19 patients
| First author (year) | Region | Study period | Sample size | Categorization of hematological factors | Main findings |
|---|---|---|---|---|---|
| Guan (2020) 16 | 552 hospitals in 30 provinces, autonomous regions, and municipalities in mainland China | 11 December 2019 – 31 January 2020 | 1099 | Lymphocytopenia: lymphocyte count of less than 1500 cells/mm3 | Lymphocytopenia was present in 83.2% of patients on admission. 92.6% (50/54) of patients with the composite primary endpoint (admission to an intensive care unit, use of mechanical ventilation, or death) presented with lymphocytopenia vs 82.5% (681/825) of patients without the primary endpoint (P = .056 a ). Severe cases presented lymphocytopenia more frequently (96.1%, 147/153) vs non‐severe cases (80.4%, 584/726); P < .001 a |
| Huang (2020) 17 | Jinyintan Hospital, Wuhan, China | 16 December 2019, to 2 January 2020 | 41 | Low lymphocyte count of <1.0 x109 lymphocytes per Liter | 85% (11/13) of patients needing ICU care presented low lymphocyte count vs 54% (15/28) of patients that did not need ICU care (P = .045). |
| Wang (2020) 19 | Zhongnan Hospital, Wuhan, China | 1 January to 3 February 2020 | 138 | Lymphocytes treated as a continuous variable, x109 per Liter | ICU cases presented with lower lymphocyte count (median:0.8, IQR: 0.5‐0.9) versis non‐ICU cases (median: 0.9, IQR: 0.6‐1.2); P = .03. Longitudinal decrease was noted in non‐survivors. |
| Wu (2020) 20 | Jinyintan Hospital, Wuhan, China | 25 December 2019, to 13 February 2020 | 201 | Lymphocytes treated as a continuous variable, x109 /mL in a bivariate Cox regression model | Lower lymphocyte count was associated with ARDS development (HR = 0.37, 95%CI: 0.21‐0.63, P < .001 in the incremental model); the association with survival did not reach significance (HR = 0.51, 95%CI: 0.22‐1.17, P = .11) |
| Young (2020) 21 | Four hospitals in Singapore | 23 January to 3 February 2020 | 18 | Lymphocytes treated as a continuous variable, x109 per L; lymphopenia was defined as <1.1 × 109/L. | Lymphopenia was present in 7 of 16 patients (39%). Median lymphocyte count was 1.1 (IQR: 0.8‐1.7) in patients that required supplemental O2 and 1.2 (IQR:0.8‐1.6) in those that did not; no statistical comparison was undertaken. |
| Fan (2020) 22 | National Centre for Infectious Diseases, Singapore | 23 January to 28 February 2020 | 69 | Lymphopenia: lymphocyte count of <0.5 × 109/L. | Lymphopenia at admission (4/9 of ICU patients vs 1/58 non‐ICU patients, P < .001) and nadir lymphopenia during hospital stay (7/9 of ICU patients vs 1/58 non‐ICU patients, P < .001) were associated with need for ICU. |
| Yang (2020) 27 | Jinyintan Hospital, Wuhan, China | 24 December 2019, to 9 February 2020 | 52 critically ill patients | Lymphocytes treated as a continuous variable (×109/L); lymphocytopenia presented but not defined | Lymphocytopenia occurred in 44 (85%) of critically ill patients, with no significant difference between survivors and non‐survivors. A numeric difference in lymphocyte count was noted in non‐survivors vs survivors (0.62 vs 0.74). |
| Zhou (2020) 31 | Jinyintan Hospital and Wuhan Pulmonary Hospital, Wuhan, China | 25 December 2019, to 31 January 2020 | 191 | Lymphocyte counts treated as a continuous variable (×109/L) in a multivariate logistic regression model | Lower lymphocyte count was associated with higher odds of death at the univariate analysis (OR = 0.02, 95%CI: 0.01‐0.08; P < .001); at the multivariate analysis, the finding lost significance (OR = 0.19, 95%CI: 0.02‐1.62; P = .13) |
| Arentz (2020) 28 | Evergreen Hospital, Washington State, USA | 20 February 2020, to 5 March 2020 | 21 ICU patients | Low lymphocyte count (less than 1000 cells/μL) | Low lymphocyte count was noted in 14/21 (67%) of critically ill patients. |
| Bhatraju (2020) 29 | Seattle region, Washington State, USA | 24 February 2020, to 9 March 2020 | 24 ICU patients | Lymphocyte counts presented as a continuous variable; definition of lymphocytopenia was not provided | Lymphocytopenia was common (75% of patients), with a median lymphocyte count of 720 per mm3 (IQR: 520 to 1375). |
| Deng (2020) 30 | Wuhan, China | Tongji Hospital and Hankou branch of Central Hospital of Wuhan, China | 1 January 2020 to 21 February 2020 | Lymphocyte counts treated as a continuous variable (×109/L) | On admission, patients in the death group exhibited significantly lower lymphocyte count (median: 0.63, IQR: 0.40‐0.79) × 109/L vs 1.00, IQR: 0.72‐1.27 × 109/L, pP < .001). Patients in the death group also exhibited lower lymphocyte/WBC ratio (median: 7.10, IQR: 4.45, 12.73% vs 23.5, IQR: 15.27‐31.25%, P < .001). The lymphocyte/WBC ratio continued to decrease during hospitalization. |
| Tan (2020) 32 | General Hospital of Central Theater Command, Wuhan, China | Not reported | 90 patients at the validation cohort | Lymphocytes at two time points: day 10‐12 from symptom onset (>20% or < 20%) and day 17‐19 (>20%, 5‐20% and < 5%). | Lymphocytes <20% on day 10–12 signal a pre‐severe disease and lymphocytes <5% on day 17‐19 denote a critical illness. |
Abbreviations: ARDS, acute respiratory distress syndrome; IQR, interquartile range.
P‐values calculated by Terpos et al., on the basis of contingency tables (Pearson's chi‐square test) in articles that did not present formal statistical comparisons.
Guan et al. provided data on the clinical characteristics of 1099 COVID‐19 cases with laboratory confirmation during the first 2 months of the epidemic in China. 16 On admission, the vast majority of patients presented with lymphocytopenia (83.2%), whereas 36.2% had thrombocytopenia, and 33.7% showed leukopenia. These hematological abnormalities were more prominent among severe vs non‐severe cases (96.1% vs 80.4% for lymphocytopenia, 57.7% vs 31.6% for thrombocytopenia, and 61.1% vs 28.1% for leukopenia). These results were consistent in four other descriptive studies that were conducted during the same period in China and included 41, 99, 138 and 201 confirmed cases with COVID‐19, respectively. 17 , 18 , 19 , 20 Specifically, Huang et al., 17 and Wang et al. 19 highlighted an association between lymphopenia and need of ICU care, whereas Wu et al. 20 showed an association between lymphopenia and acute respiratory distress syndrome (ARDS) development. Specifically, Wu et al. retrospectively analyzed possible risk factors for developing ARDS and death among 201 patients with COVID‐19 pneumonia in Wuhan, China. 20 Increased risk of ARDS during the disease course was significantly associated with increased neutrophils (P < .001), decreased lymphocytes (P < .001) in a bivariate Cox regression analysis. Increased neutrophils (P = .03) were associated with increased risk of death. 20
Furthermore, lymphopenia and was also documented in approximately 40% of the first 18 hospitalized patients with COVID‐19 in Singapore. 21 A more recent report on 69 patients confirmed the percentage of those with lymphocytopenia, whereas 20% had mild thrombocytopenia. 22 Interestingly, 69% of patients with a low lymphocyte count showed a reactive lymphocyte population including a lymphoplasmacytoid subset, which was not common in the peripheral blood of patients with SARS infection in 2003. 22 , 23 , 24 Flow cytometry did not reveal any inversion in the CD4+/CD8+ lymphocyte ratio. 22 However, functional studies have suggested that SARS‐CoV‐2 may impair the function of CD4+ helper and regulatory T‐cells and promote the initial hyperactivation which is followed by rapid exhaustion of cytotoxic CD8+ T‐cells. 25 , 26
In Singapore, Fan et al. also found that patients requiring ICU support had significantly lower lymphocyte levels (P < .001) at baseline. 22 In another retrospective study including 52 critically ill patients from Wuhan, China, lymphopenia was reported in 85% of patients. 27
Lymphopenia was also prominent among critically ill patients with COVID‐19 in Washington, USA. 28 , 29 During hospitalization, non‐survivors demonstrated a more significant deterioration in lymphopenia compared with those who survived (P < .05). 19 It has also been reported that patients with severe disease and fatal outcomes present with a decreased lymphocyte/white blood cell ratio both in admission (P < .001) and during hospitalization (P < .001) compared with those who survived. 26 , 30 Contrary to non‐survivors, survivors demonstrated a nadir of lymphocytes counted on day 7 from symptom onset and a subsequent restoration. 31 Therefore, serial assessment of lymphocyte count dynamics may be predictive of patient outcome. Tan et al have proposed a model based on lymphocyte counts at two time points; patients with less than 20% lymphocytes at days 10‐12 from the onset of symptoms, and less than 5% at days 17‐19 have the worst prognosis. 32
Recent studies have shown that myocardial injury among inpatients with COVID‐19 is associated with increased mortality. 33 , 34 In a prospective study in Wuhan, China including 416 consecutive patients 82 (19.7%) had documented myocardial injury. Compared with the others, these patients with myocardial injury had higher leukocyte (P < .001), lower lymphocyte (P < .001) and lower platelet counts (P < .001). 33 A retrospective study including 187 patients with COVID‐19 from another hospital in Wuhan showed that patients with high troponin‐T levels had leukocytosis (P < .001), increased neutrophils (P < .001) and decreased lymphocytes (P = .01). 34
A meta‐analysis of nine studies has suggested that thrombocytopenia is significantly associated with the severity of the COVID‐19 disease, with very high between‐studies heterogeneity though; a more sizeable drop in platelet counts was noted especially in non‐survivors. 35 Table 2 shows the results of main studies examining platelet counts in COVID‐19 disease.
TABLE 2.
Studies and main findings for platelet count (and platelet to lymphocyte ratio) in Covid‐19 patients
| First author (year) | Region | Study period | Sample size | Categorization of hematological factors | Main findings |
|---|---|---|---|---|---|
| Guan (2020) 16 | 552 hospitals in 30 provinces, autonomous regions, and municipalities in mainland China | 11 December 2019 – 31 January 2020 | 1099 | Thrombocytopenia was defined as a platelet count of less than 150 000/mm3 | Thrombocytopenia was present in 36.2% of patients on admission. Also, 46.6% (27/58) of patients with the composite primary endpoint (admission to an intensive care unit, use of mechanical ventilation, or death) presented with thrombocytopenia vs 35.5% (288/811) of patients without the primary endpoint (P = .091 a ). Severe cases presented thrombocytopenia more frequently (57.7%, 90/156) vs non‐severe cases (31.6%, 225/713); P < .001 a |
| Huang (2020) 17 | Jinyintan Hospital (Wuhan, China) | 16 December 2019, to 2 January 2020 | 41 | Low platelet count of <100 x109 platelets per Liter | 8% (1/13) of patients needing ICU care presented low platelet count vs 4% (1/27) of patients that did not need ICU care (P = .45). |
| Wang (2020) 19 | Zhongnan Hospital, Wuhan, China | 1 January to 3 February 2020 | 138 | Platelets treated as a continuous variable, x109 per L | No significant difference (P = .78) was noted in platelet count between ICU cases (median:142, IQR: 119‐202) vs non‐ICU cases (median: 165, IQR: 125‐188); P = .78. |
| Wu (2020) 20 | Jinyintan Hospital, Wuhan, China | 25 December 2019, to 13 February 2020 | 201 | Platelets treated as a continuous variable, x109 /mL | Platelet counts did not differ between patients with ARDS vs those without ARDS (difference: −4.00, 95%CI: −27.00 to +20.00, P = .73). Accordingly, no significant difference was noted in dead vs alive ARDS patients (P = .10). |
| Young (2020) 21 | 4 hospitals in Singapore | 23 January to 3 February 2020 | 18 | Platelets treated as a continuous variable, x109 per L | Median platelet count was 156 (IQR: 116‐217) in patients that required supplemental O2 and 159 (IQR: 128‐213) in those that did not; no statistical comparison was undertaken. |
| Fan (2020) 22 | National Centre for Infectious Diseases, Singapore | 23 January to 28 February 2020 | 69 | Low platelet count: platelet of <100 × 109/L. | Low platelets were not associated with ICU care either at admission (P = .67) or as a nadir during hospital stay (P = .69) |
| Yang (2020) 27 | Jinyintan Hospital, Wuhan, China | 24 December 2019, to 9 February 2020 | 52 critically ill patients | Platelets treated as a continuous variable (×109/L) | Platelet count noted in non‐survivors was 191 (63) and 164 (74) in survivors; no statistical tests were presented. |
| Arentz (2020) 28 | Evergreen Hospital, Washington State, USA | 20 February 2020, to 5 March 2020 | 21 ICU patients | Platelets presented as a continuous variable (×109/L) | Mean baseline platelet count was 235 (ranging between 52 and 395), whereas the reference range was 182‐369 × 109/L |
| Bhatraju (2020) 29 | Seattle region, Washington State, USA | 24 February 2020, to 9 March 2020 | 24 ICU patients | Platelet counts presented as a continuous variable (cells per mm3) | Median of lowest platelet count was 180 000 (IQR: 109000‐257 000) |
| Zhou (2020) 31 | Jinyintan Hospital and Wuhan Pulmonary Hospital, Wuhan, China | 25 December 2019, to 31 January 2020 | 191 | Platelets treated as a continuous variable (×109/L) | Median platelet count was lower in non‐survivors (165.5, IQR: 107.0‐229.0) vs survivors (220.0, IQR: 168.0‐271.0), P < .001 |
| Lippi (2020) 35 | Meta‐analysis of published studies | Studies published up to March 6, 2020 | 9 published studies | Platelets treated as a continuous variable | Platelet count was significantly lower in patients with more severe COVID‐19 (WMD −31 × 109/L, 95% CI, −35 to −29 × 109/L), with very high heterogeneity (I2 = 92%). A more substantial drop in platelets was observed in non‐survivors |
| Qu (2020) 36 | Huizhou Municipal Central Hospital, China | January 2020 to February 2020 | 30 | Platelet to lymphocyte ratio (PLR) | PLR at peak of platelets was associated with severe cases (mean ± SD: 626.27 ± 523.64 vs 262.35 ± 97.78 in non‐severe cases, P = .001). Higher PLR of patients during treatment was also associated with longer hospitalization, on average. |
Abbreviations: ARDS, acute respiratory distress syndrome; IQR, interquartile range.
P‐values calculated by Terpos et al., on the basis of contingency tables (Pearson's chi‐square test) in articles that did not present formal statistical comparisons.
Interestingly, Qu et al showed that among 30 hospitalized patients with COVID‐19, those presenting with a peak in the platelet count during the disease course had worse outcomes. 36 Interestingly, the platelet to lymphocyte ratio at the time of platelet peak emerged as an independent prognostic factor for prolonged hospitalization in the multivariate analysis. It was suggested that a high platelet to lymphocyte ratio may indicate a more pronounced cytokine storm, due to enhanced platelet activation. 36
2.2. The emerging role of biomarkers procalcitonin, ferritin and C‐reactive protein in the prognosis
Table 3 summarizes the results of studies on CRP, procalcitonin and ferritin in COVID‐19 disease. In the study by Guan et al., 16 presenting results from various provinces in China, interesting biochemical findings were described; C‐reactive protein (CRP) was elevated in 60.7% of patients. Elevated procalcitonin. Which may also be suggestive of a secondary bacterial infection complicating the clinical course of COVID‐19 disease, was found in 5.5%, and elevated lactate dehydrogenase (LDH) in 41% of patients. More severe cases showed a more marked increase compared with the non‐severe ones (81.5% vs 56.4% for CRP, 13.7% vs 3.7% for procalcitonin and 58.1% vs 37.2% for LDH). 16
TABLE 3.
Studies and main findings for biomarkers related to inflammation (CRP, ferritin, procalcitonin) in Covid‐19 patients
| Studied parameters | First author (year) | Region | Study period | Sample size | Categorization of hematological factors | Main findings |
|---|---|---|---|---|---|---|
| CRP | ||||||
| Guan (2020) 16 |
552 hospitals in 30 provinces, autonomous regions, and municipalities in mainland China |
11 December 2019 – 31 January 2020 | 1099 | Elevated CRP ≥10 mg/Liter | Disease severity was associated with elevated CRP; 81.5% (110/135) of severe cases vs 56.4% (371/658) of non‐severe cases presented with elevated CRP (P < .001a). The primary composite endpoint (admission to an intensive care unit, use of mechanical ventilation, or death) was also associated with elevated CRP (41/45, 91.1% vs 440/748, 58.8%, P < .001a). | |
| Wu (2020) 20 |
Jinyintan Hospital, Wuhan, China |
25 December 2019, to 13 February 2020 | 201 | hs‐CRP >5 vs ≤5 mg/L in a bivariate Cox regression model | Higher hs‐CRP was associated with ARDS development (HR = 4.81, 95%CI: 1.52‐15.27, P = .008). | |
| Young (2020) 21 |
Four hospitals in Singapore |
23 January to 3 February 2020 | 18 | CRP treated as a continuous variable, mg/L | Median CRP level was 65.6 (IQR: 47.5‐97.5) in patients that required supplemental O2 and 11.1 (IQR: 0.9‐19.1) in those that did not; no statistical comparison was undertaken. | |
| Deng (2020) 30 | Wuhan, China | 1 January 2020 to 21 February 2020 |
225 |
CRP treated as a continuous variable mg/L) | On admission, patients in the death group exhibited significantly higher CRP level (median: 109.25, IQR: 35.00‐170.28 mg/L vs median: 3.22 IQR: 1.04, 21.80 mg/L, P<.001). CRP levels remained high after treatment in the non‐survivors. | |
| Ferritin | ||||||
| Wu (2020) 20 |
Jinyintan Hospital, Wuhan, China |
25 December 2019, to 13 February 2020 | 201 | Serum ferritin >300 vss ≤300 ng/mL in a bivariate Cox regression model | Higher serum ferritin was associated with ARDS development (HR = 3.53, 95%CI: 1.52‐8.16, P = .003); the trend of an association with survival did not reach significance (HR = 5.28, 95%CI: 0.72‐38.48, P = .10). | |
| Zhou (2020) 31 |
Jinyintan Hospital and Wuhan Pulmonary Hospital, Wuhan, China |
25 December 2019, to 31 January 2020 | 191 | Serum ferritin >300 vs ≤300 ng/mL in a multivariate logistic regression model | Higher serum ferritin levels were associated with higher odds of death at the univariate analysis (OR = 9.10, 95%CI: 2.04‐40.58; P = .004); a multivariate analysis was not presented. | |
| Procalcitonin | ||||||
| Guan (2020) 16 |
552 hospitals in 30 provinces, autonomous regions, and municipalities in mainland China |
11 December 2019 – 31 January 2020 | 1099 | Elevated procalcitonin ≥0.5 ng/mL | Disease severity was associated with elevated procalcitonin; 13.7% (16/117) of severe cases vs 3.7% (19/516) of non‐severe cases presented with elevated procalcitonin (P < .001a). The primary composite endpoint (admission to an intensive care unit, use of mechanical ventilation, or death) was also associated with elevated procalcitonin (12/50, 24.0% vs 23/583, 3.9%, P < .001a). | |
| Huang (2020) 17 |
Jinyintan Hospital (Wuhan, China) |
16 December 2019, to 2 January 2020 | 41 | Elevated procalcitonin ≥0.5 ng/mL | 3/12 (25%) patients necessitating ICU care presented with elevated procalcitonin levels vs 0/27 non‐ICU patients. Overall, procalcitonin levels was higher in ICU vs non‐ICU patients (P = .031). | |
| Wang (2020) 19 |
Zhongnan Hospital, Wuhan, China |
1 January to 3 February 2020 | 138 | Elevated procalcitonin ≥0.05 ng/mL | 75% (27/36) of ICU patients presented with high procalcitonin vs 21.6% (22/102) of non‐ICU patients (P < .001). | |
| Zhou (2020) 31 |
Jinyintan Hospital and Wuhan Pulmonary Hospital, Wuhan, China |
25 December 2019, to 31 January 2020 | 191 | Procalcitonin treated as continuous variable (in ng/mL) in a multivariate logistic regression model | Higher serum procalcitonin levels were associated with higher odds of death at the univariate analysis (OR = 13.75, 95%CI: 1.81‐104.40; P = .011); a multivariate analysis was not presented. | |
| Arentz (2020) 28 | Evergreen Hospital, Washington State, USA | 20 February 2020, to 5 March 2020 | 21 ICU patients | Procalcitonin presented as a continuous variable (ng/mL) | Mean baseline procalcitonin was 1.8 (ranging between 0.12‐9.56 ng/mL), whereas the reference range was 0.15‐2.0 ng/mL | |
| Lippi (2020) 35 | Meta‐analysis of published studies | Studies published up to 3 March 2020 | 4 published articles | The definition of increased procalcitonin during the synthesis of studies was not declared. |
Increased procalcitonin values were associated with a nearly 5‐fold higher risk of severe infection (OR = 4.76; 95% CI: 2.74‐8.29, I2 = 34%) |
Abbreviations: ARDS, acute respiratory distress syndrome; IQR, interquartile range.
P values calculated by Terpos et al., on the basis of contingency tables (Pearson's chi‐square test) in articles that did not present formal statistical comparisons.
Accordingly in a retrospective cohort study including 191 patients with COVID‐19 from Wuhan, China, non‐survivors, as compared with survivors, presented more often with high LDH (P < .001), high procalcitonin (P < .001), increased serum ferritin levels (P < .001) and elevated IL‐6 (P < .001). 31 In the study by Wang et al., 40% of patients presented with elevated LDH. 19 Increased LDH has been associated also with higher risk of ARDS, 20 ICU support 22 and death 20 , 31 across published studies.
Higher CRP has been linked to unfavorable aspects of COVID‐19 disease, such as ARDS development, 20 higher troponin‐T levels and myocardial injury, 33 and death. 30 A meta‐analysis of four published studies showed that increased procalcitonin values were associated with a nearly 5‐fold higher risk of severe infection (OR = 4.76; 95% CI: 2.74‐8.29, I2 = 34%). 37
Regarding ferritin, Wu et al. showed that higher serum ferritin was associated with ARDS development (HR = 3.53, 95% CI: 1.52‐8.16, P = .003); the trend of an association with survival did not reach significance (HR = 5.28, 95%CI: 0.72‐38.48, P = .10). 20 At their univariate analysis, Zhou et al. supported an association between higher serum ferritin levels and death, but no multivariate analysis was presented. 31
Another emerging biomarker for COVID‐19 course is interleukin‐6 (IL‐6). In the study by Chen et al. 52% (51/99) of patients had elevated IL‐6 levels at admission. 18 Increased IL‐6 levels have been associated with increased risk of death, 20 and a gradual increase during hospitalization has been reported in non‐survivors. 31
2.3. Coagulation complications
Coagulation disorders are relatively frequently encountered among COVID‐19 patients, especially among those with severe disease. 30 , 31 In a multicenter retrospective study during the first 2 months of the epidemic in China, 260 out of 560 patients (46.4%) with laboratory confirmed COVID‐19 infection had elevated D‐dimer (≥0.5 mg/L). whereas And, the elevation was more pronounced among severe cases (59.6% vs 43.2% for non‐severe ones). 16 The D‐dimer dynamics can reflect the severity and their increased levels are associated with adverse outcomes among patients with community‐acquired pneumonia. 38 Accordingly, elevated D‐dimer (> 1.5 μg/L) was detected in 36% of patients in a descriptive study of 99 COVID‐19 cases in Wuhan, China. 18 Another retrospective study in China including 41 patients showed that D‐dimer and prothrombin time (PT) levels were higher on admission among patients requiring ICU support (median D‐dimer 2.4 mg/L for ICU vs 0.5 mg/L for non‐ICU, P = .004; median PT 12.2 seconds for ICU vs 10.7 seconds, P = .012). 17 In the study by Wang et al, which was previously described, patients requiring ICU treatment had significantly higher D‐dimers (P < .001) compared with less severe cases. 19
Patients presenting with cardiac injury in the context of COVID‐19 infection are more prone to coagulation disorders compared with those without cardiac involvement (P = .02). 33 Patients with high troponin‐T levels may present more frequently with elevated PT (P = .005), activated partial thromboplastin time (APTT) (P = .003), and D‐dimer (P < .001). 34 AMong 201 patients with COVID‐19 pneumonia, increased PT was associated with increased risk of ARDS (P < .001), whereas increased levels of D‐dimer were significantly associated with increased risk of ARDS (P < .001) and death (P = .002). 20 The difference in median levels of D‐dimer between survivors and non‐survivors was larger than that between the ARDS and non‐ARDS groups, which might suggest that DIC‐related complications may have led a subset of patients to death independently of ARDS. In a multicenter retrospective cohort study from China, increased D‐dimer levels (>1 μg/mL) were significantly associated with in‐hospital death in the multivariable analysis (P = .003). 31 Interestingly, The D‐dimer levels showed a sequential increase in time among non‐survivors compared with those who survived (P < .05). 19 , 31 In another retrospective study by Tang et al, encompassing data from 183 consecutive patients with COVID‐19, non‐survivors had significantly higher D‐dimer (P < .05), fibrin degradation products (FDP) levels (P < .05), and prolonged PT (P < .05) and APTT (P < .05) compared with survivors at initial evaluation. By the late hospitalization, the fibrinogen and AT levels were also significantly lower in nonsurvivors. 6 Interestingly, 71.4% of non‐survivors vs 0.6% of survivors fulfilled the clinical criteria for disseminated intravascular coagulation (DIC) during the disease course. The median time from admission to DIC manifestation was 4 days (range: 1‐12 days). 6 In a prospective study evaluating the coagulation profile of patients with COVID‐19; D‐dimer, FDP, and fibrinogen levels were markedly higher among patients compared with healthy controls (P < .001 for all three comparisons). Patients with severe disease showed higher values of D‐dimer and FDP than those with milder manifestation (P < .05 for both comparisons). 39
All the above indicate that D‐dimer elevation and DIC may be common in patients with severe form of COVID‐19 infection, a fact that, despite methodological limitations, became evident in a meta‐analysis of four published studies (Table 4). 40 Immune deregulation and endothelial dysfunction may be actively implicated in the underlying pathophysiology, 41 which remains to be elucidated in future studies.
TABLE 4.
Studies and main findings for D‐dimer in Covid‐19 patients
| First author (year) | Region | Study period | Sample size | Categorization of hematological factors | Main findings |
|---|---|---|---|---|---|
| Guan (2020) 16 |
552 hospitals in 30 provinces, autonomous regions, and municipalities in mainland China |
11 December 2019 – 31 January 2020 | 1099 | Elevated D‐dimer: ≥0.5 mg/L | Patients with the composite primary endpoint (admission to an intensive care unit, use of mechanical ventilation, or death) presented with elevated D‐dimer more frequently: 69.4% (34/49) vs 44.2% (226/511; P = .001 a ). Accordingly, severe cases presented elevated D‐dimer more frequently (59.6%, 65/109) vs non‐severe cases (43.2%, 195/451); P = .002 a |
| Huang (2020) 17 |
Jin Yintan Hospital (Wuhan, China) |
16 December 2019, to 2 January 2020 | 41 | D‐dimer treated as a continuous variable, in mg/L | Patients necessitating ICU care presented with higher D‐dimer levels (median: 2.4; IQR: 0.6‐14.4) vs non‐ICU patients (median: 0.5, IQR: 0.3‐0.8), P = .0042. |
| Wang (2020) 19 |
Zhongnan Hospital, Wuhan, China |
1 January to 3 February 2020 | 138 | D‐dimer treated as a continuous variable, in mg/L | ICU cases presented with higher D‐dimer level (median:414, IQR: 191‐1324) vs non‐ICU cases (median: 166, IQR: 101‐285); P < .001. Longitudinal increase was noted in non‐survivors. |
| Wu (2020) 20 |
Jinyintan Hospital, Wuhan, China |
25 December 2019, to 13 February 2020 | 201 | D‐dimer treated as a continuous variable (μg/mL) in a bivariate Cox regression model | Higher D‐dimer level was associated with ARDS development (HR = 1.03, 95%CI: 1.01‐1.04, P < .001) and poor survival (HR = 1.02, 95%CI: 1.01‐1.04, P = .002) in the incremental models. |
| Zhou (2020) 31 |
Jinyintan Hospital and Wuhan Pulmonary Hospital, Wuhan, China |
25 December 2019, to 31 January 2020 | 191 | D‐dimer greater than 1 μg/mL in a multivariate logistic regression model | Higher D‐dimer was associated with higher odds of death (OR = 18.42, 95%CI: 2.64‐128.55; P = .003) |
| Lippi (2020) 40 | Meta‐analysis of published studies | Studies published up to 4 March 2020 | 553 (4 published studies) | D‐dimer treated as a continuous variable; the definition of COVID‐19 disease Severity was not provided during the synthesis of studies |
D‐dimer values were considerably higher in COVID‐19 patients with severe disease than in those without (WMD = 2.97 mg/L; 95% CI: 2.47‐3.46 mg/L). However, heterogeneity across synthesized studies was very high (I2 = 94%). |
Abbreviations: ARDS, acute respiratory distress syndrome; IQR, interquartile range; WMD: weighted mean difference.
P‐values calculated by Terpos et al., on the basis of contingency tables (Pearson's chi‐square test) i articles that did not present formal statistical comparisons.
The scoring system for compensated and overt DIC endorsed by the International Society on Thrombosis and Hemostasis should be followed for early DIC identification. 42 A proposed treatment algorithm for managing patients with COVID‐19 and DIC is shown in Figure 1.
FIGURE 1.
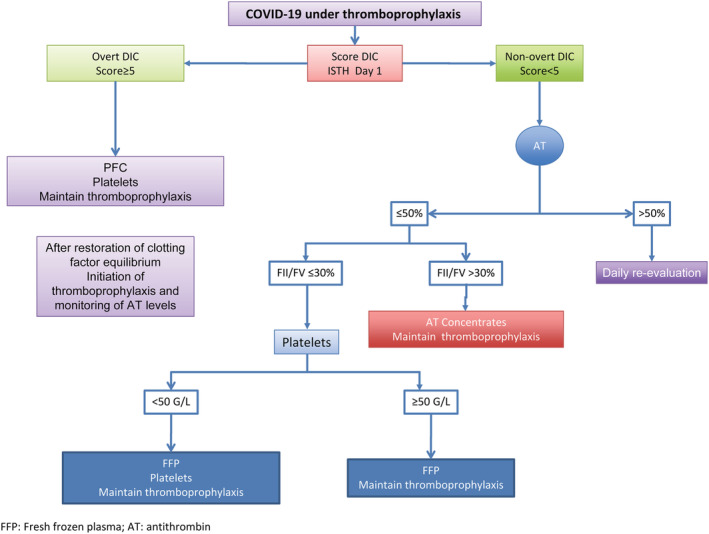
A proposed treatment algorithm for managing patients with COVID‐19 and DIC [Color figure can be viewed at wileyonlinelibrary.com]
The venous thromboembolism (VTE) risk in hospitalized COVID‐19 patients is an emerging issue. The rate of symptomatic VTE in acutely ill hospitalized medical patients gets as high as 10%. 43 Prolonged immobilization during illness, dehydration, an acute inflammatory state, presence of other cardiovascular risk factors (ie, hypertension, diabetes, obesity) or cardiovascular disease (ie, coronary artery disease, history of ischemic stroke or peripheral artery disease), previous history of VTE and classical genetic thrombophilia, such as heterozygous Factor V Leiden mutation are common comorbidities in hospitalized COVID‐19 patients, which potentially increase VTE risk. The possibility of endothelial cell activation/damage due to the virus binding to ACE2 receptor may further increase VTE risk. The release of a large amount of inflammatory mediators and the application of hormones and immunoglobulins in severe or critically ill patients may lead to an increased blood viscosity. Furthermore, mechanical ventilation, central venous catheterization, and surgery may induce vascular endothelial damage. The combination of all the above factors may lead to DVT occurrence or even the possibility of lethal PE due to thrombus migration. Thus, facing such VTE risk, the application of pharmacological thromboprophylaxis is mandatory in hospitalized COVID‐19 patients. In this context, VTE risk increase must be assessed in all acutely ill patients admitted to hospital, and thromboprophylaxis should be given to all these high‐risk patients according to current clinical practice guidelines. 44
The use of Risk Assessment Models (RAM) such as IMPROVE‐VTE in internal medicine department may be helpful. The modified IMPROVE‐VTE RAM which includes the D‐Dimers levels together with other clinical predictors of VTE enhances the precision of that score for the identification of high VTE risk patients eligible for an adapted pharmacological thromboprophylaxis. 45 Moreover, it is also important to pay attention to VTE risk in asymptomatic or ambulatory patients with mild COVID‐19 infection. Early diagnosis of PE in COVID‐19 patients with clinical manifestations of sudden deterioration of oxygenation, respiratory distress, or hypotension is of major importance for the improvement of the clinical outcomes. Although the published data are very limited, it seems reasonable that D‐dimer evaluation as well as the kinetics of their increase could offer a useful information for the research of DVT and/or PE, along with the recommended imaging techniques such as ultrasound venous echo‐doppler or bedside echocardiography. A recent small study in 25 PE suspected patients explored with Computed Tomography Pulmonary Angiography (CTPA), showed that those with confirmed PE (n = 10) had D‐dimer levels higher than 7000 ng/mL, compared to those without PE with significantly lower D‐dimer levels. 46
Low molecular weight heparins (LMWH), or unfractionated heparin (UFH) should be preferred over direct oral anticoagulants (DOACs) due to possible drug‐drug interactions with concomitant antiviral (especially anti‐HIV protease inhibitors such as ritonavir) and antibacterial (such as azithromycin) treatment. 47 Such treatments interfering with CYP3A4 and/or P‐gp pathways may increase the bleeding risk or reduce the antithrombotic effect in case of DOAC use.
In a retrospective Chinese study, including 449 severe COVID‐19 patients in Wuhan, LMWH administration among patients with markedly elevated D‐dimers or in those meeting the criteria for sepsis‐induced DIC was significantly associated with improved 28‐day overall survival, P = .017 and P = .029 for the two patient groups, users vs non‐users, respectively. 48 Furthermore, clinicians should routinely evaluate all COVID‐19 patients under heparin treatment for indices of heparin‐induced thrombocytopenia (HIT) syndrome. This is by performing the 4 T score (thrombocytopenia, timing of platelet count fall, thrombosis or other sequalae, other causes for thrombocytopenia). Although HIT incidence in this patient group has not been determined yet, there is potentially an increased risk due to the immune deregulation and the massive inflammatory syndrome induced by the viral infection, with significant neutrophil extracellular traps (NETS) and platelet factor 4 (PF4) release.
In summary, there are four important aspects in the management of COVID‐patients: (i) early diagnosis and follow‐up of DIC, by applying the ISTH score (platelet count, PT, fibrinogen, D‐dimer, antithrombin and protein C activity monitoring) which can determine prognosis and guide more appropriate critical care support); (ii) identification of patients at high risk whatever hospitalized or ambulatory; (iii) optimization of thromboprophylaxis regimen and LMWH are the first line choice drug, and (iv) the anti‐inflammatory properties of LMWH may be an added benefit in COVID 19 patients and the possible need of integrating other antithrombotic treatments such as antithrombin, and recombinant thrombomodulin that may be also helpful in this complex “immunothrombosis” process.
2.4. Blood and hematopoietic stem cell donation
Last but not least, it is of outmost importance to consider the impact of the COVID‐19 pandemic on the availability of blood products. Worldwide, insecurity and anxiety of being infected by SARS‐CoV‐2 along with measures of social isolation largely prevent blood donations. 49 Similarly, the pandemic crisis may hinder the donation of hematopoietic stem cells, as well. 50 However, the need for stem cell donors will remain constant and the need for blood donor will probably increase in order to support the critically ill COVID‐19 patients. Therefore, the authorities should reformulate the infrastructure and apply all necessary safety precautions to prevent viral transmission. Effective public awareness campaigns on the importance of maintaining an adequate national blood supply, need for blood donors, and safety of the donation process should be disseminated continuously. 51 Furthermore, they should inform and educate the younger about the priceless role of blood donation and the life‐saving value of hematopoietic stem cell donation. All of us should become aware of our collective responsibility and contribute to the well‐being of the members of our society.
3. CONCLUSIONS
In conclusion, COVID‐19 disease has prominent manifestations from the hematopoietic system and is often associated with a major blood hypercoagulability. Careful evaluation of laboratory indices at baseline and during the disease course can assist clinicians in formulating a tailored treatment approach and promptly provide intensive care to those who are in greater need. Preventive measures for thromboprophylaxis and early identification of potentially lethal complications including DIC in order to effectively intervene will improve patient outcomes, and will probably reduce the death rate overall and among infected patients without significant comorbidities. Continuous vigilance is necessary and urgent studies have to be planned to define whether optimal anticoagulation regimen with or without adjunctive antithrombotic therapies (eg, LMWH, antithrombin or thrombomodulin) may be helpful in patients with COVID‐19.
CONFLICT OF INTEREST
No relevant conflict of interest to declare.
Terpos E, Ntanasis‐Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID‐19. Am J Hematol. 2020;95:834–847. 10.1002/ajh.25829
REFERENCES
- 1. Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Driggin E, Madhavan MV, Bikdeli B, et al. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID‐19) pandemic. J Am Coll Cardiol. 2020;75(18):2352‐2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bangash MN, Patel J, Parekh D. COVID‐19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5(5):428‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mehta P, McAuley DF, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72314 cases from the chinese center for disease control and prevention. JAMA. 2020;323:1239. [DOI] [PubMed] [Google Scholar]
- 6. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;4:844‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Madjid M, Safavi‐Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020. 10.1001/jamacardio.2020.1286. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 8. Li T, Lu H, Zhang W. Clinical observation and management of COVID‐19 patients. Emerg Microbes Infect. 2020;1:687‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019‐nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Singh S, Sharma A, Arora SK. High producer haplotype (CAG) of ‐863C/A, −308G/A and ‐238G/A polymorphisms in the promoter region of TNF‐alpha gene associate with enhanced apoptosis of lymphocytes in HIV‐1 subtype C infected individuals from North India. PLoS One. 2014;9(5):e98020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liao YC, Liang WG, Chen FW, Hsu JH, Yang JJ, Chang MS. IL‐19 induces production of IL‐6 and TNF‐alpha and results in cell apoptosis through TNF‐alpha. J Immunol. 2002. Oct 15;169(8):4288‐4297. [DOI] [PubMed] [Google Scholar]
- 12. Aggarwal S, Gollapudi S, Gupta S. Increased TNF‐alpha‐induced apoptosis in lymphocytes from aged humans: changes in TNF‐alpha receptor expression and activation of caspases. J Immunol. 1999;162(4):2154‐2161. [PubMed] [Google Scholar]
- 13. Chan JF, Zhang AJ, Yuan S, et al. Simulation of the clinical and pathological manifestations of Coronavirus Disease 2019 (COVID‐19) in golden Syrian hamster model: implications for disease pathogenesis and transmissibility. Clin Infect Dis. 2020. 10.1093/cid/ciaa325. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. You B, Ravaud A, Canivet A, et al. The official French guidelines to protect patients with cancer against SARS‐CoV‐2 infection. Lancet Oncol. 2020;21(5):619‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fischer K, Hoffmann P, Voelkl S, et al. Inhibitory effect of tumor cell‐derived lactic acid on human T cells. Blood. 2007;109(9):3812‐3819. [DOI] [PubMed] [Google Scholar]
- 16. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061‐1069. 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020. 10.1001/jamainternmed.2020.0994. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic Features and Clinical Course of Patients Infected With SARS‐CoV‐2 in Singapore. JAMA. 2020;323(15):1488‐1494. https://doi.org10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fan BE, Chong VCL, Chan SSW, et al. Hematologic parameters in patients with COVID‐19 infection. Am J Hematol. 2020;95(6):E131‐E134. [DOI] [PubMed] [Google Scholar]
- 23. Chng WJ, Lai HC, Earnest A, Kuperan P. Haematological parameters in severe acute respiratory syndrome. Clin Lab Haematol. 2005;27(1):15‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee N, Hui D, Wu A, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348(20):1986‐1994. [DOI] [PubMed] [Google Scholar]
- 25. Zheng HY, Zhang M, Yang CX, et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID‐19 patients. Cell Mol Immunol. 2020;17:541‐543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID‐19 in Wuhan, China. Clin Infect Dis. 2020;ciaa248. 10.1093/cid/ciaa248. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID‐19 in Washington State. JAMA. 2020;323(16):1612‐1614. 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid‐19 in critically ill patients in the seattle region ‐ case series. N Engl J Med. 2020;382:2012‐2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Deng Y, Liu W, Liu K, et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID‐19) in Wuhan, China: a retrospective study. Chin Med J (Engl). 2020;133(11):1261‐1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020. 28; 395(10229):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tan L, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID‐19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol. 2020;e200950. 10.1001/jamacardio.2020.0950. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guo T, Fan Y, Chen M, et al. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID‐19). JAMA Cardiol. 2020;e201017. 10.1001/jamacardio.2020.1017. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID‐19) infections: a meta‐analysis. Clin Chim Acta. 2020;506:145‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Qu R, Ling Y, Zhang YH, et al. Platelet‐to‐lymphocyte ratio is associated with prognosis in patients with coronavirus disease‐19. J Med Virol. 2020. 10.1002/jmv.25767. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID‐19): a meta‐analysis. Clin Chim Acta. 2020;505:190‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Snijders D, Schoorl M, Schoorl M, Bartels PC, van der Werf TS, Boersma WG. D‐dimer levels in assessing severity and clinical outcome in patients with community‐acquired pneumonia. A secondary analysis of a randomised clinical trial. Eur J Intern Med. 2012;23(5):436‐441. [DOI] [PubMed] [Google Scholar]
- 39. Han H, Yang L, Liu R, et al. Prominent changes in blood coagulation of patients with SARS‐CoV‐2 infection. Clin Chem Lab Med. 2020. 10.1515/cclm-2020-0188. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 40. Lippi G, Favaloro EJ. D‐dimer is associated with severity of coronavirus disease 2019: a pooled analysis. Thromb Haemost. 2020;120(05):876‐878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lillicrap D. Disseminated intravascular coagulation in patients with 2019‐nCoV pneumonia. J Thromb Haemost. 2020;4:786‐787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wada H, Thachil J, Di Nisio M, et al. Guidance for diagnosis and treatment of DIC from harmonization of the recommendations from three guidelines. J Thromb Haemost. 2013;11:761‐767. [DOI] [PubMed] [Google Scholar]
- 43. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(suppl 4):e195S‐e226S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Witt DM, Nieuwlaat R, Clark NP, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018;2(22):3257‐3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Spyropoulos AC, Lipardi C, Xu J, et al. Modified IMPROVE VTE risk score and elevated D‐Dimer identify a high venous thromboembolism risk in acutely ill medical population for extended thromboprophylaxis. TH Open. 2020;4(1):e59‐e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen J, Wang X, Zhang S, et al. Findings of acute pulmonary embolism in COVID‐19 patients (in review). Lancet Infect Dis. 2020.. [Google Scholar]
- 47. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID‐19. J Thromb Haemost. 2020. 10.1111/jth.14860. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094‐1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pagano MB, Hess JR, Tsang HC, et al. Prepare to adapt: blood supply and transfusion support during the first 2 weeks of the 2019 Novel Coronavirus (COVID‐19) pandemic affecting Washington State. Transfusion. 2020;60(5):908‐911. [DOI] [PubMed] [Google Scholar]
- 50. Szer J, Weisdorf D, Querol S, Foeken L, Madrigal A. The impact of COVID‐19 on the provision of donor hematopoietic stem cell products worldwide: collateral damage. Bone Marrow Transplant. 2020. 10.1038/s41409-020-0873-x. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. WHO . Maintaining a safe and adequate blood supply during the pandemic outbreak of coronavirus disease (COVID‐19). WHO reference number: WHO/2019‐nCoV/BloodSupply/2020.1. World Health Organization 2020.


