Abstract
Background and Aims
Plant secondary metabolites play critical roles in plant stress tolerance and adaptation, and are known to be influenced by the environment and climate changes, yet the impacts and interactions of multiple climate change components are poorly understood, particularly under natural conditions.
Methods
Accumulation of phenolics and emissions of volatile organic compounds (VOCs) were assessed on heather, Calluna vulgaris, an abundant evergreen dwarf shrub in European heathlands, after 6 years of exposure to elevated CO2, summer drought and nighttime warming.
Key Results
Drought alone had the strongest effects on phenolic concentrations and compositions, with moderate effects of elevated CO2 and temperature. Elevated CO2 exerted the greatest impact on VOC emissions, mainly by increasing monoterpene emissions. The response magnitudes varied among plant tissue types and chemical constituents, and across time. With respect to interactive effects of the studied climate change components, the interaction between drought and elevated CO2 was most apparent. Drought mainly reduced phenolic accumulation and VOC emissions, while elevated CO2 mitigated such effects.
Conclusions
In natural ecosystems, co-occurring climate factors can exert complex impacts on plant secondary metabolite profiles, which may in turn alter ecosystem processes.
Keywords: Calluna vulgaris, coastal heath, climate change, drought, elevated CO2, nighttime warming, plant secondary metabolites, phenolics, tannin, temperate grassland, volatile organic compound
INTRODUCTION
Global climate change is altering ecosystem structure and function (e.g. productivity, species distributions and interactions, carbon and nutrient cycling) (DeLucia et al., 2012; Hoover et al., 2012; Ode et al., 2014), which may feedback on the climate system (Laothawornkitkul et al., 2009). An emerging theme in this context concerns how plants re-configure their metabolism to respond and adapt to novel environments (DeLucia et al., 2012; Jamieson et al., 2017).
In addition to primary metabolites, plant secondary metabolites play critical roles in plant stress adaptation, species interactions and ecosystem functioning, and are influenced by climate change (Chomel et al., 2016; Jamieson et al., 2017; Holopainen et al., 2018). Among the most intensively studied plant secondary metabolites are volatile organic compounds (VOCs) and phenolic compounds, both of which are carbon-based chemicals (Laothawornkitkul et al., 2009; Salminen and Karonen, 2011). Plant VOCs represent a large and chemically diverse group of secondary metabolites, and consist primarily of terpenoids (e.g. isoprene, monoterpenes and sesquiterpenes), fatty acid-derived volatiles (e.g. green leaf volatiles) and benzenoids (Laothawornkitkul et al., 2009; Peñuelas and Staudt, 2010). Apart from acting as potent antioxidants to protect plants from oxidative stress, plant VOCs have repeatedly been documented to mediate a wide array of ecological interactions, including attraction of pollinators and seed dispersers, plant–microbe interactions, attraction and/or repellence of herbivorous arthropods, recruitment of carnivorous arthropods, and chemical signalling between and within plants (Hare, 2011). Furthermore, plant VOCs have potential to affect atmospheric processes by serving as precursors to ozone, reducing atmospheric oxidation capacity and contributing to secondary organic aerosol formation (Laothawornkitkul et al., 2009; Harrison et al., 2013). Likewise, phenolic compounds not only protect plants from oxidative stress (e.g. photo-damage) by acting as antioxidants, but also defend plants against herbivores or pathogens by reducing plant palatability and suppressing digestive enzymes of herbivores (Salminen and Karonen, 2011). Moreover, many phenolic compounds will still persist in plant tissues following senescence and have been demonstrated to have important afterlife effects on soil processes, including litter decomposition, microbial nutrient immobilization and nutrient availability (Chomel et al., 2016).
The direction and magnitude of the effects of global climate change on plant phytochemicals vary substantially depending on climate change factors (Peñuelas and Staudt, 2010; Peñuelas et al., 2013). For example, elevated CO2 generally increases levels of phenolics and condensed tannins while exerting minimal impacts on VOC emissions (Zvereva and Kozlov, 2006; Robinson et al., 2012; Holopainen et al., 2018). In contrast, elevated temperature consistently increases VOC emissions (Peñuelas and Staudt, 2010), but often reduces levels of phenolics (Zvereva and Kozlov, 2006; Nissinen et al., 2016; Sobuj et al., 2018). The effects of drought seem to be more variable. Phenolic concentrations are found to increase under moderate drought (Rivas-Ubach et al., 2014; Holopainen et al., 2018). However, VOC emissions are generally unaffected under mild to moderate drought stress, but are suppressed under severe stress (Niinemets, 2010a, b; Eller et al., 2016; Trowbridge et al., 2019). When plants are exposed simultaneously or sequentially to multiple climatic change factors, as is the case in nature, the outcome of plant phytochemical responses becomes more complex and more difficult to predict because multiple stresses most often do not act in an additive manner (Holopainen et al., 2018).
Early experiments focused on the effects of climate change on herbaceous plants from croplands and deciduous and coniferous tree species from forest ecosystems, and utilized seedlings and saplings (Robinson et al., 2012). These plant species appear to be consistently responsive to climate change factors, particularly elevated CO2, and dominate the literature. Other ecosystems, such as grasslands and heathlands, however, are under-represented, even though these ecosystems account for substantial land cover and have co-evolved for millennia with human societies. Indeed, the effects of climate change on plant secondary metabolism have been convincingly demonstrated to be highly species/genotype-specific, and may vary dramatically over plant developmental stages with immature plants being often more responsive than mature counterparts (Couture et al., 2014, 2017). At the aspen FACE (free air carbon enrichment) facility, for example, elevated CO2 increased the synthesis of condensed tannins in aspen but not in birch (Couture et al., 2017); the CO2-induced increase in phenolic concentrations was reversed in older compared with younger aspen trees (Couture et al., 2014). Taken together, to gain a more realistic perspective of how climate change influences plant secondary metabolism – and through this, ecosystem structure and function – long-term, multifactor experiments are required.
Here, we investigated the responses of secondary metabolic profiles of heather, Calluna vulgaris, under long-term exposure to elevated CO2, drought and nighttime warming in situ. Calluna vulgaris is an abundant evergreen dwarf shrub in European heathlands and is considered to be a stress-tolerant competitor in a variety of climatic conditions and soil types (Grime et al., 1988). Typical of slow-growing woody perennials with long-lived leaves that are adapted to nutrient-poor habitats (Coley et al., 1985), C. vulgaris has been reported to contain high levels of phenolics and tannins (Iason et al., 1993; Rieger et al., 2008). Compared to numerous laboratory and field studies on the impacts of climate change on the growth, photosynthesis, water use efficiency and C : N ratio of C. vulgaris (Gordon et al., 1999; Sæbø et al., 2001; Albert et al., 2011), there are only a few studies on the secondary metabolic responses (Iason et al., 1993; Kerslake et al., 1998; Hofland-Zijlstra and Berendse, 2009), and these focus on CO2 or nitrogen enrichment and find variable effects ranging from neutral to negative.
We measured VOC emissions of C. vulgaris branches in a temperate heathland and quantified the accumulation of phenolics and condensed tannins in different plant organs (leaves, stems and flowers) over two growing seasons after 6 years of exposure to realistic climatic manipulations. We hypothesized that when acting independently, both elevated CO2 and temperature will enhance phenolic production and VOC emissions, probably by stimulating carbon assimilation and growth, and boosting activities of enzymes associated with compound synthesis. We expected drought stress to reduce phenolic production and VOC emission, via suppression of plant photosynthesis. When acting in concert, elevated CO2 and temperature are expected to show synergistic responses except with drought. While CO2 would be likely to, at least partially, mitigate drought effects, warming could aggravate these due to enhanced soil evaporation and plant transpiration.
MATERIALS AND METHODS
Study site and experimental design
The study was conducted at the CLIMAITE project (www.climaite.dk) experimental site situated 50 km northwest of Copenhagen, Denmark (55°53′N, 11°58′E). The site is a heath/grassland ecosystem on dry, nutrient-poor sandy soil. It represents unmanaged semi-natural coastal heath typical of coastal Western Europe. Mean annual air temperature is 8.0 °C and mean annual precipitation is 613 mm (Danish Meteorological Institute, www.dmi.dk). The vegetation of the site is dominated by the dwarf shrub heather [Calluna vulgaris (L.) Hull] and the perennial wavy hair grass [Deschampsia flexuosa (L.) Trin.], with a low cover of forbs, graminoids and moss species (Mikkelsen et al., 2008). The soil is sandy with an organic layer of ~5 cm depth, and a pH of 4.2. The upper 10 cm of soil has ~2.3 % C and 0.14 % N, and ammonium is the dominant available N form, 10-fold more abundant than nitrate (Larsen et al., 2011).
The experimental setup included plots with daytime elevated CO2 concentration (CO2), nighttime warming (T), 1-month induced summer drought (D) and untreated control (A) for reference. Plots were placed in pairwise octagons (6.8 m across) receiving either ambient or elevated CO2 concentration. The distances between the octagons were at least 2.5 times the octagon widths to avoid CO2 contamination from the elevated to the ambient CO2 octagons. Each octagon was divided into four parts to provide all combinations of the treatments in a split-plot design. The four subplots within each octagon were adjacent to each other, but sampling was conducted towards the centre of the plots, with at least 0.5 m distance to adjacent plots. All treatments were replicated five times (n = 5, altogether 40 plots). This long-term experiment was started in October 2005. Detailed description of the experimental setup is given by Mikkelsen et al. (2008).
The treatments were designed to represent the likely climate scenario for Denmark in 2075 with 510 ppm CO2, elevated diurnal minimum temperature and extended summer drought (but only minor changes in annual rainfall). The elevated CO2 concentration was applied at 40 cm above the ground with the FACE system running all year round. The CO2 enrichment was continuously on during the sampling in the present study. Nighttime warming was conducted by a curtain (50 cm from the ground) reflecting the infrared radiation from the plots during nighttime all year round. The curtains were retracted during rain. The mean nighttime air temperature (20 cm from the ground) was 11.3 °C (2011) and 10.4 °C (2012) in control (A), and 12.1 °C (2011) and 11.2 °C (2012) in warmed (T) plots during the measurement period (May–August, data not shown). The mean diurnal temperature in soil at −5 cm depth was 14.0 °C (2011) and 13.3 °C (2012) in control plots while it was 14.3 °C (2011) and 13.7 °C (2012) under nighttime warming during the measurement period. The drought treatment was also conducted with curtains; they were pulled over the plots automatically in case of rain during one month (May) each year, leading to extended summer drought in this treatment. The mean volumetric water content in soil at −20 cm depth was 16.0 % (2011) and 13.5 % (2012) in the control (A) plots while it was 14.5 % (2011) and 11.8 % (2012) in the drought (D) plots during the entire growing season. The air temperature at 2 m from the ground and photosynthetic photon flux density (PPFD) were continuously monitored at the site.
VOC sampling and analysis
Samples for VOC analysis were collected from intact C. vulgaris shoots in all 40 plots on 14 June, 26 July and 24–25 August, 2011 and on 29 May, 3 July and 15 August, 2012. For sampling, the shoot was carefully enclosed in a pre-cleaned (120 °C, 60 min) polyethylene (PET) bag (25 × 38 cm, Freetime, Suomen Kerta oy, Imatra, Finland). The sample was pulled from the bag through a purified stainless steel tube (Perkin Elmer, Boston, MA, USA) filled with adsorbents (Tenax TA and Carbopack B, 100 mg of each, mesh 60/80, Supelco, Bellefonte, PA, USA). Flow through the tube was set to 200 mL min−1 with an M-5 bubble calibrator (A.P. Buck, Orlando, FL, USA). Sample collection lasted 30 min. An inflow (205 mL min−1) of filtered and MnO2-scrubbed air was maintained into the bag through Teflon tubing. Temperature and relative humidity inside the bag were monitored (iButtons, DS1923-F5# Hygrochron, Maxim Integrated, San Jose, CA, USA). The sample tubes were sealed with brass caps and refrigerated until analyses.
The VOCs collected in adsorbent tubes were analysed by gas chromatography-mass spectrometry (GC-MS; Agilent 7890 A GC and 5975 C VL MSD, Agilent Technologies, Inc., Santa Clara, CA, USA) following thermal desorption as described previously (Schollert et al., 2015). The VOC samples were thermally desorbed using an ULTRA autosampler and an UNITY2 thermal desorber (Markes International, Llantrisant, UK) at 250 °C for 10 min and cryofocused at −10 °C before injection into an HP-5 capillary column (length 50 m, diameter 0.2 mm, film thickness 0.33 µm). The carrier gas was helium. The GC oven temperature was held at 40 °C for 1 min, then increased at 5 °C min−1 to 210 °C, and again at 20 °C min−1 to 250 °C. The software Enhanced ChemStation (Agilent Technologies) followed by extracting and sorting by an in-house function in Excel was used to analyse the chromatograms. VOCs were identified according to the mass spectra in the Wiley data library, verified with authentic standards where available, and quantified from total ion counts with the pure standard solution of α-pinene, isolongifolene and 3-hexenyl-acetate. The presented dataset includes compounds that were found at least in 10 % of the samples with minimum 80 % identification certainty. Owing to malfunctioning of the GC-MS device, all VOC samples collected in 2011 were lost.
Analysis of phenolic compounds and condensed tannins
After VOC sampling, the Calluna shoot was cut and dried at room temperature and used for analyses of phenolics after dry mass determination. The phenolics from milled plant material were analysed as described by Taulavuori et al. (2013). Briefly, phenolics from leaves (6–8 mg), flowers (6–8 mg) and stems (about 15 mg) were extracted using cold, 100 % methanol using a Precellys 24 homogenizer for 30 s at 5000 rpm and left to stand on an ice-bath for 15 min. The samples were then centrifuged (Eppendorf centrifuge 5415R, Eppendorf, Hamburg, Germany) for 3 min at 9500 g, and the supernatant was separated. The extraction was repeated three more times, and methanol from the combined extracts were evaporated to dryness by using a vacuum centrifuge (Eppendorf 270 concentrator). Dried samples were redissolved into 0.6 mL of milliQ water/methanol (1 : 1), and quantified using liquid chromatography-DAD (HPLC; Model 1200 Agilent Technologies) at 320 nm for flavonoids and phenolic acids and 270 nm for other phenolics (according to Sobuj et al., 2018). Ultra-high-pressure liquid chromatography-quadrupole time-of-flight mass spectrometry (UHPLC-QTOF; Model 6340 Agilent Technologies) was used for identification of compounds in addition to spectral library and literature information (Taulavuori et al., 2013; see Supplementary Data Table S1 for identification details). Chromatographic separation of compounds was performed using a 2.1 × 60-mm, 1.7-µm C18 column (Agilent technologies). The mobile phase consisted of solvent A (1.5 % tetrahydrofuran and 0.25 % acetic acid in HPLC-quality water) and solvent B (100 % methanol). The gradient elution programme was as follows: 0–1.5 min, 0 % B; 1.5–3 min, 0–15 % B; 3–6 min, 10–30 % B; 6–12 min, 30–50 % B; 12–20 min, 50–100 % B; and 20–22 min, 100–0 % B. The flow rate was 0.4 mL min−1 and the injection volume was 0.2 μL. The UHPLC injector and oven temperatures were 22 and 30 °C, respectively. The mass spectra were acquired in the 100–3000 m/z range in positive ion mode. The temperature of the drying gas and sheath gas was 350 °C, and flow rates were 12 and 11 L min−1, respectively. The nebulizer pressure was 35 psi, capillary voltage 3500 V, nozzle voltage 1000 V, fragmentor voltage 80 V, skimmer voltage 65 V and octopole voltage 750 V. The reference mass m/z 922.0098 was used for accurate mass measurements. The quantification of phenolics was based on the following standard compounds: chlorogenic acid for chlorogenic acid and neochlorogenic acid; catechin for catechin and procyanidins; hyperin for all quercetins; and kaempferol 3-glucoside for all kaempferols, callunins and tiliroside.
Condensed tannins were analysed colorimetrically by extracting 25 mg of plant material in 5 mL methanol using the vanillin/HCl method, as described previously (Graglia et al., 2001).
Near-infrared reflectance (NIR) spectroscopy
Dried and milled planned material was analysed for untargeted chemical fingerprints by a NIRSystem 6500 NIR spectrophotometer (Foss Analytical, Höganäs, Sweden). Reflectance spectra in the range 1100–2500 nm at 2-nm intervals were recorded from samples introduced in a round, spinning sample cup with a quartz window. Each sample was measured three times with repacking, with 32 scans per measurement.
Statistical analysis
We assessed the differences in emissions of VOCs and concentrations of phenolics, compound classes and individual compounds using linear mixed-effects models (LMMs) (‘lme’ function in the nlme package in R). Climatic treatments (CO2, temperature, drought), sampling dates (year, month) and their interactions were treated as fixed factors, and blocks and plots as random factors with plots nested in blocks. Treatment effects were analysed both across and within sampling dates; for phenolic compounds, leaf, flower and stem data were analysed separately.
NIR data were pre-processed using a 2nd derivative by the Savitzky–Golay algorithm, using a window size of 11, and a 2nd order polynomial fitting, removing the end-points (Rinnan et al., 2009). The data were subsequently subjected to ANOVA-simultaneous component analysis (ASCA) (Smilde et al., 2005) in Matlab 2017b (MathWorks, Natick, MA, USA) to investigate the multivariate variance in the chemical patterns. ASCA was performed stepwise with the largest variation handled first. The ASCA for each combination of year, plant part and month was performed with 1000 permutation tests in order to investigate the significance of the treatments and the interactions, as well as the block effect.
RESULTS
Responses of HPLC-phenolics and condensed tannins to climate change
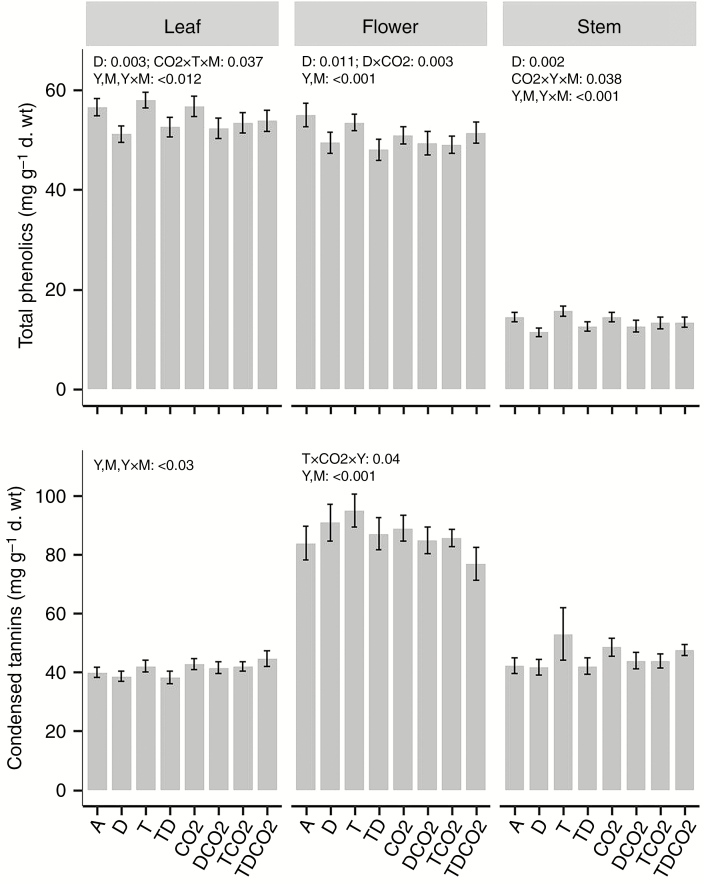
As expected, leaves, flowers and stems exhibited different phenolic profiles (Supplementary Data Fig. S1, Table S2). Stems had the most distinct phenolic profiles compared with flowers and leaves, the latter two plant tissues producing the same phenolic compounds but in different quantities (Fig. S1). Among 28 phenolic compounds used for this analysis, six compounds were found exclusively in stems while 11 compounds were unique to flowers and leaves. Stem phenolics were dominated by hyperin, while chlorogenic acid was the most abundant compound in leaves and flowers. When averaged across seasons, total HPLC-phenolic contents were similar in leaves (54.3 ± 0.7 mg g−1 d. wt) and flowers (50.9 ± 0.7) but significantly lower in stems (13.5 ± 0.4), while condensed tannin concentrations were similar in leaves (41.2 ± 0.7) and stems (45.3 ± 1.4) but significantly higher in flowers (86.6 ± 1.8).
Overall, drought effects on total HPLC-phenolic concentration were clear, but the main effects of elevated CO2 and nighttime warming were moderate. Averaged across the other treatments and over sampling times, drought decreased total HPLC-phenolic concentrations by ~7 % in leaves (F1,182 = 9.1, P = 0.003), ~5 % in flowers (F1,116 = 6.7, P = 0.011) and ~12 % in stems (F1,182 = 9.7, P = 0.002) (Fig. 1; Supplementary Data Tables S2 and S3). Such effects were most apparent at ambient CO2, but were attenuated at elevated CO2. Drought effects on total HPLC-phenolic concentrations also varied temporally both between years and seasonally within a year (Fig. S2). For both foliar and stem HPLC-phenolic concentrations, the negative drought effects were most pronounced immediately following the drought period in both years, and extended to the entire summer season in 2011, whereas drought effects on flower HPLC-phenolic concentration appeared in August in both years. While nighttime warming alone did not significantly change total HPLC-phenolic concentrations, significant interactions between drought and warming were seen for leaves on 14 June, 2011 (F1,23 = 4.7, P = 0.04), with warming negating in part the negative drought effects. There was also a significant combined effect of warming and elevated CO2 for leaves on 26 July, 2011 (F1,24 = 5.9, P = 0.023), when warming tended to increase leaf HPLC-phenolic content under ambient CO2 but decreased it under elevated CO2.
Fig. 1.
Concentrations of total HPLC-phenolics and condensed tannins averaged over two growing seasons. Bars represent mean values (mg g−1 d. wt) ± s.e. (n = 5), with P values of significant treatment effects shown above the bars. Concentrations for each growing season, and statistical results of LMMs are given in Supplementary Data Tables S2 and S3, respectively. A: ambient; D: drought; T: nighttime warming; CO2: elevated CO2; Y: year; M: month; TD, TCO2, DCO2, TDCO2: two- and three-way combinations of these climate treatments.
Compared to the total HPLC-phenolic contents, condensed tannin concentrations were less affected by the three global change components (Fig. 1). In both years, drought, particularly when acting alone, tended to decrease foliar condensed tannin concentrations immediately following the drought period. Most evident were the overall positive effects of nighttime warming on leaf condensed tannin content measured on 24 August, 2011, as well as the negative interactions between elevated CO2 and warming for flowers on 26 July and 24 August, 2011 (Supplementary Data Fig. S2). In the latter case, warming increased flower condensed tannin content under ambient CO2 but decreased it under elevated CO2 (T × CO2: P = 0.03 and 0.04 for July and August, 2011 respectively; Fig. S2, Table S4).
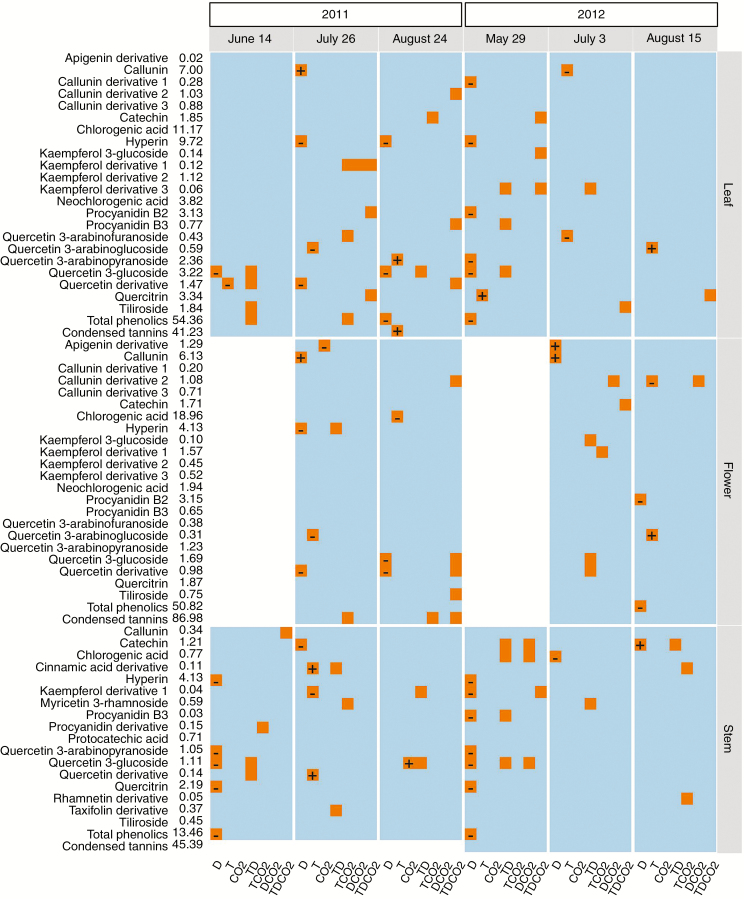
When treatment effects on individual compounds were analysed separately for each sampling time, drought remained the major factor affecting phenolic concentrations in terms of both the number of compounds that exhibited statistically significant responses and the frequency of these responses through the growing season (Fig. 2; Supplementary Data Table S4). Warming effects were less apparent, while CO2 effects were negligible. For many compounds, there were clear interactions between the environmental factors. In most cases either warming or CO2 offset drought effects, and in a few cases the combination of the three factors showed a positive or negative synergy (Table S4).
Fig. 2.
Heatmap showing significant treatment effects on concentrations of HPLC-phenolic compounds. Significant main and interactive effects at each sampling time are highlighted by orange boxes, with the direction of the main effects of CO2, warming and drought indicated by the plus (increase) and minus (decrease) signs inside the boxes, while the white space on the heatmap denotes data not available. The column of numbers following the compound names represents the mean concentrations (mg g−1 d. wt) across all treatments and sampling times. CO2: elevated CO2; T: warming; D: drought; TD, TCO2, DCO2, TDCO2: two- and three-way combinations of these climate treatments. See Supplementary Data Table S4 for detailed statistical results of the LMM analyses.
VOC emissions in climate treatments
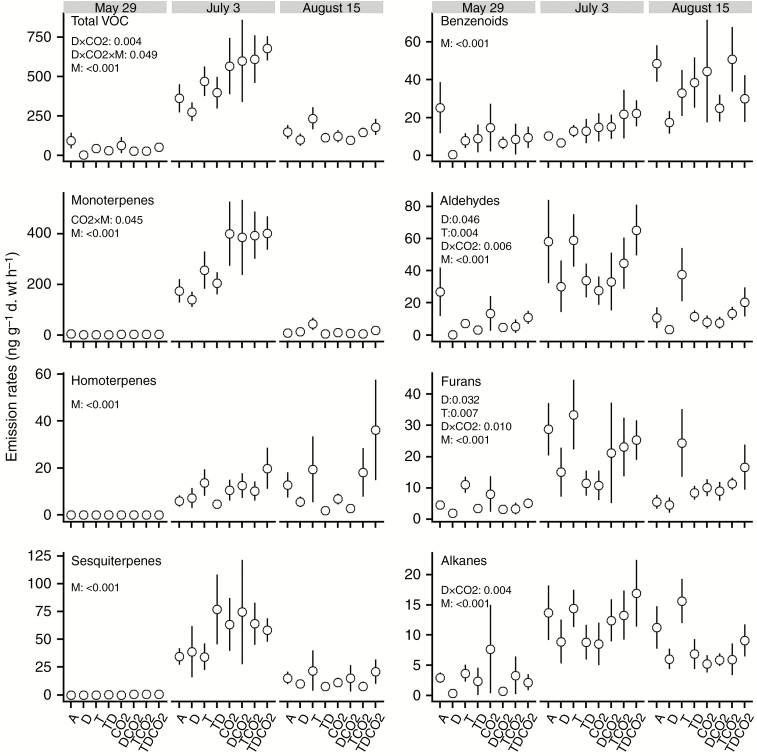
VOC emissions from C. vulgaris were dominated by monoterpenes (e.g. α-pinene and β-ocimene) and sesquiterpenes (e.g. β-caryophyllene), together accounting for ~55 % of the total VOC emissions over the season (Supplementary Data Table S5). VOC profiles changed both quantitatively and qualitatively throughout the season in 2012, with high total VOC emissions occurring on 3 July, followed by emissions on 15 August and then 29 May. This corresponded to the changes in light intensity and temperature during measurements (29 May: 371 μmol m−2 s−1, 11.2 °C; 3 July: 1126 μmol m−2 s−1, 20.6 °C; 15 August: 870 μmol m−2 s−1, 22.1 °C). Although none of the three climatic factors alone significantly affected total VOC emissions, drought significantly reduced emissions of aldehydes and furans while nighttime warming increased their emissions (Fig. 3; Table S6). The drought effects began to develop in May and persisted throughout the season. A significant interaction of CO2 and sampling time was found for monoterpene emissions, which almost doubled under elevated CO2 in early July. There were significant interactive effects of CO2 and drought on emissions of total VOCs, aldehydes, alkanes and furans, with CO2 counteracting the negative drought effect.
Fig. 3.
Mean values of VOC emissions in each climate treatment in 2012. Bars represent mean values (ng g−1 d. wt h−1) ± s.e. (n = 5), with P values of significant treatment effects shown above the bars (Supplementary Data Table S6). A comprehensive breakdown of emissions of individual compounds within each sampling time is given in Table S7. A: ambient; D: drought; T: nighttime warming; CO2: elevated CO2; M: month; TD, TCO2, DCO2, TDCO2: two- and three-way combinations of these climate treatments.
Untargeted chemical fingerprints from NIR spectroscopy
The NIR data showed that the chemical composition of the C. vulgaris plant material was clearly different for the two years. The between-year variation was followed by the variation in chemical fingerprint between stems, leaves and flowers and then by the variation between sampling months (Supplementary Data Fig. S3).
Analysis of each sampling month separately revealed several significant treatment effects (Supplementary Data Table S7). Elevated CO2 had significant or almost significant effects on the NIR spectra in both leaves and flowers. According to the loadings, the main separation in the data was due to C=C and CH2 (bonds at 1725, 1760, 2306 and 2348 cm−1), while the 2nd loading was caused by starch or cell-wall material (bonds at 1440, 1910 and 2290 cm−1) (Fig. S4). The effects of nighttime warming were less consistent, but showed significant differences for leaf chemical composition in July and August, 2011 and in May, 2012. Drought effects were significant in the sampling following the drought period.
DISCUSSION
Variation among tissues in leaf chemistry
Spatial and temporal variation in plant secondary chemistry is widespread. Several adaptive explanations have been proposed to explain this phenomenon. One of these, optimal defence theory, which predicts that defence allocation should be concentrated in tissues closely tied to plant fitness, such as reproductive parts (McKey, 1979), is supported by our results. We found higher phenolic concentrations in both flowers and leaves than stems, and higher condensed tannin concentrations in flowers than in leaves and stems. Our results are in general agreement with other observations of high tannin content in inflorescences compared to leaves (Iason et al., 1995; Hyder et al., 2002).
We also found that HPLC-phenolic and condensed tannin concentrations were relatively stable throughout the summer growing season, which is in line with an early study on temporal variation of heather phenolics (Iason et al., 1993). Unlike phenolic compounds, the untargeted chemical fingerprints based on NIR spectra and VOC emissions showed a strong variation over the growing season. Highest VOC emission was observed in July. This probably reflects the strong coupling of VOC emission, especially that of terpenoids, with temperature and irradiation (Peñuelas and Staudt, 2010; Holopainen et al., 2018).
Elevated CO2 increases monoterpene emission, but has no effects on phenolics
Contrary to our hypothesis, we found little to no effects of elevated CO2 on levels of phenolic compounds including condensed tannins. This starkly contrasts with many empirical studies and meta-analyses reporting that elevated CO2 in general increases HPLC-phenolic and condensed tannin concentrations (Veteli et al., 2007; Lindroth, 2010; Robinson et al., 2012; Sobuj et al., 2018), but agrees with other studies observing nominal CO2 effects on tissue phenolic accumulation (Holton et al., 2003; Muntifering et al., 2006).
Phenolic biosynthesis pathways or turnover metabolism of C. vulgaris may be inherently little responsive to elevated CO2 presumably because C. vulgaris is a slow-growing, stress-tolerant species, and constitutively produces high levels of a range of phenolic compounds (Grime et al., 1988; Rieger et al., 2008). A previous study on leaf condensed tannin concentrations in C. vulgaris in the same experiment as reported here documented a slight increase under elevated CO2 2 years after the initiation of CO2 fumigation (Schmidt et al., 2007). We observed similar tendencies that were not statistically significant. In agreement with this, an earlier glasshouse study on C. vulgaris found no change in foliar phenolic content after exposure to 600 ppm CO2 for 20 months (Kerslake et al., 1998). Phytochemical responses to CO2 enrichment can vary substantially with plant functional type, species and genotype (Valkama et al., 2007; Lindroth, 2010; Couture et al., 2012). These differences reflect taxonomic variation in evolutionary strategies that optimize solutions for the conflicting demands of carbon allocation toward growth, reproduction and defence.
The expected positive response of phenolics to elevated CO2 may be suppressed by low availability of soil nutrients, such as N and P, which can strongly shape plant physiological and biochemical responses to elevated CO2 (Reich et al., 2006; Robinson et al., 2012). In the heath ecosystem under investigation, previous studies have observed higher root biomass but decreased root N concentration and increased C/N ratios of photosynthetic tissue in response to elevated CO2 (Larsen et al., 2011; Arndal et al., 2013), suggesting that the vegetation, at least in CO2-enriched plots, may suffer from progressive N deficiency. Alternatively, the lack of a positive phenolic response to elevated CO2 may simply represent the ontogenetic variation in phytochemical responses to elevated CO2 given that several previous studies have demonstrated that elevated CO2 increases concentrations of phenolic compounds in the juvenile but not the mature stage (Holton et al., 2003; Couture et al., 2012, 2014).
With respect to VOC emissions, we found that monoterpene emissions doubled under elevated CO2, whilst emissions of other VOC groups (e.g. sesquiterpenes, benzenoids) were unaffected. This is in contrast to the decreased ecosystem-scale monoterpene emissions under elevated CO2 in the same experiment (Tiiva et al., 2017). However, the ecosystem plots contained a range of other vascular plants, mosses and also soil with its microbial communities, which have all potentially contributed to the net monoterpene emission reported previously (Tiiva et al., 2017). This may explain the discrepancy with our C. vulgaris shoot measurements. While a few plant-focused studies have reported increased terpenoid emissions in response to elevated CO2 (Himanen et al., 2009; Holopainen et al., 2018), most have found no changes or decreased emissions (e.g. Loreto et al., 2001; Staudt et al., 2001; Block et al., 2017). Decreased terpenoid emissions have been ascribed to CO2-induced reduction in stomatal density and conductance, as well as down-regulation of terpenoid biosynthesis at the transcriptional level (Loreto et al., 2001). Most observations of decreased terpenoid emissions come from laboratory studies, but results from field studies appear to be more complex to interpret. For instance, two field experiments on Quercus ilex grown at elevated CO2 came to opposite conclusions, indicating either inhibition (Loreto et al., 2001) or stimulation (Staudt et al., 2001) of monoterpene emissions in elevated compared to ambient CO2.
Little effect of nighttime warming on C. vulgaris phytochemicals
Temperature, a key parameter modulating plant physiological and biochemical processes, is known to stimulate plant photosynthetic rates. According to source–sink balance hypotheses (e.g. carbon/nutrient balance and growth/differentiation balance hypotheses) (Bryant et al., 1983), if resources (e.g. soil nutrients, moisture) are not limiting, accelerated photosynthesis at elevated temperature should contribute to growth rather than defence, leading to a reduction in carbon-based secondary metabolites. In partial agreement with these hypotheses, a multitude of empirical studies have demonstrated that elevated temperature reduces tissue phenolic concentrations while enhancing terpenoid emissions, although this effect is not necessarily representative of all individual compounds (Zvereva and Kozlov, 2006; Jamieson et al., 2012, 2015; Randriamanana et al., 2015; Mundim and Bruna, 2016; Escobar-Bravo et al., 2017). Increased terpenoid emissions are regarded as a result of the exponential temperature dependence of terpenoid vapour pressure and diffusion, but may also stem from the enhancement of precursor pool sizes and enzyme activities responsible for terpenoid synthesis (Niinemets, 2010a; Peñuelas and Staudt, 2010).
Surprisingly, we found little effect of nighttime warming on phenolic accumulation and VOC release except that warming increased emissions of aldehydes and furans. This is consistent with a previous study on ecosystem-level monoterpene emissions, which were only stimulated by nighttime warming in April with no effects during the main growing season (Tiiva et al., 2017). There are various reasons for the lack of temperature effects. First, compared to the active daytime warming often employed in previous studies, nighttime warming does not increase leaf surface temperature in the daytime (Albert et al., 2011). Consequently, the direct effects of nighttime warming on plant photosynthesis and metabolism may be limited. Second, reduced soil water content caused by nighttime warming (Selsted et al., 2012) may constrain phytochemical responses of C. vulgaris to temperature. Finally, C. vulgaris may be less sensitive to temperature and may show a lower responsiveness of the biosynthetic pathways related to phenolics and VOCs when variations in temperature occur in the range to which the species is adapted.
Reduced concentrations of phenolics and VOC emissions under drought stress
One-month drought in early summer (May) significantly decreased phenolic concentrations and VOC emissions immediately following the drought period but also in the following 2-month period, although the magnitude of the effects varied over each growing season and across years. The strong, negative drought effects are probably due to drought-induced reduction in photosynthesis, stomatal conductance and plant growth in C. vulgaris (Albert et al., 2011; Kongstad et al., 2012) and are consistent with reductions in ecosystem-scale monoterpene emissions (Tiiva et al., 2017), as observed earlier at the same experimental site. Our findings are in line with other studies reporting that positive, negative or no effects of drought stress on plant phytochemical responses can occur depending on the severity and duration of drought, as well as the plant species and chemical compounds in question (Niinemets, 2010a, b; Trowbridge et al., 2019). Under transient or mild drought stress, plants typically stimulate the biosynthesis of secondary metabolites with antioxidant characteristics (i.e. phenolics and terpenoids) to protect themselves from oxidative damage, whereas severe or prolonged drought stress, as observed in our experiment, can dramatically decrease the production of these compounds due to sustained inhibition of photosynthesis and impaired metabolic modulation (Niinemets, 2010a, b).
Complex interactions between elevated CO2, nighttime warming and drought
Although both elevated CO2 and nighttime warming alone had a minor impact on phytochemical responses of C. vulgaris, significant effects were manifested in their interaction with drought, as indicated by the almost equal number of compounds significantly affected by two- or three-way interactions compared to single-factor treatments. In most cases elevated CO2 tended to dampen the negative drought effects, as did the nighttime warming, whilst elevated CO2 negated the positive warming effects. Moreover, elevated CO2, nighttime warming and drought had both direct and interactive effects on the leaf chemical profiles, as revealed by the NIR-based non-targeted analysis. The magnitude of these interactions, however, varied considerably with climatic factors, plant tissues and chemical compounds, and over time, suggesting that the phytochemical responses of C. vulgaris to the combination of elevated CO2, warming and drought are complex. For example, the drought-mitigating effects of elevated CO2 were observed mainly for VOCs in peak summer, whilst the drought-mitigating effects of warming exclusively occurred for phenolics, especially in early summer.
A large number of studies have shown that elevated atmospheric CO2 can mitigate the negative effects of drought, although the magnitude of such an effect is context-dependent (Robinson et al., 2012; AbdElgawad et al., 2016; Jamieson et al., 2017; Holopainen et al., 2018). While the underlying mechanisms remain not well understood, it is clear that both physiological (e.g. decreased stomatal conductance, improved water use efficiency) and metabolic changes (e.g. increased production of antioxidants, enhanced activity of antioxidant enzymes, reduced production of active oxygen species in photorespiration) induced at elevated CO2 play important roles in protecting against drought stress (Zinta et al., 2014). In the present study, elevated CO2 reversed the negative drought effects on total VOC emissions and accumulation of phenolics. Early data from the same experiment suggest that elevated CO2 improves water use efficiency of C. vulgaris and stimulates leaf photosynthesis through the growing season (May to October) (Albert et al., 2011); this, however, does not appear to confer very effective protection against drought in terms of accumulation of phenolics, which are known to defend plants against drought-induced oxidative stress.
Warming enhances evaporation from soils, making periodic droughts worse than they would be under cooler conditions. Consequently, warming would be expected to exacerbate drought effects on plant physiological and metabolic processes. Conversely, we found that nighttime warming cancelled out the negative drought effects on phenolic concentrations in early summer. This may be due to nighttime warming stimulating photosynthesis in early summer as observed previously in this experimental site (Albert et al., 2011). Nonetheless, it should be noted that other environmental variables (e.g. the availability of light and soil nutrients) and biotic factors (e.g. plant pests and diseases) might have also played a role in the complex responses of plant defensive metabolites to the joint effects of elevated CO2, drought and nighttime warming.
In addition, care should be taken in the interpretation of the treatment effects on VOC emissions observed in the present study. The negative effects of drought as well as the mitigating effects of elevated CO2 on phenolic accumulation were consistent over the two growing seasons under investigation. While similar effects were observed on VOC emissions, they were derived from only one growing season as data from the second growing season were lost due to technical issues. Whether the treatment responses of VOC emissions could occur repeatedly across growing seasons requires future studies across multiple seasons. Previous studies (e.g. Staudt et al., 2001; Jamieson et al., 2015) as well as the present study have shown that the impacts of climate change on phytochemicals can vary both seasonally and interannually.
Ecological implications
Changes in the allocation or distribution of the different classes of defence metabolites, although highly variable, stand to shape the biodiversity and abundance of other organisms in heath ecosystems. For instance, phenolics from C. vulgaris have been shown to exhibit high radical scavenging capacity (Rieger et al., 2008), and as such they may play an important part in the plant’s protective and adaptive mechanisms developed in the harsh environments. Drought-induced reduction in phenolic concentrations may improve the palatability of C. vulgaris as a host, and so improve the performance of mammalian and insect herbivores, ultimately leading to more damage to the host plant and reduced plant fitness. In addition, changes to the quantity and composition of tissue phenolics may have a significant effect on litter decomposition and nutrient cycling, especially those of polymeric phenolics (Chomel et al., 2016). Moreover, reduction of VOC emissions during drought may render plants less apparent to herbivores and their natural enemies (Ode et al., 2014).
CONCLUSIONS
In summary, our study, performed in a natural habitat over two years following 6 years of exposure to realistic climate change manipulations, demonstrates that climate change (elevated CO2, nighttime temperature and drought frequency) influenced the overall phytochemical profiles, and also changed individual chemical constituents, which have known ecological roles. The response magnitudes, however, varied among plant tissue types and chemical constituents, and across time. Moreover, these results indicate that these responses are highly complex and dependent on co-occurring environmental factors, but also highlight that future climate change will indeed modify plant chemistry in ways that will have important impacts on ecosystem processes (e.g. plant–insect interactions, decomposition and nutrient cycling dynamics).
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: Identification of the phenolic compounds. Table S2: Phenolic concentrations under different treatments. Table S3: Statistical results of treatment effects on accumulation of phenolics. Table S4: Statistical results of treatment effects within each sampling time point. Table S5: VOC emissions under different treatments. Table S6: Statistical results of treatment effects on VOC emissions. Table S7: Statistical significance of the main and interactive effects of treatments on the chemical fingerprint based on NIR spectra. Figure S1: HPLC-phenolic profiles of different organs. Figure S2: Concentrations of total HPLC-phenolics and condensed tannins at each sampling time. Figure S3: Score plots from ASCA models showing separation of the chemical signal based on NIR spectra between tissues and sampling times. Figure S4: Score and loading plots of ASCA showing separation between CO2 treatments for the leaf chemical fingerprint based on NIR spectra.
FUNDING
This work was supported by the Academy of Finland (decisions 131969 and 272939) and European Union: An Integrated Network on Climate Research Activities on Shrubland Ecosystems (INCREASE) infrastructural project, FP7-Infrastructure-2008-1 (grant agreement 227628) to P.T., and the Danish Council for Independent Research | Natural Sciences (DFF-0602-010718) and the Villum Foundation (VKR022589) to R.R. The Villum Kann Rasmussen Foundation funded the set-up and maintenance of the field experiment.
ACKNOWLEDGEMENTS
We thank Gosha Sylvester for kindly assisting with GC-MS analyses, Sinikka Sorsa with phenolic analyses, Emilie Kann Elten, Pernille Ahlefeldt Wetterberg and Esben Vedel Nielsen with tannin analyses, Michelle Schollert, Charlotte Rasmussen and Aslak Hansen with field work, and Timo Oksanen with preparation of equipment. We would also like to thank Claus Beier and Teis Mikkelsen for access to the CLIMAITE research facility, and for site maintenance.
LITERATURE CITED
- AbdElgawad H, Zinta G, Beemster GT, Janssens IA, Asard H. 2016. Future climate CO2 levels mitigate stress impact on plants: increased defense or decreased challenge? Frontiers in Plant Science 7: 556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert KR, Ro-Poulsen H, Mikkelsen TN, Michelsen A, Van Der Linden L, Beier C. 2011. Effects of elevated CO₂, warming and drought episodes on plant carbon uptake in a temperate heath ecosystem are controlled by soil water status. Plant, Cell & Environment 34: 1207–1222. [DOI] [PubMed] [Google Scholar]
- Arndal MF, Schmidt IK, Kongstad J, Beier C, Michelsen A. 2013. Root growth and N dynamics in response to multi-year experimental warming, summer drought and elevated CO2 in a mixed heathland–grass ecosystem. Functional Plant Biology 41: 1–10. [DOI] [PubMed] [Google Scholar]
- Block A, Vaughan MM, Christensen SA, Alborn HT, Tumlinson JH. 2017. Elevated carbon dioxide reduces emission of herbivore-induced volatiles in Zea mays. Plant, Cell & Environment 40: 1725–1734. [DOI] [PubMed] [Google Scholar]
- Bryant JP, Chapin FS, Klein DR. 1983. Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 40: 357–368. [Google Scholar]
- Chomel M, Guittonny-Larchevêque M, Fernandez C, et al. 2016. Plant secondary metabolites: a key driver of litter decomposition and soil nutrient cycling. Journal of Ecology 104: 1527–1541. [Google Scholar]
- Coley PD, Bryant JP, Chapin FS., 3rd 1985. Resource availability and plant antiherbivore defense. Science 230: 895–899. [DOI] [PubMed] [Google Scholar]
- Couture JJ, Holeski LM, Lindroth RL. 2014. Long-term exposure to elevated CO2 and O3 alters aspen foliar chemistry across developmental stages. Plant, Cell & Environment 37: 758–765. [DOI] [PubMed] [Google Scholar]
- Couture JJ, Meehan TD, Lindroth RL. 2012. Atmospheric change alters foliar quality of host trees and performance of two outbreak insect species. Oecologia 168: 863–876. [DOI] [PubMed] [Google Scholar]
- Couture JJ, Meehan TD, Rubert-Nason KF, Lindroth RL. 2017. Effects of elevated atmospheric carbon dioxide and tropospheric ozone on phytochemical composition of trembling Aspen (Populus tremuloides) and paper birch (Betula papyrifera). Journal of Chemical Ecology 43: 26–38. [DOI] [PubMed] [Google Scholar]
- DeLucia EH, Nabity PD, Zavala JA, Berenbaum MR. 2012. Climate change: resetting plant–insect interactions. Plant Physiology 160: 1677–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eller AS, Young LL, Trowbridge AM, Monson RK. 2016. Differential controls by climate and physiology over the emission rates of biogenic volatile organic compounds from mature trees in a semi-arid pine forest. Oecologia 180: 345–358. [DOI] [PubMed] [Google Scholar]
- Escobar-Bravo R, Klinkhamer PG, Leiss KA. 2017. Interactive effects of UV-B light with abiotic factors on plant growth and chemistry, and their consequences for defense against arthropod herbivores. Frontiers in Plant Science 8: 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon C, Woodin SJ, Alexander IJ, Mullins CE. 1999. Effects of increased temperature, drought and nitrogen supply on two upland perennials of contrasting functional type: Calluna vulgaris and Pteridium aquilinum. New Phytologist 142: 243–258. [Google Scholar]
- Graglia E, Julkunen-Tiitto R, Shaver GR, Schmidt IK, Jonasson S, Michelsen A. 2001. Environmental control and intersite variations of phenolics in Betula nana in tundra ecosystems. New Phytologist 151: 227–236. [DOI] [PubMed] [Google Scholar]
- Grime JP, Hodgson JG, Hunt R. 1988. Comparative plant ecology: a functional approach to common British species. London: Unwin Hyman. [Google Scholar]
- Hare JD. 2011. Ecological role of volatiles produced by plants in response to damage by herbivorous insects. Annual Review of Entomology 56: 161–180. [DOI] [PubMed] [Google Scholar]
- Harrison SP, Morfopoulos C, Dani KG, et al. 2013. Volatile isoprenoid emissions from plastid to planet. New Phytologist 197: 49–57. [DOI] [PubMed] [Google Scholar]
- Himanen SJ, Nerg AM, Nissinen A, et al. 2009. Effects of elevated carbon dioxide and ozone on volatile terpenoid emissions and multitrophic communication of transgenic insecticidal oilseed rape (Brassica napus). New Phytologist 181: 174–186. [DOI] [PubMed] [Google Scholar]
- Hofland-Zijlstra JD, Berendse F. 2009. The effect of nutrient supply and light intensity on tannins and mycorrhizal colonisation in Dutch heathland ecosystems. Plant Ecology 201: 661–675. [Google Scholar]
- Holopainen JK, Virjamo V, Ghimire RP, Blande JD, Julkunen-Tiitto R, Kivimäenpää M. 2018. Climate change effects on secondary compounds of forest trees in the northern hemisphere. Frontiers in Plant Science 9:1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holton MK, Lindroth RL, Nordheim EV. 2003. Foliar quality influences tree-herbivore–parasitoid interactions: effects of elevated CO2, O3, and plant genotype. Oecologia 137: 233–244. [DOI] [PubMed] [Google Scholar]
- Hoover SE, Ladley JJ, Shchepetkina AA, Tisch M, Gieseg SP, Tylianakis JM. 2012. Warming, CO2, and nitrogen deposition interactively affect a plant–pollinator mutualism. Ecology Letters 15: 227–234. [DOI] [PubMed] [Google Scholar]
- Hyder PW, Fredrickson EL, Estell RE, Tellez M, Gibbens RP. 2002. Distribution and concentration of total phenolics, condensed tannins, and nordihydroguaiaretic acid (NDGA) in creosotebush (Larrea tridentata). Biochemical Systematics and Ecology 30: 905–912. [Google Scholar]
- Kongstad J, Schmidt IK, Riis-Nielsen T, Arndal MA, Mikkelsen TN, Beier C. 2012. High resilience in heathland plants to changes in temperature, drought, and CO2 in combination: results from the CLIMAITE experiment. Ecosystems 15: 269–283. [Google Scholar]
- Iason GR, Hartley SE, Duncan AJ. 1993. Chemical composition of Calluna vulgaris (Ericaceae): do responses to fertilizer vary with phenological stage? Biochemical Systematics and Ecology 21: 315–321. [Google Scholar]
- Iason GR, Hodgson J, Barry TN. 1995. Variation in condensed tannin concentration of a temperate grass (Holcus lanatus) in relation to season and reproductive development. Journal of Chemical Ecology 21: 1103–1112. [DOI] [PubMed] [Google Scholar]
- Jamieson MA, Burkle LA, Manson JS, Runyon JB, Trowbridge AM, Zientek J. 2017. Global change effects on plant–insect interactions: the role of phytochemistry. Current Opinion in Insect Science 23: 70–80. [DOI] [PubMed] [Google Scholar]
- Jamieson MA, Schwartzberg EG, Raffa KF, Reich PB, Lindroth RL. 2015. Experimental climate warming alters aspen and birch phytochemistry and performance traits for an outbreak insect herbivore. Global Change Biology 21: 2698–2710. [DOI] [PubMed] [Google Scholar]
- Jamieson MA, Trowbridge AM, Raffa KF, Lindroth RL. 2012. Consequences of climate warming and altered precipitation patterns for plant-insect and multitrophic interactions. Plant Physiology 160: 1719–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerslake JE, Woodin SJ, Hartley SE. 1998. Effects of carbon dioxide and nitrogen enrichment on a plant–insect interaction: the quality of Calluna vulgaris as a host for Operophtera brumata. New Phytologist 140: 43–53. [Google Scholar]
- Laothawornkitkul J, Taylor JE, Paul ND, Hewitt CN. 2009. Biogenic volatile organic compounds in the Earth system. New Phytologist 183: 27–51. [DOI] [PubMed] [Google Scholar]
- Larsen KS, Andresen LC, Beier C, et al. 2011. Reduced N cycling in response to elevated CO2, warming, and drought in a Danish heathland: synthesizing results of the CLIMAITE project after two years of treatments. Global Change Biology 17: 1884–1899. [Google Scholar]
- Lindroth RL. 2010. Impacts of elevated atmospheric CO2 and O3 on forests: phytochemistry, trophic interactions, and ecosystem dynamics. Journal of Chemical Ecology 36: 2–21. [DOI] [PubMed] [Google Scholar]
- Loreto F, Fischbach RJ, Schnitzler J-P, et al. 2001. Monoterpene emission and monoterpene synthase activities in the Mediterranean evergreen oak Quercus ilex L. grown at elevated CO2 concentrations. Global Change Biology 7: 709–717. [Google Scholar]
- McKey D. 1979. The distribution of secondary compounds within plants. In: Rosenthal GA, Janzen DH, eds. Herbivores: Their Interaction with Secondary Plant Metabolites. New York: Academic Press, 56–133. [Google Scholar]
- Mikkelsen TN, Beier C, Jonasson S, et al. 2008. Experimental design of multifactor climate change experiments with elevated CO2, warming and drought: the CLIMAITE project. Functional Ecology 22: 185–195. [Google Scholar]
- Mundim FM, Bruna EM. 2016. Is there a temperate bias in our understanding of how climate change will alter plant-herbivore interactions? A meta-analysis of experimental studies. The American Naturalist 188 Suppl 1: S74–S89. [DOI] [PubMed] [Google Scholar]
- Muntifering RB, Chappelka AH, Lin JC, Karnosky DF, Somers GL. 2006. Chemical composition and digestibility of Trifolium exposed to elevated ozone and carbon dioxide in a free-air (FACE) fumigation system. Functional Ecology 20: 269–275. [Google Scholar]
- Niinemets Ü. 2010a Mild versus severe stress and BVOCs: thresholds, priming and consequences. Trends in Plant Science 15: 145–1 53. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü. 2010b Responses of forest trees to single and multiple environmental stresses from seedlings to mature plants: past stress history, stress interactions, tolerance and acclimation. Forest Ecology and Management 260: 1623–1639. [Google Scholar]
- Nissinen K, Nybakken L, Virjamo V, Julkunen-Tiitto R. 2016. Slow-growing Salix repens (Salicaceae) benefits from changing climate. Environmental and Experimental Botany 128: 59–68. [Google Scholar]
- Ode PJ, Johnson SN, Moore BD. 2014. Atmospheric change and induced plant secondary metabolites — are we reshaping the building blocks of multi-trophic interactions? Current Opinion in Insect Science 5: 57–65. [DOI] [PubMed] [Google Scholar]
- Peñuelas J, Sardans J, Estiarte M, et al. 2013. Evidence of current impact of climate change on life: a walk from genes to the biosphere. Global Change Biology 19: 2303–2338. [DOI] [PubMed] [Google Scholar]
- Peñuelas J, Staudt M. 2010. BVOCs and global change. Trends in Plant Science 15: 133–144. [DOI] [PubMed] [Google Scholar]
- Randriamanana TR, Lavola A, Julkunen-Tiitto R. 2015. Interactive effects of supplemental UV-B and temperature in European aspen seedlings: implications for growth, leaf traits, phenolic defense and associated organisms. Plant Physiology and Biochemistry 93: 84–93. [DOI] [PubMed] [Google Scholar]
- Reich PB, Hobbie SE, Lee T, et al. 2006. Nitrogen limitation constrains sustainability of ecosystem response to CO2. Nature 440: 922–925. [DOI] [PubMed] [Google Scholar]
- Rieger G, Müller M, Guttenberger H, Bucar F. 2008. Influence of altitudinal variation on the content of phenolic compounds in wild populations of Calluna vulgaris, Sambucus nigra, and Vaccinium myrtillus. Journal of Agricultural and Food Chemistry 56: 9080–9086. [DOI] [PubMed] [Google Scholar]
- Rinnan Å, Berg Fvd, Engelsen SB. 2009. Review of the most common pre-processing techniques for near-infrared spectra. Trends in Analytical Chemistry 28: 1201–1222. [Google Scholar]
- Rivas-Ubach A, Gargallo-Garriga A, Sardans J, et al. 2014. Drought enhances folivory by shifting foliar metabolomes in Quercus ilex trees. New Phytologist 202: 874–885. [DOI] [PubMed] [Google Scholar]
- Robinson EA, Ryan GD, Newman JA. 2012. A meta-analytical review of the effects of elevated CO2 on plant–arthropod interactions highlights the importance of interacting environmental and biological variables. New Phytologist 194: 321–336. [DOI] [PubMed] [Google Scholar]
- Sæbø A, Håland Å, Skre O, Mortensen LM. 2001. Influence of nitrogen and winter climate stresses on Calluna vulgaris(L.) Hull. Annals of Botany 88: 823–828. [Google Scholar]
- Salminen J-P, Karonen M. 2011. Chemical ecology of tannins and other phenolics: we need a change in approach. Functional Ecology 25: 325–338. [Google Scholar]
- Schmidt IK, Beier C, Kongstad J, et al. 2007. Klimaændringer og processer og funktion i terrestriske økosystemer. Flora og Fauna 113: 117–128. [Google Scholar]
- Schollert M, Kivimäenpää M, Valolahti HM, Rinnan R. 2015. Climate change alters leaf anatomy, but has no effects on volatile emissions from Arctic plants. Plant, Cell & Environment 38: 2048–2060. [DOI] [PubMed] [Google Scholar]
- Selsted MB, van der Linden L, Ibrom A, et al. 2012. Soil respiration is stimulated by elevated CO2 and reduced by summer drought: three years of measurements in a multifactor ecosystem manipulation experiment in a temperate heathland (CLIMAITE). Global Change Biology 18: 1216–1230. [Google Scholar]
- Smilde AK, Jansen JJ, Hoefsloot HC, Lamers RJ, van der Greef J, Timmerman ME. 2005. ANOVA-simultaneous component analysis (ASCA): a new tool for analyzing designed metabolomics data. Bioinformatics (Oxford England) 21: 3043–3048. [DOI] [PubMed] [Google Scholar]
- Sobuj N, Virjamo V, Zhang Y, Nybakken L, Julkunen-Tiitto R. 2018. Impacts of elevated temperature and CO2 concentration on growth and phenolics in the sexually dimorphic Populus tremula (L.). Environmental and Experimental Botany 146: 34–44. [Google Scholar]
- Staudt M, Joffre R, Rambal S, Kesselmeier J. 2001. Effect of elevated CO2 on monoterpene emission of young Quercus ilex trees and its relation to structural and ecophysiological parameters. Tree Physiology 21: 437–445. [DOI] [PubMed] [Google Scholar]
- Taulavuori K, Julkunen-Tiitto R, Hyöky V, Taulavuori E. 2013. Blue mood for superfood. Natural Product Communication 8: 791–794. [Google Scholar]
- Tiiva P, Tang J, Michelsen A, Rinnan R. 2017. Monoterpene emissions in response to long-term night-time warming, elevated CO2 and extended summer drought in a temperate heath ecosystem. The Science of the Total Environment 580: 1056–1067. [DOI] [PubMed] [Google Scholar]
- Trowbridge AM, Stoy PC, Adams HD, et al. 2019. Drought supersedes warming in determining volatile and tissue defenses of piñon pine (Pinus edulis). Environmental Research Letters 14: 065006. [Google Scholar]
- Valkama E, Koricheva J, Oksanen E. 2007. Effects of elevated O3, alone and in combination with elevated CO2, on tree leaf chemistry and insect herbivore performance: a meta-analysis. Global Change Biology 13: 184–201. [Google Scholar]
- Veteli TO, Mattson WJ, Niemelä P, et al. 2007. Do elevated temperature and CO2 generally have counteracting effects on phenolic phytochemistry of boreal trees? Journal of Chemical Ecology 33: 287–296. [DOI] [PubMed] [Google Scholar]
- Zinta G, AbdElgawad H, Domagalska MA, et al. 2014. Physiological, biochemical, and genome-wide transcriptional analysis reveals that elevated CO2 mitigates the impact of combined heat wave and drought stress in Arabidopsis thaliana at multiple organizational levels. Global Change Biology 20: 3670–3685. [DOI] [PubMed] [Google Scholar]
- Zvereva EL, Kozlov MV. 2006. Consequences of simultaneous elevation of carbon dioxide and temperature for plant–herbivore interactions: a metaanalysis. Global Change Biology 12: 27–41. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.