To the Editor:
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an ongoing public health emergency of international concern. Acute respiratory distress syndrome (ARDS) develops in 3%-30% of patients with COVID-19,1 , 2 because of direct virus-induced cytopathic effects in the respiratory tract or cytokine storms triggered by the host’s immune response. Comorbidities are known to increase the risk of ARDS in SARS-CoV-2-infected patients.1
Belatacept—a CTLA4-Ig molecule designed to block the costimulatory B7-CD28 signal needed for activation of effector T cells—is used in kidney transplant recipients (KTRs) to maintain effective long-term immunosuppression while minimizing calcineurin inhibitors (CNIs)-induced nephrotoxicity. However, a recent report has shown that the use of this drug may be associated with severe opportunistic infections.3
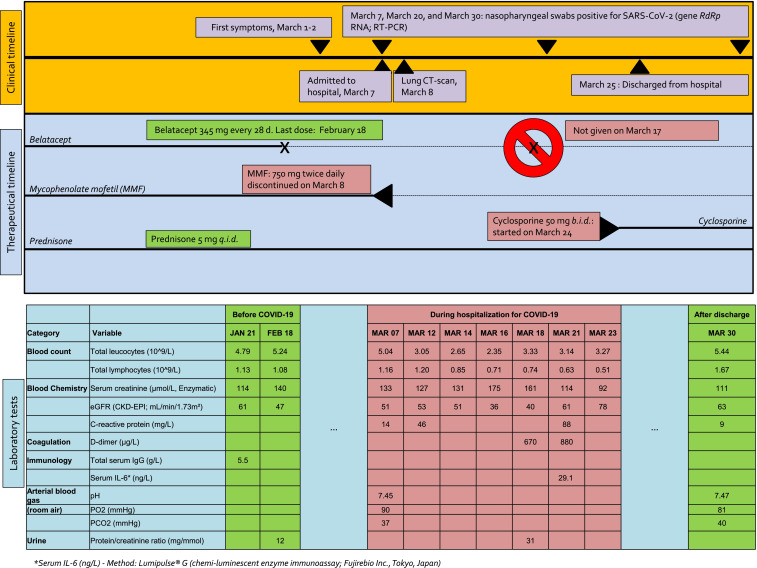
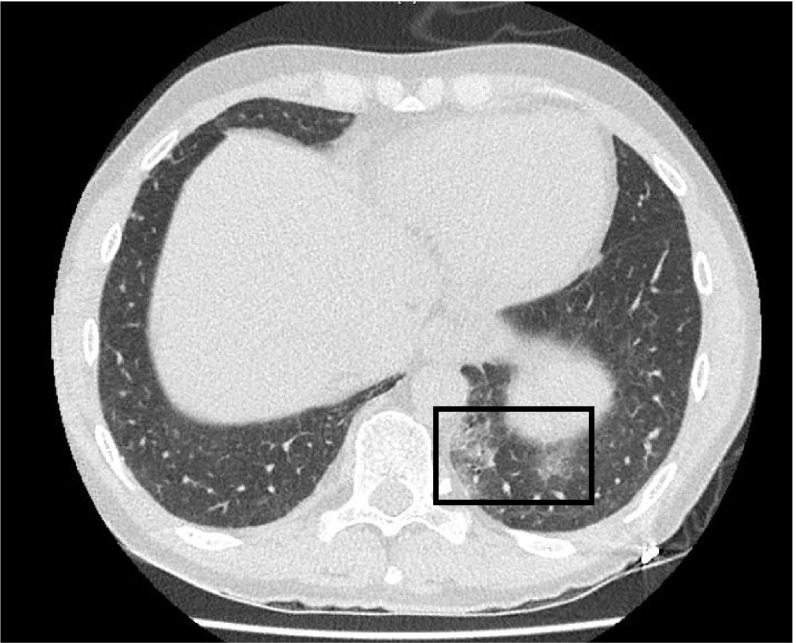
Here, we describe the first case of COVID-19 in a KTR treated with belatacept. The 58-year-old male patient—with a history of cured testicular cancer followed by renal vein thrombosis and coronary artery disease—had undergone kidney transplantation in January 2017. Because of nephrotoxicity, he was switched from a cyclosporine (CNI) to belatacept in June 2017. His maintenance immunosuppression consisted of belatacept (345 mg every 28 days), mycophenolate mofetil (MMF, 750 mg twice daily), and prednisone (5 mg/d). The last dose of belatacept was administered on February 18, 2020. The patient was referred to our center 3 weeks later (March 7, 2020) with fever (38°C), mild dyspnea, and cough 1 week after being in contact with a carrier of SARS-CoV-2 in Alsace, France. Figure 1 shows a timeline of immunosuppression management as well as the patient’s clinical course and laboratory data. Belatacept and MMF were discontinued on the day of admission. SARS-CoV-2 infection was diagnosed by RT-PCR amplification of the RdRp viral gene from a nasopharyngeal swab specimen. On admission, blood oxygen saturation on room air was 96% and remained stable during the hospitalization period. Limited peripheral pulmonary ground-glass opacities were identified on chest CT ( Figure 2). Serum IL-6 levels measured with a chemiluminescent enzyme assay were 29 ng/L, that is, markedly lower than those observed in COVID-19 patients requiring mechanical ventilation (consistently >100 ng/L; unpublished data, Dr Thomas Lavaux). The patient had a mild clinical course according to the World Health Organization interim guidance for COVID-194 with rapid recovery of respiratory symptoms after treatment of a possible bacterial superinfection. Low-dose cyclosporine (50 mg twice daily) was started on March 25. Five days after discharge, fever, cough, or breathing difficulties were absent. The results of laboratory tests were in line with those observed before SARS-CoV-2 infection (Figure 1). We are planning to withdraw cyclosporine and restart both belatacept and MMF on April 14, that is, on the date of the next scheduled belatacept infusion.
FIGURE 1.
Timeline of immunosuppression management, patient symptoms, and laboratory findings before COVID-19 infection, at time of hospitalization, and after discharge [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 2.
Lung CT scan performed 7 d after symptom onset. Evidence of minor, localized ground-glass opacities
Altogether, severe manifestations of COVID-19 due to lymphopenia-associated immunosuppression could have been feared but did not occur in this patient. ARDS caused by coronaviruses (SARS-CoV, MERS-CoV, SARS-CoV-2) is characterized by a cytokine storm, with secretion of numerous proinflammatory cytokines.5 , 6 We hypothesize that the mild clinical course of COVID-19 observed in our immunocompromised patient may have been, at least in part, related to belatacept-related blockade of massive cytokines/chemokines production. Additional clinical investigation in larger sample sizes and longer follow-up periods are required to corroborate this possibility.
Acknowledgments
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. [published online ahead of print March 11, 2020] N Engl J Med. 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed]
- 2.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. [published online ahead of print March 11, 2020] Lancet. https://linkinghub.elsevier.com/retrieve/pii/S0140673620305663. [DOI] [PMC free article] [PubMed]
- 3.Bertrand D, Chavarot N, Gatault P, et al. Opportunistic infections after conversion to belatacept in kidney transplantation. Nephrol Dial Transplant. 2020;35(2):336–345. doi: 10.1093/ndt/gfz255. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Clinical management of severe acute respiratory infection (SARI) when Covid-19 disease is suspected. Interim guidance, 13 March 2020. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Accessed April 20, 2020.
- 5.Lau SKP, Lau CCY, Chan K-H, et al. Delayed induction of proinflammatory cytokines and suppression of innate antiviral response by the novel Middle East respiratory syndrome coronavirus: implications for pathogenesis and treatment. J Gen Virol. 2013;94(12):2679–2690. doi: 10.1099/vir.0.055533-0. [DOI] [PubMed] [Google Scholar]
- 6.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.