Highlights
-
•
Patients with HFpEF and paradoxical low forward flow have a worse outcome.
-
•
Stroke volume index identifies HFpEF patients with paradoxical low flow.
-
•
Small LV cavity, atrial fibrillation and RV dysfunction affect paradoxical low flow.
Abstract
Background
Low flow (LF) in heart failure with preserved ejection fraction (HFpEF) is a paradox but is associated with worse prognosis. Determinants of LF in HFpEF have not been clarified but their assessment could corroborate recognition and definition of such a paradoxical condition.
Methods
A cohort of 193 patients hospitalized with HFpEF was retrospectively studied and divided in a group with LF (N = 45), defined by a left ventricular (LV) stroke volume index (SVI) < 30 ml/m2, and a group with normal flow (N = 148). A small LV cavity was pre-defined as LV end diastolic diameter index (EDDI) below median values (<25 mm/m2 for males and <26 mm/m2 for females). Right ventricular dysfunction (RVD) was defined as the ratio between tricuspid annular plane systolic excursion and systolic pulmonary artery pressure < 0.36 mm/mmHg. An endpoint of all-cause mortality was evaluated after a median follow-up of 2.4 years.
Results
RVD (OR = 7.4; P < 0.001), atrial fibrillation (AF) during echocardiography (OR = 3.26; P = 0.008), and small LV cavity (OR = 3.81; P = 0.003) were independently associated with LF. After adjusting for age, body mass index, systolic blood pressure, renal function, chronic obstructed pulmonary disease, use of ACE inhibitors/angiotensin receptor blockers, moderate tricuspid regurgitation, RVD), LF was associated with mortality (HR = 3.69; P < 0.001) whereas the combination of the determinants of LF was not.
Conclusion
Paradoxical LF in HFpEF is associated with small LV cavity, AF and RVD. None of the combination of different factors associated with LF could substitute direct assessment of LF status in predicting prognosis in this cohort.
1. Introduction
On the basis of the left ventricular ejection fraction (LV-EF) values, heart failure (HF) is currently differentiated in a form with preserved, mid-range and reduced LV-EF (HFpEF, HFmrEF and HFrEF, respectively) [1]. While in patients with HFrEF the LV anterograde flow, evaluated by stroke volume index (SVI), is expected to be low, this is not obvious in patients with HFpEF. Recently, Patel et al. [2] showed that, in a cohort of stable outpatients with HFpEF, there is substantial heterogeneity in the resting SVI distribution and that more than one-third of the study patients had a low-flow (LF) “paradoxical” phenotype. Lower resting SVI was independently associated with lower peak VO2 and higher NT-proBNP levels, both known markers of adverse prognosis in HF patients [2]. The issue of the LF paradoxical phenotype should also be considered for hospitalized patients with HFpEF. In previous studies we have shown that a reduced SVI is associated with a worse outcome in these patients but the clinical and echocardiographic determinants of the paradoxical HFpEF phenotype were not clarified [3]. Such an information would be important to fully understand and characterize the profile of hospitalized HFpEF patients with LF status and possibly guide their management. Therefore, in this study we sought to explore this issue.
2. Methods
Study patients. A cohort of adult patients hospitalized with HF and a LV-EF ≥50% was evaluated. Diagnosis of acute HF was established on the basis of clinical signs and symptoms and adjunctive investigations (e.g. chest X-rays) according to current guidelines [1]. This cohort is part of a wider group of 581 consecutive patients with suspected HF enrolled in a previous investigation [4]. Two-hundred and ninety-two patients were excluded because of LV-EF < 50%. At the hospital discharge, 231 patients had a confirmed diagnosis of HFpEF (diagnoses non confirmed as HFpEF were pulmonary embolism in 27 patients, chronic obstructive pulmonary disease exacerbation in 11, acute coronary syndrome in 5, pneumonia and sepsis in 10 and cardiac tamponade in 5 patients). At the moment of the echocardiographic analysis, 38 patients were excluded because of severe valve heart disease (including severe tricuspid regurgitation), defined on the basis of current guidelines [5]. Thus, the final study sample included 193 patients. All echocardiograms were performed at the central echocardiographic laboratory of our hospital.
Baseline characteristics. Baseline demographic and clinical patients’ characteristics and therapy at discharge were collected. Hypertension was defined on the basis of the use of antihypertensive drugs or of a previous diagnosis of hypertension. The first blood pressure at the time of admission was used. Results of blood test at time of admission were collected. Glomerular filtration rate (GFR) was calculated with the Cockroft-Gault formula and then normalized to a standard body surface area (BSA) of 1.73 m2. The BSA was calculated using the Mosteller formula.
If BNP or NT-proBNP had not been measured at the time of admission, the first available assay during hospitalization was used. Because either BNP or NTproBNP was available for each single patient, we pre-defined a unifying high natriuretic peptides (NatPs) category as BNP or NTproBNP above the upper limit of normality with the following cut-off values for the acute HF setting [6]: BNP > 100 pg/ml; NTproBNP > 450 pg/ml (age < 50 years), >900 pg/ml (age 50–75 years), >1800 pg/ml (age > 75 years); a 25% higher threshold was considered for patients in atrial fibrillation [6]. Heart rate and rhythm at the time of the echocardiographic examination were recorded.
Echocardiographic examination. A comprehensive echocardiographic, Doppler and color Doppler examination was performed using a GE Vivid 7 or E9 echo scanner (GE Health Care, Milwaukee, US) equipped with a 3.5 MHz transducer. Echocardiographic images were stored in digital format and analyzed using the EchoPAC software v. 201 (GE Health Care, Milwaukee, US). One trained physician did all the echocardiographic measures, according with the American Society of Echocardiography/European Association of Cardiovascular Imaging guidelines [7]. Echocardiographic analysis was performed without knowledge of clinical or hemodynamic data. Indexed LV end-diastolic diameter (EDDI) was measured at the level of the mitral valve leaflet tips on two-dimensional images [7]. Indexed LV end-diastolic and end-systolic volumes (EDVI and ESVI, respectively) were calculated from orthogonal apical views using the biplane Simpson method and LV-EF was derived from the standard equation [7]. Left atrial maximal volume index (LAVI) was calculated using the biplane method [7]. The relative wall thickness and LV mass index (LVMI) were assessed according to current guidelines and used to define the 4 following patterns of LV geometry: normal geometry, concentric remodeling, eccentric and concentric hypertrophy [7]. The mitral peak E wave velocity, peak A wave velocity and their ratio as well as the average annular peak e’ velocity and the E/e’ ratio were measured and calculated and information about normal or elevated LV filling pressure were derived according with the recommendations for the evaluation of LV diastolic function [8]. Cardiac valve regurgitations were graded conform to current guidelines [5]. The tricuspid annular plane systolic excursion (TAPSE) was measured on the M-mode tracing [9]. The systolic pulmonary artery pressure (sPAP) was calculated from peak tricuspid regurgitation jet velocity, using the simplified Bernoulli equation and combining this value with an estimate of the right atrial pressure [9]. The TAPSE/sPAP ratio was used as an index of right ventricular (RV) systolic function with a pre-specified cut-off of < 0.36 mm/mmHg to define RV dysfunction (RVD) [10]. For each Doppler-based and M-mode measurement, estimates were obtained from 3 cardiac cycles in sinus rhythm or 5 in atrial fibrillation (AF).
The LV forward stroke volume (SV) was calculated as the product of the LVOT outflow tract area and the time-velocity integral (TVI) of the aortic flow velocity, as previously described [3]. Because SV depends on BSA, it was indexed to the BSA (in m2) to obtain the SVI. A LF status was defined as a SVI < 30 ml/m2 according with previous studies [3], [11].
Endpoints and follow-up duration. The primary study endpoint was to identify clinical and echocardiographic variables associated with the LF phenotype in hospitalized HFpEF patients. As a secondary endpoint we evaluated all-cause mortality. The outcome status was determined by the hospital medical informatic platform, which is updated with patients who passed away in our country region. The median duration of the follow-up period was 2.4 years (interquartile range: 1.9–3.1 years).
Statistical analysis. Normal distribution was tested with the Kolmogorov-Smirnov test. Continuous variables were expressed as median values with 25th and 75th percentiles. Categorical variables were reported as counts and percentages. Baseline continuous variables across different subgroups were compared with the Mann-Whitney U test, and categorical variables were compared with the chi-square test or Fisher exact test, as appropriate. For the primary endpoint, an univariate logistic regression analysis was initially performed to determine the odds ratios (ORs) for the LF status, which are reported with 95% confidence intervals (CIs). Variables found to be statistically significant at the univariate analysis were included as covariates in the multivariate logistic regression analysis to find the determinants of LF. A matrix of correlation with correlation coefficients (R value) was derived to account for collinearity (R ≥ |0.5|). A score for LF accounting for the weight of the ORs from the multivariate logistic regression analysis was derived. For the secondary endpoint, a univariate Cox regression analysis was performed to determine the hazard ratios (HRs) for all-cause mortality, which are reported with 95% CIs. Variables found to be statistically significant at the univariate analysis were included as covariates in the multivariate Cox regression analysis. Because collinearity was found between determinants of LF and LF and between determinants of LF and RVD, three multivariate models were tested, one including LF and RVD (Model 1), one with determinants of LF (Model 2) and one with the score for LF (Model 3) in addition to the other significant covariates. C-statistic was used to compare the strength of the multivariate models. Estimated survival rates and 95% CIs were obtained using the Kaplan–Meier method and compared with the log-rank test. Small LV cavity was pre-defined as LV-EDDI below median values of the study population (25 and 26 mm/m2 for EDDI for males and females respectively). Data were analyzed using the IBM SPSS Statistics software, v. 24. Differences were considered statistically significant for P < 0.05. The study was approved by the local Ethics Committee.
3. Results
Patients characteristics. Patients’ characteristics are reported in Table 1 for the overall cohort and subgroups of patients with normal flow (NF) and LF. Patients with LF were the 23% of the overall HFpEF patients and their median SVI was 26 ml/m2. Compared to NF patients, those with LF had lower systolic blood pressure (SBP), higher heart rate and more AF during echocardiography, lower LVMI, LV-EDDI, EDVI and ESVI, higher sPAP, lower TAPSE and TAPSE/sPAP and higher percentage of deaths during follow-up (73%) (Table 1). Although concentric remodeling was more common in LF patients (P = 0.017), no significant difference in LV geometry was found between subgroups.
Table 1.
Baseline patient characteristics according to flow status.
|
Total N = 193 |
NF N = 148 (77%) |
LF N = 45 (23%) |
P | |
|---|---|---|---|---|
| Age (years) | 81 (73–87) | 81 (72–87) | 79 (75–87) | 0.772 |
| Males (n) | 87 (45%) | 67 (45%) | 20 (44%) | 0.922 |
| BMI (kg/m2) | 27 (24–31) | 27 (24–30) | 28 (24–32) | 0.198 |
| Hystory of HF (n) | 46 (24%) | 37 (25%) | 9 (20%) | 0.491 |
| Hystory of AF (n) | 85 (44%) | 60 (41%) | 25 (56%) | 0.076 |
| Previous diagnosis of CA (n) | 8 (4%) | 4 (3%) | 4(9%) | 0.087 |
| Hypertension (n) | 148 (77%) | 115 (78%) | 33 (73%) | 0.544 |
| Diabetes (n) | 52 (27%) | 45 (30%) | 7 (16%) | 0.049 |
| CKD (n) | 56 (29%) | 47 (32%) | 9 (20%) | 0.128 |
| CAD (n) | 59 (31%) | 44 (30%) | 15 (33%) | 0.646 |
| COPD (n) | 45 (23%) | 33 (22%) | 12 (27%) | 0.544 |
| NYHA class (n) | 0.291 | |||
| II | 12 (6%) | 8 (5%) | 4 (9%) | |
| III | 168 (87%) | 128 (87%) | 40 (89%) | |
| IV | 13 (7%) | 12 (8%) | 1 (2%) | |
| SBP (mmHg) | 145 (120–163) | 150 (130–170) | 130 (110–160) | 0.017 |
| DBP (mmHg) | 80 (70–90) | 80 (70–90) | 80 (65–90) | 0.327 |
| GFR at admission (ml/min/1.73 m2) | 43 (27–59) | 43 (26–59) | 43 (31–60) | 0.626 |
| NT-proBNP (pg/ml) | 3257 (1830–6273) | 3009 (1766–6273) | 4388 (2545–6840) | 0.215 |
| BNP (pg/ml) | 512 (309–855) | 496 (265–924) | 555 (354–795) | 0.368 |
| High NatPs (n) | 172 (92%) | 130 (92%) | 42 (95%) | 0.391 |
| Admission-to-echo time (days) | 4 (2–8) | 4 (2–8) | 4 (2–9) | 0.295 |
| HR during TTE (bpm) | 71 (63–80) | 70 (62–76) | 80 (66–90) | <0.001 |
| AF during TTE (n) | 61 (32%) | 36 (24%) | 25 (56%) | <0.001 |
| LVMI (g/m2) | 102 (90–115) | 104 (91–115) | 95 (82–108) | 0.04 |
| LV-EDVI (ml/m2) | 50 (41–60) | 52 (44–62) | 42 (37–49) | <0.001 |
| LV-ESVI (ml/m2) | 20 (16–25) | 21 (17–26) | 17 (14–21) | 0.001 |
| LV-EF (%) | 59 (55–64) | 58 (55–64) | 59 (56–65) | 0.617 |
| LV-EDDI (mm/m2) | 25 (23–28) | 26 (24–28) | 24 (22–25) | <0.001 |
| RWT | 0.42 (0.38–0.46) | 0.42 (0.37–0.45) | 0.44 (0.38–0.54) | 0.062 |
| LV geometry | 0.058 | |||
| Normal | 65 (34%) | 51 (34%) | 14 (31%) | - |
| Concentric remodeling | 47 (24%) | 30 (20%) | 17 (38%) | 0.017 |
| Eccentric hypertrophy | 36 (19%) | 32 (22%) | 4 (9%) | - |
| Concentric hypertrophy | 45 (23%) | 35 (24%) | 10 (22%) | - |
| E/A ratio | 0.9 (0.6–1.38) | 0.9 (0.65–1.3) | 0.7 (0.6–1.5) | 0.711 |
| E/e’ ratio | 12 (9–16) | 12 (9–15) | 14 (8–23) | 0.37 |
| LAVI (ml/m2) | 45 (37–55) | 45 (37–55) | 43 (36–58) | 0.862 |
| sPAP (mmHg) | 43 (35–50) | 40 (35–50) | 48 (35–58) | 0.045 |
| SVI (ml/m2) | 38 (31–46) | 40 (36–48) | 26 (23–28) | <0.001 |
| CI (l/min/m2) | 2.64 (2.17–3.19) | 2.85 (2.42–3.36) | 2 (1.69–2.23) | <0.001 |
| TAPSE (mm) | 19 (16–22) | 20 (18–23) | 15 (13–18) | <0.001 |
| Moderate MR (n) | 58 (30%) | 44 (30%) | 14 (31%) | 0.86 |
| Moderate AR (n) | 17 (9%) | 15 (10%) | 2 (4%) | 0.238 |
| Moderate TR (n) | 48 (25%) | 32 (22%) | 16 (36%) | 0.058 |
| TAPSE/sPAP (mm/mmHg) | 0.44 (0.32–0.59) | 0.49 (0.38–0.63) | 0.3 (0.22–0.37) | <0.001 |
| Beta-blockers at discharge (n) | 128 (66%) | 96 (65%) | 32 (71%) | 0.504 |
| ACEI/ARB at discharge (n) | 84 (44%) | 70 (47%) | 14 (31%) | 0.055 |
| MRA at discharge (n) | 70 (36%) | 54 (37%) | 16 (36%) | 0.909 |
| Duration of hospitalization (days) | 9 (5–14) | 9 (4–14) | 11 (6–15) | 0.242 |
| Deaths at follow-up (n) | 83 (43%) | 50 (34%) | 33 (73%) | <0.001 |
Baseline characteristics of the study population. Continuous variables are expressed as median (25th and 75th percentiles) and categorical variables as counts (frequency percentages). ACEI, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; AR, aortic regurgitation; ARB, angiotensin receptor blocker; BMI, body mass index; CA, cardiac amyloidosis; CAD, coronary artery disease; CI, cardiac index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; EDDI, end diastolic diameter index; EDVI, end diastolic volume index; EF, ejection fraction; ESVI, end systolic volume index; GFR, glomerular filtration rate; HF, heart failure; HR, heart rate; LAVI, left atrial volume index; LF, low flow; LV, left ventricular; LVMI, left ventricular mass index; MR, mitral regurgitation; MRA, mineralocorticoid receptor antagonist; NF, normal flow; NatPs, natriuretic peptides; NYHA, New York Heart Association; RWT, relative wall thickness; SBP, systolic blood pressure; sPAP, systolic pulmonary artery pressure; SVI, stroke volume index; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation; TTE, transthoracic echocardiography.
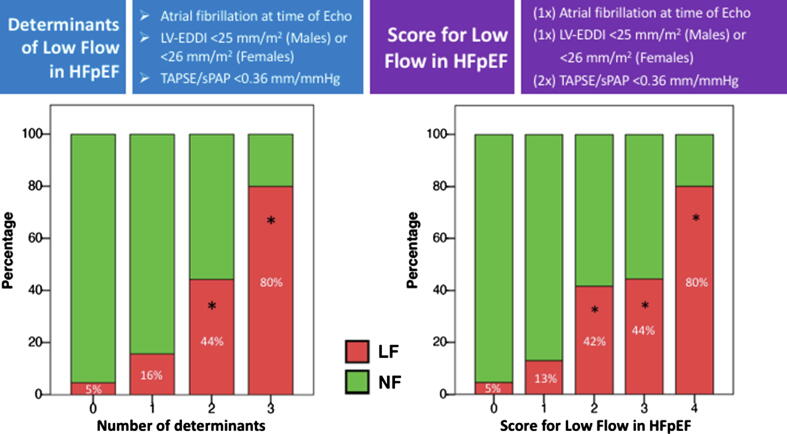
Determinants of Low Flow. Univariate and multivariate determinants of LF are reported in Table 2. On univariate logistic regression analysis, SBP, heart rate and AF during echocardiography, small LV cavity and RVD were associated with LF. On multivariate logistic regression analysis, only AF during echocardiography (OR 3.26, P = 0.008), small LV cavity (OR 3.81, P = 0.003) and RVD (OR 7.4, P < 0.001) maintained a significant association with the LF status (Table 2). No significant collinearity was found among variables (all correlation coefficients R values <|0.3|). The incremental prevalence of the LF phenotype with the growing number of independent determinants is shown in Fig. 1 (left panel). A score with the weighted LF determinants (Fig. 1, right panel) was derived accounting for the almost double OR of RVD compared to the others. A secondary logistic regression analysis was performed to investigate independent determinants of SVI ≤ 35 ml/m2 for sensitivity analysis purpose, showing similar results to that observed with the cut-off of 30 ml/m2 (Suppl Table 1).
Table 2.
Determinants of Low Flow in HFpEF at time of TTE evaluation.
| Univariate OR | P | Multivariate OR | P | |
|---|---|---|---|---|
| Age (years) | 1.01 (0.98–1.04) | 0.505 | ||
| Males (n) | 0.97 (0.49–1.89) | 0.922 | ||
| BMI (kg/m2) | 1.05 (0.99–1.11) | 0.081 | ||
| Hystory of HF (n) | 0.75 (0.33–1.7) | 0.492 | ||
| Hystory of AF (n) | 1.83 (0.94–3.6) | 0.078 | ||
| Previous diagnosis of CA (n) | 3.51 (0.84–14.66) | 0.085 | ||
| Hypertension (n) | 0.79 (0.37–1.7) | 0.544 | ||
| Diabetes (n) | 0.42 (0.18–1.02) | 0.054 | ||
| CKD (n) | 0.54 (0.24–1.21) | 0.132 | ||
| CAD (n) | 1.18 (0.58–2.41) | 0.646 | ||
| COPD (n) | 1.27 (0.59–2.73) | 0.544 | ||
| NYHA class (n) | 0.333 | |||
| SBP (mmHg) | 0.99 (0.98–1) | 0.02 | 0.093 | |
| DBP (mmHg) | 0.99 (0.97–1.01) | 0.99 | ||
| GFR at admission (ml/min/1.73 m2) | 1 (0.99–1.01) | 0.757 | ||
| High NatPs (n) | 1.94 (0.42–9.01) | 0.399 | ||
| Admission-to-echo time (days) | 1.03 (0.97–1.09) | 0.383 | ||
| HR during TTE (bpm) | 1.05 (1.03–1.08) | <0.001 | 0.114 | |
| AF during TTE (n) | 3.89 (1.94–7.81) | <0.001 | 3.26 (1.37–7.75) | 0.008 |
| LVMI (g/m2) | 0.99 (0.97–1) | 0.084 | ||
| LV-EF (%) | 6.19 (0.02–2000.05) | 0.536 | ||
| Small LV cavity | 4.34 (2.07–9.1) | <0.001 | 3.81 (1.56–9.3) | 0.003 |
| LV geometry | 0.071 | |||
| - Normal | Referent | |||
| - Concentric remodeling | 2.06 (0.89–4.78) | 0.09 | ||
| - Eccentric hypertrophy | 0.46 (0.14–1.51) | 0.197 | ||
| - Concentric hypertrophy | 1.04 (0.42–2.61) | 0.932 | ||
| High LV Pressure | 0.95 (0.43–2.13) | 0.309 | ||
| LAVI (ml/m2) | 1 (0.97–1.02) | 0.864 | ||
| Moderate MR (n) | 1.07 (0.52–2.2) | 0.86 | ||
| Moderate AR (n) | 0.41 (0.09–1.88) | 0.252 | ||
| Moderate TR (n) | 2 (0.97–4.13) | 0.061 | ||
| Right ventricular dysfunction | 10.01 (4.56–22.01) | <0.001 | 7.4 (3.13–17.49) | <0.001 |
Abbreviations as in Table 1.
Fig. 1.
Incremental prevalence of Low Flow (LF) status in HFpEF patients with the growing number of determinants (left panel) and weighted determinants (right panel) associated with LF. LV-EDDI, left ventricular end diastolic diameter index; NF, normal flow; sPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion. Overall P value < 0.001. *P < 0.05 vs. 0 and 1 risk factors.
Outcome evaluation. The cumulative survival of NF patients was 58.3%, whereas that of the LF patients was 24%. Suppl Fig. 1 shows the Kaplan-Meier survival of the overall cohort according to LF determinants (Suppl Fig. 1A), LF score (Suppl Fig. 1B) and direct echocardiographic assessment of LF (Suppl Fig. 1C). On univariate Cox regression analysis LF, LF determinants and LF score were associated with mortality (Suppl Table 2). At multivariate analysis, LF maintained significant association with mortality (HR 3.69, CIs 2.17–6.27, P < 0.001) after adjusting for other significant covariates (age, body mass index, SBP, GFR, chronic obstructed pulmonary disease, use of ACE inhibitors/angiotensin receptor blockers, moderate tricuspid regurgitation, RVD; Model 1, Table 3), whereas LF determinants did not (Model 2, Table 3). The LF score maintained significant association with mortality (HR 2.51, CIs 1.56–4.03, P < 0.001) after adjusting for age, body mass index, SBP, GFR, chronic obstructed pulmonary disease, use of ACE inhibitors/angiotensin receptor blockers and moderate tricuspid regurgitation (Model 3, Table 3). However, the multivariate model including direct assessment of LF was the strongest one in mortality prediction based on C-statistic (AUC for Model 1 = 0.823, P < 0.001; AUC for Model 3 = 0.776, P < 0.001; Table 3).
Table 3.
Multivariate Cox regression analysis with relative risk of all-cause mortality.
| Model 1 HR | P | Model 2 HR | P | Model 3 HR | P | |
|---|---|---|---|---|---|---|
| Age (per 5 years) | 1.19 (1.03–1.39) | 0.02 | 1.23 (1.06–1.42) | 0.005 | 1.27 (1.1–1.47) | 0.001 |
| BMI | 0.126 | 0.253 | 0.161 | |||
| SBP (per 10 mmHg) | 0.267 | 0.115 | 0.275 | |||
| GFR at admission (per 10 ml) | 0.8 (0.7–0.91) | 0.001 | 0.88 (0.78–0.99) | 0.027 | 0.88 (0.78–0.99) | 0.037 |
| COPD | 1.78 (1.07–2.94) | 0.026 | 0.111 | 0.269 | ||
| ACEI/ARB at discharge | 0.071 | 0.44 (0.26–0.75) | 0.002 | 0.45 (0.27–0.76) | 0.003 | |
| Moderate TR | 0.413 | 2.01 (1.25–3.23) | 0.004 | 0.497 | ||
| Right ventricular dysfunction | 1.85 (1.1–3.13) | 0.021 | Not tested | – | Not tested | – |
| Low Flow (SVI < 30 ml/m2) | 3.69 (2.17–6.27) | <0.001 | Not tested | – | Not tested | – |
| 2 LF determinants | Not Tested | – | 0.054 | Not tested | – | |
| LF score | Not Tested | – | Not tested | – | 2.51 (1.56–4.03) | <0.001 |
| Model 1 AUC | P | Model 2 AUC | P | Model 3 AUC | P | |
| C-statistic | 0.823 | <0.001 | 0.765 | <0.001 | 0.776 | <0.001 |
Abbreviations as in Table 1.
4. Discussion
Our study shows that SVI is decreased (LF phenotype) in a substantial number of patients hospitalized with HFpEF, determining a worse outcome. In these patients the LF phenotype is associated with reduced LV size, presence of AF and RVD.
The issue of paradoxical HFpEF phenotype. Identification of different phenotypes of clinical HF on the basis of LV-EF has limitations [12]. One of the most important limits is that LV-EF normalizes SV to EDV. LV-EF, therefore, does not account for low SV in patients with smaller LV cavity size. In these patients a paradoxical hemodynamic situation occurs, because ejection of blood from the LV is lower than expected in absolute terms, whereas it appears to be within normal limits in percentage. This situation is similar to that of paradoxical LF low-gradient aortic stenosis, where, despite a normal LV-EF, the small LV is responsible for the low SV [11], [13].
Prevalence and definition of LF HFpEF. Patel et al. [2] showed, in a cohort of stable outpatients with HFpEF, that 37% of the study patients had a LF phenotype. Similarly, Hachicha et al. [13] found that the paradoxical LF low-gradient pattern accounted for 35% of severe aortic stenoses with preserved LV-EF. In our study of patients hospitalized with HFpEF, the proportion of the LF phenotype was lower (23%). This is mainly related to the different SVI cut-off value utilized to define the HFpEF phenotype: <35 ml/m2 in the previously cited studies (2,13) and < 30 ml/m2 in the present study. In fact, in our investigation 79 patients (41% of the entire study cohort) had a SVI ≤ 35 ml/m2. The lower cut-off value used in our study derives from our previous observation that a SVI < 30 ml/m2 is better associated with outcome, compared to a SVI < 35 ml/m2, in patients hospitalized with HF [3]. This is also in agreement with recent observations in patients with severe aortic stenosis and normal LV-EF [11].
Determinants of the LF HFpEF. In our study, patients with the LF HFpEF phenotype were characterized by small LV (EDDI < 25 mm/m2 in males and < 26 mm/m2 in females), presence of RVD, expressed by a TAPSE/sPAP ratio < 0.36 mm/mmHg, and AF at the time of echocardiography. Interestingly, two of these factors, that is, LV-EDD and AF, were also associated with the LF phenotype in the study of Patel et al. [2] in ambulatory patients with stable HFpEF, whereas RVD has been previously found to be associated with lower SVI values in a community-based HFpEF cohort [14]. Although cause and effect cannot be established from our study, the mechanisms by which all these factors may contribute to determine the LF HFpEF phenotype are various. Regarding the LV size, it is intuitive that a smaller LV ejects a lesser amount of blood, especially when the ejection force cannot be much increased to compensate for the smaller cavity size, as in the case of a sick LV. In this study we provide, for the first time, cut-off values of LV-EDDI that can help to identify a “small” LV. This is important to precisely profiling the HFpEF patients. The mechanism by which AF acts as a determinant of the LF phenotype is most likely related to the lack of the atrial contribution to LV filling, which reduces the LV preload and end-diastolic size. Finally, although we recognize that RVD in HFpEF is mainly caused by post-capillary pulmonary hypertension, other mechanisms may contribute to RVD perpetrating LF, including direct LV under-filling due to a decreased anterograde RV-SV and impeded LV filling by increased interventricular interaction with direct compression of septum from a severely enlarged RV [15]. When all these determinants coexist, prevalence of LF HFpEF can be expected to be higher, as documented in our study cohort (Fig. 1). Also, the strength of the association between the RVD and LF HFpEF phenotype seems to be higher than the other factors (Table 2).
Effect of gender. It is known that LV cavity size is lower in females than in males [7]. In principle, therefore, an effect of gender on the relationship between LV size and LF HFpEF can be expected. In practice, however, this may not necessarily occur, depending on the measure used for assessment of LV cavity size in males and females. For example, LV-EDD is lower in females than in males, but LV-EDDI is not different or even slightly higher in females [7]. Conversely, LV-EDV and EDVI are both reduced in females [7]. In addition, the correlation between LV-EDDI and EDVI has been shown to be only moderate and even modest in females [16], thus EDVI cannot be predicted by EDDI. These observations highlight that LV-EDDI and EDVI are not interchangeable in determining a “small” LV cavity size.
Clinical implications. Assessment of LV-EF is generally the only evaluation of LV systolic function performed in patients with HF, especially in those with HFpEF. Our results, together with those of Patel at al. [2], highlight the importance of assessing also SVI in the management of patients with both stable and hospitalized HFpEF [17]. Moreover, we suggest to include measures of LV cavity size (EDDI) and RV function in the echocardiographic report to fully characterize and interpret the paradoxical HFpEF profile. Further investigations are needed to clarify whether the LF phenotype may also be a target for cardioprotective therapies in patients with HFpEF.
Study limitations and perspectives. (1) According to current guidelines [7], LV-EDD was measured at the level of the mitral valve leaflet tips on two-dimensional images. However, it has been recently shown that measurement of the EDD at the midventricular level better reflects the ellipsoid geometry of the LV and provides a more accurate estimate of the LV cavity size [18]. (2) In specific cardiomyopathies, like amyloidosis or hypertrophic cardiomyopathy, characterized by a reduced LV cavity size, paradoxical HFpEF phenotype is expected to be more prevalent. However, our study cohort was constituted by a generic group of hospitalized HFpEF patients and only 4% had confirmed diagnosis of cardiac amyloidosis, thus we could not make any specific comment on this subset of patients. (3) Strain imaging (e.g. global longitudinal strain) may reveal LV systolic dysfunction in patients with HFpEF. A future investigation is needed to clarify whether SVI is reduced in HFpEF patients characterized by impaired myocardial deformation. (4) In this study the TAPSE/sPAP ratio was used as an index of RVD instead of TAPSE alone, myocardial systolic excursion velocity (s'), and fractional area change, which have limitations. TAPSE and s’ do not reflect the contractile state of the RV but just its motion; they are also afterload dependent, as well as fractional area change. Conversely, the TAPSE/sPAP ratio examines the TAPSE versus sPAP relationship, looking at the first as a variation in length and the second as a developed pressure or force. This in vivo length-to-force relationship has been shown to provide robust clinical and prognostic insights that were stronger than those provided by TAPSE alone [10].
Conclusions. The LF phenotype, identified by a SVI < 30 ml/m2, represents about one-fourth of patients hospitalized with decompensated HFpEF and carries a worse outcome. This phenotype is associated with smaller LV cavity size, RVD and AF at the time of echocardiography. We suggest to perform the evaluation of SVI in all patients with HFpEF together with a clinical and echocardiographic profiling as discussed above on a routine basis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100539.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Ponikowski Piotr, Voors Adriaan A., Anker Stefan D., Bueno Héctor, Cleland John G.F., Coats Andrew J.S., Falk Volkmar, González-Juanatey José Ramón, Harjola Veli-Pekka, Jankowska Ewa A., Jessup Mariell, Linde Cecilia, Nihoyannopoulos Petros, Parissis John T., Pieske Burkert, Riley Jillian P., Rosano Giuseppe M.C., Ruilope Luis M., Ruschitzka Frank, Rutten Frans H., van der Meer Peter. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 2.Patel K.V., Mauricio R., Grodin J.L., Ayers C., Fonarow G.C., Berry J.D. Identifying a low-flow phenotype in heart failure with preserved ejection fraction: a secondary analysis of the RELAX trial. ESC Heart Failure. 2019;6:613–620. doi: 10.1002/ehf2.12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mele D., Pestelli G., Molin D.D., Trevisan F., Smarrazzo V., Luisi G.A. Echocardiographic evaluation of left ventricular output in patients with heart failure: a per-beat or per-minute approach? J. Am. Soc. Echocardiogr. 2020;33:135–147. doi: 10.1016/j.echo.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Mele D., Pestelli G., Dini F.L., Dal Molin D., Smarrazzo V., Trevisan F. A novel echocardiographic approach to hemodynamic phenotypes predicts outcome of patients hospitalized with heart failure. Circ. Cardiovasc. Img. 2020 doi: 10.1161/CIRCIMAGING.119.009939. [DOI] [PubMed] [Google Scholar]
- 5.Baumgartner H., Falk V., Bax J.J., De Bonis M, Hamm C., Holm P.J. 2017 ESC/EACTS guidelines for the management of valvular heart disease the task force for the management of valvular heart disease. Eur. Heart J. 2017;38:2739–2791. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 6.Ibrahim N.E., Burnett J.C., Butler J., Camacho A., Felker M., Fiuzat M. Natriuretic peptides as inclusion criteria in clinical trials. A JACC heart failure position paper. J. Am. Coll. Cardiol. HF. 2020 doi: 10.1016/j.jchf.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Lang R.M., Badano L.P., Mor-Avi V., Afilalo J., Armstrong A., Ernande L. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American society of echocardiography and the European association of cardiovascular imaging. Eur. Heart J. Cardiovasc. Imaging. 2015;16:233–271. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 8.Nagueh S.F., Smiseth O.A., Appleton C.P., Byrd B.F., Dokainish H., Edvardsen T. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European association of cardiovascular imaging. J. Am. Soc. Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Rudski L.G., Lai W.W., Afilalo J., Hua L., Handschumacher M.D., Chandrasekaran K. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American society of echocardiography. endorsed by the European association of echocardiography, a registered branch of the european society of cardiology, and the Canadian society of echocardiography. J. Am. Soc. Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Guazzi M., Bandera F., Pelissero G., Castelvecchio S., Menicanti L., Ghio S. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: an index of right ventricular contractile function and prognosis. Am. J. Physiol. Heart Circ. Physiol. 2013;305:H1373–H1381. doi: 10.1152/ajpheart.00157.2013. [DOI] [PubMed] [Google Scholar]
- 11.Rusinaru D., Bohbot Y., Ringle A., Maréchaux S., Diouf M. Tribouilloy C. Impact of low stroke volume on mortality in patients with severe aortic stenosis and preserved left ventricular ejection fraction. Eur Heart J. 2018;39:1992–1999. doi: 10.1093/eurheartj/ehy123. [DOI] [PubMed] [Google Scholar]
- 12.Mele D., Nardozza M., Ferrari R. Left ventricular ejection fraction and heart failure: an indissoluble marriage? Eur. J. Heart Fail. 2018;20:427–430. doi: 10.1002/ejhf.1071. [DOI] [PubMed] [Google Scholar]
- 13.Hachicha Z., Dumesnil J.G., Bogaty P., Pibarot P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation. 2007;115:2856–2864. doi: 10.1161/CIRCULATIONAHA.106.668681. [DOI] [PubMed] [Google Scholar]
- 14.Mohammed S.F., Hussain I., Abou Ezzeddine O.F. Right ventricular function in heart failure with preserved ejection fraction: A community-based study. Circulation. 2014;130:2310–2320. doi: 10.1161/CIRCULATIONAHA.113.008461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedberg M.K. Imaging right-left ventricular interactions. J. Am. Coll Cardiol. Img. 2018;11:755–771. doi: 10.1016/j.jcmg.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 16.Gibson P.H., Becher H., Choy J.B. Classification of left ventricular size: diameter or volume with contrast echocardiography? Open Heart. 2014;1 doi: 10.1136/openhrt-2014-000147. e000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mele D., Andrade A., Bettencourt P., Moura B., Pestelli G., Ferrari R. From left ventricular ejection fraction to cardiac hemodynamics: role of echocardiography in evaluating patients with heart failure. Heart Fail Rev. 2020;25:217–230. doi: 10.1007/s10741-019-09826-w. [DOI] [PubMed] [Google Scholar]
- 18.Chetrit M., Roujol S., Picard M.H., Timmins L., Manning W.J., Rudski L.G. Optimal technique for measurement of linear left ventricular dimensions. J. Am. Soc. Echocardiogr. 2019;32:476–483. doi: 10.1016/j.echo.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.