1.
To the Editor:
The COVID‐19 pandemic is worsening in severity globally. Singapore confirmed its first imported case of COVID‐19 on 23 Jan 2020. As of 29 March 2020, Singapore had 844 confirmed cases of COVID‐19 infection with the majority being treated in the National Centre for Infectious Diseases (NCID). Blood and blood products are precious resources and the World Health Organization (WHO) has released guidelines on the management of blood supply in response to the COVID‐19 pandemic. 1 We evaluated the indications and requirements of blood and blood products in patients with COVID‐19 infection at the NCID, as well as the monthly blood component usage at HealthCity Novena; a central campus comprising of the NCID, Renci Community Hospital and Tan Tock Seng Hospital, a 1700‐bed acute care hospital.
Out of 572 patients with COVID‐19 infection, 19 required ICU treatment. Nine out of the 572 cases required transfusions; five were male and four were female, and the median age was 70 years. None had clinical evidence of hemolysis. Here we present the clinical profiles of the nine patients who required transfusion (Figure 1).
FIGURE 1.
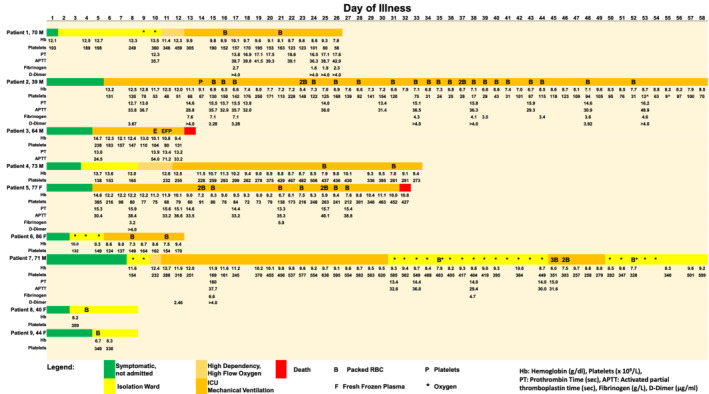
Transfusion requirements, laboratory parameters, clinical course and outcomes for patients with COVID‐19 infection. Patients 4, 6, 8, and 9 had normal PT and APTT values which were not reflected. Reference ranges: Hemoglobin (Hb): males 13.6‐16.6 g/dL; females 11.8‐14.6 g/dL. Platelets: 150‐360 × 109/L. Prothrombin time (PT): 11.7‐14.0 seconds. Activated partial thromboplastin time (APTT): 27.0‐37.0 seconds. Fibrinogen: 1.8‐4.5 g/L. D‐Dimer: <0.50 μg/mL. ( ) Symptomatic, not admitted; (
) Symptomatic, not admitted; ( ) isolation ward; (
) isolation ward; ( ) high dependency, high flow oxygen; (
) high dependency, high flow oxygen; ( ) ICU, mechanical ventilation; (
) ICU, mechanical ventilation; ( ) death; B, packed red blood cells; P, platelets; F, fresh frozen plasma; E, extracorporeal membrane oxygenation; *, oxygen; ^, platelet clumps
) death; B, packed red blood cells; P, platelets; F, fresh frozen plasma; E, extracorporeal membrane oxygenation; *, oxygen; ^, platelet clumps
Patient 1 was a 70‐year‐old male with hypertension and diabetes. He was admitted to the Intensive Care Unit (ICU) for COVID‐19 pneumonia complicated by acute respiratory distress syndrome (ARDS). He suffered a non‐ST‐elevation myocardial infarction, atrial fibrillation, a cardioembolic stroke and acute kidney injury (AKI) requiring continuous renal replacement therapy (CRRT). There was no overt bleeding. Packed red blood cell (PRBC) transfusions were initiated at the ICU physicianʼs discretion due to hemoglobin decreasing to as low as 8.1 g/dL.
Patient 2 was a 39‐year‐old male with no significant medical history. He was admitted for severe COVID‐19 pneumonia complicated by ARDS, septic shock, AKI requiring CRRT and multi‐organ failure. He suffered recurrent coffee ground nasogastric tube aspirates and was treated conservatively with intravenous esomeprazole infusions, with plans for endoscopy in the event of massive bleeding. Bone marrow examination performed for evaluation of pancytopenia showed adequate trilineage hematopoiesis. The cause of his anemia was multifactorial and attributed to anemia of inflammation and gastrointestinal bleeding. He required a total of 24 units of PRBC and 1 unit of pooled platelets.
Patient 3 was a 64‐year‐old male with asthma, hypertension, hyperlipidemia and ischemic heart disease. He was admitted for severe COVID‐19 pneumonia with ARDS and AKI requiring CRRT. Veno‐venous extracorporeal membrane oxygenation (ECMO) was initiated with no requirements for transfusions but was then abandoned when the patient developed major bleeding. Fresh frozen plasma (FFP) and pooled platelets were transfused but the patient succumbed to multi‐organ failure.
Patient 4 was a 74‐year‐old with hypertension, hyperlipidemia, previous transient ischemic attack and hypertensive nephrosclerosis. He was admitted for severe COVID‐19 pneumonia with ARDS and developed ST‐elevation myocardial infarction. Coronary angiography revealed triple vessel disease, and he was placed on medical therapy with plans for coronary artery bypass grafting when stable. Dual antiplatelet agents and low molecular weight heparin were started; thereafter he developed a drop in hemoglobin requiring 2 units of PRBC.
Patient 5 was a 77‐year‐old female with hypertension, hyperlipidemia, ischemic heart disease and pan‐atrophic gastritis confirmed on endoscopy in 2019. She was admitted for severe COVID‐19 pneumonia with ARDS, AKI requiring CRRT, melena secondary to severe gastritis on endoscopy and right sided colitis. She also developed limb gangrene and a distal limb deep vein thrombosis. She required 9 units of PRBC and unfortunately succumbed to COVID‐19 pneumonia with multiorgan failure.
Patient 6 was an 86‐year‐old female with hypertension. She was admitted for severe COVID‐19 pneumonia complicated by ARDS, acute liver and kidney injury. She had worsening anemia attributed to the severe infection and developed bleeding from a central line site, requiring 2 units of PRBC.
Patient 7 was a 71‐year‐old male with type 2 diabetes mellitus. He was admitted for severe COVID‐19 pneumonia with ARDS complicated by right upper limb deep vein thrombosis secondary to a right central venous line placement. He was anticoagulated but developed recurrent frank per rectal bleeding even after anticoagulation was discontinued. Angiography did not show any active bleeding, but colonoscopy revealed cytomegalovirus (CMV) proctitis with CMV inclusion bodies seen on rectal stromal cells, requiring treatment with intravenous ganciclovir. His retroviral screen was negative.
Patients 8 and 9 were middle aged pre‐menopausal females who had iron deficiency anemia, with symptoms of anemia exacerbated by COVID‐19 pneumonia. They had one transfusion of PRBC each and iron replacement was started.
Most patients with mild COVID‐19 infection did not require blood transfusions. Only 0.36% (two out of 553) of non‐ICU COVID‐19 patients required PRBC transfusion. However, 36.8% (seven out of 19) of ICU patients required PRBC transfusion, with lesser requirements for FFP and platelet transfusion. Most transfusions consisted of red cell concentrates (total of 48 units of PRBC) and occurred mainly in the ICU setting. Three out of seven ICU patients suffered from severe gastrointestinal bleeding, requiring high volumes of PRBC transfusion. Xiao et al 2 showed fecal‐oral transmission of the SARS‐CoV‐2 virus as an additional route of transmission, by means of entry into gastrointestinal epithelial cells by the angiotensin converting enzyme 2 (ACE2) protein. They demonstrated mucosal damage to the esophagus with SARS‐CoV‐2 positive staining in tissues obtained from the esophagus, stomach, duodenum and rectum. This may possibly explain why in the sickest of patients with COVID‐19 infection, gastrointestinal mucosal damage from SARS‐CoV‐2 infection, stress/uremic gastritis, or opportunistic CMV infection causing colitis may predispose to a higher red cell transfusion requirement in the critical care setting.
There is also a minority of the non‐ICU patients who require transfusions. These were pre‐menopausal females who had iron deficiency anemia whose symptoms of anemia were exacerbated by concurrent COVID‐19 infection. The benefit of transfusions in these patients must be carefully weighed against risk of transfusion associated complications. Iron replacement therapy with an aim to improving erythropoiesis is a more appropriate treatment approach. Prudent use of blood products based on Patient Blood Management (PBM) guidelines should be practiced.
The use of FFP and platelet transfusions was minimal in our patients with COVID‐19 infections, with one patient having major bleeding while being on ECMO. Increased D‐dimer values were observed; however, this parameter is nonspecific and known to be raised in hospitalized patients with inflammation and liver disease. As none had clinical diagnosis of disseminated intravascular coagulopathy (DIC) or met the International Society on Thrombosis and Hemostasis criteria 3 for overt DIC (score ≥ 5), we did not see any use of fibrinogen concentrates or cryoprecipitate. Platelet transfusions were used to mitigate peri‐procedural risks of bleeding. We did not use specialized blood products in our patients with COVID‐19 infection such as factor VIII concentrates, 4‐Factor prothrombin complex concentrates or recombinant Factor VIIa. The main limitation of our review is the small numbers of patients requiring transfusion and hence no major trends can be drawn from this.
The total number of blood products transfused at the HealthCity Novena campus was lower in February 2020 and March 2020 as compared to the preceding 12 months. A mean of 1270 PRBC units/month in 2019 was transfused, as compared to 1063 PRBC units/month (16% decrease) in February and March 2020. There was also a reduction in FFP from 245 to 193 units/month (21.2% decrease) and platelet products from 197 to 166 units/month (15.7% decrease). While Tan Tock Seng Hospital still admits non COVID‐19 patients, the postponing of elective surgeries and lower bed occupancy rates during February and March 2020 has resulted in decreased demand for blood products. This was also observed from March 2003 to May 2003 during the height of the Severe Acute Respiratory Syndrome (SARS) epidemic, where Tan Tock Seng Hospital was then the designated public hospital in Singapore to manage only patients with suspected and confirmed SARS. The blood transfusion requirements during that period showed a 66.5% decline from a mean 941 monthly red cell units transfused in 2002, to 315 monthly red cell units transfused, and a corresponding decline in mean monthly usage of FFP and platelet transfusions from 500 units FFP and 289 platelet products in 2002, to 211 units FFP (57.8% decrease) and 155 platelet products (46.3% decrease).
The challenges faced by blood banks during an infectious pandemic are securing and protecting the blood supply. 4 While the demand for blood and blood products may decrease during a pandemic due to postponement of elective surgeries, measures such as physical distancing and a complete lockdown of cities, provinces or countries in an attempt to curb the spread of infection may result in a larger decline in blood supply and an overall shortage of blood products. This was observed in April 2003 to July 2003 in Beijing, China during the SARS outbreak where it was necessary to import blood and blood products from other Chinese provinces to ensure availability for clinical use in patients. 5 Hospitals should have in place an emergency blood management plan in preparedness planning for sustainability and safety of blood supply.
In summary, most patients with mild COVID‐19 infection do not require blood transfusions. A subset of critically ill patients with severe COVID‐19 infection in the ICU, especially those with overt gastrointestinal bleeding, requires mostly PRBC transfusion, with lesser requirements for FFP and platelet products.
2. CONFLICT OF INTEREST
All authors declare no competing interests. This study was approved by the National Healthcare Group Domain Specific Review Board (DSRB).
ACKNOWLEDGEMENTS
We salute the efforts of all healthcare workers and the support of their families during this pandemic. We thank the Department of Hematology and Department of Laboratory Medicine, Blood Transfusion Service, Tan Tock Seng Hospital for the support in the collation of data.
REFERENCES
- 1.Maintaining a safe and adequate blood supply during the pandemic outbreak of coronavirus disease (COVID‐19) WHO Interim guidance, March 2020. https://www.who.int/publications‐detail/maintaining‐a‐safe‐and‐adequate‐blood‐supply‐during‐the‐pandemic‐outbreak‐of‐coronavirus‐disease‐(covid‐19).
- 2. Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS‐CoV‐2. Gastroenterology. 2020;158(6):1831‐1833.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID‐19. J Thromb Haemostasis. 2020;18(5):1023‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gschwender AN, Gillard L. Disaster preparedness in the blood bank. Am Soc Clin Lab Sci. 2017;30(4):250‐257. 10.29074/ascls.30.4.250. [DOI] [Google Scholar]
- 5. Shan H, Zhang P. Viral attacks on the blood supply: the impact of severe acute respiratory syndrome in Beijing. Transfusion. 2004;44:467‐469. 10.1111/j.0041-1132.2004.04401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


