Abstract
The reaction mechanism of the urethane formation for both aliphatic and aromatic isocyanates in the presence of organotin dicarboxylate as a catalyst is investigated theoretically and experimentally. Modelling on a dispersion corrected DFT level of theory (B3LYP-D3) shows that an alkoxide complex is formed between organotin dicarboxylate and alcohol. This complex is the dominant catalyst for the urethane formation reaction. In this study, the interaction between the alkoxide complex and isocyanate through N-coordination is considered. By using thermochemical data, it is possible to show that aliphatic isocyanates can be more sensitive to the carboxylic ligand content of the organotin compound as a catalyst in urethane formation in non-polar solvents compared to aromatic isocyanates. The interactions of carboxylic acid, which is formed as an intermediate in the catalysis process, with isocyanate and the effects on the catalytic process are also discussed.
Keywords: Inorganic chemistry, Organic chemistry, Organotin dicarboxylate, Computational, Urethane, Catalysis, DFT B3LYP-D3
Inorganic chemistry, Organic chemistry, Organotin dicarboxylate; Computational; Urethane; Catalysis; DFT B3LYP-D3
1. Introduction
Organotin dicarboxylates have widely been used in urethane synthesis for the reaction between isocyanate and alcohol [1]. These catalysts are used for both aliphatic and aromatic isocyanates in the preparation of urethanes. Due to their wide range of applications, many studies have proposed mechanisms using classical methods [2, 3, 4, 5]. However, based on computational and experimental results, we have recently proposed mechanisms for the catalytic processes of the formation of urethanes. Organotin dicarboxylate was used as catalyst in combination with aliphatic and aromatic isocyanates [6, 7, 8]. In this work, the DFT B3LYP functional was used with LAN2DZ and 6-31G∗ basis sets for tin and light elements, respectively, and both geometrical and thermodynamic data were used to explain experimental findings. These mechanisms are different from what had been suggested previously based on using only classical methods [9]. Our calculations also revealed that the N-coordinated interaction has a lower transition state energy compared to the O-coordinated interaction in the insertion step [8].
It is well known that the hybrid B3LYP functional overestimates the enthalpy of formation when molecular sizes increase [10] and when heavy elements such as Sn are included in compounds [11]. However, DFT functionals such as M06-2X [12] or the dispersion corrected B3LYP-D3 [13] give more accurate thermochemical data compared to B3LYP. The B3LYP-D3 functional has already been used in investigations of organotin compounds [14]. We assume the B3LYP-D3 functional will give more realistic values for thermochemical data for organotin compounds that are comparable with what is reported experimentally [15] for organotin dicarboxylate catalysed urethane formation. Since the B3LYP-D3 functional takes weak dispersion interactions into account, it will help to keep interacting associated molecules together. That is particularly critical for modelling multistep reactions pathways that includes multi-molecular interactions.
It has been reported in the literature that during the organotin dicarboxylate catalysis of urethane formation, the catalyst will first be converted to an alkoxide by interacting with alcohol [16], while the alkoxide becomes the dominant catalyst. In our previous publication [6], we have shown experimentally and computationally that organotin dicarboxylate does not undergo free alkoxide formation when interacting with alcohol. In these investigations, we used a dibutyltin dicarboxylate for experimental work and dimethyl tin dicarboxylate for computational work. However, the simulations showed that the two methyl groups did not configure into an axial position which would have prevented an SN2 type interaction. This effect was further refined in the present investigation. A similar effect is observed when simulating SN2 substitution of HCN with methyl iodide [17], where a configuration inversion can be found from start to finish.
It is also reported that for reactions involving organometallic catalysts, the interaction between the reactants and the catalyst can take place from different directions [18]. Depending on the activation energies of the transition states and stability of complexes formed as intermediates in the reaction, the mechanisms could be selected. However, using the results, it should be possible to explain the experimental trends.
In this paper, urethane catalysis with organotin dicarboxylate as a catalyst will be further investigated based on DFT/B3LYP-D3 calculations. The theoretical studies will be combined with experimental NCO depletion results and 1H-NMR results. The following two steps will be considered: 1) The formation of the organotin alkoxide complex which acts as the dominant catalyst, 2) The interaction between the organotin alkoxide complex with isocyanate to form the urethane and regenerate the active catalyst. The effect of the carboxylic acid interaction with isocyanate on the organotin dicarboxylate catalysis during the urethane formation will also be discussed.
2. Computational
2.1. Computational methods
In this work, DFT/B3LYP-D3 was used with an EPC LANL2DZ basis set for tin and the Pople 6-31G∗ basis sets for all other elements. All calculations were performed using Gaussian 09 [19] and Spartan 14 [20] software packages. Methyl alcohol, methyl isocyanate and dimethyl tin dicarboxylate were used as model compounds in these calculations.
2.2. Transition state location
Transition states were obtained by using procedures available in the software package Gaussian 09 [19]. Depending on the case under investigation, an appropriate method was selected. In general, the transition state was determined by bringing the reaction centres close together while optimizing the total energy of the system in a step – by – step process. If a chemical reaction was taking place during the optimization process, the energy went through a maximum. Then the structure at the maximum energy was further refined using the Berny optimization procedure [21] to locate the transition state. However, in some situations, it was necessary to use QST2 or QST3 optimization procedures [22, 23]. The transition state was confirmed by using frequency energy calculations, resulting in one single negative frequency. However, the successful completion of a transition state search did not necessarily guarantee that the right transition structure that connects the reactants and products was found. The transition structure was further confirmed by carrying out intrinsic reaction coordinate calculations [24] to determine reactants and product. The structure related to minimum energy on the product side for a corresponding transition state is considered as the pre-complex for that reaction.
2.3. Solvent effects
In practical applications, most of the chemical reactions take place in solvent media. It is also well known that solvents have significant effects on reaction mechanisms [25]. In the present paper, the conductor-like polarizable continuum model (CPCM) solvent model [26] was used to simulate solvent effects on the products formed, and their stabilities were discussed. Toluene and water are selected to simulate the effect of a non-polar and polar solvent, respectively.
2.4. Thermochemical parameters
Thermochemical properties of selected structures at 298.15 K and 1 atm were determined by carrying out frequency calculations [27].
In the present paper, the difference in thermochemical parameters such as enthalpy (ΔH) and Gibbs free energy (ΔG) were calculated with respect to starting material for the selected structure.
3. Experimental section
3.1. Materials
Hexamethylene diisocyanate was obtained from Bayer, Germany. Phenyl isocyanate, Cyclohexyl isocyanate, n-butanol, xylene, diethylene glycol monomethyl ether (DEGMME), DBTDA and acetic acid were obtained from Merck, Germany. DBTDB was obtained from Gelest Inc., USA.
3.2. NCO depletion
HDI was reacted with diethylene glycol monomethyl ether in xylene at 25 °C using different organotin compounds. In this experiment, 0.209 equivalents of each reactant were used with 0.183∙10−3 mol L−1 tin concentration of different organotin compounds as a catalyst or as specified. The total initial volume of the mix was maintained at 138 mL.
Phenyl isocyanate was reacted with diethylene glycol monomethyl ether in xylene at 25 °C using different organotin compounds. In this experiment, 0.209 equivalents of each reactant were used with 0.037∙10−3 mol L−1 tin concentration of different organotin compounds as a catalyst or as specified. The total initial volume of the mix was maintained at 138 mL.
Samples were drawn from the reaction mix at 10 min intervals and the % NCO was determined as to the method specified in ASTM D 2572-97 [28].
3.3. NCO depletion rate determination
NCO depletion with time data for the reaction between isocyanate and alcohol in the presence of different catalyst data were obtained from previous experiments [6, 7]. By using this data, reaction rates at selected points for each reaction were calculated to determine the NCO depletion rate with time. The calculations were done using OriginPro software [29]. Then the rate data was plotted against time.
3.4. 1H-NMR spectroscopy
Cyclohexyl isocyanate was reacted with n-butanol and sec-butanol at stoichiometric ratio and samples were subject to 1H-NMR analysis using chloroform-d as the solvent. Similarly, DBTBA (dibutyltin butoxy acetate) was first mixed with sec-butanol and then reacted with cyclohexyl isocyanate at (1:1:1) mole ratio to form urethane. Then the product was subject to 1H-NMR analysis using chloroform-d as a solvent. DBTBA was synthesised by reacting with DBTDA (dibutyltin diacetate) + DBTDB (dibutyltin dibutoxide) at a (1:1) mole ratio by heating at 60 °C overnight [30]. The product was used without further purification. More details of the preparation of these compounds are given in our previous publication [7].
NMR spectra were recorded on Bruker DRX 400 MHz NMR spectrometer at ambient temperature at 399.89 MHz (1H) with chemical shifts (δ, ppm) reported relative to the solvent peaks of the deuterated solvent.
4. Results and discussion
4.1. Alkoholysis reaction
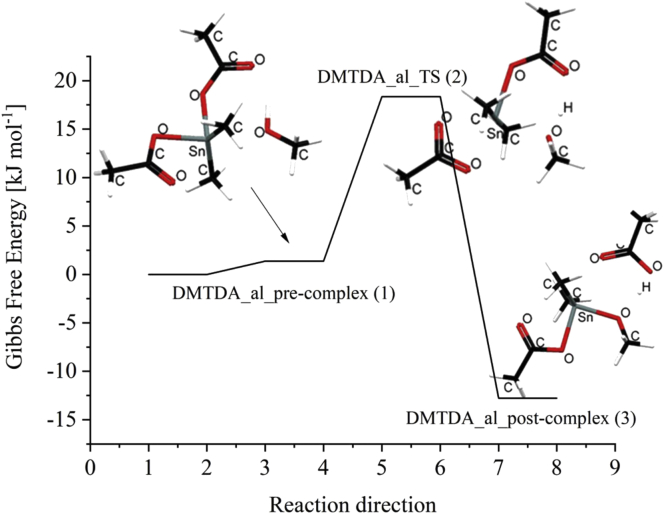
In order to simulate the alcoholysis reaction, the interaction between methyl alcohol and dimethyl tin dicarboxylate was investigated. Previously we have used the interaction between methyl alcohol and dimethyl tin dicarboxylate by reducing the distance between the oxygen of alcohol and tin atom step by step while optimizing the energy [6]. These simulations did not result in a SN2 type interaction with a valid transition state. However, in the present investigation, the interaction was simulated by optimizing the transition state as a starting point. The transition state was built by orienting the two methyl groups in an axial position and placing the alcohol oxygen directing towards the tin centre and the OH hydrogen towards the carboxylic oxygen. Figure 1 shows the complete free energy diagram of the interacting path. The molecular assembly between organotin dicarboxylate and alcohol at position (1) cannot interact with an isocyanate to give a urethane. As to the energy diagram, position (3) has the lowest free energy; therefore, it can be taken as the active species to interact with isocyanate. Therefore, in the present investigation, we have omitted the condition (a) and condition (b) stages and considered only (1), (2) and (3). However, if an axial orientation for the substituents on the tin atom is not possible due to their bulkiness, then the reaction may follow our previous mechanism.
Figure 1.
Complete free energy diagram for the interaction alkoxide formation.
Figure 2 shows the transition state DMTDA_al_TS1(2) for this interaction. It shows that on one side the alcohol molecule is complexed with the organotin dicarboxylate shown as the DMTDA_al_pre-complex (1); on the product side the alkoxide is formed by alcohol oxygen bonding with the tin atom shown as the DMTDA_al_post-complex(3). The DMTDA_al_post-complex(3) contains no free alkoxide, but a complex between an alkoxide and carboxylic acid can be found instead. This may be the reason why no free alkoxide absorption peak in the FTIR spectra could be found previously [6]. It is also interesting to see that the free energy of the alkoxide complexed carboxylic acid is lower than the alcohol complex, as shown in Figure 2. Table 1 gives enthalpy and Gibbs free energy data for the interaction in the gas phase.
Figure 2.
Molecular structures of DMTDA_al_pre-complex (1), transition state DMTDA_al_TS (2), and DMTDA_al_post complex (3) and free energy diagram for the alcoholysis of organotin dicarboxylate.
Table 1.
Enthalpy and Gibbs free energy differences for alcoholysis of organotin di- carboxylate.
| Complex | Enthalpy ΔH [kJ mol−1] |
Gibbs Free energy ΔG [kJ mol−1] |
|---|---|---|
| DMTDA_al_pre-complex (1) | -51.28 | 1.37 |
| DMTDA_al_TS1 (2) | -39.49 | 18.35 |
| DMTDA_al_post-complex (3) | -58.55 | -12.79 |
Table 2 shows the enthalpy and Gibbs free energies calculated in polar (water) and non-polar (toluene) media. It can be seen that the free energy difference between DMTDA_al_pre-complex (1) and DMTDA_al_post-complex (3) of the interaction between DMTDA and methanol is higher in water (-7.79 kJ mol−1) than toluene (-10.28 kJ mol−1). However, as the transition state energy of DMTDA_al_TS1 (2) is less than 80 kJ mol−1, both complexes can exist at ambient temperature. The DMTDA_al_post-complex(3) is taken as the dominant catalyst, and the N-coordinated interaction of the isocyanate with the catalyst as the main reaction pathway is considered.
Table 2.
Enthalpy and Gibbs free energy differences for alcoholysis of organotin dicarboxylate in solvent media.
| Complex | toluene |
water |
||
|---|---|---|---|---|
| Enthalpy ΔH [kJ mol−1] | Gibbs Free energy ΔG [kJ mol−1] | Enthalpy ΔH [kJ mol−1] |
Gibbs Free energy ΔG [kJ mol−1] | |
| DMTDA_al_pre-complex (1) | -45.02 | 11.78 | -37.92 | 10.96 |
| DMTDA_al_TS1 (2) | -30.57 | 30.63 | -22.25 | 30.77 |
| DMTDA_al_post-complex (3) | -49.36 | 1.50 | -40.06 | 3.17 |
4.2. Methyl isocyanate N-coordinated interaction with alcoholised organotin dicarboxylate complex
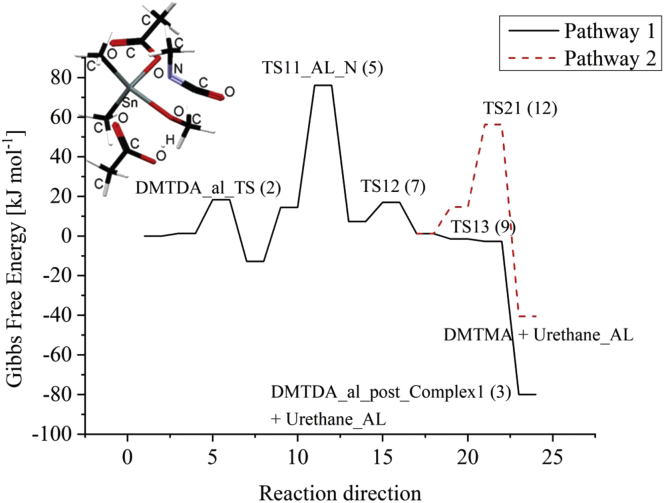
We assume that the major part of the alcoholised organotin dicarboxylate is the alkoxide complex that interacts with the methyl isocyanate. The interaction can be either in N- or O- coordinated orientation. The transition state for O-coordinated interactions for both aliphatic and aromatic isocyanates gave higher activation free energies compared to N-coordinated interactions [8]. Therefore, we will focus on the N-coordinated form. Figure 3 shows the free energy diagram for the interaction. Figure 3 also shows the N-coordinated transition state TS11_AL_N (5) for methyl isocyanate. The interaction will lead to the formation of urethane following two different pathways, as explained in the following paragraph. Tables 3 and 4 show the enthalpy and Gibbs free energy differences for the intermediate and products formed during these interactions.
Figure 3.
Molecular structure of transition state TS11_AL_N (5) for aliphatic isocyanate and free energy diagram for urethane formation for methyl isocyanate using DMTDA as catalyst.
Table 3.
Enthalpy and Gibbs free energy differences for pathway 1 of aliphatic isocyanate and alcohol reaction when DMTDA use as a catalyst.
| Molecular structure | Enthalpy ΔH [kJ mol−1] |
Gibbs Free energy ΔG [kJ mol−1] |
|---|---|---|
| DMTDA_al_pre_complex (1) | -51.28 | 1.37 |
| DMTDA_al_TS (2) | -39.49 | 18.35 |
| DMTDA_al_post_complex (3) | -58.55 | -12.79 |
| Pre-complex11 (4) | -80.21 | 14.42 |
| TS11_AL_N (5) | -32.52 | 76.12 |
| Post_min_TS11 (6) | -100.04 | 7.35 |
| TS12 (7) | -93.60 | 17.02 |
| Pre-complex_acid (8) | -107.10 | 1.27 |
| TS13 (9) | -113.38 | -1.40 |
| Post_min_TS13 (10) | -115.55 | -2.61 |
| DMTDA_al_post_complex1(3) +Urethane_AL | -177.53 | -80.02 |
Table 4.
Enthalpy and Gibbs free energy differences for pathway 2 of aliphatic isocyanate and alcohol reaction when DMTDA use as a catalyst.
| Molecular structure | Enthalpy ΔH [kJ mol−1] |
Gibbs Free energy ΔG [kJ mol−1] |
|---|---|---|
| Pre-complex_alcohol (11) | -86.20 | 14.70 |
| TS21 (12) | -54.35 | 56.28 |
| DMTMA + Urethane_AL | -89.88 | -40.52 |
In partway 1, the interaction will lead to the formation of urethane by an interaction between the organotin carbamate formed with the free carboxylic acid molecule by first forming the pre-complex_acid (8). In pathway 2, the organotin carbamate will interact with alcohol which is present in stoichiometric excess by first forming the pre_complex_alcohol (11) to give the free organotin alkoxide and the urethane. The two pathways will be governed by the stability of complexes (8) and (11), respectively, formed between free carboxylic acid and the organotin carbamate or the alcohol and the organotin carbamate. It can be seen from Tables 3 and 4 that the free energy difference for the formation of complex (8) (1.3 kJ mol−1) is significantly less than of complex (11) (14.7 kJ mol−1).
It is also interesting to see that the Gibbs free energy for the transition state TS13 (9) for the interaction between carboxylic acid and tincarbamate is less than transition state TS21 (12) for the interaction between alcohol and tincarbamate. This difference is 57.7 kJ mol−1. From these results, it is evident that the aliphatic isocyanate can be more sensitive to the carboxylate ligand content on the organotin compound.
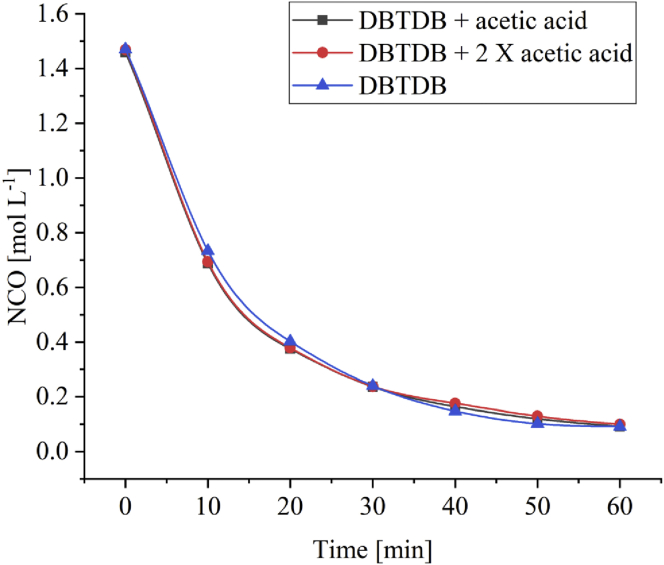
The depletion of the NCO content with time is shown in Figure 4 for hexamethylene diisocyante as aliphatic isocyanate during the urethane formation using different catalysts when xylene is used as solvent. The DBTDB + 2 X acetic acid combination used as catalyst shows the highest depletion rate, whereas pure DBTDB as a catalyst has the lowest depletion rate. In all experiments, the tin content was maintained at a constant level. These results are similar to previous results [6], where different aliphatic isocyanate monomers were used. Therefore, the results further confirm the sensitivity of the acetic acid ligands on the reaction rate of the aliphatic isocyanate.
Figure 4.
NCO depletion with time for the reaction between hexamethylene diisocyante and DEGMME in the presence of different catalysts using xylene as the solvent.
4.3. Phenyl isocyanate N-coordinated interaction with alcoholised organotin dicarboxylate complex
In the following work, phenyl isocyanate was interacted with alcoholised organotin dicarboxylate, DMTDA_al_post_complex (3) as an N-coordination interaction. Figure 5 shows the free energy diagram for the interaction, and also the transition state TS11_AR_N (14) for the N-coordinated interaction between DMTDA alcohol complex and phenyl isocyanate. Similar to methyl isocyanate, the interaction could follow two different pathways. In pathway 1, the alcoholised organotin dicarboxylate is regenerated, while in pathway 2 the organotin alkoxide is formed along with urethane. Tables 5 and 6 show the enthalpies and Gibbs free energies for the intermediates and products that were formed. It can be seen that in pre-complex_acid (17) (formed between carboxylic acid and organotin carbamate) and pre-complex_alcohol (22) (formed between alcohol and organotin carbamate), the free energy difference (11.3 kJ mol−1) is less compared to what was found for aliphatic isocyanate (13.4 kJ mol−1).
Figure 5.
Molecular structure of transition state TS11_AR_N (14) for aliphatic isocyanate and free energy diagram for urethane formation for phenyl isocyanate using DMTDA as catalyst.
Table 5.
Enthalpy and Gibbs free energy differences for pathway 1 of phenyl isocyanate and alcohol reaction when DMTDA use as a catalyst.
| Molecular structure | Enthalpy ΔH [kJ mol−1] |
Gibbs Free energy ΔG [kJ mol−1] |
|---|---|---|
| DMTDA_MA complex (1) | -51.28 | 1.37 |
| DMTDA_al_TS (2) | -39.49 | 18.35 |
| DMTDA_al_post_complex (3) | -58.55 | -12.79 |
| Pre-complex11 (13) | -79.24 | 17.33 |
| TS11_AR_N (14) | -21.08 | 89.28 |
| Post_min_TS11 (15) | -84.01 | 22.28 |
| TS12 (16) | -80.81 | 30.38 |
| Pre-complex_acid (17) | -92.67 | 16.16 |
| TS13 (18) | -95.05 | 18.96 |
| Post_min_TS13 (19) | -96.64 | 15.47 |
| Post_min_al_complex (20) | -168.50 | -0.63 |
| TS14 (21) | -170.66 | 1.04 |
| DMTDA_al_post_complex1(3) +Urethane_AR | -174.30 | -75.74 |
Table 6.
Enthalpy and Gibbs free energy differences for pathway 2 of phenyl isocyanate and alcohol reaction when DMTDA use as a catalyst.
| Molecular structure | Enthalpy ΔH [kJ mol−1] |
Gibbs Free energy ΔG [kJ mol−1] |
|---|---|---|
| Pre-complex_alcohol (22) | -71.37 | 27.41 |
| TS21 (23) | -39.63 | 69.56 |
| DMTMA(24)+ Urethane_AR | -86.67 | -36.25 |
It is also seen from Tables 5 and 6 that the free energy difference for transition state TS13 (18) (interaction between carboxylic acid and tin carbamate) and transition state TS21 (23) (interaction between alcohol and tin carbamate) 50.6 kJ mol−1 is less than for the aliphatic system (57.68 kJ mol−1). Therefore, in an environment where alcohol is in significant excess, the chances of following pathway 2 are higher to result in the formation of organotin alkoxide and urethane for the aromatic system compared to the aliphatic system. This may make aromatic isocyanates less sensitive to carboxylic ligand content on the organotin catalysts. Therefore, by considering all thermochemic data, it can be concluded that an aliphatic isocyanate will be more sensitive to a carboxylic ligand content attached on the tin catalyst than aromatic isocyanate.
Figure 6 shows the NCO depletion with time for different organotin compounds when used as a catalyst in urethane formation of aromatic isocyanate in xylene. In all experiments, the tin content was maintained at a constant level. These results are similar to what we obtained in our previous experiments [6], and it further confirms the effect that the carboxylic ligand content in the catalyst is negligible to the reaction rate of aromatic isocyanates.
Figure 6.
NCO depletion with time for the reaction between Phenyl isocyanate and DEGMME in the presence of different catalyst using xylene as solvent.
4.4. Solvent effects in selection of reaction path
For both aliphatic and aromatic systems, the insertion step transition states were optimized for both N-coordinated and O-coordinated interactions in a polar and non-polar medium. Table 7 shows the enthalpy and Gibbs free energy differences for these transition states. From the results, it can be concluded that the most probable interaction is through N-coordinated interactions. However, for the aromatic isocyanate, the transition state free energy difference for N-coordinated and O-coordinated interactions is less than for the aliphatic isocyanate. Therefore, it may be possible for aromatic isocyanates to go through O-coordinated interactions depending on the steric effects on each interacting molecule.
Table 7.
Enthalpy and Gibbs free energy for transition states in polar and non-polar medium.
| Molecular structure | Enthalpy ΔH [kJ mol−1] |
Gibbs Free energy ΔG [kJ mol−1] |
|---|---|---|
| TS11 AL_N_T (25) | -21.27 | 90.98 |
| TS11 AL_N_W (26) | -10.58 | 92.86 |
| TS11 AL_O_T (27) | 7.76 | 123.75 |
| TS11 AL_O_W (28) | 15.57 | 122.84 |
| TS11 AR_N_T (29) | -10,01 | 103.90 |
| TS11 AR_N_W (30) | 0.26 | 105.99 |
| TS11 AR_O_T (31) | 6.87 | 124.12 |
| TS11 AR_O_W (32) | 15.19 | 124.29 |
Tables 8 and 9 show the Gibbs free energies for the complex formation between carboxylic acid and alcohol with organotin carbamate for aliphatic and aromatic systems in different solvents. The difference in the Gibbs free energy between the pre_complex_acid T(33) and pre_complex_alcohol T(37) for the aliphatic system is higher in non-polar solvents (10.2 kJ mol−1) compared to the pre_complex_acid W(34) and pre_complex_alcohol W(38) in a polar solvent (5.8 kJ mol−1). This means that although the concentration of carboxylic acid is low, it is still possible to compete with alcohol and interact with organotin carbamate in non-polar solvents. Since the difference between pre_complex_acid W(34) and pre_complex_alcohol W(38) is less in a polar solvent, the reaction may follow pathway 2, leading to alkoxide formation in the presence of significant excess of alcohol. It is also interesting to see that moving from a non-polar to a polar solvent, the difference between the transition state TS13 T(35), TS21 T(39) is 56.3 kJ mol−1 and TS13 W(38), TS21 W(40) 55.8 kJ mol−1, respectively, indicating that in an environment with an excess of alcohol, the chances of forming the alkoxide is higher. However, for an aromatic system, the difference in the Gibbs free energy for the complexes pre_complex_acid T(41) and pre_complex_alcohol T(45) (8.9 kJ mol−1) is slightly less compared to the aliphatic system (10.2 kJ mol−1). This means that chances of carboxylic acid reacting with organotin carbamate in an environment with a high concentration of alcohol is less compared to an aliphatic system. It is also seen that the difference in Gibbs free energy for the formation of pre_complex_acid W(42) and pre_complex_alcohol W(46) (2.1 kJ mol−1) for the aromatic system is also less in polar solvents compared to pre_complex_acid W(34) and pre_complex_alcohol W(37) (5.8 kJ mol−1) for an aliphatic system.
Table 8.
Enthalpy and Gibbs free energy differences for the interaction with organotin carbamate with carboxylic acid and alcohol in solvent environment for aliphatic system.
| Complex | Toluene |
Water |
||
|---|---|---|---|---|
| Enthalpy ΔH [kJ mol−1] |
Gibbs Free energy ΔG [kJ mol−1] |
Enthalpy ΔH [kJ mol−1] |
Gibbs Free energy ΔG [kJ mol−1] |
|
| Pre-complex_acid T(33) and W(34) | -97.33 | 13.44 | -90.02 | 15.14 |
| TS13T(35) and W(36) | -103.19 | 13.73 | -92.80 | 15.36 |
| Pre-complex_alcohol T(37) and W(38) | -81.32 | 23.66 | -78.14 | 20.97 |
| TS21T(39) and W(40) | -44.97 | 70.05 | -35.57 | 71.13 |
Table 9.
Enthalpy and Gibbs free energy differences for the interaction with organotin carbamate with carboxylic acid and alcohol in solvent environment for aromatic system.
| Complex | Toluene |
Water |
||
|---|---|---|---|---|
| Enthalpy ΔH [kJ mol−1] |
Gibbs Free energy ΔG [kJ mol−1] |
Enthalpy ΔH [kJ mol−1] |
Gibbs Free energy ΔG [kJ mol−1] |
|
| Pre-complex_acid T(41) and W(42) | -84.99 | 27.56 | -77.19 | 28.24 |
| TS13 T(43) and W(44) | -86.32 | 32.37 | -77.43 | 33.50 |
| Pre-complex_alcohol T(45) and W(46) | -66.69 | 36.44 | -71.49 | 30.29 |
| TS21 T(47) and W(48) | -31.78 | 80.36 | -24.23 | 80.66 |
Similar to the aliphatic system, when moving from a non-polar to a polar solvent, the difference the in transition state energy for TS13 T(43), TS21 T(47) is 48.0 kJ mol−1 and TS13 W(44), TS21 W(48)) is 47.2 kJ mol−1, respectively. This is less than for the aliphatic system (56.3 and 55.3 kJ mol−1, respectively). Therefore, moving from a non-polar to polar medium, the partway is more controlled by the pre-complex that is formed with the tincarbamate for both aliphatic and aromatic systems. It can also be said that in a polar medium, the dominant catalyst will be the alkoxide for both types of isocyanates.
Thermochemical data also show that the reaction between a carboxylic acid and an organotin carbamate for an aromatic system is low in an environment with a significant excess of alcohol compared to the carboxylic acid concentration is generated from the catalyst. Therefore, for an aromatic system, the chance of following pathway 2 for both polar and non-polar system is higher, which is in agreement with experimental results [6, 7].
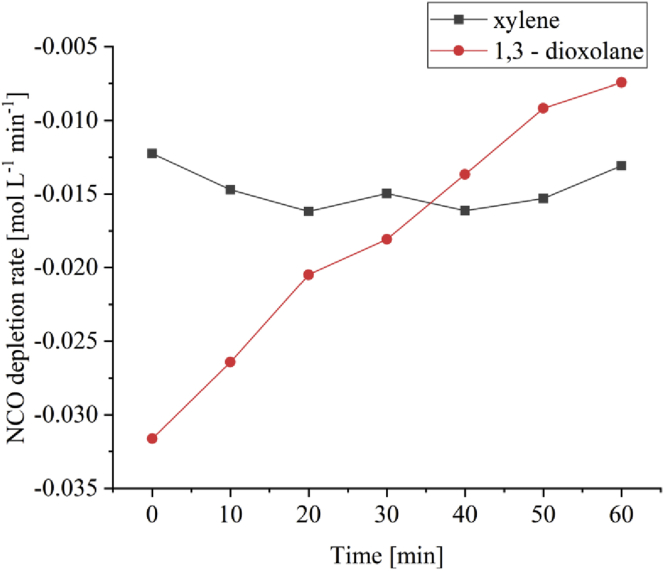
We have also reported that a primary aliphatic isocyanate has a higher reactivity in a polar solvent compared to a non-polar solvent [7]. Figure 7 shows the NCO depletion rate vs time plots for an aliphatic isocyanate in urethane formation when DBTDA is used as the catalyst in different solvents using calculated from raw data obtained from our previous work [7, 8]. When xylene is used as the solvent, the rate is constant with time, which may be due to the reaction rate been less sensitive to the concentration of the reactants. However, by using 1,3-dioxolane as the solvent, the results show a change from lower to a higher depletion rate with increasing time. The reaction rate seems to be more sensitive to reactants in the mix. This may be due to the interaction of the alcohol with carbamate that is formed to liberate the urethane when alkoxide is the dominant catalyst in a polar medium, as explained previously.
Figure 7.
NCO depletion rate with time for the reaction between Desmodur N3390 BA/SN and n-butanol as stoichiometric amounts in different solvents when DBTDA was used as a catalyst (0.183・10−3 mol L−1 tin content).
Urethane can also be formed if the isocyanate interacts with un-complexed alkoxide. Although the thermochemical data show that most of the DMTMA should be found as a dimer (DMTMA_dimer), it could also be found as a complex with alcohol (DMTMA_al) or in its free form (DMTMA). Table 10 shows the enthalpies and Gibbs free energies for the DMTMA_dimer and the DMTMA_al complex in non-polar (DMTMA_dimer_T(49), DMTMA_al_T(51)) and polar (DMTMA_dimer_W(50), DMTMA_al_W(52)) media. The Gibbs free energies indicate that these products are less stable in a polar medium which suggests that the concentration of the un-complexed DMTMA is higher in a polar medium than a non-polar medium.
Table 10.
Enthalpy and Gibbs free energy differences for DMTMA complexes.
| Complex | Toluene |
Water |
||
|---|---|---|---|---|
| Enthalpy ΔH [kJ mol−1] |
Gibbs Free energy ΔG [kJ mol−1] |
Enthalpy ΔH [kJ mol−1] |
Gibbs Free energy ΔG [kJ mol−1] |
|
| DMTMA_dimer_T(49) and W(50) | -119.33 | -62.76 | -113.11 | -58.74 |
| DMTMA_al_T(51) and W(52) | -40.90 | 3.04 | -37.89 | 5.54 |
Considering the Curtin Hammett law [31], the isocyanate can interact with free DMTMA, also forming the carbamate and subsequently urethane. Therefore, both transition states DMTMA_al_NCO_TSW(54) and DMTMA_NCO_TSW(56), shown in Table 11, can take place to give urethane. These free energy data show the resultant DMTMA_al_NCO_TSW(54) and DMTMA_NCO_TSW(56)) of the interactions in the polar medium will have a higher catalytic action compared if the reaction pass through a reaction TS11 AL_N_T(25) pathway suggested in a non-polar medium. This is further confirmed by 1H-NMR spectroscopy.
Table 11.
Transition state energies for aliphatic isocyanate interacting with different organotin catalyst complexes.
| Complex | Toluene |
Water |
||
|---|---|---|---|---|
| Enthalpy ΔH [kJ mol−1] |
Gibbs Free energy ΔG [kJ mol−1] |
Enthalpy ΔH [kJ mol−1] |
Gibbs Free energy ΔG [kJ mol−1] |
|
| TS11 AL_N_T (25) and W(26) | -21.27 | 90.98 | (-10.58) | (92.87) |
| DMTMA_al_NCO_TS T(53) and W(54) | (-22.40) | (84.95) | -14.20 | 92.45 |
| DMTMA_NCO_TS T(55) and W(56) | (1.61) | (56.23) | 5.60 | 59.34 |
4.5. 1H-NMR analysis
The product mix that was formed by reacting DBTBA + CHI + sec-butanol was analysed using 1H-NMR spectroscopy. Figure 8 shows the results of the analysis. Figure 8 a) shows the resonance at 4.05 ppm of the hydrogens on the carbon attached to the alkoxy oxygen on n-butanol that formed urethane with CHI. Figure 8 b) shows the resonance at 4.73 ppm of the hydrogen on the carbon attached to the alkoxy oxygen of sec-butanol that formed urethane with CHI. By comparing these two with the 1H-NMR of the reaction product of DBTBA + CHI + sec-butanol at (1:1:1) mole ratio shows the resonance of the hydrogens as marked in Figure 8 c). The NMR spectrum of DBTBA and urethane from CHI and n-butanol showed that hydrogen resonance on butoxy carbon from urethane interferes with hydrogens resonance on carbon of butoxy group of DBTBA.
Figure 8.
1H NMR spectra on the carbon link to alkoxy oxygen a) CHI + n-butanol, b) CHI + sec-butanol, c) DBTBA + CHI + sec-butanol and d) DBTBA.
However, by comparing the marked peak areas (1:1) in c), it is possible to say that both products could be formed in the reaction. This could be due to the formation of urethane by a) isocyanate interacting with the organotin alkoxide and b) through a tri-molecular interaction involving the free alcohol as suggested previously [7]. Figure 8 d) shows the 1H-NMR resonance at 4.06 ppm for the hydrogen on the carbon next to the oxygen of the butoxy group of DBTBA.
4.6. The effect of the reaction between isocyanate and carboxylic acid on organotin dicarboxylate catalysis of urethane formation
It is reported that isocyanates react with carboxylic acid forming an amide [32, 33]. The reaction goes through two stages. First, it forms an adduct to give an N-carboxyanhydride which then undergoes a condensation reaction by the elimination of carbon dioxide to give an amide. The first step of the reaction is a low energy equilibrium controlled reaction. Modelling of organotin dicarboxylate catalysis of urethane formation showed that carboxylic acid is detached from the tin centre to give an organotin carbamate. The liberated carboxylic acid can associate with the organotin carbamate or move to the medium and interact with isocyanate to give an N-carboxyanhydride.
Tables 12 and 13 show the enthalpy and Gibbs free energy data for the pre-complex and transition state for the formation of N-carboxyanhydride from aliphatic isocyanate (methyl isocyanate) and aromatic isocyanate (phenyl isocyanate) in different solvents.
Table 12.
Enthalpy and Gibbs free energy differences for the reaction between methyl isocyanate and acetic acid.
| Complex | Toluene |
Water |
||
|---|---|---|---|---|
| Enthalpy ΔH [kJ mol−1] | Gibbs Free energy ΔG [kJ mol−1] | Enthalpy ΔH [kJ mol−1] |
Gibbs Free energy ΔG [kJ mol−1] | |
| aliphatic_iso_acid_pre complex T(57), W(58) | -23.94 | 9.46 | -22.93 | 10.17 |
| aliphatic_iso_acid TS1 T(59), W(60) | 16.99 | 67.05 | 21.48 | 71.68 |
| aliphatic_N-carbxyanhydride T(61), W(62) | -68.60 | -16.47 | -67.02 | -13.27 |
Table 13.
Enthalpy and Gibbs free energy differences for the reaction between phenyl isocyanate and acetic acid.
| Complex | Toluene |
Water |
||
|---|---|---|---|---|
| Enthalpy ΔH [kJ mol−1] | Gibbs Free energy ΔG [kJ mol−1] | Enthalpy ΔH [kJ mol−1] |
Gibbs Free energy ΔG [kJ mol−1] | |
| aromatic_iso_acid_pre complex T(63), W(64) | -23.89 | 13.71 | -21.90 | 15.72 |
| aromatic_iso_acid TS1 T(65), W(66) | 17.57 | 69.92 | 22.30 | 74.34 |
| aromatic_N-carboxyanhydride T(67), W(68) | -62.25 | -8.65 | -60.66 | -8.22 |
It can be seen that the differences in the Gibbs free energy data for the isocyanate and acid interaction pre-complex (Table 8) of pre-complex_acid T(33) and W(34) and aliphatic_iso_pre complex T(57) and W(58) (Table 12) are -3.98 kJ mol−1 and -4.97 kJ mol−1, respectively, for the aliphatic isocyanate. Similarly, comparing the Gibbs free energy data of the aromatic isocyanates of Pre_complex_acid T(41) and W(42) (Table 9) with the aromatic_iso_pre_complex T(63) and W(64) (Table 13) results in differences of -13.85 kJ mol−1 and -12.52 kJ mol−1, respectively. This indicates that the aromatic system will have a higher chance of losing of carboxylic acid from the tin centre (organotin carbamate/carboxylic acid).
Comparing the transition state Gibbs free energy of TS T(35) and W(36) (Table 8) with TS T(59) and W(60) (Table 12) for the aliphatic system results in differences of 53.32 kJ mol−1 and 56.32 kJ mol−1, respectively. Similarly for the aromatic system, the Gibbs free energy for TS13 T(43) and W(44) from Table 9 and with T(65) and W(66) from Table 13 give differences of 37.55 kJ mol−1 and 40.84 kJ mol−1, respectively. This data also suggests that the aromatic isocyanate will lead to the formation of N-carboxyanhydride by reacting with carboxylic acid from the tin centre (organotin carbamate/carboxylic acid) more compared to the aliphatic isocyanate in the catalysis of urethane formation using organotin dicarboxylate. Therefore, the aromatic system will lose more carboxylic acid from the tin centre (organotin carbamate/carboxylic acid).
The Gibbs free energy differences between the transition state T(35), W(36) and the N-carboxyanhydride T(61), W(62) for the aliphatic system are -30.2 kJ mol−1 and -28.63 kJ mol−1, respectively. Similarly, for an aromatic system, the Gibbs free energy differences from Tables 9 and 13, T(43), W(44) and (T67) W(68) are -41.02 kJ mol−1 and -41.72 kJ mol−1 in toluene and water medium, respectively. This means the N-carboxylic anhydride formation is more probable for the aromatic system compared to the aliphatic system, allowing more carboxylic acid being available for organotin carbamate to react in the aliphatic system.
By considering the data mentioned above, it can be said that in the case of the aliphatic system (organotin carbamate/carboxylic acid) is less chance of losing carboxylic acid to the medium compared to the aromatic system (organotin carbamate/carboxylic acid) formed from the organotin dicarboxylate catalyst. This data further supports our argument that in organotin dicarboxylate catalysis of aliphatic isocyanates in urethane formation, the carboxylic acid will stay with the organotin catalyst and contribute to the catalysis.
5. Conclusions
Computational studies reveal that alcohol can interact with organotin dicarboxylate to give a complex that could catalyse urethane formation. The complex contains alkoxide, but it is associated with a carboxylic acid, which in contrast to what was stated before [16]. The complex can interact with isocyanate resulting in an N-coordinated interaction to give an organotin carbamate. The transition state shows low free energy (76.12 kJ mol−1 in the gas phase) that could be activated at room temperature.
For the catalytic cycle, Gibbs free energy data show the stability of the association between the organotin carbamate and the free carboxylic acid that is coordinated with the tin centre is higher for aliphatic isocyanate than aromatic isocyanate in non-polar medium, regenerating the starting organotin dicarboxylate. The transition states Gibbs free energy difference between the carboxylic acid and alcohol association with organotin carbamate for an aromatic system is less than that of an aliphatic system in urethane formation. Therefore, in an environment with a high concentration of alcohol, the aromatic system will follow pathway 2, forming the organotin alkoxide, which will function as a catalyst in both polar and non-polar medium. Aliphatic isocyanates will be more sensitive to the carboxylic ligand content in the tin catalyst than aromatic isocyanates. This effect is more pronounced in non-polar systems than in polar systems. The reaction between free carboxylic acid that is formed as an intermediate in the catalysis can react with isocyanate present in the medium. This effect is more likely for an aromatic system than for the aliphatic system. Therefore when considering both scenarios, a) the relative reactivity of alcohol and carboxylic acid with organotin carbamate, and b) the loss of free carboxylic acid to the medium due to its reaction with isocyanate, an aliphatic isocyanate will be more sensitive to carboxylic acid ligand than an aromatic isocyanate in the urethane formation when organotin dicarboxylate is used as a catalyst.
Declarations
Author contribution statement
Ransi Devendra: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Neil R. Edmonds: Conceived and designed the experiments; Wrote the paper.
Tilo Söhnel: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
Ransi Devendra was supported by Uroxsys Ltd., Auckland, New Zealand.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We also thank the New Zealand eScience Infrastructure (NeSI) for providing high-performance computing facility to carry out the computational work, the University of Auckland for providing access to the HPC facilities and the technical support teams at Gaussian and Wavefunction for their valuable help. The authors also thank Florence Sennour ENSCBP Bordeaux, France, for some of the experimental work during her internship at Uroxsys Ltd.
References
- 1.Szycher M. first ed. Taylor & Francis; 1999. Szycher's Handbook of Polyurethanes. [Google Scholar]
- 2.Bloodworth A.J., Davies A.G. Organometallic reactions. Part I. The addition of tin alkoxides to isocyanates. J. Chem. Soc. 1965:5238. [Google Scholar]
- 3.Robins J. Structural effects in metal ion catalysis of isocyanate–hydroxyl reactions. J. Appl. Polym. Sci. 1965;9:821. [Google Scholar]
- 4.Smith H.A. Catalysis of the formation of urethanes. J. Appl. Polym. Sci. 1963;7:85. [Google Scholar]
- 5.Van Der Weij F.W. Kinetics and mechanism of urethane formation catalyzed by organotin compounds. I. The reaction of phenyl isocyanate with methanol in dibutyl ether under the action of dibutyltin diacetate. J. Polym. Sci. Polym. Chem. Ed. 1981;19:381. [Google Scholar]
- 6.Devendra R., Edmonds N.R., Söhnel T. Computational and experimental investigations of the urethane formation mechanism in the presence of organotin(IV) carboxylate catalysts. J. Mol. Catal. Chem. 2013;366:126. [Google Scholar]
- 7.Devendra R., Edmonds N.R., Söhnel T. Organotin carboxylate catalyst in urethane formation in a polar solvent: An experimental and computational study. RSC Adv. 2015;5:48935. [Google Scholar]
- 8.Devendra R., Edmonds N.R., Söhnel T. Insight into the mechanism of the catalysis of urethane formation by organotin(IV) dicarboxylate. React. Kinet. Mech. Catal. 2018;124:487. [Google Scholar]
- 9.Gielen M., Davies A.G., Pannell K., Tiekink E. Wiley; 2008. Tin Chemistry: Fundamentals, Frontiers, and Applications. [Google Scholar]
- 10.Lu L., Hu H., Hou H., Wang B. An improved B3LYP method in the calculation of organic thermochemistry and reactivity. Comput. Theor. Chem. 2013;1015:64. [Google Scholar]
- 11.Adams M.R., Bushnell E.A.C., Bruce Grindley T., Boyd R.J. Organotin bond dissociation energies: An interesting challenge for contemporary computational methods. Comput. Theor. Chem. 2014;1050:7. [Google Scholar]
- 12.Zhao Y., Truhlar D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008;120:215. [Google Scholar]
- 13.Grimme S. Density functional theory with London dispersion corrections. Wiley Interdiscipl. Rev. Comput. Mol. Sci. 2011;1:211. [Google Scholar]
- 14.Şirikci G., Ancın N.A., Öztaş S.G. Theoretical studies of organotin(IV) complexes derived from ONO-donor type schiff base ligands. J. Mol. Model. 2015;21 doi: 10.1007/s00894-015-2764-4. [DOI] [PubMed] [Google Scholar]
- 15.Draye A.C., Tondeur J.J. Activation parameters of an organotin-catalyzed alcohol-isocyanate reaction. Main Group Met. Chem. 1999;22:497. [Google Scholar]
- 16.Houghton R.P., Mulvaney A.W. Mechanism of tin(IV) -catalysed urethane formation. J. Organomet. Chem. 1996;518:21. [Google Scholar]
- 17.Hehre W.J.A. 2003. Guide to Molecular Mechanics and Quantum Chemical Calculations; Wavefunction, Incorporated. [Google Scholar]
- 18.Sameera W.M.C., Maeda S., Morokuma K. Computational Catalysis Using the Artificial Force Induced Reaction Method. Acc. Chem. Res. 2016;49:763. doi: 10.1021/acs.accounts.6b00023. [DOI] [PubMed] [Google Scholar]
- 19.Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Scalmani G., Barone V., Mennucci B., Petersson G.A., Nakatsuji H., Caricato M., Li X., Hratchian H.P., Izmaylov A.F., Bloino J., Zheng G., Sonnenberg J.L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery J.A., J., Peralta J.E., Ogliaro F., Bearpark M., Heyd J.J., Brothers E., Kudin K.N., Staroverov V.N., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J.C., Iyengar S.S., Tomasi J., Cossi M., Rega N., Millam J.M., Klene M., Knox J.E., Cross J.B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann E., Yazyev O., Austin A.J., Cammi R., Pomelli C., Ochterski J.W., Martin R.L., Morokuma K., Zakrzewski V.G., Voth G.A., Salvador P., Dannenberg J.J., Dapprich S., Daniels A.D., Farkas Ö., Foresman J.B., Ortiz J.V., Cioslowski J., Fox D.J. Gaussian, Inc.; Wallingford CT: 2009. Gaussian 09. 2013. [Google Scholar]
- 20.Spartan‘ 10. Wavefunction, Inc.; Irvine, CA: 2013. [Google Scholar]
- 21.Schlegel H.B. Optimization of equilibrium geometries and transition structures. J. Comput. Chem. 1982;3:214. [Google Scholar]
- 22.Peng C., Ayala P.Y., Schlegel H.B., Frisch M.J. Using redundant internal coordinates to optimize equilibrium geometries and transition states. J. Comput. Chem. 1996;17:49. [Google Scholar]
- 23.Peng C., Bernhard Schlegel H. Combining Synchronous Transit and Quasi-Newton Methods to Find Transition States. Isr. J. Chem. 1993;33:449. [Google Scholar]
- 24.Fukui K. The path of chemical reactions - The IRC approach. Acc. Chem. Res. 1981;14:363. [Google Scholar]
- 25.Maskill H. Wiley-Blackwell; 2006. The Investigation of Organic Reactions and Their Mechanisms. [Google Scholar]
- 26.Barone V., Cossi M., Tomasi J. A new definition of cavities for the computation of solvation free energies by the polarizable continuum model. J. Chem. Phys. 1997;107:3210. [Google Scholar]
- 27.Foresman J.B., Frisch A.E., Gaussian I. Gaussian, Inc.; 1996. Exploring Chemistry with Electronic Structure Methods. [Google Scholar]
- 28.ASTM-D2572-97; ASTM International . 1997. West Conshohocken, Pennsylvania 19428-2959. USA. [Google Scholar]
- 29.OriginPro, OriginLab Corporation, Northampton, MA, USA. www.originlab.com
- 30.Davies A.G., Harrison P.G. Organotin chemistry. Part IV. The preparation of compounds of the composition R2Sn(OMe)X. J. Chem. Soc. C: Org. Chem. 1967;298 [Google Scholar]
- 31.Burés J., Armstrong A., Blackmond D.G. Curtin-hammett paradigm for stereocontrol in organocatalysis by diarylprolinol ether catalysts. J. Am. Chem. Soc. 2012;134:6741. doi: 10.1021/ja300415t. [DOI] [PubMed] [Google Scholar]
- 32.Gürtler C., Danielmeier K. A catalyst system for the reaction of carboxylic acids with aliphatic isocyanates. Tetrahedron Lett. 2004;45:2515. [Google Scholar]
- 33.Xiao H., Xiao H.X., Frisch K.C., Malwitz N. Kinetic studies of the reactions between isocyanates and carboxylic acids. High Perform. Polym. 1994;6:235. [Google Scholar]