Abstract
This data paper aims to provide data on the effect of the process settings on the fouling of an electrodialysis pilot installation treating a sodium chloride solution (0.1 M and 0.2 M) in the presence of humic acid (1 g/L). This data was used by “Colloidal fouling in electrodialysis: a neural differential equations model” [1] to construct a predictive model and provides interpretive insights into this dataset. 22 electrodialysis fouling experiments were performed where the electrical resistance over the electrodialysis stack was monitored while varying the crossflow velocity (2.0 cm/s - 3.5 cm/s) in the compartments, the current applied (1.41 A - 1.91 A) to the stack and the salt concentration in the incoming stream. The active cycle was maintained for a maximum of 1.5 h after which the polarity was reversed to remove the fouling layer. Additional data is gathered such as the temperature, pH, flow rate, conductivity, pressure in the different compartments of the electrodialysis stack. The data is processed to remove the effect of temperature fluctuations and some filtering is performed. To maximise the reuse potential of this dataset, both raw and processed data are provided along with a detailed description of the pilot installation and sensor locations. The data generated can be useful for researchers and industry working on electrodialysis fouling and the modelling thereof. The availability of conductivity and pH in all compartments is useful to investigate secondary effects of humic acid fouling such as the eventual decrease in membrane permselectivity or water splitting effects introduced by the fouling layer.
Keywords: Electrodialysis, colloidal fouling, humic acid, ion-exchange membranes, process settings, bio-based
Specifications table
| Subject | Filtration and separation |
| Specific subject area | Electrodialysis fouling |
| Type of data | Tables Code |
| How data were acquired | The data consist of time series of an electrodialysis pilot installation that processes a sodium chloride solution with humic acid and is subject to fouling. Each of the experiments is performed at a different crossflow velocity, applied current and the salt concentration in the fouling solution, following an experimental design. Different inline sensors monitor the state of the ED pilot; • Electric potential (M 3000 - Delta electronika, the Netherlands) • Conductivity & temperature (Indumax CLS50D - Endress + Hauser, USA) • Flow rate (Promag 55S DN15 - Endress + Hauser, USA) • pH (Orbisint CPS11D - Endress + Hauser, USA) • Pressure (Cerabar MPMC51 – Endress + Hauser, USA) The data acquisition and control is handled by Mefias®, a LabVIEW-based control software (VITO, Belgium). |
| Data format | Raw Filtered |
| Parameters for data collection | Ambient temperature (measured), Data acquired with a sampling period of 15 seconds for all sensors. |
| Data source location | Flemish institution of technological research (VITO), Mol, Belgium |
| Data accessibility | Repository name: A dataset on ion-exchange membrane fouling by humic acid during electrodialysis Data identification number: 10.5281/zenodo.3551928 Direct URL to data: http://doi.org/10.5281/zenodo.3551928 The code used to process and analyse this dataset is published on Github: https://github.com/Beramos/Data-on-ion-exchange-membrane-fouling-by-humic-acid-during-electrodialysis |
| Related research article | Bram De Jaegher, Wim De Schepper, Arne Verliefde, Ingmar Nopens, Colloidal fouling in electrodialysis: a neural differential equations model, Desalination, In Press |
Value of the data
-
•
Ion-exchange membrane fouling is still an important hurdle to overcome when processing bio-based process streams through electrodialysis. Previous research showed that the fouling rate strongly depends on the process settings [2]. This dataset enables the quantification of these effects.
-
•
Researchers and industry aiming to improve the fouling resistance of electrodialysis can make use of this dataset to explore the effect of the process settings on the fouling rate during electrodialysis. Furthermore, researchers aiming to develop mathematical models for fouling prediction can make use of this data.
-
•
The availability of conductivity and pH measurements before and after all compartments can be used to investigate the secondary effects of humic acid fouling such as the decrease in membrane permselectivity or water splitting effects introduced by the fouling layer.
-
•
This data is collected with the use for model training, calibration and validation in mind and provides more consistency, reproducibility and more measured quantities than previous work [2].
1. Data Description
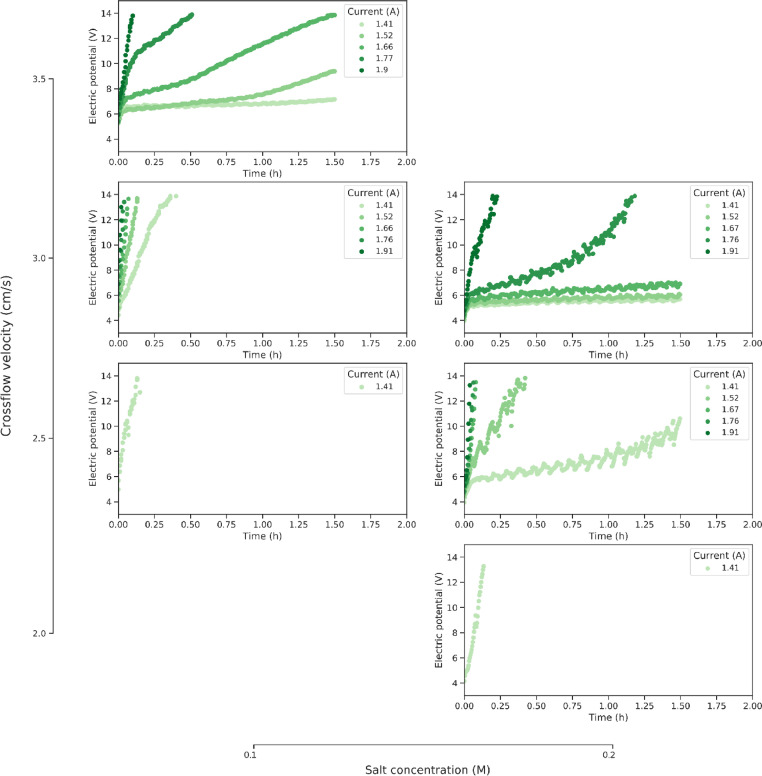
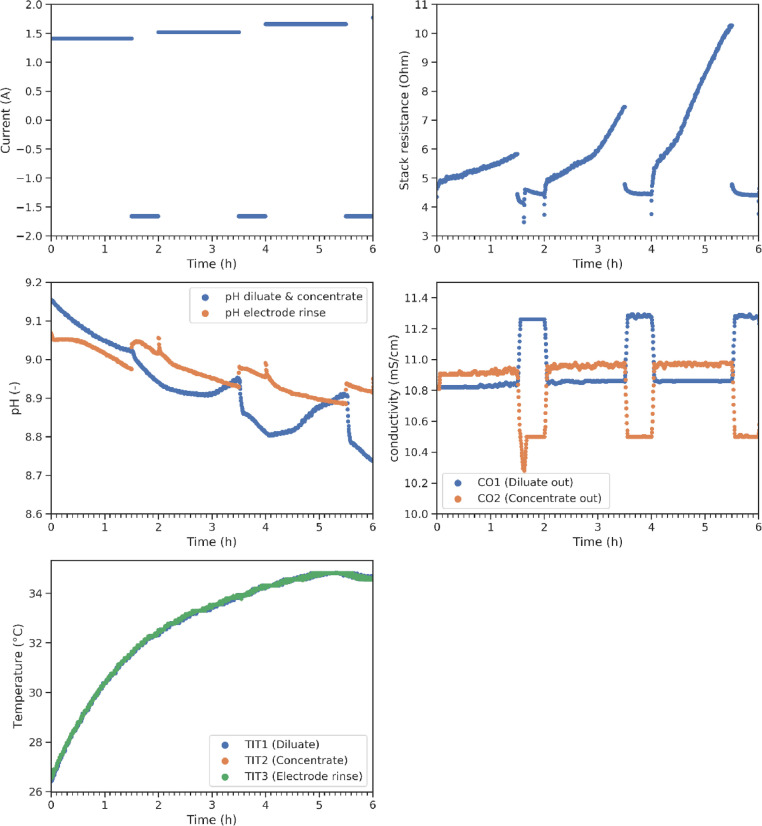
The data consists of time series of different sensors throughout an electrodialysis pilot installation that processes a salt solution in the presence of humic acid. Humic acid fouling is described in a set of 22 experiments, performed under varying process settings as indicated by Fig. 4. Fig. 1 shows the evolution of the stack resistance for all experimental conditions. The y-axis depicts the stack resistance and the x-axis the time. The rows represent the different crossflow velocities tested and the columns the salt concentration. Next to this, a lot of additional sensor data is collected. A subset of the data is illustrated in Fig. 2 where three reversal cycles are displayed at an increasing current. The alternation of the active and cleaning cycles is shown in Fig. 2a where the applied current is visualised in time. Next to the stack resistance (derived from the stack potential and applied current (Fig. 2a), there is data on the pH of the feed streams and the electrolyte rinsing solution (Fig. 2c). The conductivity is also monitored at 5 locations, two of which are visualised in Fig. 2d. Lastly, the temperature of the different circuits is monitored at 3 different locations (Fig. 2e). The data consists of both raw data and the processed data after temperature compensation (Eqs. 1-3), filtering (Eq. 4) and resampling. All data is available on a data repository on Zenodo and the code to reproduce the figures in this paper from the dataset is published on Github.
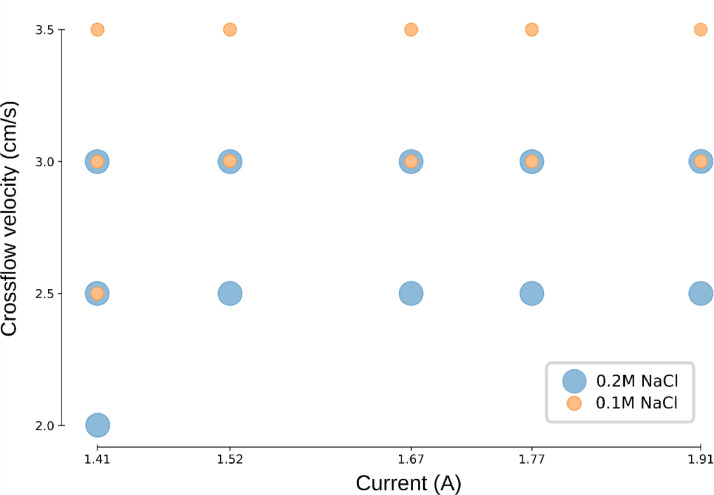
Fig. 4.
Experimental design of the 22 experiments performed in this study. Each point represents an experiment and the three degrees of freedom are the crossflow velocity (y-axis), the current density applied (x-axis) and the concentration of NaCl added to the fouling solution (size and colour of the markers).
Fig. 1.
A graphical summary of the performed experiments. The stack resistance in function of time for different currents (colour). The subfigures indicate different levels of crossflow velocity (vertical direction) and salt concentration (horizontal direction).
Fig. 2.
A subset of the sensor data on the applied current (a), the stack resistance (b), the pH (c), the conductivity (d) and the temperature (e) acquired by the various sensors during experimentation.
2. Experimental design, materials and methods
2.1. Electrodialysis pilot installation
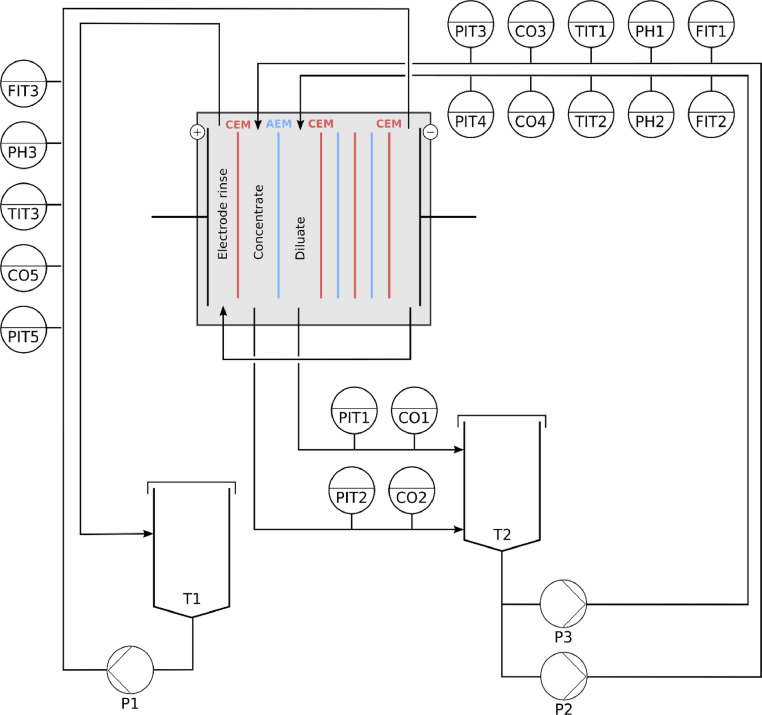
An automated ED pilot installation (VITO, Belgium) is used to generate the data in a series of electrodialysis fouling experiments. The ED installation consists of three circulation loops separating the concentrate, diluate and electrode rinsing solutions. The diluate and concentrate compartments are connected to the same storage tank to ensure a constant salt concentration. The electrolyte rinsing circuit is fed by a 0.1M solution of Na2SO4 in a separate tank and pumped to the anode and cathode compartments in series. The concentrate, diluate and electrolyte rinsing circuit are driven by three separate pumps (GM-V IWAKI USA - PID controlled). In the pilot, the temperature, pH, flow rate, conductivity, pressure and electric potential are measured at several locations in the pilot installation, a detailed overview of the sensor locations is shown in Fig. 3. An overview of the different sensors along with the manufacturers’ information is provided in Table 1. The data acquisition and control is handled by Mefias®, a LabVIEW-based control software (VITO, Belgium). An FT-ED(R)-100-4 (ED100, FuMA-Tech, Germany) ED stack with three cell pairs is built of alternating cation- and anion-exchange membranes, starting and ending with a cation-exchange membrane. The cation- and anion-exchange membranes used in this study are homogeneous PCA-SK and PCA-SA membranes (PCA GmBh, Germany), respectively, both with a surface area of 100cm2. The ED stack is powered by a power supply operated as a galvanostat (SM 3000 - Delta electronika) [1].
Fig. 3.
Piping and Instrumentation diagram of the ED pilot used in this study.
Table 1.
An overview of the sensors in the pilot installation along with information on the identifier in the P&ID, the manufacturer and the units of the measured signal.
| Parameter | Identifiers | Manufacturer | Unit |
|---|---|---|---|
| Electric potential | EPx | M 3000 - Delta electronika, the Netherlands | (V) |
| Conductivity | COx | Indumax CLS50D - Endress + Hauser, USA | (mS/cm) |
| Temperature | TITx | Indumax CLS50D - Endress + Hauser, USA | (°C) |
| pH | PHx | Orbisint CPS11D - Endress + Hauser, USA | (-) |
| Flowrate | FITx | Promag 55S DN15 - Endress + Hauser, USA | (l/h) |
| Pressure | PITx | Cerabar MPMC51 – Endress + Hauser, USA | (bar) |
2.2. Experimental Design
For every experiment the following procedure was followed, a NaCl solution is prepared at a certain concentration (Fig. 4 - colour) and humic acid is added to a concentration of 1 g/L. Next, the electrodialysis pilot installation is run at a certain crossflow velocity and current density (Fig. 4 - x/y axis). Following this procedure, 22 experiments were performed at a different crossflow velocity, applied current and the salt concentration in the fouling solution, according to the experimental design depicted in Fig. 4. The time series consists of the electrical resistance of the stack as a measure of the fouling severity [2] complemented by the data from the above-mentioned inline sensors. Humic acid sodium salt (Sigma-Aldrich) is used as the fouling component in 1g/L solution.
2.3. Data-processing and filtering
The data sampling period for data-acquisition is 15 seconds for all sensors. Each of the experiments presented in Fig. 4. As time progresses the resistance of the stack increases and as the power supply is operated in a galvanostatic manner the potential applied to system increases. For some of the experimental conditions presented in Fig. 4 the fouling intensity was severe and the maximum potential of 15V was reached before 1.5h and are terminated early. Consequently, the length of the experiments varies from a few minutes to a maximum of 1.5h (360 data points). After termination or after 1.5h the polarity of the electrodes is inverted for 0.5h to clean the membranes and bring the system back to its original state. To remove the internal and external temperature effects on the stack resistance a non-linear temperature compensation is performed which takes into account the effect of temperature on water viscosity and ion diffusivity [3],
| (1) |
with R20 the compensated resistance, R the measured resistance. A and B are temperature-dependent parameters and are computed as follows [3],
| (2) |
| (3) |
with Tthe temperature (°C). By applying this compensation, the effect of temperature fluctuations is eliminated. Filtering of the data was performed with a simple moving-average filter with a window size of 10 data points,
| (4) |
Finally, the data is resampled to yield 20 time points spanning each experiment.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships which have, or could be perceived to have, influenced the work reported in this article.
Acknowledgements
This work was supported by the Flemish institute for technological research (VITO).
References
- 1.De Jaegher B., De Schepper W., Verliefde A., Nopens I. C., Colloidal fouling in electrodialysis: a neural differential equations model, Desalination, In Press.
- 2.Korngold E., de Körösy F., Rahav R., Taboch M.F. Fouling of anion-selective membranes in electrodialysis. Desalination. 1970;8(2):195–220. doi: 10.1016/S0011-9164(00)80230-1. ISSN 00119164. [DOI] [Google Scholar]
- 3.Atkins P., de Paula J. In: tenth edn. Freeman W.H., editor. 9780716787594. 2014. (Physical Chemistry: Thermodynamics, Structure, and Change). ISBN. [Google Scholar]