Highlights
-
•
Coccidioides meningitis is a difficult to treat invasive infection.
-
•
Voriconazole is an option for the treatment of refractory Coccidiodes meningitis.
-
•
Adjuvant interferon may play an important role in the treatment of refractory Coccidioides meningitis.
Keywords: Coccidioidomycosis, Meningitis, Gamma-interferon
Abstract
Coccidioides meningitis (CM) is a challenging infection, given the limited penetration to the cerebrospinal fluid of conventional antifungals, resulting in a high risk of recurrence. We present the first case of a successfully treated persistent CM with voriconazole and adjuvant INF-γ 1b.
Introduction
Coccidioides meningitis (CM) is an invasive infection caused by Coccidioides immitis and Coccidioides posadasii. Advanced age, comorbidities and disorders of the immunologic response increase the risk for invasive coccidioidomycosis [1]. Although new azoles have become available in the last decade, there is little information on treatment of CM with voriconazole (VRC). We report a case of persistent CM successfully treated with VRC and interferon-gamma (INF-γ) adjuvant therapy.
Case report
A 63-year-old female with past medical history of type 2 diabetes mellitus and hypertension was admitted to the emergency department in 2010 for dry cough and fever. A chest X-ray showed mediastinal lymphadenopathy and a lymph node biopsy reported necrotizing granulomas. Special stains and culture were positive for Coccidioides spp. She reported a trip to Lubbock, Texas few months earlier. Diagnosis of coccidioidomycosis was made and antifungal treatment was started with fluconazole (FLC) 400 mg daily for two months and 200 mg FLC daily for 1 year due to renal function impairment (CrCl<50 mL/min).
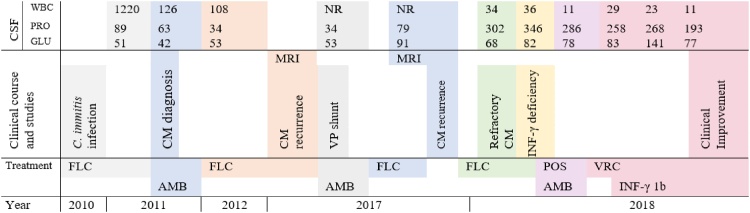
After stopping FLC in 2011 she developed fever and meningeal signs. A lumbar puncture (LP) was performed and the cerebrospinal fluid (CSF) showed WBC: 2025 cells/mm3, lymphocytes: 84 %, protein: 103 mg/mL, glucose: 53 mg/mL and positive CSF antibodies for Coccidioides spp leading to the diagnosis of CM. Treatment and clinical course are summarized in Fig. 1. She was treated with one week of IV liposomal amphotericin B (L-AmB) 5 mg/kg daily and then switched to oral FLC 800 mg daily, secondary to acute kidney injury. After two weeks of treatment, suppressive lifelong therapy with FLC 200 mg daily was indicated.
Fig. 1.
Treatments and studies.
CM: Coccidioides meningitis FLC: Fluconazole AmB: Amphotericin B MRI: Magnetic resonance, POS: Posaconazole, VP: Ventriculo-peritoneal CSF: Cerebrospinal fluid WBC: White blood cells (cells/mm3) Pro: Proteins (mg/dL) Glu: Glucose (mg/dL) NR: Not reported.
In follow-up visits no new symptoms were noticed until 2017, when she consulted for frequent falls and memory loss. An MRI revealed hydrocephalus. When the LP was performed, the opening pressure was elevated, pleocytosis was observed and recurrence of CM was presumed. She underwent a ventriculoperitoneal (VP) shunt placement and was started on IV l-AmB 5 mg/kg daily for 14 days and then switched to FLC 400 mg daily. Several months later, the patient was still complaining of frequent falling. A new MRI revealed leptomeningeal enhancement with surrounding inflammatory changes and the LP showed relapse of CM. Oral FLC 800 mg daily was started without improvement in CSF variables, leading to diagnosis of refractory persistent CM. She was assessed for immunodeficiencies. Serum levels of INF-γ were assessed (ARUP labs, Salt Lake City, USA) and were found to be low <5 pg/mL. This value was later confirmed to be <5 pg/mL. Additional workup for other CNS infections were negative.
On February 2018, she was admitted to our hospital for worsening neurological symptoms. Salvage therapy was started with IV L-AmB 5 mg/kg. VRC was avoided as the patient reported visual hallucinations. In the follow up visit one month later, she was found to be afebrile, conscious and ambulated with assistance. On February 2018 therapy was switched for VRC 6 mg/kg twice daily for the first 24 h and then 200 mg two times a day. On April 2018, INF-γ 1b once a week was initiated as adjunctive therapy, and levels were monitored.
After 6 months of therapy, VRC level was elevated (6.7 μg/mL) and she developed hallucinations, so the dose was reduced to 150 mg twice daily in August 2018 with subsequent levels of 2.4 μg/mL in October 2018. INF-γ 1b levels increased gradually up to 18 pg/mL in December 2018 with good tolerance. After 10 months of combination therapy, the patient gained weight, no hallucinations or headaches were reported.
Discussion
Meningeal coccidioidomycosis is known to have a high rate of relapse after initial treatment [1]. Current guidelines recommend oral FLC 400−1200 mg as the first line treatment, with subsequent lifelong FLC suppressive therapy [2]. However, indications for the management of refractory infection have not been well elucidated. Intrathecal AmB has been recommended as rescue therapy, but it was avoided given the high risk of toxicity. Itraconazole was also considered, but its toxicity profile, poor bioavailability and interactions with other drugs limit its use [3].
VRC is a triazole with better in-vitro antifungal activity compared to FLC [4]. In fact, in one study and few case reports, VRC is to be considered for the treatment of CM after therapeutic failure with FLC [[3], [4], [5], [6]]. In one of these reports [4], IV VRC was used initially, and then switched to oral therapy for several months, resulting in a decrease of the inflammatory parameters in the CSF with significant improvement of symptoms. Although VRC penetrates into the CNS at a similar rate of FLC, it is notable for adverse events such as neurotoxicity [4,6].
An effective host immune response to Coccidioides spp. infection requires INF-γ and IL-12 for the activation of B-cells, T-cells and phagocytes [7]. INF-γ promotes the immune response against intracellular pathogens in T-cells. Multiple animal studies and one case report describe a role of adjuvant INF-γ therapy for coccidioidomycosis [7]. Following failure of treatment, low levels of INF-γ were detected in our patient, so the option of adjuvant therapy with INF-γ 1b was considered. After 8 months of adjunctive therapy with VRC and INF-γ 1b, notable improvement was observed.
This case gives insight into probable therapeutic effects of this molecule in CM. However, further studies of INF-γ 1b as adjunctive therapy for patients with refractory CM are required.
Ethics statement
Written informed consent was given by the patient to publish the information in this case report. Ethics committee approval does not apply for this work.
Funding
This study was funded by the Grant A Star foundation.
Contribution
RH and AM took care of the patient and developed the treatment strategy. AM, RH and AD gathered the clinical data and structured the discussion.
CRediT authorship contribution statement
Alejandro De la Hoz: Conceptualization, Writing - original draft, Writing - review & editing. Alexandre Malek: Conceptualization, Writing - original draft. Rodrigo Hasbun: Conceptualization, Supervision, Writing - review & editing.
Declaration of Competing Interest
AD and AM have no conflicts of interest, RH is speaker for Merck® and Biofire® and consultant to Gilead®.
Acknowledgements
None.
Contributor Information
Alejandro De la Hoz, Email: adelahoz@javeriana.edu.co.
Alexandre Malek, Email: Alexandre.E.Malek@uth.tmc.edu.
Rodrigo Hasbun, Email: Rodrigo.Hasbun@uth.tmc.edu.
References
- 1.Nguyen C., Barker B.M., Hoover S., Nix D.E., Ampel N.M., Frelinger J.A. Recent advances in our understanding of the environmental, epidemiological, immunological, and clinical dimensions of coccidioidomycosis. Clin Microbiol Rev. 2013;26:505–525. doi: 10.1128/CMR.00005-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galgiani J.N., Ampel N.M., Blair J.E., Catanzaro A., Geertsma F., Hoover S.E. Infectious Diseases Society of America (IDSA) clinical practice guideline for the treatment of coccidioidomycosis. Clin Infect Dis. 2016;(63):e112–e146. doi: 10.1093/cid/ciw360. [DOI] [PubMed] [Google Scholar]
- 3.Kim M.M., Vikram H.R., Kusne S., Seville M.T., Blair J.E. Treatment of refractory coccidioidomycosis with voriconazole or posaconazole. Clin Infect Dis. 2011;53:1060–1066. doi: 10.1093/cid/cir642. [DOI] [PubMed] [Google Scholar]
- 4.Cortez K.J., Walsh T.J., Bennett J.E. Successful treatment of coccidioidal meningitis with voriconazole. Clin Infect Dis. 2003;36:1619–1622. doi: 10.1086/375235. [DOI] [PubMed] [Google Scholar]
- 5.Proia L.A., Tenorio A.R. Successful use of voriconazole for treatment of Coccidioides meningitis. Antimicrob Agents Chemother. 2004;48:2341. doi: 10.1128/AAC.48.6.2341.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freifeld A., Proia L., Andes D., Badour L.M., Blair J., Spellberg B. Voriconazole use for endemic fungal infections. Antimicrob Agents Chemother. 2009;53:1648–1651. doi: 10.1128/AAC.01148-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duplessis C.A., Tilley D., Bavaro M., Hale B., Holland S.M. Two cases illustrating successful adjunctive interferon-γ immunotherapy in refractory disseminated coccidioidomycosis. J Infect. 2011;63:223–228. doi: 10.1016/j.jinf.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]