Abstract
Eating out of phase with daily circadian rhythms induces metabolic desynchrony in peripheral metabolic organs and may increase chronic disease risk. Time-restricted eating (TRE) is a dietary approach that consolidates all calorie intake to 6- to 10-h periods during the active phase of the day, without necessarily altering diet quality and quantity. TRE reduces body weight, improves glucose tolerance, protects from hepatosteatosis, increases metabolic flexibility, reduces atherogenic lipids and blood pressure, and improves gut function and cardiometabolic health in preclinical studies. This review discusses the importance of meal timing on the circadian system, the metabolic health benefits of TRE in preclinical models and humans, the possible mechanisms of action, the challenges we face in implementing TRE in humans, and the possible consequences of delaying initiation of TRE.
Subject Areas: Biological Sciences, Chronobiology, Nutrition
Graphical Abstract
Biological Sciences; Chronobiology; Nutrition
Introduction
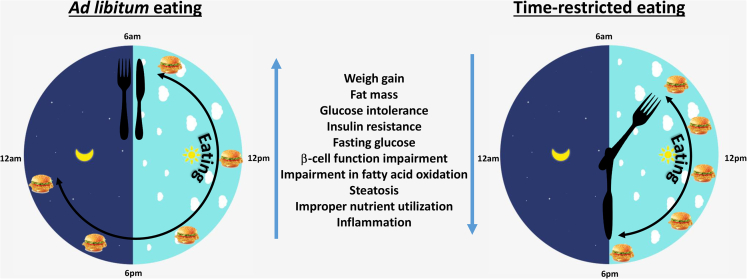
Lifestyle-induced metabolic diseases, such as type 2 diabetes (T2D) and cardiovascular disease, are often associated with obesity, reductions in physical activity and increased consumption of energy-dense foods. Accumulating evidence suggests that when we eat may be another contributing factor to chronic disease progression (Andrzejczak et al., 2011). Lengthened daily eating patterns, in excess of 14 h/day, were evident in studies conducted in the USA and India, with less than 25% of caloric intake occurring prior to 1 pm (Gill and Panda, 2015, Gupta et al., 2017). Time-restricted eating (TRE, also known as time-restricted feeding, TRF) is a novel dietary tool that recommends individuals shorten the duration of the daily eating window, without altering calorie intake or diet quality. TRE restores circadian rhythms and imparts pleiotropic metabolic benefits in animal models (Chaix et al., 2014, Delahaye et al., 2018, Gill et al., 2015, Hatori et al., 2012, Olsen et al., 2017, Villanueva et al., 2019, Wang et al., 2018, Woodie et al., 2018). TRE also reduces body weight and fat mass, improves glucose tolerance and reduces blood pressure in humans, particularly in those with overweight or obesity (Figure 1) (Gabel et al., 2018, Gill and Panda, 2015, Hutchison et al., 2019, Sutton et al., 2018, Wilkinson et al., 2019). The studies to date in humans are limited in size and duration, and the effectiveness and acceptability of TRE in the general population remains unclear. The majority of TRE studies have also initiated the eating window early in the active phase, presumably to maximize the metabolic benefits. This review will discuss the metabolic benefits of TRE in preclinical models and the possible mechanisms of action. We also discuss the likely challenges of implementing TRE in humans and the possible consequences of delaying initiation of TRE.
Figure 1.
TRE Metabolic Benefits
Regulation of Central and Peripheral Clock Machinery
Circadian rhythms are ubiquitous periodic oscillations in internal biological process that direct behavior and metabolism such as hormonal signaling, body temperature, nutrient absorption, and metabolism (Dongen, 2017, Espelund et al., 2005, Panda et al., 2002, Reppert and Weaver, 2002). At the molecular level, circadian rhythms arise from tightly controlled autonomous interlocked genetic transcriptional feedback loop that involves circadian locomotor output cycles kaput (clock) and brain and muscle ARNT like protein 1 (bmal1) as positive transcriptional factors for period (per1, per2, per3) and cryptochrome (cry1, cry2) genes (extensively reviewed in Hastings et al., 2018). The translation products of per and cry dimerize and act as negative regulators by inhibiting clock and bmal1. An additional feedback loop involves the transcriptional regulation of bmal1 by retinoic acid related orphan receptor (rorα) and nuclear receptor subfamily 1, group D, member 1(rev-erbα). One cycle of this feedback loop takes ~24 h and is the basis of circadian rhythms in many organisms. The suprachiasmatic nucleus (SCN) is considered the master regulator of circadian rhythms and is primarily entrained by the light-dark cycle. This feedback loop also operates in peripheral tissues, including the liver, skeletal muscle, adipose tissue, pancreas, and intestine, which are not directly entrained by light (Reppert and Weaver, 2002, Yoo et al., 2004).
Peripheral clocks are exquisitely sensitive to the fasting-feeding cycle and, as discussed in the next section, can be uncoupled from the central clock through modifications in meal delivery (Damiola et al., 2000). At the molecular level, fasting increases the AMP/ATP ratio, activating 5′ AMP-activated protein kinase (AMPK). This in turn phosphorylates serine71 of cry1, reducing its stability (Lamia et al., 2009). AMPK also regulates the activity of Casein kinase I epsilon via its phosphorylation at serine389, which is a critical regulator of per phosphorylation and stability (Meng et al., 2008). Nicotinamide adenine dinucleotide (NAD+) is a cofactor of several key pathophysiological enzymes and an absolute requirement of sirtuin 1 (SIRT1, a NAD+-dependent histone deacetylase). The majority of cellular NAD+ comes from its salvage pathway where nicotinamide phosphoribosyltransferase (NAMPT) is the rate-limiting enzyme (Poljsak, 2018, Zhang et al., 2017). Fasting activates NAMPT, thus increasing the cellular availability of NAD+, activating SIRT1. Activated SIRT1 has been shown to directly bind with clock:bmal1 and repress the transcription of per2 (Ramsey et al., 2009). Thus, fasting also reduces both transcription and stability of per and cry, which de-represses clock:bmal1 targets and increases their amplitude (Lamia et al., 2009, Um et al., 2007). SIRT1 has also been described to regulate the acetyltransferase activity of clock (Doi et al., 2006, Nakahata et al., 2008). In contrast, the mechanistic target of rapamycin (mTOR), a nutrient-activated serine/threonine protein kinase, is activated during the fed state. This post-transcriptionally induces cry1 through an unknown mechanism (Ramanathan et al., 2018). Feeding also suppressed NAMPT function, reduced cellular NAD+, inactivating SIRT1. This abrogated SIRT1-mediated suppression of clock:bmal1 and increased per2 transcription (Ramsey et al., 2009). Hence, fasting increases the positive limb of circadian clock (clock and bmal1), whereas feeding increases the negative limb of circadian clock (cry and per).
Mealtime Is a Strong Entraining Cue of Peripheral Clocks and Subsequently Impacts Metabolism and Risk Factors for Chronic Disease
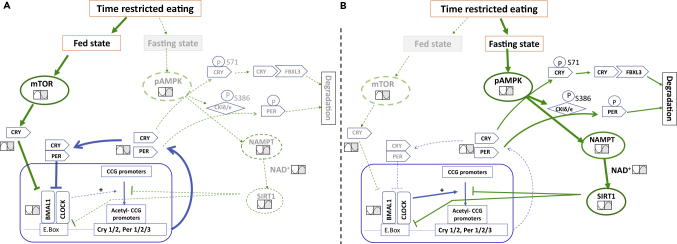
Disrupted feeding therefore has a marked effect on the expression of the molecular clock in peripheral tissues (Jiang and Turek, 2017) and uncouples this from the SCN (Damiola et al., 2000). For example, restricting food access solely to the light phase in mice, when this nocturnal animal normally sleeps, completely reversed the phase of the circadian clock in liver, stomach, intestine, heart, pancreas, and kidney, without affecting the phase in the SCN (Damiola et al., 2000, Davidson et al., 2003). Simply delaying meals by 4 h also resulted in a phase shift in the circadian clock in mouse liver of a similar length (Shimizu et al., 2018). Delaying a single breakfast meal by 5 h also delayed expression of genes under per control in human adipose tissue (Wehrens et al., 2017). Conversely, studies have shown that rhythmic feeding was sufficient to maintain circadian rhythms of clock genes in peripheral tissues during constant light or darkness or following lesion of SCN (Hamaguchi et al., 2015, Kolbe et al., 2019, Novakova et al., 2011). These findings show that cues from the fasting-feeding cycle are more powerful entraining cues for peripheral clocks than the light-dark cycle (Figure 2) (Damiola et al., 2000, Wang et al., 2017).
Figure 2.
TRE Regulation of Peripheral Circadian Clocks
TRE can reprogram circadian clock in the fasting state via AMPK and in the fed state via mTOR.
(A) In the fed state, nutrient availability activates mTOR. Activated mTOR induces Cry, which represses Clock:Bmal1.
(B) In the fasting state, nutrient depletion activates AMPK that directly and indirectly enhances phosphorylation on cry, and per. Phosphorylation is key for degradation of these proteins. Next, AMPK can activate SIRT1 activity via NAMPT. SIRT1 binds with clock:bmal1 and represses the transcription of per2. The acetyltransferase activity of clock is counteracted by SIRT1. Blue arrows represent core clock machinery, green arrows represent effect of TRE. AMPK:,AMP-activated protein kinase; bmal1, brain and muscle arnt like 1; CCG, clock-controlled genes; CK, casein kinase; clock, circadian locomotor output cycle kaput; cry, cryptochrome; mTOR, mechanistic target of rapamycin; NAD, nicotinamide adenine dinucleotide; NAMPT, nicotinamide phosphoribosyl transferase; per, period; SIRT1, sirtuin1.
Disrupting molecular clocks by altering feeding behaviors has subsequent impacts on metabolism in animal models. Reversing the phase of clock genes in peripheral organs by daytime restricted feeding was associated with weight gain, dyslipidemia, and fatty liver as compared with animals that were pair-fed to an equivalent calorie level solely during the active phase (Bray et al., 2013, Yasumoto et al., 2016). This also reversed the phase of several genes involved in glucose homeostasis such as glut2, pyruvate kinase, glucokinase, and glycogen synthase in liver and several genes involved in lipid homeostasis such as acetyl CoA carboxylase, diacylglycerol-O-acyltransferase, medium chain acylCoA dehydrogenase in liver, muscle, and epididymal fat (Bray et al., 2013, Yasumoto et al., 2016). Daytime restricted feeding also reversed the phase of insulin, leptin, and ghrelin in plasma compared with mice fed only during nighttime (Yasumoto et al., 2016).
Similarly, a rotating light cycle (a mimic of shift work) altered the phase and reduced oscillation of clock genes in liver and caused higher weight gain, increased hepatosteatosis, and reduced β cell function and glucose-stimulated insulin secretion (Christie et al., 2018, Gale et al., 2011, Zhong et al., 2019). Chow fed mice under rotating light cycle also had altered phase of insulin and corticosterone in plasma and transcription factors FOXO1, PPARa, and PPARϒ in liver (Zhong et al., 2019), whereas, high-fat diet (HFD)-fed mice under rotating light cycle completely lost the rhythmic expression of lipogenic gene acylCoA carboxylase in liver (Christie et al., 2018).
Furthermore, delaying the feeding phase by just 4 h shifted peripheral clocks and increased weight gain in rats exposed to HFD (Shimizu et al., 2018). Meal delay also delayed the peak of several genes involved in glucose homeostasis, such as glucokinase, glucose 6 phosphatase, and phosphoenol pyruvate carboxykinase, and several genes and transcription factors involved in lipid homoeostasis, such as SREBP, PPARa, fatty acid synthase, carnitine palmitoyl transferase, and malic enzyme in liver. Likewise, meal delay also delayed the peak time of insulin, free fatty acids, and bile acids in plasma and circadian rise in body temperature (Shimizu et al., 2018).
Health Effects of TRE in Animal Models and Humans
TRE Induces Pleiotropic Metabolic Health Benefits in Animal Models of Obesity and Aging
TRE, defined here as the provision of food for up to 12 h during the active phase, is commonly known as TRF in animal studies to depict the eating window or food availability is externally controlled. TRE limited weight and fat gain and protected nocturnal mice and diurnal flies from the metabolic consequences of HFD (Chaix et al., 2014, Delahaye et al., 2018, Gill et al., 2015, Hatori et al., 2012, Olsen et al., 2017, Sundaram and Yan, 2016, Villanueva et al., 2019, Woodie et al., 2018). This included protection from inflammation (Sherman et al., 2011) and immune responses (Cisse et al., 2018) and enhanced bile acid synthesis facilitating cholesterol excretion (Chaix et al., 2014) and reduced cholesterol levels (Delahaye et al., 2018). TRE also prevented age- and HFD-induced reductions in cardiac contractile function (Gill et al., 2015, Tsai et al., 2013) in mice and flies and restored HFD-induced loss of gastric vagal afferent mechanosensitivity (Kentish et al., 2018). TRE restored HFD-induced dampening of the circadian rhythms in the gut microbiome (Hu et al., 2019, Zarrinpar et al., 2014) and circadian rhythms in fatty acid oxidation (Chaix et al., 2019, Hatori et al., 2012). Thus, TRE has pleiotropic metabolic benefits to protect against chronic disease in mice and flies and importantly was able to reverse the consequences of obesity (Chaix et al., 2014) and aging (Duncan et al., 2016). The beneficial effects were also evident when TRE was implemented 5 days per week and food access was allowed ad libitum during weekends (Chaix et al., 2014, Olsen et al., 2017) in HFD-fed mice.
At the molecular level, TRE increased the amplitude of expression of AMPK and mTOR (Hatori et al., 2012, Sherman et al., 2012) (Figure 2) and NAMPT in the liver of mice that were fed HFD (Chaix et al., 2019). TRE also increased the amplitude of ribosomal protein phospho-S6 in skeletal muscle during the active phase (Chaix et al., 2014), suggesting increased mTOR activation during feeding. TRE increased the amplitude of cry1 and per1 in the liver of mice fed chow (Greenwell et al., 2019) and restored the amplitude of bmal1, cry1, per2, and rev-erbα in mice that were fed HFD (Hatori et al., 2012). In liver, TRE reduced the amplitude of pyruvate carboxylase and glucose 6-phosphatase, and increased glucokinase during the active phase (Chaix et al., 2014, Hatori et al., 2012), potentially underpinning reductions in hepatic glucose production and increased glucose utilization. TRE also reduced the amplitude of genes fatty acid synthase, stearoyl CoA desaturase, and fatty acid elongase during the active phase and increased the amplitude of hepatic triglyceride lipase during the inactive phase, which was associated with reduced lipid storage and increased triglyceride hydrolysis.
Importantly, Chaix et al. examined the effects of TRE in mice that were deficient in cry1 and cry2 at the whole-body level or deficient in bmal1 and reverbα & β only in liver. In this study, TRE was effective to restore robust rhythms in genes involved in energy metabolism and nutrient utilization in the liver from all knockouts, as well as nutrient signaling pathways with higher AMPK and mTOR function (higher pS6 levels during feeding) in fasting and fed states, respectively. TRE also protected knockouts from HFD-induced weight gain, glucose intolerance, hepatic steatosis, and dyslipidemia (Chaix et al., 2019). This study proves that sustaining daily rhythms in the fasting and feeding cycle is sufficient to maintain metabolic homeostasis, independently of circadian clocks (Chaix et al., 2019).
From studies to date, it is difficult to separate whether TRE improves health, independently of changes in calorie intake. Certainly, some studies have suggested this (Chaix et al., 2014, Chaix et al., 2019, Hatori et al., 2012). However, food intake is difficult to measure accurately, and other studies have shown lower calorie consumption in TRE mice that are fed a HFD (Delahaye et al., 2018, Sundaram and Yan, 2016) and marked weight loss initially in response to TRE (Kentish et al., 2018, Sundaram and Yan, 2016). Thus, some of the metabolic benefits of TRE may well be mediated by calorie restriction and weight loss. However, a recent study in mice showed that TRE improved glucose tolerance and reduced HOMA-IR in rats following high fat-high sugar diet, without any weight loss (Woodie et al., 2018). A recent human study also supported the notion that TRE imparts metabolic benefits independently of changes in body weight (Sutton et al., 2018).
TRE Improves Metabolic Health Outcomes in Humans
Several TRE protocols with daily meal intakes prescribed from 4 to 13 h have been trialed in people of normal weight and overweight (summarized in Table 1), although all of these trials are short-term (4 days to 16 weeks) and conducted in a small number of participants. The majority of studies report modest reductions in body weight and fat mass (Anton et al., 2019, Chow et al., 2020, Gabel et al., 2018, Gill and Panda, 2015, LeCheminant et al., 2013, Moro et al., 2016, Stote et al., 2007, Wilkinson et al., 2019) and waist circumference (Kesztyus et al., 2019). TRE also reduced plasma triglycerides and inflammatory markers (LeCheminant et al., 2013, Moro et al., 2016), blood pressure, and atherogenic lipids (Gabel et al., 2020, Wilkinson et al., 2019). Some studies have also reported significant improvement in glucose control (Hutchison et al., 2019, Jamshed et al., 2019), although this was not universally observed (Wilkinson et al., 2019). TRE for 12 weeks did not alter gut microbiome in humans (Gabel et al., 2020).
Table 1.
List of Time-Restricted Eating Trials in Humans and Their Major Findings
| Study | Participants | Trial length | Design | Intervention, Meal Time | Major Findings |
|---|---|---|---|---|---|
| (Carlson et al., 2007, Stote et al., 2007) | n = 15 (10 females, 5 males), normal weight | 8 weeks | Cross-over | TRE: one isocaloric meal (5 pm–9 pm) Control: three meals/day |
↓ Body weight, fat mass, blood pressure, glucose tolerance ↑ Fasting glucose |
| (LeCheminant et al., 2013) | n = 27 males, normal weight | 2 weeks | Cross-over | TRE: 13-h TRE (6 am–7 pm) Control: AL |
↓ 0.4 kg Body weight (vs ↑ 0.6 kg control condition) |
| (Gill and Panda, 2015) | n = 8 (3 females, 5 males), overweight | 16 weeks | Within participant | TRE: 10–11 h (self-selected) Baseline: >14 h |
↓ Body weight |
| (Moro et al., 2016) | n = 34 males, normal weight | 8 weeks | Randomized controlled | TRE: 8 h (1 pm–8 pm) Control: 12 h (8 am–8 pm) |
↓ Fat mass, fasting glucose, fasting insulin, total testosterone, IGF-1 |
| (Tinsley et al., 2017) | n = 18 resistance trained males (10: RT-TRE; 8: RT-AL) | 8 weeks | Randomized controlled | TRE: 4 h (anytime 4 pm to midnight) for 4 days a week Control: AL |
↔ Body weight, fat mass |
| (Gabel et al., 2018) | n = 23 (20 females, 3 males), obese | 12 weeks | Historical control | TRE: 8 h (10 am–6 pm) Control: AL |
↓ Body weight and blood pressure ↔ Fat mass, fasting glucose, LDL cholesterol, TG |
| (Sutton et al., 2018) | n = 8 males, overweight | 5 weeks | Cross-over | eTRE: 6 h (8 am–2 pm, dinner before 3 pm) Control: 12 h |
↓ Fasting TG, desire to eat in the evening ↑ Insulin sensitivity, β cell responsiveness ↔ Body weight |
| (Jamshed et al., 2019, Ravussin, 2019) | n = 11 (4 females and 7 males), overweight | 4 days | Cross-over | TRE: 6 h (8 am–2 pm) Control: 12 h (8 am–8 pm) |
↓ Mean 24-h glucose, glycemic excursions, morning ghrelin, desire to eat ↑ Daytime EE, metabolic flexibility, fullness, plasma ketones |
| (Hutchison et al., 2019) | n = 15 males, overweight | 1 week | Cross-over | eTRE: 9 h (8 am–5 pm) dTRE: 9 h (12 pm–9 pm) Baseline: AL |
↓ Body weight, fasting TG, and hunger ↓ Mean fasting glucose by CGM in eTRE ↑ Glucose tolerance |
| (Tinsley et al., 2019) | n = 40 females, resistance trained | 8 weeks | Randomized controlled | TRE: 8 h (12 pm–8 pm) TRE plus β-hydroxy β-methyl butyrate |
↓ Fat mass ↑ Muscle performance |
| (Anton et al., 2019) | n = 10 (6 females, 4 males), overweight, ≥65 years | 4 weeks | Within participant | TRE: 8 h Baseline: AL |
↓ Body weight |
| (Wilkinson et al., 2019) | n = 19 (6 females, 13 males), overweight | 12 weeks | Within participant | TRE: 10 h (self-selected, dinner before 8 pm) Baseline: ≥ 14 h |
↓ Body weight, fat mass, waist circumference, blood pressure, plasma cholesterol ↔ Fasting glucose, HbA1c, HOMA-IR, fasting insulin |
| (Kesztyus et al., 2019) | N = 40 (31 females, 9 males), with abdominal obesity | 3 months | Within participant | TRE: 8 -9 h Baseline: AL |
↓Waist circumference, HbA1c |
| (Parr et al., 2020) | n = 11 males, overweight | 5 days | Cross-over | TRE: 8 h (10 am–6 pm) Extended eating: 15 h (7 am–10 pm) |
↓ Night-time glucose, glucose and insulin iAUC after lunch ↔ Daytime glucose ↑ TG after lunch |
| (Chow et al., 2020) | N = 20 (17 females, 3 males), overweight | 12 weeks | Randomized controlled | TRE: 8 h Non-TRE: AL |
↓Body weight, lean mass, and visceral fat mass. |
| (Gabel et al., 2020) | N = 14, overweight | 12 weeks | Within participant | TRE: 8 h (10 am–6 pm) Baseline: AL |
↓Body weight, fat mass, systolic blood pressure ↔ Gut microbiome |
n, number; TRE, time restricted eating; RT, resistance trained; AL, ad libitum; IGF, insulin like growth factor; TG, triglycerides; LDL, low-density lipoprotein; EE, energy expenditure; SIRT1, sirtuin1; mTOR, mechanistic target of rapamycin; CGM, continuous glucose monitoring; eTRE, early TRE; dTRE, delayed TRE; HbA1c, glycosylated hemoglobin; HOMA-IR, homeostatic model assessment of insulin resistance; iAUC, incremental area under the curve; ↓, reduced; ↑, increased; ↔, no change.
Most of the studies performed in humans have observed a reduction in self-reported energy intake (Gabel et al., 2018, Gill and Panda, 2015, Wilkinson et al., 2019), which may account for some of the beneficial weight and health effects. However, in a highly controlled, cross-over feeding trial, 5 weeks of early (e) TRE (dinner before 3 pm) increased insulin sensitivity and β cell responsiveness and reduced oxidative stress as compared with the control condition in the absence of energy restriction and weight loss (Sutton et al., 2018), and increased fasting triglyceride as a physiological response to the increased fasting duration. Four days of eTRE (8 am–2 pm) also reduced fasting and postprandial glucose and increased daytime energy expenditure and the expression of SIRT1, clock genes, and genes involved in autophagy in blood (Jamshed et al., 2019, Ravussin, 2019). Five days of TRE (10 am–5 pm) also reduced night-time glucose in participants who were overweight (Parr et al., 2020). These studies suggest that TRE could be a promising tool for the improvement of metabolic outcomes in the general population.
Challenges in Translating TRE to Humans
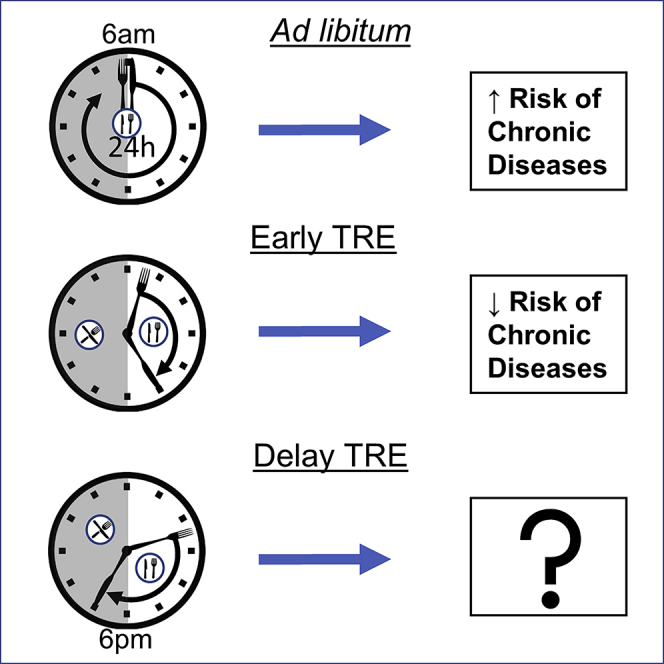
TRE is a simple approach that could be highly beneficial in primary practice, since it does not require extensive nutrition knowledge or significant time commitment to convey to the patient in need, unlike current dietary practice guidelines (Australian Dietary Guideline, 2013). However, the majority of TRE interventions in animal models and in humans have been initiated early in the active phase (Chaix et al., 2014, Gabel et al., 2018, Gill and Panda, 2015, Hatori et al., 2012, Jamshed et al., 2019, Olsen et al., 2017, Ravussin, 2019, Sutton et al., 2018). Here, we discuss possible challenges with the translation of eTRE in the general population and the possible outcomes of delayed TRE (dTRE, i.e., allowing food consumption for identical time lengths late in the day).
Early morning is likely to be the optimal time to initiate TRE to maximize the metabolic benefits. For example, insulin sensitivity and glucose uptake are higher at the beginning of the active phase in nocturnal mice (Basse et al., 2018, Rudic et al., 2004) and diurnal humans (Sonnier et al., 2014). Similarly, lipid absorption in the intestine (Douris et al., 2011) and de novo lipogenesis in the liver are higher during the active phase in mice (Gilardi et al., 2014). Cholesterol and bile acid synthesis are also elevated early during the active phase (Chaix et al., 2014). Furthermore, findings from observational and epidemiological studies suggest that breakfast skippers are also more likely to be overweight, have poorer glucose control, and develop T2D as compared with people who identify as breakfast consumers (Bi et al., 2015). Although other observational studies have reported that skipping breakfast, without eating late, does not link to obesity, suboptimal glycemic control, or poorer metabolic health (Azami et al., 2019, Nakajima and Suwa, 2015, Okada et al., 2019). Women who were overweight were randomized to high-calorie breakfast versus high-calorie dinners, where the high-calorie breakfast group lost more body weight and had greater reductions in waist circumference, fasting glucose, and fasting insulin (Jakubowicz et al., 2013). In another study, individuals with T2D were provided with a three-meal-per-day diet (light dinner before 8 pm) or an isocaloric six-meal diet (heavy dinner and snacks continued until 11 pm) for 12 weeks. The three-meal diet reduced body weight, glycosylated hemoglobin, and therapeutic insulin dose and significantly lowered hyperglycemic episodes by continuous glucose monitoring. Clock gene expression in blood samples also showed higher oscillation in the three-meal diet (Jakubowicz et al., 2019). Eating breakfast and lunch only also reduced body weight, plasma glucose, and hepatic fat more than eating six meals spread throughout the day (Kahleova et al., 2014). Together, these studies show that consuming meals earlier in the day are optimal for weight control and improvements in glycemic profile under isocaloric conditions.
Implementing TRE early in the morning may be challenging in the general population both biologically and socially. There is large endogenous circadian variation in hunger, with peak in the evening and nadir in the morning (Qian et al., 2019, Scheer et al., 2013). This is because ghrelin, a hormone secreted by stomach that increases feelings of hunger, is under circadian regulation and is at biological nadir in the morning (Espelund et al., 2005) and peaks in the afternoon. Furthermore, family and communal get-togethers are essential factors to increase social bonding, feeling of physical and mental well-being, and overall happiness in humans. Group eating and food sharing are considered the easiest way to strengthen family and community bonds capable of providing social and emotional support (Dunbar, 2017). However, many social events are typically geared toward evening. Delaying the start time of TRE may overcome both of these issues, but the metabolic consequences of dTRE are not clear. As described earlier, fasting and feeding are known regulators of peripheral molecular clocks. Thus, it is likely that delaying TRE will delay peripheral clocks in metabolic organs. This was seen in recent human studies where skipping breakfast delayed per rhythms in adipose tissue (Loboda et al., 2009, Wehrens et al., 2017). However, whether there is a net consequence of a short phase delay in clocks on metabolic health in humans is currently unknown.
In athletes undertaking resistance training, dTRE (12-8 pm) reduced fat mass without altering fat-free mass and improved muscle performance (Tinsley et al., 2019). Blood glucose, insulin, total testosterone, and IGF-1 were also reduced in this study. However, severe time restriction, whereby all food intake was limited to one large daily meal eaten between 5 and 9 pm, impaired glucose tolerance in the following morning (Carlson et al., 2007). dTRE also failed to show any benefits in glycemic profile and body weight reduction when meal intake was limited to 4 h in the evening (anytime between 4 pm and midnight) for 4 days per week (Tinsley et al., 2017). However, the irregular patterning of meals in that study could also have contributed to this result (Farshchi et al., 2004).
Only two animal studies have directly compared eTRE with dTRE. In one study, mice underwent 6 h of TRE with HFD given either during the first half (ZT12-18) or second half (ZT18-24) of the night for 8 weeks. Body weight gain and insulin resistance as measured by HOMA-IR were higher in dTRE than in eTRE. However, both TREs equally improved glucose tolerance compared with ad libitum (Delahaye et al., 2018). The fasting length prior to the glucose assessment was not standardized in that study (7–16 h depending on intervention), which may have contributed to the results as glucose tolerance is higher after 18 h of fasting versus 6 h of fasting (Andrikopoulos et al., 2008). In another study, rats were fed for 12 h during night whether at ZT12-24 (eTRE) or ZT16-4 (dTRE) for 2 weeks. Despite similar caloric consumption, body weight gain was higher in the dTRE group and dTRE also delayed the phase of clock, bmal1, per1, cry2, and rev-erbα by 2 h and that of cry1 by 4 h in liver (Shimizu et al., 2018). The amplitudes of those genes were also lower in dTRE. However, the study was of short duration and did not include an ad libitum fed group. We conducted a preliminary study comparing dTRE with eTRE in men with obesity. This study showed that dTRE produced similar improvements in glucose tolerance as eTRE (Hutchison et al., 2019). However, when glycemic measurements were made by continuous glucose monitoring, only eTRE significantly reduced fasting glucose versus baseline. This reduction was at trend level for dTRE versus baseline, and there was no statistical difference in this improvement between TRE groups (Hutchison et al., 2019). The impact on clock genes was not examined. Larger trials comparing effects of eTRE versus dTRE are warranted.
Studies in mice have shown that the beneficial effects of TRE are dose dependent, with greater reductions in body weight, fat mass, and improvement in glucose tolerance when a 9-h protocol was implemented versus 12 and 15 h (Chaix et al., 2014, Sundaram and Yan, 2016). The optimal TRE time frame to recommend for people has not been tested. Clear improvements have been noted after 6-, 8-, 9-, and 10-h protocols (Gabel et al., 2018, Hutchison et al., 2019, Sutton et al., 2018, Wilkinson et al., 2019). It is likely that the greater time restriction would result in greater weight losses, which may maximize the metabolic benefits. However, very short feeding windows could also reduce adherence or result in poorer food choices, if the individual feels under too great a time pressure. Extending the eating window beyond 12 h is unlikely to have major beneficial metabolic effects (LeCheminant et al., 2013).
Conclusion and Future Directions
TRE initiated early in the active phase shows pleiotropic metabolic benefits in animal models of diet-induced obesity and aging. Short-term TRE trials in humans have shown modest reductions in body weight and improved cardio-metabolic health in people who are overweight or obese, suggesting that TRE may be a promising therapeutic tool. However, these studies are limited in number, sample size, and study duration. The feasibility of implementing early TRE in the general population on a daily basis is unclear, and the effects of delaying TRE to increase the potential translatability and acceptability of this dietary approach are unknown. Large-scale, long-term trials are warranted to determine if TRE is a viable alternative to current practice dietary guidelines.
Resource Availability
Lead Contact
A/Prof Leonie K Heilbronn, Adelaide Medical School, University of Adelaide, Adelaide 5000, SA, Australia.
Email: leonie.heilbronn@adelaide.edu.au.
Materials Availability
Not applicable.
Data and Code Availability
Not applicable.
Acknowledgments
Author Contributions
P.R. searched the literature, and both authors contributed to data interpretation and preparation of the manuscript. L.K.H. had primary responsibility for the final publication.
References
- Andrikopoulos S., Blair A.R., Deluca N., Fam B.C., Proietto J. Evaluating the glucose tolerance test in mice. Am. J. Physiol. Endocrinol. Metab. 2008;295:E1323–E1332. doi: 10.1152/ajpendo.90617.2008. [DOI] [PubMed] [Google Scholar]
- Andrzejczak D., Kapala-Kempa M., Zawilska J.B. [Health consequences of shift work] Przegl Lek. 2011;68:383–387. [PubMed] [Google Scholar]
- Anton S.D., Lee S.A., Donahoo W.T., McLaren C., Manini T., Leeuwenburgh C., Pahor M. The effects of time restricted feeding on overweight, older adults: a pilot study. Nutrients. 2019;11:1500. doi: 10.3390/nu11071500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Australian Dietary Guideline . 2013. NHMRC.https://www.nhmrc.gov.au/about-us/publications/australian-dietary-guidelines#block-views-block-file-attachments-content-block-1 [Google Scholar]
- Azami Y., Funakoshi M., Matsumoto H., Ikota A., Ito K., Okimoto H., Shimizu N., Tsujimura F., Fukuda H., Miyagi C. Long working hours and skipping breakfast concomitant with late evening meals are associated with suboptimal glycemic control among young male Japanese patients with type 2 diabetes. J. Diabetes Investig. 2019;10:73–83. doi: 10.1111/jdi.12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basse A.L., Dalbram E., Larsson L., Gerhart-Hines Z., Zierath J.R., Treebak J.T. Skeletal muscle insulin sensitivity show circadian rhythmicity which is independent of exercise training status. Front. Physiol. 2018;9:1198. doi: 10.3389/fphys.2018.01198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi H., Gan Y., Yang C., Chen Y., Tong X., Lu Z. Breakfast skipping and the risk of type 2 diabetes: a meta-analysis of observational studies. Public Health Nutr. 2015;18:3013–3019. doi: 10.1017/S1368980015000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray M.S., Ratcliffe W.F., Grenett M.H., Brewer R.A., Gamble K.L., Young M.E. Quantitative analysis of light-phase restricted feeding reveals metabolic dyssynchrony in mice. Int. J. Obes. (Lond.) 2013;37:843–852. doi: 10.1038/ijo.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson O., Martin B., Stote K.S., Golden E., Maudsley S., Najjar S.S., Ferrucci L., Ingram D.K., Longo D.L., Rumpler W.V. Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal-weight middle-aged men and women. Metabolism. 2007;56:1729–1734. doi: 10.1016/j.metabol.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaix A., Lin T., Le H.D., Chang M.W., Panda S. Time-restricted feeding prevents obesity and metabolic syndrome in mice lacking a circadian clock. Cell Metab. 2019;29:303–319 e304. doi: 10.1016/j.cmet.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaix A., Zarrinpar A., Miu P., Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014;20:991–1005. doi: 10.1016/j.cmet.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow L.S., Manoogian E.N.C., Alvear A., Fleischer J.G., Thor H., Dietsche K., Wang Q., Hodges J.S., Esch N., Malaeb S. Time-restricted eating effects on body composition and metabolic measures in humans with overweight: a feasibility study. Obesity (Silver Spring) 2020;28:860–869. doi: 10.1002/oby.22756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie S., Vincent A.D., Li H., Frisby C.L., Kentish S.J., ORielly R., Wittert G.A., Page A.J. A rotating light cycle promotes weight gain and hepatic lipid storage in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2018;315:G932–G942. doi: 10.1152/ajpgi.00020.2018. [DOI] [PubMed] [Google Scholar]
- Cisse Y.M., Borniger J.C., Lemanski E., Walker W.H., 2nd, Nelson R.J. Time-restricted feeding alters the innate immune response to bacterial endotoxin. J. Immunol. 2018;200:681–687. doi: 10.4049/jimmunol.1701136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiola F., Le Minh N., Preitner N., Kornmann B., Fleury-Olela F., Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson A.J., Poole A.S., Yamazaki S., Menaker M. Is the food-entrainable circadian oscillator in the digestive system? Genes Brain Behav. 2003;2:32–39. doi: 10.1034/j.1601-183x.2003.00005.x. [DOI] [PubMed] [Google Scholar]
- Delahaye L.B., Bloomer R.J., Butawan M.B., Wyman J.M., Hill J.L., Lee H.W., Liu A.C., McAllan L., Han J.C., van der Merwe M. Time-restricted feeding of a high fat diet in C57BL/6 male mice reduces adiposity, but does not protect against increased systemic inflammation. Appl. Physiol. Nutr. Metab. 2018;43:1033–1042. doi: 10.1139/apnm-2017-0706. [DOI] [PubMed] [Google Scholar]
- Doi M., Hirayama J., Sassone-Corsi P. Circadian regulator CLOCK is a histone acetyltransferase. Cell. 2006;125:497–508. doi: 10.1016/j.cell.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Dongen SMJAHGPAV 2017, shift work: disrupted circadian rhythms and sleep—implications for health and well-being. Curr. Sleep Med. Rep. 2017;3:9. doi: 10.1007/s40675-017-0071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douris N., Kojima S., Pan X., Lerch-Gaggl A.F., Duong S.Q., Hussain M.M., Green C.B. Nocturnin regulates circadian trafficking of dietary lipid in intestinal enterocytes. Curr. Biol. 2011;21:1347–1355. doi: 10.1016/j.cub.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar R.I.M. Breaking bread: the functions of social eating. Adapt. Hum. Behav. Physiol. 2017;3:198–211. doi: 10.1007/s40750-017-0061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan M.J., Smith J.T., Narbaiza J., Mueez F., Bustle L.B., Qureshi S., Fieseler C., Legan S.J. Restricting feeding to the active phase in middle-aged mice attenuates adverse metabolic effects of a high-fat diet. Physiol. Behav. 2016;167:1–9. doi: 10.1016/j.physbeh.2016.08.027. [DOI] [PubMed] [Google Scholar]
- Espelund U., Hansen T.K., Hojlund K., Beck-Nielsen H., Clausen J.T., Hansen B.S., Orskov H., Jorgensen J.O., Frystyk J. Fasting unmasks a strong inverse association between ghrelin and cortisol in serum: studies in obese and normal-weight subjects. J. Clin. Endocrinol. Metab. 2005;90:741–746. doi: 10.1210/jc.2004-0604. [DOI] [PubMed] [Google Scholar]
- Farshchi H.R., Taylor M.A., Macdonald I.A. Regular meal frequency creates more appropriate insulin sensitivity and lipid profiles compared with irregular meal frequency in healthy lean women. Eur. J. Clin. Nutr. 2004;58:1071–1077. doi: 10.1038/sj.ejcn.1601935. [DOI] [PubMed] [Google Scholar]
- Gabel K., Hoddy K.K., Haggerty N., Song J., Kroeger C.M., Trepanowski J.F., Panda S., Varady K.A. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: a pilot study. Nutr. Healthy Aging. 2018;4:345–353. doi: 10.3233/NHA-170036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabel K., Marcell J., Cares K., Kalam F., Cienfuegos S., Ezpeleta M., Varady K.A. Effect of time restricted feeding on the gut ` in adults with obesity: a pilot study. Nutr. Health. 2020 doi: 10.1177/0260106020910907. 260106020910907. [DOI] [PubMed] [Google Scholar]
- Gale J.E., Cox H.I., Qian J., Block G.D., Colwell C.S., Matveyenko A.V. Disruption of circadian rhythms accelerates development of diabetes through pancreatic beta-cell loss and dysfunction. J. Biol. Rhythms. 2011;26:423–433. doi: 10.1177/0748730411416341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilardi F., Migliavacca E., Naldi A., Baruchet M., Canella D., Le Martelot G., Guex N., Desvergne B., Cycli X.C. Genome-wide analysis of SREBP1 activity around the clock reveals its combined dependency on nutrient and circadian signals. PLoS Genet. 2014;10:e1004155. doi: 10.1371/journal.pgen.1004155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S., Le H.D., Melkani G.C., Panda S. Time-restricted feeding attenuates age-related cardiac decline in Drosophila. Science. 2015;347:1265–1269. doi: 10.1126/science.1256682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S., Panda S. A smartphone app reveals erratic diurnal eating patterns in humans that can Be modulated for health benefits. Cell Metab. 2015;22:789–798. doi: 10.1016/j.cmet.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwell B.J., Trott A.J., Beytebiere J.R., Pao S., Bosley A., Beach E., Finegan P., Hernandez C., Menet J.S. Rhythmic food intake drives rhythmic gene expression more potently than the hepatic circadian clock in mice. Cell Rep. 2019;27:649–657.e5. doi: 10.1016/j.celrep.2019.03.064. [DOI] [PubMed] [Google Scholar]
- Gupta N.J., Kumar V., Panda S. A camera-phone based study reveals erratic eating pattern and disrupted daily eating-fasting cycle among adults in India. PLoS One. 2017;12:e0172852. doi: 10.1371/journal.pone.0172852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi Y., Tahara Y., Hitosugi M., Shibata S. Impairment of circadian rhythms in peripheral clocks by constant light is partially reversed by scheduled feeding or exercise. J. Biol. Rhythms. 2015;30:533–542. doi: 10.1177/0748730415609727. [DOI] [PubMed] [Google Scholar]
- Hastings M.H., Maywood E.S., Brancaccio M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat. Rev. Neurosci. 2018;19:453–469. doi: 10.1038/s41583-018-0026-z. [DOI] [PubMed] [Google Scholar]
- Hatori M., Vollmers C., Zarrinpar A., DiTacchio L., Bushong E.A., Gill S., Leblanc M., Chaix A., Joens M., Fitzpatrick J.A. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D., Mao Y., Xu G., Liao W., Ren J., Yang H., Yang J., Sun L., Chen H., Wang W. Time-restricted feeding causes irreversible metabolic disorders and gut microbiota shift in pediatric mice. Pediatr. Res. 2019;85:518–526. doi: 10.1038/s41390-018-0156-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison A.T., Regmi P., Manoogian E.N.C., Fleischer J.G., Wittert G.A., Panda S., Heilbronn L.K. Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: a randomized crossover trial. Obesity (Silver Spring) 2019;27:724–732. doi: 10.1002/oby.22449. [DOI] [PubMed] [Google Scholar]
- Jakubowicz D., Barnea M., Wainstein J., Froy O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity (Silver Spring) 2013;21:2504–2512. doi: 10.1002/oby.20460. [DOI] [PubMed] [Google Scholar]
- Jakubowicz D., Landau Z., Tsameret S., Wainstein J., Raz I., Ahren B., Chapnik N., Barnea M., Ganz T., Menaged M. Reduction in glycated hemoglobin and daily insulin dose alongside circadian clock upregulation in patients with type 2 diabetes consuming a three-meal diet: a randomized clinical trial. Diabetes Care. 2019;42:2171–2180. doi: 10.2337/dc19-1142. [DOI] [PubMed] [Google Scholar]
- Jamshed H., Beyl R.A., Della Manna D.L., Yang E.S., Ravussin E., Peterson C.M. Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients. 2019;11:1234. doi: 10.3390/nu11061234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P., Turek F.W. Timing of meals: when is as critical as what and how much. Am. J. Physiol. Endocrinol. Metab. 2017;312:E369–E380. doi: 10.1152/ajpendo.00295.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahleova H., Belinova L., Malinska H., Oliyarnyk O., Trnovska J., Skop V., Kazdova L., Dezortova M., Hajek M., Tura A. Eating two larger meals a day (breakfast and lunch) is more effective than six smaller meals in a reduced-energy regimen for patients with type 2 diabetes: a randomised crossover study. Diabetologia. 2014;57:1552–1560. doi: 10.1007/s00125-014-3253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentish S.J., Hatzinikolas G., Li H., Frisby C.L., Wittert G.A., Page A.J. Time-restricted feeding prevents ablation of diurnal rhythms in gastric vagal afferent mechanosensitivity observed in high-fat diet-induced obese mice. J. Neurosci. 2018;38:5088–5095. doi: 10.1523/JNEUROSCI.0052-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesztyus D., Cermak P., Gulich M., Kesztyus T. Adherence to time-restricted feeding and impact on abdominal obesity in primary care patients: results of a pilot study in a pre-post design. Nutrients. 2019;11:2854. doi: 10.3390/nu11122854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbe I., Leinweber B., Brandenburger M., Oster H. Circadian clock network desynchrony promotes weight gain and alters glucose homeostasis in mice. Mol. Metab. 2019;30:140–151. doi: 10.1016/j.molmet.2019.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamia K.A., Sachdeva U.M., DiTacchio L., Williams E.C., Alvarez J.G., Egan D.F., Vasquez D.S., Juguilon H., Panda S., Shaw R.J. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science. 2009;326:437–440. doi: 10.1126/science.1172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeCheminant J.D., Christenson E., Bailey B.W., Tucker L.A. Restricting night-time eating reduces daily energy intake in healthy young men: a short-term cross-over study. Br. J. Nutr. 2013;110:2108–2113. doi: 10.1017/S0007114513001359. [DOI] [PubMed] [Google Scholar]
- Loboda A., Kraft W.K., Fine B., Joseph J., Nebozhyn M., Zhang C., He Y., Yang X., Wright C., Morris M. Diurnal variation of the human adipose transcriptome and the link to metabolic disease. BMC Med. Genomics. 2009;2:7. doi: 10.1186/1755-8794-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Q.J., Logunova L., Maywood E.S., Gallego M., Lebiecki J., Brown T.M., Sladek M., Semikhodskii A.S., Glossop N.R.J., Piggins H.D. Setting clock speed in mammals: the CK1 epsilon tau mutation in mice accelerates circadian pacemakers by selectively destabilizing PERIOD proteins. Neuron. 2008;58:78–88. doi: 10.1016/j.neuron.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro T., Tinsley G., Bianco A., Marcolin G., Pacelli Q.F., Battaglia G., Palma A., Gentil P., Neri M., Paoli A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl Med. 2016;14:290. doi: 10.1186/s12967-016-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata Y., Kaluzova M., Grimaldi B., Sahar S., Hirayama J., Chen D., Guarente L.P., Sassone-Corsi P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K., Suwa K. Association of hyperglycemia in a general Japanese population with late-night-dinner eating alone, but not breakfast skipping alone. J. Diabetes Metab. Disord. 2015;14:16. doi: 10.1186/s40200-015-0147-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novakova M., Polidarova L., Sladek M., Sumova A. Restricted feeding regime affects clock gene expression profiles in the suprachiasmatic nucleus of rats exposed to constant light. Neuroscience. 2011;197:65–71. doi: 10.1016/j.neuroscience.2011.09.028. [DOI] [PubMed] [Google Scholar]
- Okada C., Imano H., Muraki I., Yamada K., Iso H. The association of having a late dinner or bedtime snack and skipping breakfast with overweight in Japanese women. J. Obes. 2019:2439571. doi: 10.1155/2019/2439571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen M.K., Choi M.H., Kulseng B., Zhao C.M., Chen D. Time-restricted feeding on weekdays restricts weight gain: a study using rat models of high-fat diet-induced obesity. Physiol. Behav. 2017;173:298–304. doi: 10.1016/j.physbeh.2017.02.032. [DOI] [PubMed] [Google Scholar]
- Panda S., Antoch M.P., Miller B.H., Su A.I., Schook A.B., Straume M., Schultz P.G., Kay S.A., Takahashi J.S., Hogenesch J.B. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Parr E.B., Devlin B.L., Radford B.E., Hawley J.A. Delaying breakfast as a modified time-restricted 2 feeding protocol for improving glycemic control and 3 encouraging dietary adherence for men with 4 overweight/obesity: a randomized controlled trial. Nutrients. 2020;12:505. doi: 10.3390/nu12020505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poljsak B. NAMPT-mediated NAD biosynthesis as the internal timing mechanism: in NAD+ world, time is running in its own way. Rejuvenation Res. 2018;21:210–224. doi: 10.1089/rej.2017.1975. [DOI] [PubMed] [Google Scholar]
- Qian J., Morris C.J., Caputo R., Garaulet M., Scheer F. Ghrelin is impacted by the endogenous circadian system and by circadian misalignment in humans. Int. J. Obes. (Lond) 2019;43:1644–1649. doi: 10.1038/s41366-018-0208-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan C., Kathale N.D., Liu D., Lee C., Freeman D.A., Hogenesch J.B., Cao R., Liu A.C. mTOR signaling regulates central and peripheral circadian clock function. PLoS Genet. 2018;14:e1007369. doi: 10.1371/journal.pgen.1007369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey K.M., Yoshino J., Brace C.S., Abrassart D., Kobayashi Y., Marcheva B., Hong H.K., Chong J.L., Buhr E.D., Lee C. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science. 2009;324:651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravussin E. Early time-restricted feeding reduces appetite and increases fat oxidation but does not affect energy expenditure in humans. Obesity (Silver Spring) 2019;27:1244–1254. doi: 10.1002/oby.22518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert S.M., Weaver D.R. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Rudic R.D., McNamara P., Curtis A.M., Boston R.C., Panda S., Hogenesch J.B., Fitzgerald G.A. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer F.A., Morris C.J., Shea S.A. The internal circadian clock increases hunger and appetite in the evening independent of food intake and other behaviors. Obesity (Silver Spring) 2013;21:421–423. doi: 10.1002/oby.20351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman H., Frumin I., Gutman R., Chapnik N., Lorentz A., Meylan J., le Coutre J., Froy O. Long-term restricted feeding alters circadian expression and reduces the level of inflammatory and disease markers. J. Cell Mol. Med. 2011;15:2745–2759. doi: 10.1111/j.1582-4934.2010.01160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman H., Genzer Y., Cohen R., Chapnik N., Madar Z., Froy O. Timed high-fat diet resets circadian metabolism and prevents obesity. FASEB J. 2012;26:3493–3502. doi: 10.1096/fj.12-208868. [DOI] [PubMed] [Google Scholar]
- Shimizu H., Hanzawa F., Kim D., Sun S., Laurent T., Umeki M., Ikeda S., Mochizuki S., Oda H. Delayed first active-phase meal, a breakfast-skipping model, led to increased body weight and shifted the circadian oscillation of the hepatic clock and lipid metabolism-related genes in rats fed a high-fat diet. PLoS One. 2018;13:e0206669. doi: 10.1371/journal.pone.0206669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnier T., Rood J., Gimble J.M., Peterson C.M. Glycemic control is impaired in the evening in prediabetes through multiple diurnal rhythms. J. Diabetes Complications. 2014;28:836–843. doi: 10.1016/j.jdiacomp.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Stote K.S., Baer D.J., Spears K., Paul D.R., Harris G.K., Rumpler W.V., Strycula P., Najjar S.S., Ferrucci L., Ingram D.K. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am. J. Clin. Nutr. 2007;85:981–988. doi: 10.1093/ajcn/85.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram S., Yan L. Time-restricted feeding reduces adiposity in mice fed a high-fat diet. Nutr. Res. 2016;36:603–611. doi: 10.1016/j.nutres.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Sutton E.F., Beyl R., Early K.S., Cefalu W.T., Ravussin E., Peterson C.M. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018;27:1212–1221.e3. doi: 10.1016/j.cmet.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinsley G.M., Forsse J.S., Butler N.K., Paoli A., Bane A.A., La Bounty P.M., Morgan G.B., Grandjean P.W. Time-restricted feeding in young men performing resistance training: a randomized controlled trial. Eur. J. Sport Sci. 2017;17:200–207. doi: 10.1080/17461391.2016.1223173. [DOI] [PubMed] [Google Scholar]
- Tinsley G.M., Moore M.L., Graybeal A.J., Paoli A., Kim Y., Gonzales J.U., Harry J.R., VanDusseldorp T.A., Kennedy D.N., Cruz M.R. Time-restricted feeding plus resistance training in active females: a randomized trial. Am. J. Clin. Nutr. 2019;110:628–640. doi: 10.1093/ajcn/nqz126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai J.Y., Villegas-Montoya C., Boland B.B., Blasier Z., Egbejimi O., Gonzalez R., Kueht M., McElfresh T.A., Brewer R.A., Chandler M.P. Influence of dark phase restricted high fat feeding on myocardial adaptation in mice. J. Mol. Cell Cardiol. 2013;55:147–155. doi: 10.1016/j.yjmcc.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um J.H., Yang S., Yamazaki S., Kang H., Viollet B., Foretz M., Chung J.H. Activation of 5-AMP-activated kinase with diabetes drug metformin induces casein kinase Iepsilon (CKIepsilon)-dependent degradation of clock protein mPer2. J. Biol. Chem. 2007;282:20794–20798. doi: 10.1074/jbc.C700070200. [DOI] [PubMed] [Google Scholar]
- Villanueva J.E., Livelo C., Trujillo A.S., Chandran S., Woodworth B., Andrade L., Le H.D., Manor U., Panda S., Melkani G.C. Time-restricted feeding restores muscle function in Drosophila models of obesity and circadian-rhythm disruption. Nat. Commun. 2019;10:2700. doi: 10.1038/s41467-019-10563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.B., Loh D.H., Whittaker D.S., Cutler T., Howland D., Colwell C.S. Time-restricted feeding improves circadian dysfunction as well as motor symptoms in the Q175 mouse model of huntingtons disease. eNeuro. 2018;5 doi: 10.1523/ENEURO.0431-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., van Spyk E., Liu Q., Geyfman M., Salmans M.L., Kumar V., Ihler A., Li N., Takahashi J.S., Andersen B. Time-restricted feeding shifts the skin circadian clock and alters UVB-induced DNA damage. Cell Rep. 2017;20:1061–1072. doi: 10.1016/j.celrep.2017.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrens S.M.T., Christou S., Isherwood C., Middleton B., Gibbs M.A., Archer S.N., Skene D.J., Johnston J.D. Meal timing regulates the human circadian system. Curr. Biol. 2017;27:1768–1775.e3. doi: 10.1016/j.cub.2017.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson M.J., Manoogian E.N.C., Zadourian A., Lo H., Fakhouri S., Shoghi A., Wang X., Fleischer J.G., Navlakha S., Panda S., Taub P.R. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab. 2019;31:92–104.e5. doi: 10.1016/j.cmet.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodie L.N., Luo Y., Wayne M.J., Graff E.C., Ahmed B., ONeill A.M., Greene M.W. Restricted feeding for 9h in the active period partially abrogates the detrimental metabolic effects of a Western diet with liquid sugar consumption in mice. Metabolism. 2018;82:1–13. doi: 10.1016/j.metabol.2017.12.004. [DOI] [PubMed] [Google Scholar]
- Yasumoto Y., Hashimoto C., Nakao R., Yamazaki H., Hiroyama H., Nemoto T., Yamamoto S., Sakurai M., Oike H., Wada N. Short-term feeding at the wrong time is sufficient to desynchronize peripheral clocks and induce obesity with hyperphagia, physical inactivity and metabolic disorders in mice. Metabolism. 2016;65:714–727. doi: 10.1016/j.metabol.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Yoo S.H., Yamazaki S., Lowrey P.L., Shimomura K., Ko C.H., Buhr E.D., Siepka S.M., Hong H.K., Oh W.J., Yoo O.J. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci. U S A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrinpar A., Chaix A., Yooseph S., Panda S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. 2014;20:1006–1017. doi: 10.1016/j.cmet.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.Q., Van Haandel L., Xiong M., Huang P., Heruth D.P., Bi C., Gaedigk R., Jiang X., Li D.Y., Wyckoff G. Metabolic and molecular insights into an essential role of nicotinamide phosphoribosyltransferase. Cell Death Dis. 2017;8:e2705. doi: 10.1038/cddis.2017.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L.X., Li X.N., Yang G.Y., Zhang X., Li W.X., Zhang Q.Q., Pan H.X., Zhang H.H., Zhou M.Y., Wang Y.D. Circadian misalignment alters insulin sensitivity during the light phase and shifts glucose tolerance rhythms in female mice. PLoS One. 2019;14:e0225813. doi: 10.1371/journal.pone.0225813. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.