Abstract
Post-mortem studies consistently show evidence of reduced synaptic protein levels in patients with schizophrenia. Clinically high-risk subjects show a steeper decrease in grey matter thickness and in vitro modeling using patient-derived cells implicate excessive synaptic pruning during neurodevelopment as a part of the schizophrenia pathophysiology. However, it is unclear to what extent synapse elimination is present during various stages of the disease, which is of clinical importance as in a real-world setting most subjects received their first-episode psychosis (FEP) diagnosis not until their mid-twenties. In the present study, we measured cerebrospinal fluid (CSF) concentrations of the two pre-synaptic proteins synaptosomal-associated protein 25 (SNAP-25) and synaptotagmin-1 (SYT-1), both of which are increased in conditions of ongoing synaptic degeneration, in 44 FEP subjects (mean age 29.9 years) and 21 healthy controls (25.9 years) using immunoprecipitation mass spectrometry. Neither protein was found to differ between healthy controls and patients, and they showed no correlation with symptom ratings, cognitive performance or antipsychotic medication. Additional studies in high-risk subjects in the early prodromal phase will be needed to address if excessive synapse destruction occurs before the development of overt psychotic symptoms.
Abbreviations: FEP, first-episode psychosis; HC, healthy controls; GAF, Global Assessment of Functioning; PANSS, the Positive and Negative Syndrome Scale; CGI, Clinical Global Impression; BMI, body mass index; DUP, duration of untreated psychosis; BACS-SC, Brief Assessment of Cognition in Schizophrenia Symbol Coding; BVMT-R, Brief Visuospatial Memory Test-Revised; CPT-IP, Continuous Performance Test-Identical Pairs; HVLT-R, Hopkins Verbal Learning Test-Revised; LNS, Letter-Number Span; MSCEIT, Mayer–Salovey– Caruso Emotional Intelligence Test; NAB: MAZES, Neuropsychological Assessment Battery: Mazes; TMT, Trail Making Test; WMS-III, Wechsler Memory Scale-3rd Edition
Keywords: Schizophrenia, Synapse pruning, SNAP-25, SYT-1
Introduction
Synaptic remodeling is a dynamic process of ultimate importance for brain functions and behavior. Although synapse elimination is generally considered to peak in adolescence, postmortem studies indicate that this process in healthy individuals may continue at least into the third decade of life (Petanjek et al., 2011). Schizophrenia is suggested to involve a developmentally disrupted neuronal connectivity of the brain involving e.g. between temporal and frontal regions, as well as hippocampus (Lawrie et al., 2002; Meyer-Lindenberg et al., 2005; Stephan et al., 2009). Postmortem and genetic studies suggest a reduced spine density in schizophrenia (Blennow et al., 1996; Davidsson et al., 1999; Harrison, 1999; Glausier and Lewis, 2013). In addition, magnetic resonance imaging (MRI) studies of high-risk subjects indicate that the decline in grey matter thickness occurs already in late adolescence, although this technique does not allow for direct visibility of synaptic degeneration (Lawrie and Abukmeil, 1998; Levitt et al., 2010). A recent positron emission tomography study found lower density of synaptic vesicle glycoprotein 2A (SV2A) across several brain regions in patients with chronic schizophrenia compared to healthy volunteers (Onwordi et al., 2020). Notably, according to a recent study, patient-derived cellular models (Sellgren et al., 2019) show a hyperactive synaptic pruning secondary to increased copy numbers of the long form of the schizophrenia risk gene complement factor 4A (C4AL).
Synaptosomal-associated protein (SNAP)-25 and synaptotagmin (SYT)1 are situated in synaptic terminals of nerve cells, and essential for neurotransmitter release. A number of previous post-mortem studies show abnormalities of these proteins in schizophrenia (Gabriel et al., 1997; Young et al., 1998; Sokolov et al., 2000; Knable et al., 2004; Ramos-Miguel et al., 2015). Further, these protein appears to interact with antipsychotic treatment (Gil-Pisa et al., 2012). In addition, SNAP-25 and SYT1 in the cerebrospinal fluid (CSF) are generally suggested to reflect synaptic destruction (Brinkmalm et al., 2014b; Ohrfelt et al., 2016). Clinical in vivo evidence for an increased synaptic pruning in schizophrenia is sparse. Here, we clinically assess synapse remodeling in the disease by analyzing CSF SNAP-25 and SYT1, recently suggested as biomarkers to reflect synaptic degeneration in Alzheimer’s disease (Brinkmalm et al., 2014a; Ohrfelt et al., 2016). In this study all soluble forms of SNAP-25 (SNAP-25tot), as well as the longer soluble forms including at least amino acid 32-40 (SNAP-25aa40), and SYT-1 were analyzed in well-characterized first-episode psychosis (FEP) patients and healthy controls collected from a longitudinal study of schizophrenia.
Experimental procedures
Patients
The Karolinska Schizophrenia Project(KaSP) enrolls drug-naïve first-episode psychosis pateints and age- matched healthy controls in a outpatient cinical program. 44 FEP Patients who met criteria of schizophrenia, delusional disorder, brief psychotic disorder, psychotic disorder not otherwise specified or schizoaffective syndrome according to Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) were enrolled. After 1.5 years of reassessment, the following diagnoses were made: schizophrenia (n = 27), psychotic disorder not otherwise specified (n = 5), delusional disorder (n = 6), brief psychotic disorder (n = 1), schizoaffective syndrome (n = 3). Two patients recovered from the psychotic episode. The exclusion criteria were neurologic or severe somatic disease, substance abuse, or antipsychotic treatment >1 month (one patient that was treated for 57 days was included as an exception). 14 patients had a history of psychiatric illness, other than psychosis, before enrollment. Additional brain magnetic resonance imaging (MRI) was performed to exclude major brain abnormalities. Clinical characterization was conducted during the first 10 days of enrollment with the Positive and Negative Syndrome Scale (PANSS), the Global Assesment of Functioning (GAF), the Clinical Global Impression (CGI). Alcohol Use Disorders Identification Test (AUDIT) and Drug Use Disorders Identification Test (DUDIT) were used to screen for substance abuse. For most patients, GAF, PANSS, cognitive testing and lumbar puncture were all performed within a 10-day period, whereas seven of the patients underwent these investigations during a period from 14 to 40 days.
Controls
Twenty-one healthy volunteers were recruited by advertisement. Exclusion criteria were previous or current substance abusement evaluated with AUDIT and DUDIT, psychiatric illness evaluated by The Mini International Neuropsychiatric Interview (MINI), or first-degree relatives with psychotic illness. None of the control subjects were on any pharmacological medication at the time of the study. Routine laboratory blood and urine tests and as well as MRI were conducted to exclude abnormal physical conditions. For all healthy controls, cognitive test session and lumbar puncture were all performed within 15 days.
Lumbar puncture
Most lumbar punctures were performed between 07.45 and 10.00 a.m. after a night of rest and avoidance of physical activity at least 8 h before sampling. Due to clinical routines, morning sampling was not possible in the remaining FEP patients (n = 18). To control for this confounding factor, seven controls also underwent lumbar puncture during the same time interval (that is, 1030 and 1315 h).
A disposable atraumatic needle (22 G Sprotte, Geisingen, Germany) was inserted at the L4-L5 level with the subject in the right decubitus position. CSF was allowed to drip into a plastic polypropylene tube, protected from light, and then centrifuged in a 5810 R, Eppendorf AG (Hamburg, Germany), at 1200 rpm (350 g) for 10 min after the puncture. Cell-free CSF was prepared and stored in aliquots at −80 °C within one hour of sampling until analyzed. All samples underwent routine analyzes to exclude blood contamination and inflammatory/infectious disorders (cell counts, immunoglobulin G and albumin concentrations and oligoclonal bands.
Analysis of SNAP-25 and SYT-1
SNAP-25 and SYT1 was measured by immunoprecipitation mass spectrometry (IP-MS). The SNAP‐25/synaptotagmin‐1 assay consists of enrichment with immunoprecipitation (IP) followed by quantitation with liquid chromatography/tandem mass spectrometry (LC‐MS/MS). The IP was performed with automated magnetic-particle processing in a 96‐well plate format on a KingFisher™ Flex System (Thermo Fisher Scientific). CSF samples (200 μL) were incubated 90 min at 22 °C with mouse monoclonal antibodies clone 41.1 (Synaptic Systems) and SMI81 (Biosite) added (0.5 g/L) to IgG‐coated magnetic beads (Dynabeads M‐280 Sheep anti‐Mouse IgG (Thermo Fisher scientific)). After washing in 0.025 % Tween in PBS, PBS and 50 mM NH4HCO3, captured proteins were eluted with 0.5 % aqueous formic acid, and dried in a vacuum centrifuge overnight. The plate was stored at −20 °C until use. Digestion was performed by reconstituting the sample in a mixture of Trypsin/Lys‐C (Promega) in 50 mM NH4HCO3 and incubating at 37 °C overnight. Each sample was subsequently transferred to LC‐vials (SUN‐SRi) and loaded into the LC autosampler. Stable heavy isotope‐labelled standards (IS) were added to the sample before IP (SYT1 MS Standard C13 and N15-labeled recombinant protein (NP_001129278, Origene), and SNAP-25 Ac-2-16[RR] (AQUA Ultimate Thermo Fisher Scientific)) and at reconstitution (SNAP-25 Ac-2-16[L], 18-30[R], 32-40 [K] (AQUA Ultimate Thermo Fisher Scientific)). High‐resolution parallel reaction monitoring (HR‐PRM) analyses were performed on a Q Exactive quadrupole–orbitrap mass spectrometer (Thermo Fisher Scientific) coupled to an Ultimate 3000 standard liquid chromatography system (Thermo Fisher Scientific). The samples (50 μL) were loaded directly onto a Hypersil GOLD HPLC C18 column (Thermo Fisher Scientific) with 0.1 % aqueous formic acid at 300 μL/min. The mass spectrometer was set to acquire scheduled pairs or triplets of fragmentation scans (PRM scans) in profile mode, allowing simultaneous detection of the CSF peptide and the corresponding IS. LC–MS/MS raw files acquired with Xcalibur software version 2.2 SP1.48 (Thermo Fisher Scientific) were imported into Pinpoint software version 1.3.0 (Thermo Fischer Scientific), where LC–MS peak areas were generated. CSF levels of SNAP‐25tot (Ac-2-16), SNAP‐25aa40 (32–40) and SYT‐1 (215–223) were calculated by multiplying the ratio of the LC–MS peak areas of with the concentration of the corresponding IS. SNAP‐25tot corresponds to the total level of all soluble forms of SNAP‐25 containing the SMI81 epitope Ac-2-16, whereas SNAP‐25aa40 corresponds to the level of the longer soluble forms (including Ac-2-11 and at least amino acids 32–40). SYT-1 corresponds to the levels of all soluble SYT-1 forms that contain the epitope of 41.1 which includes the area around the N-terminal portion of the first C2-domain (specifically amino acids 215–223).
Cognition
All participants underwent MATRICS Consensus Cognitive Battery (MCCB) test to evaluate cognitive function which was performed by a qualified psychologist. This battery included ten tests that measure seven cognitive domains. The following tests were used: attention/vigilance (Continuous Performance Test-Identical Pairs), speed of processing (Category Fluency: Animal Naming, Trail Making Test: Part A), verbal learning (Hopkins Verbal Learning Test-Revised), working memory (Wechsler Memory Scale: Spatial Span) and visual learning (Brief Visuospatial Memory Test-Revised).
Statistical analysis
The normality of data was determined by the D’Agostino and Pearson omnibus normality test. Unpaired t-test and Chi-squre test were used to compare numerical and categorical variables respectively. Comparison of the cognitive tests between healthy controls and patients was performed giving an alpha-threshold of 0.005 after Bonferroni-corrected for multiple testing. Symptom ratings were highly correlated, and therefore not corrected for repeated measure. We used regression model to study potential confounding factors in predicting disease status and association between synapse markers and different variables. All data were performed using Prism version 5.0 (GraphPad Software, La Jolla, CA, USA) and SPSS Statistics version 20.0 (IBM, Armonk, NY, USA). Significance level was set to P < 0.05, and all reported P-values are two-sided.
Results
Demographic and clinical characteristics of FEP patients and healthy controls are presented in Table 1. There was no significant difference in sex distribution or body mass index (BMI) between FEP patients and controls, while age and nicotine users were statistically different. For cognitive tests, most domains were significantly different between the groups. Almost half of the patients were under antipsychotic treatment at the time of CSF sampling (mean number of days on treatment: 15.9 ± 2.7 days). The most commonly used antipsychotics in our cohort were olanzapine, aripiprazole, risperidone, and quetiapine.
Table 1.
Demographics and clinical characteristics.
| Characteristics | HC (n = 21) | FEP patients (n = 44) | P |
|---|---|---|---|
| Age (years) | 25.9 ± 1.1 | 29.9 ± 1.3 | 0.04a |
| Gender (male/female) | 11/10 | 27/17 | 0.49b |
| BMI (kg/m2) | 22.2 ± 0.6 (20) | 22.5 ± 1.0 (43) | 0.82a |
| Nicotine users % | 0% | 30 % (11/37) | 0.006b |
| DUP (months) | – | 9.7 ± 1.8 (36) | – |
| Medicine | |||
| Antipsychotics | – | 47 %(21) | – |
| Benzodiazepines | – | 28 % | – |
| Zopiclone | – | 23 % | – |
| Antidepressants | – | 10 % | – |
| Antiepileptic drugs | – | 3% | – |
| PANSS | |||
| PANSS positive symptoms | – | 19.5 ± 0.8 | – |
| PANSS negative symptoms | – | 16.0 ± 1.1 | – |
| PANSS general symptoms | – | 37.9 ± 1.8 | – |
| PANSS total | – | 73.4 ± 3.2 | – |
| GAF symptoms | – | 31.8 ± 7.1 | – |
| GAF functioning | – | 41.6 ± 1.7 | – |
| CGI score | – | 4.3 ± 0.2 | – |
| Cognitive test* | |||
| TMT | 23.2 ± 1.1 | 31.4 ± 2.0(n = 39) | 0.00083* |
| BACS_SC | 61.2 ± 1.8 | 47.2 ± 2.1 | 3.063e-06* |
| HVLT_R | 28.7 ± 0.7 | 24.3 ± 0.8 | 8.496e-05* |
| WMS_II_SS | 18.5 ± 0.6 | 16.0 ± 0.5 | 0.00289* |
| LNS | 15.5 ± 0.6 | 13.4 ± 0.5 | 0.00858 |
| NAB_MAZES | 22.9 ± 0.9 | 19.28 ± 0.9 | 0.00689 |
| BVMT_R | 29.5 ± 1.1 | 22.6 ± 1.1 | 3.475e-05* |
| Fluency | 25.2 ± 1.3 | 22.0 ± 0.9 | 0.04453 |
| MSCEIT_ME | 98.0 ± 1.2 | 89.1 ± 2.0 | 0.00046* |
| CPT_IP | 61.6 ± 27.3 | 2.3 ± 0.1 | 0.04195 |
Values are given as mean ± S.E.M. Abbreviations: BMI, body mass index; DUP, duration of untreated psychosis; PANSS, Positive and Negative Syndrome Scale; GAF, Global Assessment of Functioning; CGI, Clinical Global Impression; FEP, first-episode psychosis; HC, healthy controls; BACS-SC, Brief Assessment of Cognition in Schizophrenia Symbol Coding; BVMT-R, Brief Visuospatial Memory Test-Revised; CGI, Clinical Global Impression; CPT-IP, Continuous Performance Test-Identical Pairs; GAF, Global Assessment of Functioning; HVLT-R, Hopkins Verbal Learning Test-Revised; PANSS, Positive and Negative Syndrome Scale; TMT, Trail Making Test; WMS-III, Wechsler Memory Scale-3rd Edition.
Unpaired t-test.
X2 test.
Significant after Bonferroni-correction, α-value = 0.005.
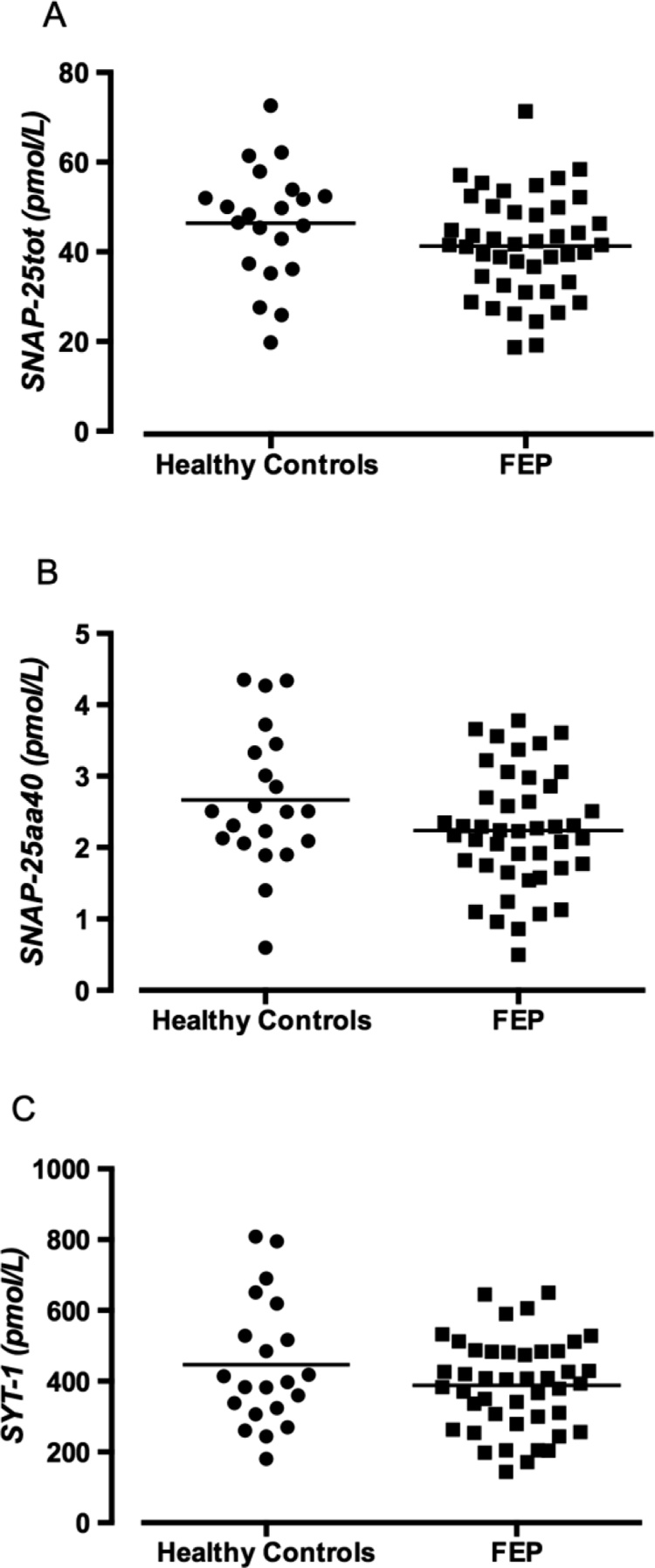
CSF concentrations of synapse markers in FEP patients and healthy controls
CSF levels of SNAP‐25tot, SNAP‐25aa40, or SYT-1 were not significantly affected by age, sex, or BMI in the combined FEP and control group, or in each group analysed separately (Table 2). FEP patients using nicotine, or an antipsychotic, displayed similar levels as the remaining FEP patients (Table 2). No statistical differences between controls and FEP patients regarding CSF levels of SNAP‐25tot (46.4 ± 2.8 pmol/L vs. 41.3 ± 1.7 pmol/L, P = 0.104), SNAP‐25aa40 (2.7 ± 0.2 pmol/L vs. 2.2 ± 0.1 pmol/L, P = 0.065), or SYT-1 (446.3 ± 39.1 pmol/L vs. 387.9 ± 19.4 pmol/L, P = 0.137) were observed, although a trend for reduced CSF levels of SNAP‐25aa40 in patients was evident (Fig. 1). Analyses adjusted for age and sex also displayed similar results (SNAP‐25tot: OR = 0.965, P = 0.139; SNAP‐25aa40: OR = 0.540, P = 0.056; SYT-1: OR = 0.997; P = 0.175). Furthermore, when restricting the analysis to patients diagnosed with schizophrenia at 1.5 years of follow up (n = 27), still no significant differences compared to controls were observed. SNAP‐25tot (HS vs. FEP: 46.4 ± 2.8 pmol/L vs. 41.7 ± 2.2 pmol/L, P = 0.193), SNAP‐25aa40 (2.7 ± 0.2 pmol/Lvs. 2.2 ± 0.2 pmol/L, P = 0.117), or SYT-1 (446.3 ± 39.1 pmol/L vs. 399.8 ± 27.6 pmol/L, P = 0.337).
Table 2.
Correlation between CSF concentrations of synapse markers and demographic parameters.
| SNAP25tot |
SNAP‐25aa40 |
SYT-1 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| FEP | HC | FEP + HC | FEP | HC | FEP + HC | FEP | HC | FEP+ HC | |
| Age | r =-0.03 | r =-0.10 | r =-0.09 | r = 0.10 | r = 0. 03 | r = 0.16 | r = -0.12 | r = 0.05 | r = -0.11 |
| P = 0.87 | P = 0.67 | P = 0.47 | P = 0.53 | P = 0.89 | P = 0.90 | P = 0.43 | P = 0.83 | P = 0.38 | |
| Gender | r = -0.04 | r = 0.20 | r = 0.03 | r = -0.04 | r = 0. 20 | r = 0.02 | r = 0.02 | r = 0.20 | r = 0.07 |
| P = 0.80 | P = 0.37 | P = 0.83 | P = 0.78 | P = 0.40 | P = 0.86 | P = 0.89 | P = 0.40 | P = 0.57 | |
| BMI | r = -0.17 | r = 0.06 | r=-0.12 | r = -0.08 | r = 0. 08 | r=-0.05 | r = -0.20 | r = 0.08 | r = -0.13 |
| P = 0.27 | P = 0.80 | P = 0.34 | P = 0.63 | P = 0.76 | P = 0.70 | P = 0.20 | P = 0.73 | P = 0.32 | |
| Use of nicotinea | P = 0.59 | n/a | n/a | P = 0.23 | n/a | n/a | P = 0.69 | n/a | n/a |
| Use of antipsychoticsa | P = 0.46 | n/a | n/a | P = 0.15 | n/a | n/a | P = 0.50 | n/a | n/a |
Unpaired t-test otherwise Pearson correlation.HC, healthy controls.
Fig. 1.
Synapse markers in the CSF from healthy controls (n = 21) and FEP patients (n = 44). The three panels depict the levels of the two soluble forms of SNAP-25tot (A), SNAP‐25aa40 (B) and SYT-1 (C). Each point represents the concentration of a single CSF sample and the horizontal lines represent the mean value for each group. Statistical differences between controls and FEP patients were determined using Unpaired t-test, P = 0.104 (A), P = 0.065 (B), P = 0.137 (C), respectively.
No correlations between CSF markers and symptom ratings, or cognitive performance in FEP patients were found (Table 3). However, as expected, the synaptic markers showed a strong interrelationship (SNAP‐25tot vs. SNAP‐25aa40, r = 0.801, SNAP‐25tot vs. SYT-1, r = 0.880, SYT-1 vs. SNAP‐25aa40, r = 0.849).
Table 3.
Correlation between CSF markers and symptom severity or cognitive functions in FEP patients.
| SNAP-25tot | SNAP-25aa40 | SYT-1 | ||
|---|---|---|---|---|
| PANSS | ||||
| PANSS positive symptoms | 0.289 | 0.179 | 0.261 | |
| PANSS negative symptomsa | 0.264 | 0.276 | 0.127 | |
| PANSS general symptoms | 0.268 | 0.159 | 0.206 | |
| PANSS total | 0.292 | 0.198 | 0.222 | |
| Severity of illness | ||||
| GAF symptoma | −0.087 | −0.131 | −0.139 | |
| GAF functioning | −0.157 | −0.064 | −0.154 | |
| CGIa | 0.111 | 0.226 | 0.195 | |
| Cognitive test* | ||||
| TMTa | 0.066 | −0.109 | −0.102 | |
| BACS_SC | −0.011 | 0.052 | 0.014 | |
| HVLT_R | −0.125 | −0.071 | −0.055 | |
| WMS_II_SS | −0.080 | −0.147 | −0.111 | |
| LNS | −0.175 | −0.012 | −0.020 | |
| NAB_MAZESa | 0.026 | 0.018 | 0.021 | |
| BVMT_R | 0.020 | 0.001 | 0.008 | |
| Fluency | 0.091 | −0.015 | 0.032 | |
| MSCEIT_ME | −0.040 | −0.047 | −0.066 | |
| CPT_IP | −0.022 | 0.075 | −0.023 | |
Bonferroni correction was used in the Cognitive test, giving an α-threshold of *P < 0.005 (0.05/10).
Spearman correlation otherwise Pearson’s correlation applied, results represented the r value. Abbreviations: BACS-SC, Brief Assessment of Cognition in Schizophrenia Symbol Coding; BVMT-R, Brief Visuospatial Memory Test-Revised; CGI, Clinical Global Impression; CPT-IP, Continuous Performance Test-Identical Pairs; GAF, Global Assessment of Functioning; HVLT-R, Hopkins Verbal Learning Test-Revised; PANSS, Positive and Negative Syndrome Scale; TMT, Trail Making Test; WMS-III, Wechsler Memory Scale-3rd Edition.
Discussion
Recent studies in Alzheimer’s disease show promising results for both SNAP-25 and SYT1 in the CSF as biomarkers in the early stages of the disease, reflecting synapse degeneration(Brinkmalm et al., 2014a; Ohrfelt et al., 2016). Notably, these proteins appear to reveal the ongoing synapse degeneration rather than the presence of synapses per se. The results of the present study show no significant differences in CSF SNAP-25 or SYT-1 between FEP patients and healthy controls, although the levels of SNAP‐25aa40 tended to be lower in FEP patients compared to healthy controls. As expected, the levels of SNAP-25 were highly correlated with those of SYT-1, as both proteins act together as a functional unit regulating neurotransmitter exocytosis(Zhang et al., 2002). The results of the present study differ from those of Thompson and coworkers(Thompson et al., 2003), who found an increase in CSF SNAP-25 in patients with schizophrenia compared to healthy controls, independent of treatment with haloperidol. This discrepancy may be related to differences between FEP patients and patients with chronic schizophrenia, or methods in the evaluation of SNAP-25.
Previous experimental studies show that the antipsychotics haloperidol or clozapine downregulate the gene expression of SNAP-25 in the cornu ammonis 3 and cingulate gyrus, respectively (von Wilmsdorff et al., 2018). The present clinical study though, does not provide evidence for an effect of antipsychotics on CSF SNAP-25, although the power of our analysis is relatively low in this regard.
A possible explanation for the negative finding here may be that a phase of excessive synapse elimination occurs well before the appearance of FEP. The mean age in our patient sample was close to 30 years and we noted no effect on SNAP‐25tot, SNAP‐25aa40, or SYT-1 levels by age in FEP patients or healthy controls. Our results mimic post-mortem data from healthy controls showing only a slight decrease in synapse density within this age range (Petanjek et al., 2011). Thus, schizophrenia-related hyperpruning may predominately occur during the physiological peak in synaptic pruning that takes place in late adolescence. At the stage when the subjects show FEP symptoms, a pathophysiological increase in synapse elimination might have been normalized, and therefore undetectable by analysis of the SNAP-25 or SYT1 biomarkers. A putative spacial difference in synapse elimination in the brain may further complicate a true evaluation of pruning as a pathophysiological factor or the disease.
A drug that is suggested to affect synapse remodeling is the antibiotic minocycline. Hyperpruning of synapses by microglial cells appears to be counteracted by the antibiotic minocycline, as shown both in animal models (Miyazaki et al., 2016) and in schizophrenia-derived in vitro models (Sellgren et al., 2019). Further, electronic health records show that minocycline, when given in late adolescence, is associated with reduced risk to develop schizophrenia later in life (Sellgren et al., 2019). Several clinical trials with minocycline show that add-on treatment with the drug is associated with symptom relief regarding total, general and negative symptoms when compared to monotherapy (Solmi et al., 2017; Xiang et al., 2017), although a recent randomized controlled trial of minocycline in subjects with schizophrenia spectrum disorder showed no benefits (Deakin et al., 2018). Notably though, all clinical trials with minocycline so far have been evaluated on patients with chronic schizophrenia. Thus, to design successful clinical trials of therapeutic interventions targeting synapse elimination, it is crucial to identify to what extent this process prolongs into currently clinically identifiable stages of the disease. Aligning with clinical studies showing that cognitive deficits in schizophrenia are already established before the prodromal phase of psychosis, and image studies showing a steep decline in grey matter thickness in high clinical risk adolescents later developing psychosis, our results highlight the importance of identifying high-risk subjects already during adolescence for successful therapeutic interventions targeting synapse elimination and cognitive symptoms.
The present study holds several limitations. Analysis of SNAP-25 and SYT-1 in CSF does not allow evaluation of regional concentrations of the compounds or a putative contribution of non-neuronal origin. Previous post-mortem studies provide a heterogeneous picture regarding SNAP-25 or SYT-1 in schizophrenia, including region-specific differences where an elevation of SNAP-25 is observed in prefrontal association cortex (Thompson et al., 1998) whereas decreased levels have been found in the inferior temporal cortex (Thompson et al., 1998) the cerebellum (Mukaetova-Ladinska et al., 2002), and in hippocampus (Young et al., 1998; Ebdrup et al., 2010; Osimo et al., 2019). Thus, we cannot entirely exclude that a modest regional pathophysiological synaptic remodeling occurs in FEP patients that is not reflected in the CSF, at least not within the current study due to lack of power. Studies using molecular imaging and radioligands for synaptic markers could shed further light on this issue (Onwordi et al., 2020). Similarly, we cannot exclude presence of non-neuronal SNAP-25 in the CSF levels. Also, SNAP-25 is predominately expressed in glutamatergic neurons, with sparse expression in GABAergic terminals, and may therefore not typically reflect elimination of inhibitory synapses (Verderio et al., 2004; Matteoli et al., 2009). Finally, the power to detect potential differences was limited due to the relatively small number of healthy controls. Also, the difference in mean age between patients and healthy controls may blur the interpretation of current data.
Taken together, our results highlight the importance of early identification of high-risk schizophrenia subjects for therapeutic interventions aimed at reducing hyperactive synaptic pruning.
Consent for publication
All authors approve the publicationof this manuscript.
Ethics
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving human subjects/patients were approved by Regional Ethics Committee in Stockholm. All subjects included had given written informed consent after receiving written and oral information.
Funding
This work was supported by grants from Psykiatrifonden, the Swedish Research Council (SE: 2017-00875; HZ: 2018-02532; KB: 2017-00915), the Swedish Brain Foundation, Åhlén-siftelsen, Märta Lundqvists Stiftelse, Svenska Läkaresällskapet, Petrus och Augusta Hedlunds Stiftelse, Torsten Söderbergs Stiftelse, the AstraZeneca-Karolinska Institutet Joint Research Program in Translational Science, Söderbergs Königska Stiftelse, Professor Bror Gadelius Minnesfond, Knut och Alice Wallenbergs stiftelse, Marianne och Markus Wallenberg Foundation, Psykiatrifonden, Hjärnfonden (SE, HZ, KB), Stockholm Country Council (ALF, PPG), Center for Psychiatry Research, Swedish Alzheimer Foundation (KB: AF-742881), European Research Council (HZ: 681712), Swedish State Support for Clinical Research (HZ: ALFGBG-720931; KB: ALFGBG-715986), and KID-funding from the Karolinska Institutet.
CRediT authorship contribution statement
Chengai Xu: Data curation, Validation, Writing - review & editing. Carl M. Sellgren: Writing - original draft. Helena Fatouros-Bergman: Data curation, Validation, Writing - review & editing. Fredrik Piehl: Data curation, Validation, Writing - review & editing. Kaj Blennow: Conceptualization, Methodology, Formal analysis, Investigation. Henrik Zetterberg: Conceptualization, Methodology, Formal analysis, Investigation. Ann Brinkmalm: Conceptualization, Methodology, Formal analysis, Investigation. Alexander Frizell Santillo: Data curation, Validation, Writing - review & editing. Sofia Lundgren: Data curation, Validation, Writing - review & editing. Simon Cervenka: . Göran Engberg: Resources, Writing - review & editing, Supervision. Sophie Erhardt: Resources, Writing - review & editing, Supervision.
Conflicts of interest
Sophie Erhardt has received grant support from AstraZeneca and Jansen Pharmaceuticals as principal investigator and has been a speaker for Roche Pharmaceuticals, Astra Zeneca, Eli Lilly and Bristol Myers Squibb. Carl Sellgren is a co-founder of Outermost Therapeutics Inc. HZ has served at scientific advisory boards of Roche Diagnostics, Wave, Samumed and CogRx, has given lectures in symposia sponsored by Alzecure and Biogen, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg. KB has served as a consultant or at advisory boards for Alector, Alzheon, CogRx, Biogen, Lilly, Novartis and Roche Diagnostics, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, a venture-based platform company at the University of Gothenburg, all unrelated to the work presented in this paper.
There are no commercial associations that might pose a conflict of interest in connection with the manuscript. No funding sources had any role in the study design, in the collection, analysis, and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
None of the other authors declare any competing interests.
Acknowledgements
We thank all the patients and healthy volunteers for their willingness to participate in the study and the research coordinators Maria Lee, Minna Juntura, Henrik Gregemark, Joachim Eckerström, Maria Adolfsson and Martin Szabo for their excellent work in recruiting and taking care of patients and healthy volunteers, as well as for their handling and maintenance of the clinical data base. The helpfulness of the staff at the participating psychiatric units in Stockholm (Prima Vuxenpsykiatri, Psykiatri Nordväst and Norra Stockholms Psykiatri) is also gratefully acknowledged.
Sophie Erhardt, Göran Engberg, and Carl Sellgren designed the study. Chengai Xu, Göran Engberg, Carl Sellgren and Sophie Erhardt drafted the report. Chengai Xu was responsible for the statistical analyses. Helena Fatouros-Bergman and Fredrik Piehl collected all clinical data. Kaj Blennow, Ann Brinkmalm and Henrik Zetterberg performed the analysis of synase markers. All authors contributed to the interpretation of the results, provided critical revision of the report and approved the final manuscript.
Appendix A
Members of the Karolinska Schizophrenia Project (KaSP) consortium: Farde L1, Flyckt L1, Engberg G2, Erhardt S2, Fatouros-Bergman H1, Cervenka S1, Schwieler L2, Piehl F3, Agartz I1,4,5, Collste K1, Sellgren CM2, Victorsson P1, Malmqvist A2, Hedberg M2, Orhan F2
1Centre for Psychiatry Research, Department of Clinical Neuroscience, Karolinska Institutet, & Stockholm Health Care Services, Stockholm County Council, Stockholm, Sweden; 2Department of Physiology and Pharmacology, Karolinska Institutet, Stockholm, Sweden; 3Neuroimmunology Unit, Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden; 4 NORMENT, Division of Mental Health and Addiction, Oslo University Hospital & Institute of Clinical Medicine, University of Oslo, Oslo, Norway; 5Department of Psychiatry, Diakonhjemmet Hospital, Oslo, Norway.
References
- Blennow K., Davidsson P., Gottfries C.G., Ekman R., Heilig M. Synaptic degeneration in thalamus in schizophrenia. Lancet (London, England) 1996;348:692–693. doi: 10.1016/S0140-6736(05)65124-0. [DOI] [PubMed] [Google Scholar]
- Brinkmalm A., Brinkmalm G., Honer W.G., Frolich L., Hausner L., Minthon L., Hansson O., Wallin A., Zetterberg H., Blennow K., Ohrfelt A. SNAP-25 is a promising novel cerebrospinal fluid biomarker for synapse degeneration in Alzheimer’s disease. Mol. Neurodegener. 2014;9:53. doi: 10.1186/1750-1326-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmalm A., Brinkmalm G., Honer W.G., Moreno J.A., Jakobsson J., Mallucci G.R., Zetterberg H., Blennow K., Ohrfelt A. Targeting synaptic pathology with a novel affinity mass spectrometry approach. Mol. Cell. Proteom.: MCP. 2014;13:2584–2592. doi: 10.1074/mcp.M114.040113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidsson P., Gottfries J., Bogdanovic N., Ekman R., Karlsson I., Gottfries C.G., Blennow K. The synaptic-vesicle-specific proteins rab3a and synaptophysin are reduced in thalamus and related cortical brain regions in schizophrenic brains. Schizophrenia Res. 1999;40:23–29. doi: 10.1016/s0920-9964(99)00037-7. [DOI] [PubMed] [Google Scholar]
- Deakin B., Suckling J., Barnes TRE Byrne K., Chaudhry I.B., Dazzan P., Drake R.J., Giordano A., Husain N., Jones P.B., Joyce E., Knox E., Krynicki C., Lawrie S.M., Lewis S., Lisiecka-Ford D.M., Nikkheslat N., Pariante C.M., Smallman R., Watson A., Williams S.C.R., Upthegrove R., Dunn G. The benefit of minocycline on negative symptoms of schizophrenia in patients with recent-onset psychosis (BeneMin): a randomised, double-blind, placebo-controlled trial. Lancet Psychiatry. 2018;5:885–894. doi: 10.1016/S2215-0366(18)30345-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebdrup B.H., Glenthoj B., Rasmussen H., Aggernaes B., Langkilde A.R., Paulson O.B., Lublin H., Skimminge A., Baare W. Hippocampal and caudate volume reductions in antipsychotic-naive first-episode schizophrenia. J. Psychiatry Neurosci.: JPN. 2010;35:95–104. doi: 10.1503/jpn.090049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel S.M., Haroutunian V., Powchih P., Honer W.G., Davidson M., Davies P., Davis K.L. Increased concentrations of presynaptic proteins in the cingulate cortex of subjects with schizophrenia. Arch. Gen. Psychiatry. 1997;54:559–566. doi: 10.1001/archpsyc.1997.01830180077010. [DOI] [PubMed] [Google Scholar]
- Gil-Pisa I., Munarriz-Cuezva E., Ramos-Miguel A., Uriguen L., Meana J.J., Garcia-Sevilla J.A. Regulation of munc18-1 and syntaxin-1A interactive partners in schizophrenia prefrontal cortex: down-regulation of munc18-1a isoform and 75 kDa SNARE complex after antipsychotic treatment. Int. J. Neuropsychopharmacol. 2012;15:573–588. doi: 10.1017/S1461145711000861. [DOI] [PubMed] [Google Scholar]
- Glausier J.R., Lewis D.A. Dendritic spine pathology in schizophrenia. Neuroscience. 2013;251:90–107. doi: 10.1016/j.neuroscience.2012.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P.J. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122(Pt 4):593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- Knable M.B., Barci B.M., Webster M.J., Meador-Woodruff J., Torrey E.F. Molecular abnormalities of the hippocampus in severe psychiatric illness: postmortem findings from the Stanley Neuropathology Consortium. Mol. Psychiatry. 2004;9:609–620. doi: 10.1038/sj.mp.4001471. [DOI] [PubMed] [Google Scholar]
- Lawrie S.M., Abukmeil S.S. Brain abnormality in schizophrenia. A systematic and quantitative review of volumetric magnetic resonance imaging studies. Br. J. Psychiatry: J. Ment. Sci. 1998;172:110–120. doi: 10.1192/bjp.172.2.110. [DOI] [PubMed] [Google Scholar]
- Lawrie S.M., Buechel C., Whalley H.C., Frith C.D., Friston K.J., Johnstone E.C. Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol. Psychiatry. 2002;51:1008–1011. doi: 10.1016/s0006-3223(02)01316-1. [DOI] [PubMed] [Google Scholar]
- Levitt J.J., Bobrow L., Lucia D., Srinivasan P. A selective review of volumetric and morphometric imaging in schizophrenia. Curr. Top. Behav. Neurosci. 2010;4:243–281. doi: 10.1007/7854_2010_53. [DOI] [PubMed] [Google Scholar]
- Matteoli M., Pozzi D., Grumelli C., Condliffe S.B., Frassoni C., Harkany T., Verderio C. The synaptic split of SNAP-25: different roles in glutamatergic and GABAergic neurons? Neuroscience. 2009;158:223–230. doi: 10.1016/j.neuroscience.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A.S., Olsen R.K., Kohn P.D., Brown T., Egan M.F., Weinberger D.R., Berman K.F. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch. Gen. Psychiatry. 2005;62:379–386. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- Miyazaki S., Hiraoka Y., Hidema S., Nishimori K. Prenatal minocycline treatment alters synaptic protein expression, and rescues reduced mother call rate in oxytocin receptor-knockout mice. Biochem. Biophys. Res. Commun. 2016;472:319–323. doi: 10.1016/j.bbrc.2016.02.109. [DOI] [PubMed] [Google Scholar]
- Mukaetova-Ladinska E., Hurt J., Honer W.G., Harrington C.R., Wischik C.M. Loss of synaptic but not cytoskeletal proteins in the cerebellum of chronic schizophrenics. Neurosci. Lett. 2002;317:161–165. doi: 10.1016/s0304-3940(01)02458-2. [DOI] [PubMed] [Google Scholar]
- Ohrfelt A., Brinkmalm A., Dumurgier J., Brinkmalm G., Hansson O., Zetterberg H., Bouaziz-Amar E., Hugon J., Paquet C., Blennow K. The pre-synaptic vesicle protein synaptotagmin is a novel biomarker for Alzheimer’s disease. Alzheimer’s Res. Ther. 2016;8:41. doi: 10.1186/s13195-016-0208-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onwordi E.C., Halff E.F., Whitehurst T., Mansur A., Cotel M.C., Wells L., Creeney H., Bonsall D., Rogdaki M., Shatalina E., Reis Marques T., Rabiner E.A., Gunn R.N., Natesan S., Vernon A.C., Howes O.D. Synaptic density marker SV2A is reduced in schizophrenia patients and unaffected by antipsychotics in rats. Nat. Commun. 2020;11:246. doi: 10.1038/s41467-019-14122-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osimo E.F., Beck K., Reis Marques T., Howes O.D. Synaptic loss in schizophrenia: a meta-analysis and systematic review of synaptic protein and mRNA measures. Mol. Psychiatry. 2019;24(4):549–561. doi: 10.1038/s41380-018-0041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petanjek Z., Judas M., Simic G., Rasin M.R., Uylings H.B., Rakic P., Kostovic I. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc. Natl. Acad. Sci. U. S. A. 2011;108:13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Miguel A., Beasley C.L., Dwork A.J., Mann J.J., Rosoklija G., Barr A.M., Honer W.G. Increased SNARE protein-protein interactions in orbitofrontal and anterior cingulate cortices in schizophrenia. Biol. Psychiatry. 2015;78:361–373. doi: 10.1016/j.biopsych.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellgren C.M., Gracias J., Watmuff B., Biag J.D., Thanos J.M., Whittredge P.B., Fu T., Worringer K., Brown H.E., Wang J., Kaykas A., Karmacharya R., Goold C.P., Sheridan S.D., Perlis R.H. Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat. Neurosci. 2019;22:374–385. doi: 10.1038/s41593-018-0334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov B.P., Tcherepanov A.A., Haroutunian V., Davis K.L. Levels of mRNAs encoding synaptic vesicle and synaptic plasma membrane proteins in the temporal cortex of elderly schizophrenic patients. Biol. Psychiatry. 2000;48:184–196. doi: 10.1016/s0006-3223(00)00875-1. [DOI] [PubMed] [Google Scholar]
- Solmi M., Veronese N., Thapa N., Facchini S., Stubbs B., Fornaro M., Carvalho A.F., Correll C.U. Systematic review and meta-analysis of the efficacy and safety of minocycline in schizophrenia. CNS Spectr. 2017;22:415–426. doi: 10.1017/S1092852916000638. [DOI] [PubMed] [Google Scholar]
- Stephan K.E., Friston K.J., Frith C.D. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophrenia Bull. 2009;35:509–527. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson P.M., Sower A.C., Perrone-Bizzozero N.I. Altered levels of the synaptosomal associated protein SNAP-25 in schizophrenia. Biol. Psychiatry. 1998;43:239–243. doi: 10.1016/S0006-3223(97)00204-7. [DOI] [PubMed] [Google Scholar]
- Thompson P.M., Kelley M., Yao J., Tsai G.C., van Kammen D.P. Elevated cerebrospinal fluid SNAP-25 in schizophrenia. Biol. Psychiatry. 2003;53:1132–1137. doi: 10.1016/s0006-3223(02)01599-8. [DOI] [PubMed] [Google Scholar]
- Verderio C., Pozzi D., Pravettoni E., Inverardi F., Schenk U., Coco S., Proux-Gillardeaux V., Galli T., Rossetto O., Frassoni C., Matteoli M. SNAP-25 modulation of calcium dynamics underlies differences in GABAergic and glutamatergic responsiveness to depolarization. Neuron. 2004;41:599–610. doi: 10.1016/s0896-6273(04)00077-7. [DOI] [PubMed] [Google Scholar]
- von Wilmsdorff M., Manthey F., Bouvier M.L., Staehlin O., Falkai P., Meisenzahl-Lechner E., Schmitt A., Gebicke-Haerter P.J. Effects of haloperidol and clozapine on synapse-related gene expression in specific brain regions of male rats. Eur. Arch. Psychiatry Clin. Neurosci. 2018;268:555–563. doi: 10.1007/s00406-018-0872-8. [DOI] [PubMed] [Google Scholar]
- Xiang Y.Q., Zheng W., Wang S.B., Yang X.H., Cai D.B., Ng C.H., Ungvari G.S., Kelly D.L., Xu W.Y., Xiang Y.T. Adjunctive minocycline for schizophrenia: a meta-analysis of randomized controlled trials. Eur. Neuropsychopharmacol.: J. Eur. Coll. Neuropsychopharmacol. 2017;27:8–18. doi: 10.1016/j.euroneuro.2016.11.012. [DOI] [PubMed] [Google Scholar]
- Young C.E., Arima K., Xie J., Hu L., Beach T.G., Falkai P., Honer W.G. SNAP-25 deficit and hippocampal connectivity in schizophrenia. Cereb. Cortex (New York, NY: 1991) 1998;8:261–268. doi: 10.1093/cercor/8.3.261. [DOI] [PubMed] [Google Scholar]
- Zhang X., Kim-Miller M.J., Fukuda M., Kowalchyk J.A., Martin T.F. Ca2+-dependent synaptotagmin binding to SNAP-25 is essential for Ca2+-triggered exocytosis. Neuron. 2002;34:599–611. doi: 10.1016/s0896-6273(02)00671-2. [DOI] [PubMed] [Google Scholar]