Abstract
Background and Aims
Understorey species in temperate deciduous woodlands such as wild daffodil (Narcissus pseudonarcissus) and common snowdrop (Galanthus nivalis) have complex dormancy: seeds that are shed in late spring require warm summer temperatures for embryo elongation and dormancy alleviation, but then cooler temperatures for germination in autumn. As seasons warm and tree canopies alter, how will different seasonal temperature sequences affect these complex dormancy responses?
Methods
The effect of different sequences of warmer (+5 °C), current or cooler (–5 °C) seasons (summer to spring) on seed germination patterns over seven successive seasons were investigated, with all sequences combined factorially to determine the consequences of differential seasonal temperature change for the temporal pattern of germination (and so seedling recruitment).
Key Results
Little (<1 %, G. nivalis) or no (N. pseudonarcissus) seed germination occurred during the first summer in any treatment. Germination of N. pseudonarcissus in the first autumn was considerable and greatest at the average (15 °C) temperature, irrespective of the preceding summer temperature; germination was also substantial in winter after a warmer autumn. Germination in G. nivalis was greatest in the warmest first autumn and influenced by preceding summer temperature (average > warmer > cooler); the majority of seeds that germinated over the whole study did so during the two autumns but also in year 2’s cooler summer after a warm spring.
Conclusions
Warmer autumns and winters delay first autumn germination of N. pseudonarcissus to winter but advance it in G. nivalis; overall, warming will deplete the soil seed bank of these species, making annual seed influx increasingly important for recruitment and persistence. This study provides a comprehensive account of the effects of temperature changes in different seasons on seed germination in these early spring-flowering geophytes and consequently informs how these and other temperate woodland species with complex seed dormancy may respond to future climate change.
Keywords: Amaryllidaceae, climate change, daffodil, Galanthus nivalis L, geophyte, morphophysiological dormancy, Narcissus pseudonarcissus L, seed germination, snowdrop, temperature, temperate woodland
INTRODUCTION
Biodiversity is under direct and indirect pressure from an increasing human population, through habitat loss, invasive alien species, overexploitation of species, pollution and global climate change (Millennium Ecosystem Assessment, 2005). Warming due to anthropogenic emissions of greenhouse gases is anticipated to continue throughout the remainder of the 21st century, with more frequent high temperature extremes and more variable rainfall (IPCC, 2013). The weakening of the Atlantic Meridional Overturning Circulation (AMOC) during the 20th century (Rahmstorf et al., 2015) is also likely to continue in the 21st century (IPCC, 2013). Even though a collapse of the AMOC in the 21st century is very unlikely (IPCC, 2013), if it were to occur, the whole of the Northern Hemisphere would cool and further warming would be delayed (Drijfhout, 2015), as has occurred in the past (Henry et al., 2016).
The phenology (timing of leaf appearance, flowering and fruiting) of many plant species has been advanced by warming (Parmesan and Hanley, 2015), with earlier spring species being more sensitive (Menzel et al., 2006). British plant species, on average, flower earlier than previously and flowering is especially sensitive to temperature in the previous month, providing a strong biological signal of climate change (Fitter and Fitter, 2002). Early spring-flowering plants, which exploit the narrow time period prior to canopy closure, would have the greatest evolutionary pressure to respond to a changing climate by commencing development at the earliest opportunity (McEwan et al., 2011). Acclimation to climate change within a habitat is necessary for forest understorey plants that cannot quickly alter their range of distribution to enable them to persist (De Frenne et al., 2011). Woodland geophytes such as the wild daffodil (Narcissus pseudonarcissus) and common snowdrop (Galanthus nivalis) occupy a very distinct seasonal niche whereby they flower and shed seeds as the woodland canopy develops during the period from winter through to late spring (Newton et al., 2013). This narrow temporal window might provide a severe constraint to such species in the future under climate change, especially since future warming is unlikely to be uniform across regions and seasons (Christensen et al., 2007). In Narcissus, earlier-flowering cultivars have been shown to be advanced most by warming (Bock et al., 2015); similarly, flowering in G. nivalis was also advanced by warming (Maak and von Storch, 1997; Sparks et al., 2000).
Whilst the effects of global warming involve responses to environmental change across a range of plant life history processes, those which impact recruitment are especially important (Cochrane et al., 2019). Temperatures during seed development and maturation, as well as post-harvest environmental conditions, influence seed dormancy (Cochrane, 2019). Temperature also directly affects seed dormancy, survival and germination (Roberts, 1988; Batlla and Benech-Arnold, 2015) and is an important factor in plant establishment by seed–seedling cycles (Woodward, 1988; Cochrane, 2016). Thus, there is a high risk that seed germination in the soil seed bank will be compromised by climate change (Cochrane et al., 2015), particularly by extreme events such as heatwaves or summer drought (Giménez-Benavides et al., 2018).
Seeds are not necessarily shed at the best time for seedling establishment (Murdoch and Ellis, 2000). Seed dormancy, the inability of a seed to germinate in a specified period of time in environments usually favourable for germination (Baskin and Baskin, 2004), has therefore evolved to prevent germination in environments favourable for germination when the probability of seedling establishment, growth and survival is low (Vleeshouwers et al., 1995; Fenner and Thompson, 2005). Seed dormancy is a risk-spreading mechanism which may be compromised by climate change and so affect population dynamics and species persistence (Ooi et al., 2009). In contrast to the general advancement of flowering, the consequences of warming for loss of dormancy and germination within single habitats are species specific and can differ considerably (Hoyle et al., 2013; Orsenigo et al., 2015).
When investigating seed dormancy and ecology, fresh seeds are vital to ensure that it is the ecology of seed dormancy on shedding which is considered (Baskin et al., 2006). This is because physiological changes may occur during storage which reduce seed dormancy and alter germination requirements (Benech-Arnold and Sánchez, 1995; Egli, 1995; Vertucci and Farrant, 1995; Probert, 2000). This is particularly so in the moist temperate woodland species N. pseudonarcissus (daffodil) and G. nivalis (snowdrop). The seeds of these amaryllids are shed during spring or early summer at high moisture content (Newton et al., 2013). Seeds of Amaryllidaceae have linear embryos (Martin, 1946; Werker, 1997), with embryo elongation occurring within the seed after shedding and prior to germination. This phenomenon is termed morphological dormancy (Baskin and Baskin, 2014). Yet, as there is no arrest in embryo elongation, an alternative view is that dormancy, if present, is physiological (Newton et al., 2015; Vandelook et al., 2019). Nonetheless, ‘morphophysiological’ dormancy, where embryo elongation occurs and a physiological block must be overcome prior to germination, has been widely reported in Narcissus seeds (e.g. Vandelook and van Assche, 2008; Herranz et al., 2013a, b; Newton et al., 2013; Copete et al., 2014; Herranz et al., 2015). Seeds with morphophysiological dormancy typically have quite precise and often differing temperature requirements for embryo elongation, loss of dormancy and then germination (Baskin and Baskin, 2014). In G. nivalis and N. pseudonarcissus, embryo elongation after the seeds are shed can continue for more than a year (Newton et al., 2013). Warm summer temperatures followed by cooler autumns promote germination in the first, second or even third and fourth autumn–winter periods after shedding (Newton et al., 2015).
Different responses to warming amongst species are driven by seed dormancy traits, such that those with deep seed dormancy are the most affected because reduced primary and secondary dormancy results in greater likelihood of germination soon after dispersal (Bernareggi et al., 2016). Earlier or later germination are both theoretically possible with warmer temperatures in species with complex dormancy-breaking patterns, because they will depend upon which season’s response is the most affected by warming (Walck et al., 2011). The response may also depend upon which season experiences the greater increase in temperature. Moreover, the effect of warmer or cooler temperature in one season might not be detected until a later season in seeds with complex temperature requirements for dormancy alleviation and germination.
The impacts of anthropogenic climate change on seeds and seedlings, and consequently plant establishment, are understudied phases in the life cycle of plants (Parmesan and Hanley, 2015). The response of seed dormancy and germination, the crux of the transition from seed to seedling and so plant recruitment, of the woodland geophytes N. pseudonarcissus and G. nivalis was not known. Sustainable forest management can reduce (and indeed sometimes reverse) land degradation and can support mitigation and adaptation to climate change, slowing a decline in biodiversity (IPCC, 2019). These geophytes represent an important component of temperate woodland understorey species, typified by complex seed dormancy-breaking requirements and poor ability to disperse to and colonize new habitats, and are thus a focal constituent of temperate woodland ecology (Blandino, 2017). Our aim was to determine the responses of germination to contrasting climate change scenarios, season by season, from shedding onwards in these exemplars. We tested the hypotheses within each species that (1) seasonal temperature affects germination in that season; (2) seasonal temperature affects germination in subsequent seasons; and (3) the most suitable temperatures for promotion of germination vary amongst the four seasons.
MATERIALS AND METHODS
Study sites and seed collection
Capsules from at least 30 individual plants of each species were collected on 31 May 2007 from a wild population of N. pseudonarcissus in the Loder Valley Nature Reserve (51°03'29''N, 00°05'33''W); or on 19 May 2008 from an introduced population of G. nivalis at Wakehurst (51°04'07''N, 00°05'16''W). Capsules were held at 75 % relative humidity and 15 °C, representative of mean ambient study site conditions (Newton et al., 2013), until the germination test treatments began on 4 June 2007 for N. pseudonarcissus. Germination tests began on the day of collection for G. nivalis. Only fully formed seeds from naturally dehiscing capsules were selected for study.
Experimental design and selected temperature regimes
A factorial move-along experimental design was adopted to explore the potential effects of different seasonal (summer, autumn, winter and spring) warming and/or cooling on seed germination.
The average experimental temperature for each season was that value closest (in 5 °C increments from 0 °C) to the average of the mean daily temperature weighted by photoperiod (‘seasonal mean’) between 1 December and 28/29 February for winter, 1 March and 31 May for spring, 1 June and 31 August for summer, and 1 September and 30 November for autumn. The mean weighted daily temperature (Tw, °C) was calculated from Wakehurst temperature (T) records over a 2 year period (2004–2005) as:
The average treatment temperatures selected were: summer 20 °C (seasonal mean = 19.2 °C); autumn 10 °C (seasonal mean = 11.6 °C); winter 5 °C (seasonal mean = 4.6 °C); and spring 10 °C (seasonal mean = 11.0 °C). A year of the four average seasons was thus 20–10–5–10 °C. Seasonal temperatures were average, 5 °C warmer (25, 15, 10 and 15 °C, respectively) or 5 °C cooler (15, 5, 0 and 5 °C, respectively), providing a total of 81 different annual sequences of seasonal temperature. As the study sites (Loder Valley Nature Reserve and Wakehurst) are approx. 1 km apart, the same temperature sequences were used for seeds from each population. Seeds of both species were placed directly into summer temperatures (as they were shed in late May). Every possible factorial move-along combination of seasonal temperature was provided in a complete ‘year’ with the number of treatments (nt) = 81 (3 × 3 × 3 × 3), each represented by a single 50 seed germination test. Hence, the first summer’s three treatments were represented by 27 replicate germination tests each, the first autumn’s nine treatments by nine replicate germination tests each, the first winter’s 27 treatments by three replicate germination tests each and the first spring’s 81 different treatments (and all subsequent seasons in later years) were each represented by a single test. The interseasonal temperature changes imposed were broadly compatible with temperature variation measured near the collection sites: variation in daily temperature over 1 to 5 d periods included 1–8 swings of >10 °C each year between 2004 and 2015 (Supplementary data Fig. S1). Treatments were cycled through seven 84 d seasons, except for the second autumn for N. pseudonarcissus which was 112 d.
Germination tests
Seeds were sown on 1 % distilled water agar (Microbiological Agar Granules, Merck, Darmstadt, Germany) in 10 mm-diameter wells of Nunc multiwell dishes (Scientific Laboratory Supplies Ltd, Nottingham, UK). The latter were used to avoid fungal spread between seeds during long test periods (Newton et al., 2013). Dishes were placed in clear plastic bags in cooled incubators (LMS Ltd, Sevenoaks, UK) at the specified temperatures with lighting provided by 30 W cool white fluorescent bulbs. Based on known seed dispersal ecology and germination requirements for these species (Newton et al., 2015), germination tests for N. pseudonarcissus were conducted in the light (8 h d–1) and for G. nivalis in the dark, by double-wrapping dishes in aluminium foil prior to incubation. Seeds of N. pseudonarcissus were checked for germination at regular intervals under a flow hood (Capitarhood, Bigneat Ltd, Waterlooville, UK) and seeds of G. nivalis in a dark room under a dim, safe, green light comprising 15–20 W cool white fluorescent tubes covered with three layers of no. 39 (primary green) Cinemoid film (Probert and Smith, 1986). Seeds were transferred to fresh agar at least every 84 d. They were scored as germinated when the radicle was at least 2 mm long. At the end of the experiment, ungerminated seeds were dissected to determine whether they remained white and firm, and so probably viable.
Data analysis
Analyses were carried out in Genstat for Windows Eleventh Edition (VSN International Ltd, Hemel Hempstead, UK). Logistic regression analysis (i.e. fitting of a generalized linear model with logit link function and binomial error distribution) was used to determine the significance of the effect of different temperatures within each season on the final proportion of seeds that germinated at the end of each season.
RESULTS
Narcissus pseudonarcissus
No seeds of N. pseudonarcissus germinated during the first summer in any regime (Figs 1 and 2). Overall, the principal effect of the autumn temperature on cumulative germination throughout the 616 d study was striking (Fig. 1): under cold autumns of 5 °C (left-side columns, Fig. 1) total germination was lower than average (10 °C) (central columns, Fig. 1) or warm (15 °C) autumns (right-side columns, Fig. 1). Almost all germination occurred during 5 and 10 °C autumn periods, or 5 and 10 °C winter periods following 15 °C in autumn, in both years. Logistic regression analysis of final germination confirmed that the effects of summer, autumn and winter temperatures were significant (Supplementary data Table S1). Autumn was the most influential, followed by winter, then summer, with spring having the least effect on germination (compare residual deviances, Supplementary data Table S1).
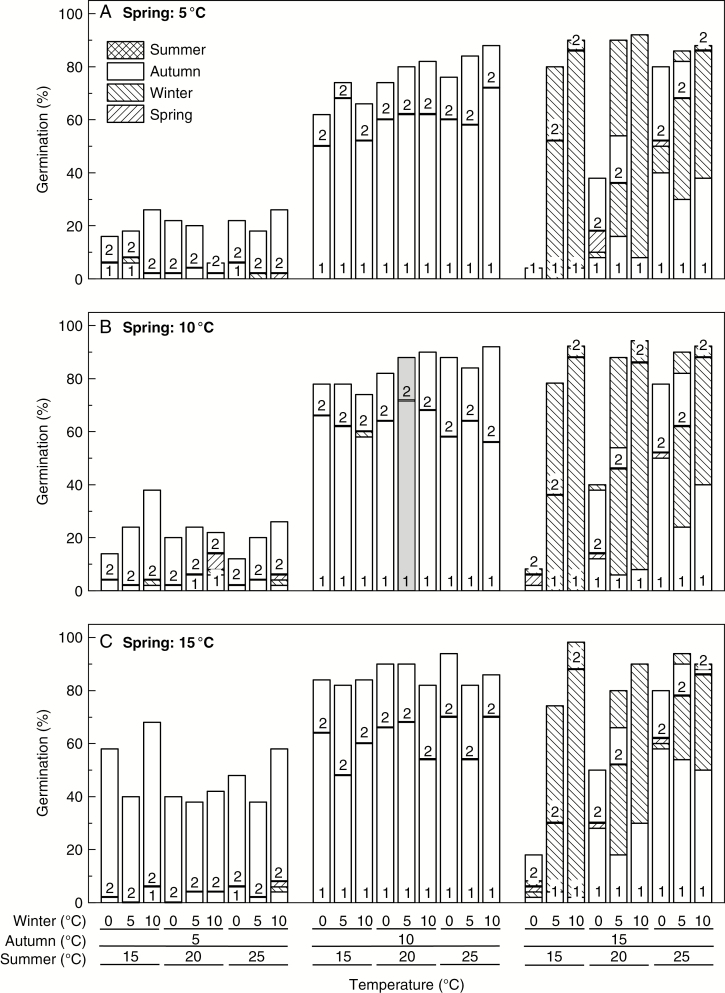
Fig. 1.
Cumulative seed germination (%, n = 50 seeds) of Narcissus pseudonarcissus under a (A) cool (5 °C), (B) average (10 °C) or (C) warm (15 °C) spring with all possible combinations of summer [cool (15 °C), average (20 °C) or warm (25 °C)], autumn [cool (5 °C), average (10 °C) or warm (15 °C)] and winter [cool (0 °C), average (5 °C) or warm (10 °C)] temperatures over 616 d with 84 or 112 d duration of the seasons. See the Materials and Methods for durations of the seasons. Year 1 (1 in column) comprised four seasonal periods from summer onwards; year 2 (2 in column) ended at the end of winter. The control (average seasonal temperatures) result is highlighted by light grey shading (centre of figure).
Fig. 2.
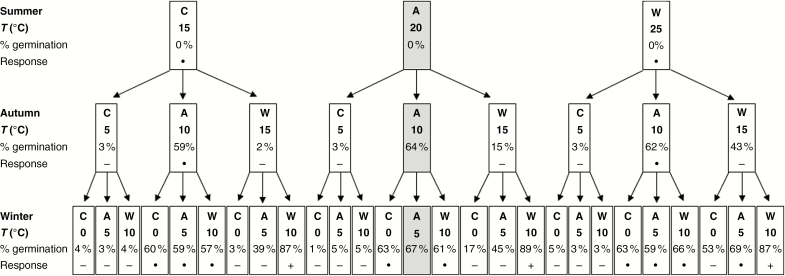
Cumulative seed germination (%) of Narcissus pseudonarcissus at the end of summer (nt = 27 treatments), autumn (nt = 9 treatments) and winter (nt = 3 treatments) in year 1 for average (A), cooler (C) or warmer (W) seasonal temperatures (T). Data are from Fig. 1. Germination was not significantly different (∙) or was significantly lower (−) or higher (+) than the control (logistic regression, P = 0.05). The control (average seasonal temperatures) result is highlighted by light grey shading (centre of figure).
Final germination in the average seasonal treatment (20–10–5–10 °C) after 616 d was 88 % (nt = 1) (Fig. 1) when almost all seeds capable of germinating had done so: only 4 % of ungerminated seeds remained viable (Supplementary data Table S2). Only 16 temperature combinations resulted in germination greater than the average seasonal treatment, eight of which combined a warm autumn (15 °C) with a warm winter (10 °C) (Fig. 1). In contrast, a cool winter (0 °C) after a warm autumn (15 °C) preceded by a cool summer (15 °C) had the lowest final germination (10 ± 4.2 %, nt = 3), with 83 ± 1.3 % (nt = 3) viable seeds remaining (Supplementary data Table S2). Final germination in treatments with a cool autumn (5 °C) was also comparatively low (30 ± 3.0 %, nt = 27) (Fig. 1), with 61 ± 3.1 % (nt = 27) viable seeds remaining (Supplementary data Table S2). Treatments with a cool winter (0 °C) following an average summer (20 °C) and a warm autumn (15 °C) similarly resulted in the germination of less than half of the population (43 ± 3.7 %, nt = 3) (Fig. 1), with 49 ± 3.3 % (nt = 3) viable seeds left (Supplementary data Table S2).
Figure 2 summarizes the cumulative results after the first three seasons. Germination during the first autumn was greatest at the average 10 °C (62 ± 1.3 %, nt = 27), irrespective of the preceding summer temperature (logistic regression, nt = 27, P = 0.240) which had a negligible effect (Fig. 2). Germination at the cooler autumn of 5 °C was negligible (3 ± 0.4 %, nt = 27), and germination was also not affected by summer temperature (logistic regression, nt = 27, P = 0.907). At the warmer autumn of 15 °C, however, germination was affected significantly by preceding summer temperature (logistic regression, nt = 27, P < 0.001), with greater germination the warmer the summer: 43 % (± 3.8 %, nt = 9) after 25 °C; 15% (± 3.0 %, nt = 9) after 20 °C; and only 2 % (± 0.6 %, nt = 9) after 15 °C (Fig. 2).
Further germination occurred during winter at 10 °C (warm) or 5 °C (average) after a warm autumn of 15 °C (Figs 1 and 2), but little in any other winter treatment. Cumulative germination at the end of the first winter was greatest in all treatments in which a warm winter of 10 °C followed a warm autumn of 15 °C (88 ± 0.7%, nt = 9) (Figs 1 and 2).
Negligible germination occurred in spring (<1 %, nt = 81) or the second summer (<1 %, nt = 81), irrespective of treatment (Fig. 1). During the second autumn, further germination (20 ± 1.4 %, nt = 27) occurred in the average autumn (10 °C) and in the cool autumn (5 °C, 26 ± 3.0 %, nt = 27), but not thereafter (Fig. 1). With a warm second autumn (15 °C), however, germination occurred then and/or in the subsequent winter, in a similar pattern to the previous year.
In the first autumn, most germination was observed at 10 °C and least at 5 °C, irrespective of the summer to autumn drop in temperature, whereas germination in 15 °C autumns increased as this seasonal drop increased from 0 to 10 °C (Fig. 2; Table 1). These patterns (as a percentage of seeds remaining) were repeated in the second autumn, with the exception of 5 °C which provided 24 % higher, on average, germination than the first autumn (Table 1). The patterns for winter germination were similar across years, with the biggest drop in temperature providing the greatest germination of remaining seeds within every winter regime (Table 1).
Table 1.
Effect of autumn or winter temperature and drop in temperature from the previous season (summer in the case of autumn, autumn in the case of winter) on the germination of Narcissus pseudonarcissus seeds during autumn (year) 1, winter 1, autumn 2 and winter 2, calculated as a percentage (mean ± s.e., nt = 9) of those seeds that had not germinated at the start of each season
| Temperature drop (°C) | Germination temperature (°C) | |||||
|---|---|---|---|---|---|---|
| Autumn 1 | Autumn 2 | |||||
| 5 | 10 | 15 | 5 | 10 | 15 | |
| 0 | – | – | 02 ± 0.6 | – | – | 01 ± 1.2 |
| 5 | – | 59 ± 2.4 | 15 ± 3.0 | – | 41 ± 5.5 | 17 ± 4.5 |
| 10 | 3 ± 0.8 | 64 ± 1.8 | 43 ± 3.8 | 31 ± 6.8 | 57 ± 3.9 | 36 ± 8.1 |
| 15 | 3 ± 0.7 | 62 ± 2.3 | – | 23 ± 4.3 | 63 ± 4.5 | – |
| 20 | 3 ± 0.8 | – | – | 27 ± 5.0 | – | – |
| Winter 1 | Winter 2 | |||||
| 0 | 5 | 10 | 0 | 5 | 10 | |
| 0 | – | 01 ± 0.3 | 00 ± 0.0 | – | 00 ± 0.0 | 01 ± 0.5 |
| 5 | 0 ± 0.0 | 00 ± 0.0 | 84 ± 2.0 | 0 ± 0.0 | 00 ± 0.0 | 30 ± 9.0 |
| 10 | 0 ± 0.0 | 42 ± 3.7 | – | 0 ± 0.0 | 54 ± 6.1 | – |
| 15 | 3 ± 1.8 | – | – | 1 ± 0.4 | – | – |
Of the seeds that germinated, most germination (58 %) occurred at 10 °C, followed by 26 % at 5 °C, 16 % at 15 °C and <1 % at 0 °C, with no germination at either 20 or 25 °C. Overall, 62 ± 3.3 % of seeds germinated, with 29 ± 3.3 % viable and 9 ± 0.3 % non-viable on dissection at the end of the investigation (nt = 81, Supplementary data Table S2). The number of non-viable seeds may have been influenced by summer temperature (logistic regression, nt = 81, P = 0.061), with the possibility of slightly more non-viable seeds the warmer the summer.
Galanthus nivalis
There was negligible germination during the first summer in G. nivalis. The majority of seeds that germinated did so during the autumn (years 1 and 2), but with some summer germination in year 2 at the (cool) 15 °C after the (warm) spring of 15 °C. Almost no seeds germinated (<1 %, nt = 81) during the first summer in any treatment (Figs 3 and 4), and few over the entire 588 d study in regimes combining cold autumns (5 °C) with cool and average springs (5 and 10 °C, left-side columns, Fig. 3A, B). Cumulative germination benefitted from a warm autumn (15 °C, Fig. 3).
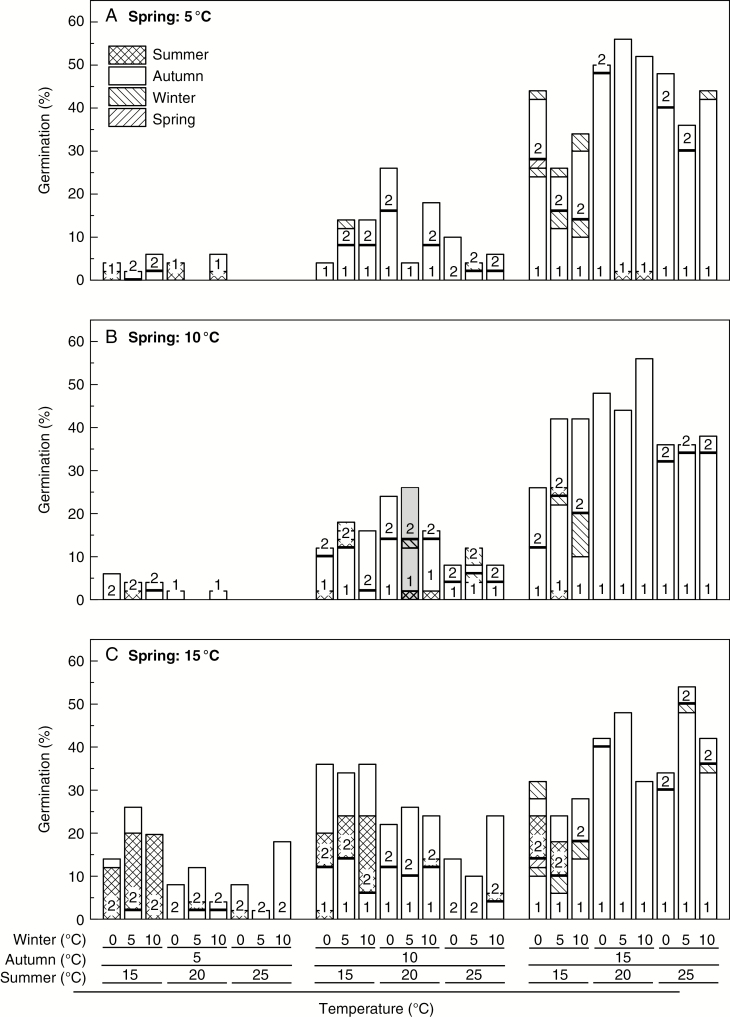
Fig. 3.
Cumulative seed germination (%, n = 50 seeds) of Galanthus nivalis under a (A) cool (5 °C), (B) average (10 °C) or (C) warm (15 °C) spring with all possible combinations of summer [cool (15 °C), average (20 °C) or warm (25 °C)], autumn [cool (5 °C), average (10 °C) or warm (15 °C)] and winter [cool (0 °C), average (5 °C) or warm (10 °C)] temperatures over 588 d with 84 d duration of seasons. Note the restricted y-axis. Year 1 (1 in column) comprised four seasonal periods from summer onwards; year 2 (2 in column) ended at the end of winter. The control (average seasonal temperatures) result is highlighted by light grey shading (centre of figure).
Fig. 4.
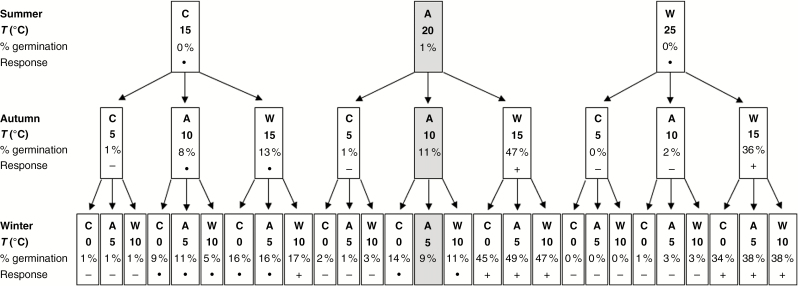
Cumulative seed germination (%) of Galanthus nivalis at the end of summer (nt = 27 treatments), autumn (nt = 9 treatments) and winter (nt = 3 treatments) for average (A), cooler (C) or warmer (W) seasonal temperatures (T). Data are from Fig. 3. Germination was not significantly different (∙) or was significantly lower (−) or higher (+) than the control (logistic regression, P = 0.05). The control (average seasonal temperatures) result is highlighted by light grey shading (centre of figure).
Logistic regression analysis of final germination for G. nivalis confirmed that autumn temperature affected germination (Supplementary data Table S3) and had the most influence, followed by spring, then summer, with winter having the least effect (compare residual deviances, Supplementary data Table S3). Germination was greatest at a warm autumn of 15 °C (32 ± 3.0 %, nt = 27, year 1), but was influenced by the preceding summer temperature (logistic regression, nt = 27, P < 0.001): greatest (47 ± 2.6 %, nt = 9) when preceded by an average summer (20 °C), less (36 ± 2.0 %, nt = 9) when preceded by a warm summer (25 °C) and least (13 ± 1.9 %, nt = 9) after a cool summer (15 °C; Fig. 4; Table 2). Negligible germination occurred during the following winter (<1 %, nt = 81) or spring (<1 %, nt = 81), irrespective of temperature regime (Fig. 3). In the second autumn, some further germination occurred at warm (15 °C, 6 ± 1.2 %, nt = 27), average (10 °C, 8 ± 1.0 %, nt = 27) and cool (5 °C, 3 ± 0.8 %, nt = 27) seasonal temperatures, with little germination (<1 %, nt = 81) during the second winter.
Table 2.
Effect of autumn or winter temperature and drop in temperature from the previous season (summer in the case of autumn, autumn in the case of winter) on the germination of Galanthus nivalis seeds during autumn (year) 1, winter 1, autumn 2 and winter 2, calculated as a percentage (mean ± s.e., nt = 9) of those seeds that had not germinated at the start of each season
| Temperature drop (°C) | Germination temperature (°C) | |||||
|---|---|---|---|---|---|---|
| Autumn 1 | Autumn 2 | |||||
| 5 | 10 | 15 | 5 | 10 | 15 | |
| 0 | – | – | 13 ± 1.9 | – | – | 15 ± 2.4 |
| 5 | – | 08 ± 1.3 | 47 ± 2.6 | – | 09 ± 2.4 | 01 ± 0.5 |
| 10 | 1 ± 0.4 | 11 ± 1.2 | 36 ± 2.0 | 3 ± 0.8 | 10 ± 1.8 | 07 ± 1.3 |
| 15 | 1 ± 0.5 | 02 ± 0.6 | – | 2 ± 1.2 | 08 ± 2.1 | – |
| 20 | 0 ± 0.0 | – | – | 3 ± 2.0 | – | – |
| Winter 1 | Winter 2 | |||||
| 0 | 5 | 10 | 0 | 5 | 10 | |
| 0 | – | 0 ± 0.0 | 0 ± 0.0 | – | 0 ± 0.0 | 0 ± 0.0 |
| 5 | 0 ± 0.0 | 0 ± 0.3 | 3 ± 1.2 | 0 ± 0.0 | 1 ± 0.5 | 1 ± 0.6 |
| 10 | 0 ± 0.0 | 2 ± 0.7 | – | 0 ± 0.0 | 0 ± 0.3 | – |
| 15 | 1 ± 0.4 | – | – | 1 ± 0.7 | – | – |
Final germination in average seasons (20–10–5–10 °C) after 588 d was 26 % (nt = 1) (Fig. 3), with 20 % of the remaining seeds viable (Supplementary data Table S4). Germination in treatments with warm (15 °C) autumns (with the exception of 15–15–5–15 °C with 24 % germination) was equal to or greater than average seasons (Fig. 3), with only 3 ± 1.0 % (nt = 27) viable seeds remaining (Supplementary data Table S4). In contrast, germination in a cool autumn of 5 °C was lower than that in average seasons (Fig. 3) (with the exception of 15–5–5–15 °C with 26 % germination), with 41 ± 3.1 % (nt = 27) viable seed remaining (Supplementary data Table S4).
Of the seeds that germinated, most germination (66 %) occurred at 15 °C, followed by 25 % at 10 °C, 7 % at 5 °C, 1 % at 20 °C and <1 % at both 0 and 25 °C. Overall, 21 ± 1.9 % of seeds germinated, with 24 ± 2.2 % viable and a much larger percentage non-viable (55 ± 1.4 %) at the end of the investigation (nt = 81, Supplementary data Table S4). The non-viable proportion was not influenced by summer temperature (logistic regression, nt = 81, P = 0.442).
DISCUSSION
The periods required for germination were considerable in both N. pseudonarcissus and G. nivalis, extending to >500 d in some individual seeds, whilst the temporal pattern of germination and the final value were affected greatly by the sequence of temperatures amongst seasons (Figs 1 and 3). The failure of seeds to germinate in the first summer (Figs 1 and 3) and their considerable germination, more in N. pseudonarcissus than in G. nivalis, with the drop in temperature in the first autumn and winter (Tables 1 and 2) matched the expected response given their complex temperature requirements for germination (Newton et al., 2013, 2015).
Although shared selection pressures can result in the convergence of germination syndromes in temperate forest herbs from different families (Vandelook et al., 2019), the responses of seed germination in species with similar germination syndromes to warming can vary. For example, the temperate forest understorey plants Anemone nemorosa and Milium effusum showed contrasting responses of seed germination to warming: germination was consistently enhanced in A. nemorosa but not in M. effusum (De Frenne et al., 2011). Warming increased the germination of both N. pseudonarcissus and G. nivalis in the current study, but with key seasonal differences due to different optimal temperatures for seed germination for each species. Warming in autumn improved germination in that season in G. nivalis (optimum germination temperature of 15 °C, Fig. 4) but reduced it in N. pseudonarcissus (optimum germination temperature of 10 °C, Fig. 2), whilst warming in the winter promoted germination in N. pseudonarcissus but not in G. nivalis. Warming across all three seasons would be expected to extend the period of germination in N. pseudonarcissus from the autumn alone towards autumn and winter, whilst, in G. nivalis, germination would probably occur earlier in the autumn of the first year (Fig. 4).
Seasonal changes in the dormancy of seeds in soil have been well documented in temperate plants (e.g. Baskin and Baskin, 1988). Dormancy in winter annuals and perennials can be induced by cold temperatures, whilst dormancy is relieved during warm summer temperatures before germination in autumn (Baskin and Baskin, 1988, 1994; Baskin et al., 2002; Copete et al., 2009; Newton et al., 2015). After the winter treatments, little (G. nivalis) or no (N. pseudonarcissus) germination occurred in the spring or second summer (Figs 1 and 3), which was also found in germination studies in N. pseudonarcissus seeds collected from both different populations and in different years and in G. nivalis seeds collected in different years (Newton et al., 2015). The failure to germinate in the first summer with a flush of germination in the autumn can be attributed to primary dormancy at shedding and its subsequent alleviation by warm temperatures. Whilst seeds that did not germinate may have retained primary dormancy throughout the first four seasons, the cold winter temperatures are more likely to have induced secondary dormancy as seeds failed to germinate at temperatures suitable for germination in the spring (Newton et al., 2015). This consequently delayed germination until the second autumn (with secondary dormancy lost in the second summer). Induction of secondary dormancy by low temperatures has been observed in Papaver rhoeas (Baskin et al., 2002) and N. hispanicus (Copete et al., 2011), for example.
In both species, on average, viable seeds remained at the end of the study in most treatments, as also observed by Newton et al. (2015) in studies with different populations (N. pseudonarcissus) and from different years (N. pseudonarcissus and G. nivalis), with the notable exception of the warmest summer–autumn–winter combination (25–15–10–X °C). In general, seed germination in N. pseudonarcissus tended to be greater overall (Figs 1–4), with considerably more dead seeds at the end of the study in G. nivalis (Supplementary data Tables S2 and S4). The results imply that soil seed bank depletion would be driven rather more by germination in N. pseudonarcissus and rather more by seed death in G. nivalis. Warming will deplete these species’ medium-term presence in the soil seed bank and so annual seed influx will become even more important to the persistence of these species in the future. Thompson and Cox (1978) similarly predicted a reduction in the reserve population of dormant seeds in the soil in response to higher summer temperatures in Hyacinthoides non-scripta, a later spring flowering temperate woodland geophyte. A reduction in the soil seed bank in response to a warmer climate has also been observed in alpine plants (Mondoni et al., 2015).
According to Christensen et al. (2007), the risk of summer drought is likely to become more severe in central Europe and the Mediterranean, with climate change posing a greater threat of desiccation to both N. pseudonarcissus and G. nivalis seeds. This is not likely to affect N. pseudonarcissus seed viability, as seeds are tolerant to drying throughout the warm summer period (Newton et al., 2015). However, as G. nivalis seeds are sensitive to drying (Newton et al., 2015), increased risk of summer drought may negatively impact seed viability and hence the species’ ability to regenerate from seed. Drying near the end of the summer period had the effect of preventing germination in a proportion of N. pseudonarcissus seeds in the subsequent autumn – probably by inducing dormancy – which was alleviated by warm temperatures in the second summer season, resulting in germination in the second autumn (Newton et al., 2015). This may function to counteract the increased germination predicted to occur in N. pseudonarcissus during the first autumn due to warmer temperatures. Dormancy cycling through the imposition and alleviation of secondary dormancy within buried seeds of Echinocactus platyacanthus was similarly observed by Aragón-Gastélum et al. (2018) whilst studying the impact of warming in the Chihuahuan Desert.
Narcissus pseudonarcissus is native to the Western Mediterranean and all southern Europe, and was probably introduced to Britain in the fourth and fifth centuries by the Romans (Church, 1908). Galanthus nivalis is native to southern, central and western Europe, introduced into the UK where it has naturalized extensively from escaped garden plants (Church, 1908). The origin of both species from regions with warmer climates than the UK may explain why the best germination is observed at warmer rather than average autumn temperatures for West Sussex. Either the environment in West Sussex has not been sufficiently stressful to drive selection for germination at appreciably cooler temperatures than the Mediterranean, or the populations have not existed in this environment for long enough for adaptation to have taken place. However, even though their origin and germination temperature optima may suggest that these species will fare better in future, given that the soil seed bank is likely to be depleted more quickly, they may be at greater risk of one-off events such as extreme rainfall.
Extrapolation from single population studies to the possible behaviour of the species requires caution, as population size, as well as temperature and precipitation during seed development, for example, can affect seed dormancy and dormancy release (Simpson, 1990; Gutterman, 1992, 1997). Although this specific experimental design with all factorial move-along permutations was not repeated on different populations of the same species, similar partial factorial move-along temperature conditions during germination tests with seeds collected both in different years and from different populations (Newton et al., 2015) showed broadly similar germination response patterns to those reported here.
Other studies that have considered how future changes in average temperatures might influence germination have often focused on temperature optima for germination and involved germinating non-dormant seeds on temperature gradient plates (e.g. Fernández-Pascual et al., 2015; Cochrane, 2016). However, this approach is too basic for species with complex dormancy, where seasonal changes in temperature may influence subsequent germination and recruitment.
Conclusions
The experimental approach devised here enabled the effect of different permutations of seasonal warming and/or cooling to be ascertained, not simply warming (or cooling) over the whole year. This is pertinent: at high latitudes, greater warming is likely in winter than other seasons (IPCC, 2013); and whilst warmer temperatures on average promoted germination of seeds of these two woodland geophytes eventually, the effect varied considerably with the particular season(s) of warming, species and cumulative number of seasons. Warming over the summer alone had little effect on seed germination in the first autumn for N. pseudonarcissus but reduced it in G. nivalis; warming in autumn alone reduced germination in the first autumn in N. pseudonarcissus but increased it in G. nivalis; warming in winter alone had little or no effect on germination in either species in the first year. Whilst the response of seed dormancy and germination to warming in these temperate woodland geophytes was both temporally and species dependent, warming by 5 °C did not expose a tipping point in the regulation of dormancy loss and germination by temperature. Hence, the responses of these two species to anthropogenic climate change would be considered conventional rather than aberrant by Parmesan and Hanley (2015).
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: results from logistic regression analyses, showing the relative importance of seasons on germination of Narcissus pseudonarcissus seeds. Table S2: the cumulative number of germinated Narcissus pseudonarcissus seeds and condition of non-germinated seeds at the end of the investigation, determined by dissection. Table S3: results from logistic regression analyses, showing the relative importance of seasons on germination of Galanthus nivalis seeds. Table S4: the cumulative number of germinated Galanthus nivalis seeds and condition of non-germinated seeds at the end of the investigation, determined by dissection. Figure S1: mean daily temperatures at Wakehurst, West Sussex, from 2004 to 2015.
FUNDING
This work was supported by the Millennium Commission, The Wellcome Trust and Orange plc. The Royal Botanic Gardens, Kew receives grant-in-aid from Defra, UK.
ACKNOWLEDGEMENTS
We thank the Statistical Advisory Service, University of Reading, for advice on logistical regression, John Adams and Nicola Keogh for laboratory support, David Hardman and Stephen Robinson for permission to collect seeds, and Robin Probert, Paul Neve and Alistair Murdoch for helpful discussions.
LITERATURE CITED
- Aragón-Gastélum JL, Flores J, Jurado E, et al. 2018. Potential impact of global warming on seed bank, dormancy and germination of three succulent species from the Chihuahuan Desert. Seed Science Research 28: 312–318. [Google Scholar]
- Baskin CC, Baskin JM. 1988. Germination ecophysiology of herbaceous plant species in a temperate region. American Journal of Botany 75: 286–305. [Google Scholar]
- Baskin CC, Baskin JM. 2014. Seeds: ecology, biogeography, and evolution of dormancy and germination, 2nd edn.London: Academic Press. [Google Scholar]
- Baskin CC, Milberg P, Andersson L, Baskin JM. 2002. Non-deep simple morphophysiological dormancy in seeds of the weedy facultative winter annual Papaver rhoeas. Weed Research 42: 194–202. [Google Scholar]
- Baskin CC, Thompson K, Baskin JM. 2006. Mistakes in germination ecology and how to avoid them. Seed Science Research 16: 165–168. [Google Scholar]
- Baskin JM, Baskin CC. 1994. Nondeep simple morphophysiological dormancy in seeds of the mesic woodland winter annual Cordalis flavula (Fumariaceae). Bulletin of the Torrey Botanical Club 121: 40–46. [Google Scholar]
- Baskin JM, Baskin CC. 2004. A classification system for seed dormancy. Seed Science Research 14: 1–16. [Google Scholar]
- Batlla D, Benech-Arnold RL. 2015. A framework for the interpretation of temperature effects on dormancy and germination in seed populations showing dormancy. Seed Science Research 25: 147–158. [Google Scholar]
- Benech-Arnold RL, Sánchez RA. 1995. Modelling weed seed germination. In: Kigel J, Galili GM, eds. Seed development and germination. New York: Dekker Inc., 545–566. [Google Scholar]
- Bernareggi G, Carbognani M, Mondoni A, Petraglia A. 2016. Seed dormancy and germination changes of snowbed species under climate warming: the role of pre- and post-dispersal temperatures. Annals of Botany 118: 529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandino C. 2017. Comparative seed biology of European temperate forest herbs. PhD thesis, University of Pavia, Italy. [Google Scholar]
- Bock A, Sparks TH, Estrella N, et al. 2015. Climate sensitivity and variation in first flowering of 26 Narcissus cultivars. International Journal of Biometeorology 59: 477–480. [DOI] [PubMed] [Google Scholar]
- Christensen JH, Hewitson B, Busuioc A, et al. 2007. Regional climate projections. In: Solomon S, Qin D, Manning M, et al. , eds. Climate change 2007: the physical science basis. Contribution of working group I to the fourth assessment report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press, 847–940. [Google Scholar]
- Church AH. 1908. Types of floral mechanism. Part I. Oxford: Clarendon Press. [Google Scholar]
- Cochrane A. 2016. Can sensitivity to temperature during germination help predict global warming vulnerability? Seed Science Research 26: 14–29. [Google Scholar]
- Cochrane A. 2019. Multi-year sampling provides insight into the bet-hedging capacity of the soil-stored seed reserve of a threatened Acacia species from Western Australia. Plant Ecology 220: 241–253. [Google Scholar]
- Cochrane A, Hoyle GL, Yates CJ, Wood J, Nicotra AB. 2015. Climate warming delays and decreases seedling emergence in a Mediterranean ecosystem. Oikos 124: 150–160. [Google Scholar]
- Cochrane A, Nicotra A, Ooi M. 2019. Climate change: alters plant recruitment from seed. Austral Ecology 44: 931–934. [Google Scholar]
- Copete E, Herranz JM, Ferrandis P, Baskin CC, Baskin JM. 2011. Physiology, morphology and phenology of seed dormancy break and germination in the endemic Iberian species Narcisssus hispanicus (Amaryllidaceae). Annals of Botany 107: 1003–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copete E, Herranz JM, Copete MÁ, Ferrandis P. 2014. Interpopulation variability on embryo growth, seed dormancy break, and germination in the endangered Iberian daffodil Narcissus eugeniae (Amaryllidaceae). Plant Species Biology 29: e72–e84. [Google Scholar]
- Copete MÁ, Herranz JM, Ferrandis P. 2009. Seed germination ecology of the endemic Iberian winter annuals Iberis pectinata and Ziziphora aragonensis. Seed Science Research 19: 155–159. [Google Scholar]
- De Frenne P, Brunet J, Shevtsova A, et al. 2011. Temperature effects on forest herbs assessed by warming and transplant experiments along a latitudinal gradient. Global Change Biology 17: 3240–3253. [Google Scholar]
- Drijfhout S. 2015. Competition between global warming and an abrupt collapse of the AMOC in Earth’s energy imbalance. Scientific Reports 5: 14877. doi: 10.1038/srep14877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli GH. 1995. Seed germination in soil: dormancy cycles. In: Kigel J, Galili GM, eds. Seed development and germination. New York: Dekker Inc., 529–543. [Google Scholar]
- Fenner M, Thompson K. 2005. The ecology of seeds. Cambridge: Cambridge University Press. [Google Scholar]
- Fernández-Pascual E, Seal CE, Pritchard HW. 2015. Simulating the germination response to diurnally alternating temperatures under climate change scenarios: comparative studies on Carex diandra seeds. Annals of Botany 115: 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitter AH, Fitter RSR. 2002. Rapid changes in flowering time in British plants. Science 296: 1689–1691. [DOI] [PubMed] [Google Scholar]
- Giménez-Benavides L, Escudero A, Garcia-Camacho R, et al. 2018. How does climate change affect regeneration of Mediterranean high-mountain plants? An integration and synthesis of current knowledge. Plant Biology 20: 50–62. [DOI] [PubMed] [Google Scholar]
- Gutterman Y. 1992. Maternal effects on seeds during development. In: Fenner M, ed. Seeds: the ecology of regeneration in plant communities. Wallingford, UK: CABI Publishing, 27–59. [Google Scholar]
- Gutterman Y. 1997. Genotypic, phenotypic and opportunistic germination strategies of some common desert annuals compared with plants with other seed dispersal and germination strategies. In: Ellis RH, Black M, Murdoch AJ, Hong TD eds. Basic and applied aspects of seed biology. Dordrecht: Kluwer Academic, 611–622. [Google Scholar]
- Henry LG, McManus JF, Curry WB, Roberts NL, Plotrowski AM, Keigwin LD. 2016. North Atlantic ocean circulation and abrupt climate change during the last glaciation. Science 353: 470–474. [DOI] [PubMed] [Google Scholar]
- Herranz JM, Copete E, Ferrandis P. 2013a Non-deep complex morphophysiological dormancy in Narcissus longispathus (Amaryllidaceae): implications for evolution of dormancy levels within section Pseudonarcissi. Seed Science Research 23: 141–155. [Google Scholar]
- Herranz JM, Copete MÁ, Ferrandis P. 2013b Environmental regulation of embryo growth, dormancy breaking and germination in Narcissus alcaracensis (Amaryllidaceae), a threatened endemic Iberian daffodil. The American Midland Naturalist 169: 147–167. [Google Scholar]
- Herranz JM, Copete E, Copete MÁ, Ferrandis P. 2015. Germination ecology of the endemic Iberian daffodil Narcissus radinganorum (Amaryllidaceae). Dormancy induction by cold stratification or desiccation in late stages of embryo growth. Forest Systems 24: e013. doi: 10.5424/fs/2015241-06197. [Google Scholar]
- Hoyle GL, Venn SE, Steadman KJ, et al. 2013. Soil warming increases plant species richness but decreases germination from the alpine soil seed bank. Global Change Biology 19: 1549–1561. [DOI] [PubMed] [Google Scholar]
- IPCC 2013. Climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the Intergovernmental Panel on Climate Change. Stocker TF, Qin D, Plattner G, et al. , eds. Cambridge: Cambridge University Press. [Google Scholar]
- IPCC 2019. IPCC special report on climate change, desertification, land degradation, sustainable land management, food security, and greenhouse gas fluxes in terrestrial ecosystems. Summary for policymakers. Approved Draft. https://www.ipcc.ch/site/assets/uploads/2019/08/Edited-SPM_Approved_Microsite_FINAL.pdf (5 September 2019).
- Maak K, von Storch H. 1997. Statistical downscaling of monthly mean air temperature to the beginning of flowering of Galanthus nivalis L. in Northern Germany. International Journal of Biometeorology 41: 5–12. [Google Scholar]
- Martin AC. 1946. The comparative morphology of seeds. The American Midland Naturalist 36: 513–660. [Google Scholar]
- McEwan RW, Brecha RJ, Geiger DR, John GP. 2011. Flowering phenology change and climate warming in southwestern Ohio. Plant Ecology 212: 55–61. [Google Scholar]
- Menzel A, Sparks TH, Estrella N, et al. 2006. European phenological response to climate change matches the warming pattern. Global Change Biology 12: 1969–1976. [Google Scholar]
- Millennium Ecosystem Assessment 2005. Ecosystems and human well-being: biodiversity synthesis. Washington, DC: World Resources Institute. [Google Scholar]
- Mondoni A, Pedrini S, Bernareggi G, et al. 2015. Climate warming could increase recruitment success in glacier foreland plants. Annals of Botany 116: 907–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch AJ, Ellis RH. 2000 Dormancy, viability and longevity. In: Fenner M, ed. Seeds: the ecology of regeneration in plant communities, 2nd edn.Wallingford, UK: CABI Publishing, 183–214. [Google Scholar]
- Newton RJ, Hay FR, Ellis RH. 2013. Seed development and maturation in early spring-flowering Galanthus nivalis and Narcissus pseudonarcissus continues post-shedding with little evidence of maturation in planta. Annals of Botany 111: 945–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton RJ, Hay FR, Ellis RH. 2015. Ecophysiology of seed dormancy and the control of germination in early spring-flowering Galanthus nivalis and Narcissus pseudonarcissus (Amaryllidaceae). Botanical Journal of the Linnean Society 177: 246–262. [Google Scholar]
- Ooi MKJ, Auld TD, Denham AJ. 2009. Climate change and bet-hedging: interactions between increased soil temperatures and seed bank persistence. Global Change Biology 15: 2375–2386. [Google Scholar]
- Orsenigo S, Abeli T, Rossi G, et al. 2015. Effects of autumn and spring heat waves on seed germination of high mountain plants. PLoS One 10: e0133626. doi: 10.1371/journal.pone.0133626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmesan C, Hanley ME. 2015. Plants and climate change: complexities and surprises. Annals of Botany 116: 849–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probert RJ. 2000. The role of temperature in the regulation of seed dormancy and germination. In: Fenner M, ed. Seeds: the ecology of regeneration in plant communities, 2nd edn.Wallingford, UK: CABI Publishing, 261–292. [Google Scholar]
- Probert RJ, Smith RD. 1986. The joint action of phytochrome and alternating temperatures in the control of seed germination in Dactylis glomerata. Physiologia Plantarum 67: 299–304. [Google Scholar]
- Rahmstorf S, Box JE, Feulner G, et al. 2015. Exceptional twentieth-century slowdown in Atlantic Ocean overturning circulation. Nature Climate Change 5: 475–480. [Google Scholar]
- Roberts EH. 1988. Temperature and seed germination. Symposia of the Society for Experimental Biology 42: 109–132. [PubMed] [Google Scholar]
- Simpson GM. 1990. Seed dormancy in grasses. Cambridge: Cambridge University Press. [Google Scholar]
- Sparks TH, Jeffree EP, Jeffree CE. 2000. An examination of the relationship between flowering times and temperature at the national scale using long-term phenological records from the UK. International Journal of Biometeorology 44: 82–87. [DOI] [PubMed] [Google Scholar]
- Thompson PA, Cox SA. 1978. Germination of the bluebell (Hyacinthoides non-scripta (L.) Chouard) in relation to its distribution and habitat. Annals of Botany 42: 51–62. [Google Scholar]
- Vandelook F, van Assche JA. 2008. Temperature requirements for seed germination and seedling development determine timing of seedling emergence of three monocotyledonous temperate forest spring geophytes. Annals of Botany 102: 865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandelook F, Van de Vyver A, Carta A. 2019. Three phylogenetically distant shade-tolerant temperate forest herbs have similar seed germination syndromes. Folia Geobotanica 54: 73–84. [Google Scholar]
- Vertucci CW, Farrant JM. 1995. Acquisition and loss of desiccation tolerance. In: Kigel J, Galili GM eds. Seed development and germination. New York: Dekker Inc., 237–272. [Google Scholar]
- Vleeshouwers LM, Bouwmeester HJ, Karssen CM. 1995. Redefining seed dormancy: an attempt to integrate physiology and ecology. Journal of Ecology 83: 1031–1037. [Google Scholar]
- Walck JL, Hidayati SN, Dixon KW, Thompson K, Poschlod P. 2011. Climate change and plant regeneration from seed. Global Change Biology 17: 2145–2161. [Google Scholar]
- Werker E. 1997. Seed anatomy. Berlin: Gebrüder Borntraeger. [Google Scholar]
- Woodward FI. 1988. Temperature and the distribution of plant species. Symposia of the Society for Experimental Biology 42: 59–75. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.