Abstract
Background and Aims
Plants have the potential to adjust the configuration of their hydraulic system to maintain its function across spatial and temporal gradients. Species with wide environmental niches provide an ideal framework to assess intraspecific xylem adjustments to contrasting climates. We aimed to assess how xylem structure in the widespread species Nothofagus pumilio varies across combined gradients of temperature and moisture, and to what extent within-individual variation contributes to population responses across environmental gradients.
Methods
We characterized xylem configuration in branches of N. pumilio trees at five sites across an 18° latitudinal gradient in the Chilean Andes, sampling at four elevations per site. We measured vessel area, vessel density and the degree of vessel grouping. We also obtained vessel diameter distributions and estimated the xylem-specific hydraulic conductivity. Xylem traits were studied in the last five growth rings to account for within-individual variation.
Key Results
Xylem traits responded to changes in temperature and moisture, but also to their combination. Reductions in vessel diameter and increases in vessel density suggested increased safety levels with lower temperatures at higher elevation. Vessel grouping also increased under cold and dry conditions, but changes in vessel diameter distributions across the elevational gradient were site-specific. Interestingly, the estimated xylem-specific hydraulic conductivity remained constant across elevation and latitude, and an overwhelming proportion of the variance of xylem traits was due to within-individual responses to year-to-year climatic fluctuations, rather than to site conditions.
Conclusions
Despite conspicuous adjustments, xylem traits were coordinated to maintain a constant hydraulic function under a wide range of conditions. This, combined with the within-individual capacity for responding to year-to-year climatic variations, may have the potential to increase forest resilience against future environmental changes.
Keywords: Elevational gradient, latitudinal gradient, Nothofagus pumilio, vessel diameter distributions, vessel grouping, xylem anatomy
INTRODUCTION
Climate change is impacting forest ecosystems worldwide, increasing the frequency of mortality episodes and promoting shifts in species’ distribution ranges (Kelly and Goulden, 2008; Jump et al., 2009; Allen et al., 2015). Trees are expected to increase their elevational limit as the climate becomes warmer, and in fact, some shifts in treeline elevation have been reported and linked to global warming (Harsch et al., 2009; Du et al., 2018). However, climate warming may be differentially perceived in forest ecosystems with contrasting climatic regimes, because temperature increases combined with variations in water availability may either mitigate or enhance the impacts of warming on vegetation, even promoting downward treeline shifts or diminishing growth rates if soil moisture availability crosses a critical minimum threshold (Sigdel et al., 2018; Fajardo et al., 2019a). In addition, the occurrence of extreme climatic events is not necessarily linked to vegetation shifts, because stabilizing demographic processes may minimize and counteract the effects of climatic extremes, increasing the resilience of forest ecosystems (Lloret et al., 2012). In this context of evidence regarding the impacts of climate change on vegetation, we need to increase our knowledge about tree adjustments to climate under natural conditions.
Disentangling potential responses of vegetation to climate change may benefit from an understanding of plants’ hydraulic adjustments across broad natural gradients. In isohydric species (i.e. those that show strong regulation of leaf water potential), hydraulic supply is coordinated with photosynthetic rates, because without a steady water input replacing water losses by evapotranspiration, leaves are forced to close their stomata to avoid desiccation at the expense of reductions in carbon uptake (Meinzer et al., 1990; Mencuccini, 2003; Martínez-Vilalta and García-Forner, 2016). Anisohydric species, by contrast, maintain stomata more open during drought episodes and, as a result, are expected to sustain carbon assimilation for longer (McDowell et al., 2008), although in some cases they may be as carbon-constrained as isohydric plants (García-Forner et al., 2017). Regardless, the coupling between hydraulics and carbon acquisition may in turn alter the carbon balance in the tree. Studies on plant hydraulics at the xylem level have demonstrated the potential of xylem to adjust its structure to environmental variations by modifications of vessel size, number and distribution, or by vessel clustering (Hacke et al., 2006). In cold or dry environments, the average diameter of conductive vessels tends to be smaller (but see Fajardo et al., 2019b), because frost and drought increase cavitation risk and smaller vessels are more resistant. This reduction in mean vessel size may be compensated for by an increase in vessel density, which helps to maintain hydraulic conductivity at decreasing water potentials, thus increasing xylem safety. These general trends were early observed within genera or within regional floras across latitude (Baas, 1973; van der Graaff and Baas, 1974; van der Oever et al., 1981; Noshiro and Baas, 1998), elevation (van der Graaff and Baas, 1974; van der Oever et al., 1981) or moisture gradients (Carlquist and Hoekman, 1985). However, hydraulic conductivity is related to the fourth power of vessel radius (Hacke and Sperry, 2001; Tyree and Zimmermann, 2002), and as a consequence, the contribution of larger vessels to hydraulic conductivity is disproportionately higher than that of smaller vessels. Therefore, increases in safety usually imply reductions in efficiency, defining a trade-off for the functionality of the hydraulic system (Sperry, 2003). The universality of this trade-off is not conclusive: some species may show simultaneously reduced safety and low efficiency (Maherali et al., 2004; Gleason et al., 2016), others may be highly resistant to cavitation while having relatively wide and potentially efficient vessels (Hacke et al., 2017), and others may maintain xylem efficiency in combination with different levels of safety (García-Cervigón et al., 2018).
Deviations from the safety–efficiency trade-off may be related to different factors. For instance, a given mean vessel diameter may be reached through different vessel diameter distribution curves. A higher frequency of smaller vessels in cold environments has been related to increased protection against freeze–thaw embolism (Davis et al., 1999; Fisher et al., 2007; Medeiros and Pockman, 2014). Also, shifts in vessel diameter distributions from unimodal to bimodal (i.e. from diffuse porous to semi-ring porous) have been described in cold sites (Schreiber et al., 2015). Bimodal distributions are considered an adaptation to environments with abundant water availability in spring due to snow melt (when the earlywood is produced), a dry continental climate during the summer and favourable conditions in autumn to form the latewood (Hacke et al., 2017). Otherwise, the degree of vessel clustering may contribute to increase or reduce safety levels without the need of varying the vessel size. Increases in vessel grouping reported with increasing water limitation suggest a potential role of this trait in increasing the safety of the hydraulic system (López et al., 2005; Robert et al., 2009; Trifilò et al., 2014). This increase in safety would be related to the fact that a high degree of vessel grouping may improve the hydraulic redundancy by allowing a blocked vessel to be bypassed through one or more connected vessels from the same group that still remain functional. However, an increase in vessel grouping can also be related to vulnerability to drought-induced cavitation (Scholz et al., 2013), because increasing contact surface among grouped vessels may enhance the risk of cavitation spreading by the aspiration of air through the pit pores between contiguous vessels (Alder et al., 1997; Brodersen et al., 2013).
Although xylem traits may respond to environmental shifts, there is also considerable variation within a plant (Hacke et al., 2017). This within-individual variation may be due to intrinsic features such as tree height or ontogeny (Fonti et al., 2010; Olson et al., 2014; Fajardo et al., 2019b), but the use of standardized sampling procedures may help to control these factors (Carrer et al., 2015; Lechthaler et al., 2019). However, in perennial plants, there is another source of within-individual variability that does not depend on the part of the tree where the sample is acquired, but on the fact that xylem traits respond to yearly and monthly variations in climate (e.g. Fonti et al., 2010; Alla and Camarero, 2012; Gea-Izquierdo et al., 2013; Rita et al., 2015; Castagneri et al., 2017). The overall xylem hydraulic functionality results from the combination of vessel traits developed in different growth rings, each ring responding to climatic conditions in the current and previous years. As a result, year-to-year variability in xylem traits may be compensated for at the organ level (i.e. whole branch or trunk). If this compensation occurs, this would imply the absence of clear responses of xylem traits to environmental shifts, as has been observed for leaf traits (Herrera et al., 2015; Herrera, 2017), indicating high within-individual resilience.
For the work described here, our goal was to characterize the xylem anatomical traits related to the hydraulic function in branches of the widespread species Nothofagus pumilio across a broad latitudinal gradient and at different elevations in Chile, ranging from closed forests up to the treeline. Different climatic regimes occur across the whole latitudinal gradient where this species prevails in the southern Andes, with contrasting levels of water availability over the year. The elevational gradient is related to variations in average temperature. We specifically investigated whether (1) xylem structure in N. pumilio varies with combined gradients of temperature and moisture under natural field conditions, and (2) what is the contribution of within-individual variation to xylem responses across environmental gradients. According to current theory, we hypothesized that xylem structure would be arranged towards increased levels of safety at colder and drier sites. Also, we expected shifts in vessel diameter distributions from unimodal to bimodal (i.e. from diffuse-porous to semi-ring porous) when increasing elevation and moisture deficit.
MATERIALS AND METHODS
Study species and sampling sites
Nothofagus pumilio (Poepp. et Endl.) Krasser (Nothofagaceae) is a deciduous broadleaved tree species that extends through a wide latitudinal and elevational range in the southern Andes of Chile and Argentina (Fajardo and Piper, 2011). Its broad distribution provides a unique opportunity to study adjustment of xylem traits to contrasting climates. We selected five sites across the Chilean Andes, from 36° to 54°SS, comprising nearly the whole latitudinal range of the species (Fig. 1). At each site, N. pumilio trees were studied at four elevations covering the last 300 m of the treeline ecotone, resulting in a total of 20 plots (Table 1). From lowest to highest, these elevations corresponded to closed and mature forests (CF), intermediate stands between closed forest and the timberline (IN), timberline (TB) and treeline (TL). The treeline was defined as the uppermost limit of individual trees with an upright growth form of at least 3 m (Körner, 2003).
Fig. 1.
Location (A) and climatic diagrams (B) of the five sites sampled in Chile. Green areas in the map indicate the world distribution of Nothofagus pumilio, according to Amigo and Rodríguez-Guitián (2011). Climatic diagrams were plotted using the climatol R package (Guijarro 2016) and contain data for the period 1981–2010 generated with the ClimateSA v1.0 software package, available at http://tinyurl.com/ClimateSA (Hamann et al. 2013). Black numbers beside the left y-axis indicate maximum and minimum average temperatures of the hottest and coldest months, respectively. Note that months are arranged to keep the summer period in the central zone of the plots.
Table 1.
Environmental characteristics of the study plots and sample size.
| Site | Plot | Altitude (m a.s.l.) | Latitude | Longitude | MAT (°C) | MDs | n |
|---|---|---|---|---|---|---|---|
| Termas | t4 (TL) | 2090 | 36°54′22.60″ | 71°23′37.73″ | 5.3 | −41.2 | 8 |
| t3 (TB) | 1980 | 36°54′25.85″ | 71°23′58.50″ | 5.8 | −44.0 | 8 | |
| t2 (IN) | 1890 | 36°54′20.79″ | 71°24′18.10″ | 6.2 | −47.6 | 8 | |
| t1 (CF) | 1793 | 36°54′23.05″ | 71°24′26.74″ | 6.7 | −50.4 | 6 | |
| Antillanca | a4 (TL) | 1330 | 40°47′1.09″ | 72°11′25.62″ | 5.9 | 182.4 | 10 |
| a3 (TB) | 1220 | 40°46′36.22″ | 72°11′46.60″ | 6.4 | 177.4 | 7 | |
| a2 (IN) | 1090 | 40°46′24.75″ | 72°12′07.54″ | 7.0 | 174.4 | 8 | |
| a1 (CF) | 1000 | 40°46′46.25″ | 72°12′32.84″ | 7.4 | 172.0 | 8 | |
| Coyhaique | c4 (TL) | 1220 | 45°31′4.20″ | 72°02′30.44″ | 4.2 | 129.0 | 6 |
| c3 (TB) | 1120 | 45°31′11.94″ | 72°02′46.12″ | 4.7 | 127.2 | 7 | |
| c2 (IN) | 1035 | 45°31′6.90″ | 72°02′59.17″ | 5.1 | 127.0 | 7 | |
| c1 (CF) | 940 | 45°31′18.11″ | 72°03′05.63″ | 5.6 | 125.2 | 6 | |
| O’Higgins | h4 (TL) | 1125 | 48°29′48.3″ | 72°31′23.00″ | 3.7 | 67.0 | 8 |
| h3 (TB) | 1000 | 48°29′07.7″ | 72°30′37.90″ | 4.4 | 63.8 | 6 | |
| h2 (IN) | 893 | 48°29′02.3″ | 72°31′44.50″ | 4.9 | 62.6 | 7 | |
| h1 (CF) | 757 | 48°29′18.6″ | 72°31′43.40″ | 5.6 | 57.6 | 6 | |
| Karukinka | f4 (TL) | 625 | 54°20.257′ | 68°49.339′ | 3.2 | 72.8 | 7 |
| f3 (TB) | 560 | 54°19.667′ | 68°49.111′ | 3.5 | 71.0 | 8 | |
| f2 (IN) | 488 | 54°19.471′ | 68°49.505′ | 3.8 | 70.2 | 8 | |
| f1 (CF) | 410 | 54°19.065′ | 68°49.137′ | 4.2 | 67.8 | 8 |
Four populations were sampled per study site: CF, closed forest; IN, intermediate stand; TB, timberline; TL, treeline. MAT, mean annual temperature; MDs, summer moisture deficit index; n, sample size. The more positive the MDs values, the higher the moisture availability. Climatic data were generated with the ClimateSA v1.0 software package (Hamann et al. 2013) for the period 1981–2010.
Despite the common temperature gradient in the elevational limit, the five sites experience different hydric environments that range from Mediterranean-like to temperate climate conditions (Fig. 1, Table 1; Fajardo, 2018). At the northernmost site (Termas de Chillán, hereafter Termas), the treeline occurs at 2080 m a.s.l. and annual precipitation is 1520 mm [Las Trancas weather station, Dirección General de Aguas (DGA), 2005–2014, 1250 m a.s.l.], with a marked water deficit in summer. The following site was in the Antillanca area within the Puyehue National Park (hereafter Antillanca), where the treeline occurs at 1340 m a.s.l. and annual precipitation is c. 4000 mm (Aguas Calientes weather station, 1980–2000). In these two sites the soils are derived from andesitic rocks of volcanic origin. At the third site (Coyhaique National Reserve, hereafter Coyhaique), the treeline occurs at 1215 m a.s.l. Annual precipitation is 890 mm (Coyhaique National Reserve weather station, DGA, 2004–2013, 400 m a.s.l.) and the soil is derived from aeolian volcanic ash deposits. The fourth site was situated near Villa O’Higgins (hereafter O’Higgins), where the treeline occurs at 1120 m a.s.l., and annual precipitation is 1050 mm (Villa O’Higgins weather station, DGA, 2000–2007, 270 m a.s.l.). At the southernmost site (Karukinka Private Reserve in Tierra del Fuego, hereafter Karukinka), the treeline occurs at 630 m a.s.l. Annual precipitation here is 565 mm (Lago Deseado weather station, DGA, 2006–2013, 400 m a.s.l.) and the soil is of granitic origin. At all sites, most precipitation falls as snow from May to September.
Sampling design
Sampling was conducted in late January 2013 at Termas and Antillanca, in late January to early February 2014 at Coyhaique and Karukinka, and in early February 2017 at O’Higgins. At each plot, six to nine individuals located at least 30 m apart were sampled (Table 1). For each tree, and at ~2–4 m height, we cut one terminal, sun-exposed branch of 2 m length with fully expanded leaves using a 5.6-m telescoping pole (ARS Corporation, Sakai, Japan; Fajardo, 2018). This standardization of branch length minimizes xylem anatomical and hydraulic differences related to distance to the branch tip (Carrer et al., 2015). To avoid sampling tension wood, which would otherwise give a misleading appreciation of wood anatomical features, we selected branches that were always terminal, straight and with an angle >45° regarding the main stem. For each branch, we selected and cut one 2-cm-long piece of wood (always sampled at the bottom end of the 2-m-long branch) that was labelled and placed in a refrigerator for transportation. In the laboratory, branch sections were used to measure vessel anatomical traits.
Quantitative wood anatomy
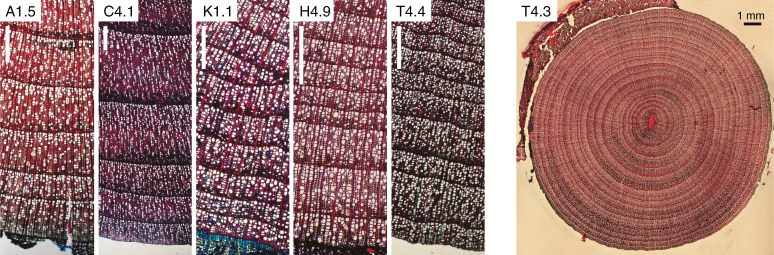
Xylem anatomical analyses followed the protocol proposed by von Arx et al. (2016). Anatomical wood cross-sections of 10 μm thickness from branch sections were produced with a sledge microtome (Gärtner et al., 2015). These cross-sections were then placed on a slide and stained with Alcian blue (1 % solution in acetic acid) and safranin (1 % solution in ethanol). The cross-sections were then dehydrated using a series of ethanol solutions of increasing concentration, washed with xylol and permanently preserved by embedding them in Eukitt glue. Overlapping images covering a complete radius from pith to bark were captured with a Nikon D90 digital camera mounted on a Nikon Eclipse 50i optical microscope with 40× magnification and merged to a single image using PTGUI v8.3.10 Pro (New House Internet Services B.V., Rotterdam, the Netherlands; Fig. 2).
Fig. 2.
Cross-sections of Nothofagus pumilio branches. The five outermost rings of individuals from the five study sites (A: Antillanca, C: Coyhaique, K: Karkinka, H: O’Higgins, T: Termas) at closed forests (1) and at the treeline (4) are shown. White scale bars represent 500 µm. Note the concentric growth and the central pith in the complete cross-section, which ensures that no reaction wood is being analysed.
Vessel anatomical features and ring width were measured using ROXAS v3.0 (von Arx and Dietz, 2005), a specific image-analysis tool based on Image-Pro Plus (Media Cybernetics, Silver Spring, MD, USA). The specific procedure is detailed in the Supplementary Data (Methods S1). From each image, we analysed a linear subsample of the last five complete annual rings to evaluate xylem anatomy, having in this way five repeated measurements per individual (Fig. 2). We first measured the area of all vessels on each ring, obtained the diameter of each vessel (assuming circularity) and estimated its potential hydraulic conductivity (Kh) following Nonweiler (1975) and considering its ovality. Based on size and vessel spatial distribution, we then obtained seven anatomical traits per ring: (1) the average lumen area of all vessels (mean vessel area, MVA, μm2); (2) the vessel density (VD, no. mm–2); (3) the vessel composition index, a measure of vessel size distribution obtained as the quotient of mean vessel lumen area to vessel density (MVA/VD, Zanne et al., 2010); (4) percentage of conductive area (CA), obtained as the cumulative area of all counted vessels divided by the ring area; (5) the mean number of vessels with contiguous cell walls, considering solitary vessels as a group of 1 (vessel grouping index, VGI, von Arx et al., 2013); (6) the percentage of solitary vessels with respect to all vessels in the ring (vessel solitary fraction, VSF); and (7) an anatomy-based estimate of specific hydraulic conductivity (Ks, m2 s–1 MPa–1), which is the hydraulic conductivity per unit area (see equations in Olano et al., 2017). We also measured ring width as the mean distance between two consecutive ring borders corrected for inclined ring boundaries. Some images were discarded from image analyses due to deficient quality. Overall, we processed 147 images, each corresponding to a different individual (Table 1).
Climatic data
We used the ClimateSA v1.0 software package (Hamann et al., 2013) to generate data for the period 1981–2010 for each of the 20 sampling plots, available at http://tinyurl.com/ClimateSA. Climatic data have been developed with the parameter-elevation regressions on an independent-slopes model (PRISM), an expert interpolation approach described by Daly et al. (2008) which uses physiographic information to better predict climate patterns in mountainous terrains. We obtained data for mean annual temperature (MAT) and monthly mean temperature and precipitation between December and February (summer in the southern hemisphere). Monthly data were used to calculate an index of summer moisture deficit (MDs), obtained by subtracting twice the mean temperature (in °C) to precipitation (in mm) during the growing season, from December to February (Gaussen and Bagnouls, 1953).
Statistical analyses
Variation in vessel diameter distributions.
We used Gaussian kernel density estimation to compare distributions of vessel lumen diameters among sites and elevations, following Schreiber et al. (2015). Kernel density estimation is a non-parametric procedure that allows the probability density function of a variable to be estimated based on data from a finite sample (Silverman, 1986). Bandwidth for the kernel density estimator in the R density() function was calculated according to Scott (1992). To further describe the shape of these curves, we also tested multimodality, kurtosis and skewness using, respectively, the functions dip.test(), kurtosis() and skewness() from the R packages diptest (Maechler, 2016) and e1071 (Meyer et al., 2017).
Effects of climate gradients on xylem traits.
To evaluate the effects of temperature and site-related moisture deficit conditions on xylem traits, we fitted separate linear mixed-effects models (LMMs) for each measured trait. Explanatory fixed factors included MAT per plot, MDs and their interaction. These two climatic variables represent average conditions per site, but vessel traits are also expected to respond to year-to-year variations in climate (Fonti et al., 2010; Alla and Camarero, 2012; Rita et al., 2015; Castagneri et al. 2017). Because data on annual climate per site were not available, we used ring width as a proxy for yearly climatic conditions given its high responsiveness to annual variations in temperature and moisture (Lara et al., 2005). Therefore, ring width was also included as a fixed covariate representing variations in yearly climatic conditions. In addition, we included a dummy variable to consider the ring to which each observation belonged, in sequential numbers from 1 (the inner ring) to 5 (the outer ring), in order to incorporate the tapering effect on variation in vessel traits. To account for the grouping structure of our data, we included tree as a random factor nested in plot nested in site. Autocorrelation structures of first order were considered to incorporate the intrinsic correlation structure of the data, because xylem traits in a given year are potentially correlated with xylem traits in the previous year (Fritts, 1976). Models were fitted using Restricted Maximum Likelihood (REML) and normalized residuals were extracted. Residuals were checked for normality and homoscedasticity, refitting models with different variance covariates in those cases when model assumptions were not accomplished. We allowed revised models to have (1) different residual spread per site, (2) larger residual spread as the response variable increases (proportional) and (3) a combination of both. We compared revised models using the Akaike Information Criterion (AIC), selected models that minimized AIC value and checked again for normality and homoscedasticity of residuals. Optimal models were refitted with REML (Zuur et al., 2009). We used the nlme package (Pinheiro et al., 2019) in the R environment (R Core Team, 2019) to fit LMMs, the function r2beta() from the r2glmm package (Jaeger, 2017) to obtain R2 per model using the Nakagawa and Schielzeth (2013) approach, and the varcomp() function in the ape package (Paradis and Schliep, 2019) to obtain the proportion of variance around the mean attributed to the random effects (i.e. site, elevation and individual).
Effects of climate gradients on global xylem anatomy.
We used multivariate analyses to evaluate the effects of temperature and moisture gradients on global variation of xylem traits. First, we visualized global variation of xylem traits by performing a principal component analysis (PCA) on an individual × xylem traits matrix. We then used canonical ordination techniques to evaluate the effects of average climate conditions per site (i.e. MAT, MDs and their interaction) on xylem traits, also including ring width as a proxy for yearly climatic variations. Specifically, we performed a redundancy analysis (RDA), which combines multiple regression with PCA, to relate the dependent matrix (individual × xylem traits) to an explanatory matrix [MAT, MDs and ring width (RW)] following the same structure as for the fixed factors in LMMs (i.e. RW + MAT × MDs). The significance of the whole model and that of each explanatory variable were evaluated by means of Monte Carlo tests with 9999 permutations. Then, to assess the contribution of each explanatory variable to the dependent matrix, we performed partial redundancy analyses (pRDAs). For each pRDA, the dependent matrix was constrained by each of the explanatory variables and controlled for the remaining variables. Adjusted R2 values were obtained with the function RsquareAdj(). Multivariate analyses were performed with the vegan package (Oksanen et al., 2019) in the R environment (R Core Team, 2019).
RESULTS
Vessel diameter distributions
In total, 371 236 vessels were measured in the last five rings of the 147 analysed images. Vessel diameters ranged from 4 to 49 µm and their arrangement was diffuse-porous, with a slight tendency to semi-ring porosity (Fig. 2) that varied depending on the plot. Non-unimodal distributions occurred in almost half of the plots, particularly at Coyhaique and Karukinka, whereas vessel diameter distributions were all unimodal at O’Higgins (Fig. 3). In all cases, a first peak on density curves was evident at around 11 µm, corresponding to the diameter of latewood vessels, whereas a second peak occurred at around 20 µm, suggesting this was the average diameter for earlywood vessels. Despite significant variations in the multimodality of density curves, no clear trend was detected towards increasing elevation (i.e. decreasing temperature) or water availability. The only common pattern observed in all sites, except Karukinka, was an increase in the frequency of larger vessels at closed forest plots as indicated by a larger diameter at which 80 % of conductivity was reached (Fig. 3). Vessel diameter distributions differed among plots within sites and elevations (significant paired Kolmogorov–Smirnov tests, data not shown) and were in all cases flat (platykurtic) and right-skewed (Supplementary Data Table S1).
Fig. 3.
Gaussian kernel density estimates of vessel diameter in Nothofagus pumilio by site and elevation considering five rings per individual. CF: closed, low-elevation forest, IN: intermediate stands, TB: timberline, TL: treeline. Black dotted lines intersect the x-axis at 11 µm, highlighting a first peak on density curves. Coloured dashed lines indicate the diameter at which 80 % of xylem-specific estimated conductivity is reached. Asterisks in the upper right corner of some panels indicate significant Hartigan’s dip statistic (D) tests, confirming non-unimodal distributions (see Supplementary Data Table S1 for detailed output of D tests). Sites are ordered by increasing summer moisture deficit from left to right.
Response of xylem traits to climate gradients
LMMs showed significant effects of average climate conditions per site (i.e. temperature and summer moisture deficit) and of the interaction between them on vessel density, on combined trait indices (vessel composition index and percentage of conductive area) and on vessel grouping (vessel grouping index and vessel solitary fraction; Table 2, Fig. 4). Vessel density and the percentage of conductive area decreased with increasing average temperature and moisture availability, although their relationship with temperature shifted from negative to positive at the most humid site (Antillanca, Fig. 4). Vessel composition index showed the opposite trend, increasing with temperature and water availability, particularly at the driest site (Termas), thus indicating that higher average temperature and water availability favoured shifts in vessel distributions towards larger vessels and/or at lower density. This result was partially supported by vessel diameter distributions (Fig. 3), as a higher abundance of larger vessels was observed in closed, lower-elevation forests (i.e. warmer sites), but no clear trend was observed with increasing moisture availability. Regarding grouping, vessels were more grouped (higher vessel grouping index and lower vessel solitary fraction) in cold and dry sites, although the relationship with temperature shifted from negative to positive with increasing moisture availability (Fig. 4). Significant effects of yearly variations in climate (represented by ring width) were also detected for all xylem traits, except those related to vessel grouping (Table 2).
Table 2.
Sign of significant coefficients estimated for explanatory factors (i.e. fixed effects) in linear mixed models (LMMs) that were fitted for xylem traits (VD, MVA, MVD/VD, CA, Ks, VGI, VSF) of Nothofagus pumilio at five sites across the Andes mountains.
| VD | MVA | MVA/VD | CA | K s | VGI | VSF | |
|---|---|---|---|---|---|---|---|
| Tapering | – | + | + | – | – | – | + |
| RW | – | + | + | – | – | (–) | n.s. |
| MAT | – | (+) | + | – | n.s. | – | + |
| MDs | – | n.s. | + | – | n.s. | – | + |
| MAT × MDs | + | n.s. | – | + | n.s. | + | – |
| Model R2 | 0.378 | 0.197 | 0.378 | 0.190 | 0.070 | 0.138 | 0.115 |
Trait values in the five outermost rings of each individual were considered (n = 730). Tapering represents the effect of ontogeny on xylem traits, coded from 1 (data from the inner ring) to 5 (the outer ring). Ring width (RW) was included as a surrogate of yearly climatic variations in xylem anatomy. Random effects included tree nested in plot nested in site. Symbols in parentheses indicate significant effects at P < 0.1. VD, vessel density; MVA/VD, vessel composition index; CA, percentage of conductive area; Ks, estimated xylem-specific hydraulic conductivity; VGI, vessel grouping index; VSF, vessel solitary fraction; RW, ring width; MAT, mean annual temperature; MDs, moisture deficit in summer (Dec-Feb); n.s., non-significant coefficient. See Supplementary Data Table S2 for detailed output on LMMs.
Fig. 4.
Predicted values for anatomical traits in Nothofagus pumilio in response to temperature for different sites with varying moisture availability (see Table 2). Ring width was kept constant for predictions. Sites are ordered by increasing summer moisture deficit, from Antillanca (the wettest site) to Termas (the driest site). Plots correspond to: (A) VD: vessel density; (B) CA: conductive area; (C) MVA/VD: vessel composition index; (D) VGI: vessel grouping index; and (E) VSF: vessel solitary fraction.
Variance partitioning across sites, plots and individuals showed an overwhelming proportion of variance due to the individual in most xylem traits (Fig. 5). Within-individual variation was particularly high in the vessel composition index, for which it reached 80 %, although vessel density and mean vessel area showed values over 50 % as well.
Fig. 5.
Variance partitioning of the LMMs adjusted for xylem traits of Nothofagus pumilio across three nested ecological scales: five sites (summer moisture deficit), four plots per site of comparable elevations (mean annual temperature) and six to nine individuals per plot (tree). Proportions represent the variance around the mean due to each ecological scale. See trait abbreviations in the legend to Fig. 3.
Global response of xylem anatomy to climate gradients
The first two axes of the PCA accounted for 92.9 % of the global variance of xylem traits (Fig. 6). The first axis showed positive loads of vessel density, the percentage of conductive area, Ks and the vessel grouping index, and negative loads of the vessel solitary fraction. The second axis showed positive loads of mean vessel area and the vessel composition index. The RDA was significant (F = 82.500, P < 0.001), and climate (i.e. MAT, MDs and their interaction) together with ring width explained 30.7 % of the variance in xylem traits. Fractions explained by climatic variables and ring width were all significant in pRDAs (Table 3), but the contribution of ring width to the explained variance was much higher (18.7 %) than that of climatic factors (between 3.7 and 3.8 %; Table 3). The shared contribution to the explained variance was just 1.0 %.
Fig. 6.
Biplot of the principal component analysis (PCA) performed on vessel traits of Nothofagus pumilio showing the position of rings per individual along the two first PCA axes. The percentage of variance explained by each axis is indicated. Sites are ordered by increasing summer moisture deficit, from the wettest (Antillanca) to the driest site (Termas). Squares correspond to closed forests, triangles to intermediate stands, diamonds to timberlines, and circles to treelines. VD: vessel density, MVA: mean vessel area, MVA/VD: vessel composition index, CA: percentage of conductive area, Ks: anatomy-based estimate of xylem-specific hydraulic conductivity, VGI: vessel grouping index, VSF: vessel solitary fraction.
Table 3.
Results of the permutation tests for evaluating the significance of each of the variables included in the explanatory matrix in the redundancy analysis (RDA) (F and P) and in partial RDAs (Fp and Pp), and the proportion of variance explained by each variable when controlling for the remaining variables in partial RDAs (R2adj). RW, ring width; MAT, mean annual temperature; MDs, summer moisture deficit index.
| F | P | F p | P p | R 2 adj | |
|---|---|---|---|---|---|
| RW | 266.937 | <0.001 | 198.280 | <0.001 | 0.1869 |
| MAT | 21.103 | <0.001 | 40.041 | <0.001 | 0.0370 |
| MDs | 1.759 | 0.165 | 39.514 | <0.001 | 0.0365 |
| MAT × MDs | 40.200 | <0.001 | 40.200 | <0.001 | 0.0371 |
DISCUSSION
In this study, we assessed xylem configuration in N. pumilio across a wide latitudinal gradient covering almost its entire distribution range and combined with elevational gradients ranging from closed forests to the treeline. Across this comprehensive study system, most xylem traits responded to temperature, moisture and their combination, but the estimated xylem-specific hydraulic conductivity (Ks) remained constant. Within-individual variation accounted for a high proportion of the explained variance in xylem responses across the study system, suggesting high individual capacity for responding to year-to-year climatic variations. In the following paragraphs we elaborate on plausible explanations for these patterns.
Conductivity remains constant across climatic gradients
Elevational changes in xylem traits suggested increased safety levels against freeze–thaw embolism at higher elevation. Mean vessel area tended to decrease at higher elevation and this agreed with our expectations, as narrower vessels are less likely to cavitate (Sperry, 2003; Fisher et al., 2007). Reductions in vessel diameter with lower temperature were accompanied by increases in vessel density, which also increased with lower moisture availability. These adjustments in vessel density suggest higher safety levels in colder sites, but also in drier locations, which agrees with adjustments observed for vessel density in other angiosperm species and floristic groups in relation to temperature (Noshiro and Baas, 1998; Medeiros and Pockman, 2014) and moisture gradients (Carlquist and Hoekman, 1985; Villar-Salvador et al., 1997; García-Cervigón et al., 2018). According to these studies, variations in vessel density are accompanied by variations in vessel diameter. In our case, vessel density was negatively correlated with mean vessel area (Pearson’s r = –0.497, P < 0.001), indicating that when N. pumilio produces wider vessels, it compensates for this with lower density. The observed trend towards increasing frequency of larger vessels at closed, low-elevation forests supports this interpretation, as well as observed trends in the vessel composition index and in the percentage of conductive area. Increased vessel density at colder and drier sites was reflected in a higher percentage of conductive area. This higher fraction of conductive area was, in fact, achieved through increases in vessel density rather than in vessel diameter, because the vessel composition index (obtained as mean vessel area divided by vessel density) was lower at colder and drier sites.
Vessels in N. pumilio showed higher grouping levels under cold and dry conditions (i.e. when the cavitation risk due to frost and drought is supposed to be higher). This observation agrees with studies suggesting that vessel grouping plays a role in increasing the safety of the hydraulic system (López et al., 2005; Robert et al., 2009; Trifilò et al., 2014), because a higher degree of vessel grouping may improve the hydraulic redundancy by providing alternative pathways for water transport when a vessel is blocked by an embolism. Our results corroborate those observed for the congeneric N. antarctica across a gradient of water availability (García-Cervigón et al., 2018). However, this similarity with a congeneric species contrasts with the fact that a coexisting species with different vessel arrangement and from a distant phylogenetic origin (Embothrium coccineum, Proteaceae) shows the inverse pattern (García-Cervigón et al., 2018). This suggests that the response of vessel grouping to environmental conditions might depend on the specific vessel arrangement shown by different plant lineages, although further studies across phylogenetic groups need to test this hypothesis.
In spite of the variability in vessel density and size, and in vessel grouping, xylem-specific estimated hydraulic conductivity (Ks) remained constant across sites and elevations, suggesting that similar levels of hydraulic efficiency can be reached regardless of temperature or moisture in N. pumilio. This result agrees with observations from the congeneric N. antarctica (García-Cervigón et al., 2018), in which Ks remains constant across a strong rainfall gradient in combination with different levels of safety. Our results, therefore, did not support the existence of a safety–efficiency trade-off in relation to climatic variations, but rather that xylem traits are coordinated towards homeostasis in xylem function across a wide range of climatic conditions (Borghetti et al., 2016).
Temperature and moisture gradients have combined effects on xylem traits
Our sampling design allowed us to detect the combined effects of temperature and moisture on xylem configuration. Vessel density and the fraction of conductive area increased, whereas the vessel composition index decreased with lower temperature, but these relationships shifted at the most humid site (Antillanca). Contrasting responses of secondary growth to temperature depending on the climatic regime have been reported at the treeline in the Himalayas (Sigdel et al., 2018). However, the combined effects of temperature and moisture on vessel traits have been scarcely explored under natural conditions and, when done, these combined effects have not been detected (e.g. Villar-Salvador et al., 1997). Our results, instead, suggest that variations in temperature may trigger contrasting responses in xylem traits depending on moisture availability. A possibility is that responses to temperature are not always linear, as assumed in LMMs, and that this non-linearity is related to the climatic regime of the study area. The lack of clear trends in vessel diameter distribution curves with either elevation or moisture supports this interpretation, as well as the fact that different combinations of temperature and moisture resulted in similar average population values of vessel traits (Supplementary Data Fig. S1). Regardless, our results, linked to the fact that temperature and its combination with moisture significantly explained global variation on xylem traits in the RDAs, highlight the need to consider both factors simultaneously when evaluating xylem responses to climatic gradients.
Shifts in vessel diameter distributions provide additional potential for xylem adjustment
The arrangement of vessels in N. pumilio (diffuse-porous, with a slight tendency to semi-ring porosity) was similar as in other species from the same genus (Dettmann et al., 2013; García-Cervigón et al., 2018). This vessel arrangement may provide an opportunity for anatomical adjustments that range from unimodal (i.e. diffuse-porous) to bimodal (i.e. semi-ring porous) depending on the environment (Schreiber et al., 2015; Hacke et al., 2017). Although our results regarding multimodality of vessel diameter distribution curves did not follow clear trends with either elevation or latitude, we did observe non-unimodal distributions at closed, low-elevation forests in three out of the five study sites, which was contrary to our expectations of bimodal distributions at higher elevation (Schreiber et al., 2015). These non-unimodal distributions may be related to the fact that higher air temperatures experienced at low-elevation forests reduce the freeze–thaw embolism risk and allow trees to produce wider vessels to maximize water transport. The higher frequency of larger vessels observed at low-elevation forests in all sites (except the coldest Karukinka site) in fact supports this interpretation, although this effect might also be related to highest tree-to-tree competition in closed forests. Individuals with larger vessels would be able to extract a larger share of available soil water in spring more rapidly, as previously shown for alpine plants (Olano et al., 2013), thus outcompeting individuals with smaller vessels. At the same time, however, the higher temperatures experienced at closed, low-elevation forests combined with similar rainfall levels to those recorded at higher elevation plots may increase the summer moisture deficit, thus favouring the production of smaller vessels in the late growing season and leading to vessel arrangements that tend toward semi-ring porosity (Detmann et al., 2013; Hacke et al., 2017).
Within-individual variation modulates xylem responses across climatic gradients
Within-individual variation accounted for an overwhelming proportion of variance in the response of xylem traits to environment (see Fig. 5 and Table 3), particularly in the vessel composition index, vessel density and mean vessel area. This suggests that most of the observed variation in xylem traits was related to within-individual responses to year-to-year environmental fluctuations, rather than to average site conditions, and that these responses were mainly driven by adjustments of vessel density and size. This partially agrees with results from studies evaluating long-term responses of xylem traits to year-to-year climatic variations. In some of these studies, vessel density is more responsive to yearly climatic fluctuations than vessel diameter (Gea-Izquierdo et al., 2013; Rita et al., 2015) but in other cases, the inverse occurs and vessel density barely varies among years (Alla and Camarero, 2012). These studies usually work with few populations under contrasting conditions (e.g. two sites in Gea-Izquierdo et al., 2013; Rita et al., 2015; three sites across a gradient in Castagneri et al., 2017; two sites and two sexes in Olano et al., 2017), and thus it is possible that combined responses of vessel density and size are not detected due to the small number of populations under study. In our case, working with 20 different populations provided a better estimation of the role played by within-individual adjustments in driving forest responses to environmental gradients. It is also possible that observed adjustments in xylem anatomical traits across elevation and latitude are linked to yearly variations in the timing and duration of cambial production (Pacheco et al., 2015; Castagneri et al., 2017), although this possibility should be further studied. Regardless, our results suggest that within-individual responsiveness to yearly climatic fluctuations may increase forest resilience against large-scale climatic changes. At the same time, our results highlight the need to resolve the relative importance of within-individual vs. environmental-related variation to really understand the level of pressure that environment may push on xylem adjustments across species’ distribution ranges.
CONCLUSIONS
The widespread species Nothofagus pumilio adjusted its xylem anatomy in response to both elevation and latitude, and to their combination. However, despite the existence of noticeable adjustments, xylem traits were coordinated to maintain hydraulic function under a wide range of conditions. This revealed the homeostatic capacity of xylem and suggests that the hydraulic system of N. pumilio may have enough adaptive potential to face forecasted climatic trends, thus allowing this species to keep within its current elevational and latitudinal limits. Our results also indicate that the potential for adapting to climatic change may be strongly determined by the within-individual capacity for responding to year-to-year variability in environmental conditions. Thus, the scaling from individual trees to the forest level may strongly determine increases in forest resilience against environmental changes.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Methods S1: Details of image analyses. Table S1: Statistics describing the shape of vessel diameter distributions of N. pumilio. Table S2: Summary of LMM coefficients. Figure S1: Pairwise comparisons of xylem traits mean population values between sites and elevations.
FUNDING
This work was supported by the Chilean Fondo Nacional de Desarrollo Científico y Tecnológico [FONDECYT projects 1120171 and 1160329 to A.F.]; BBVA foundation [to J.J.C.]; the Spanish MINECO [project CGL2017-87309-P to J.M.O. and Juan de la Cierva-Incorporación grant IJCI-2017-34052 to A.I.G.C.] and Comunidad de Madrid [project REMEDINAL TE-CM (S2018/EMT-4338) to A.I.G.C.].
ACKNOWLEDGEMENTS
We thank Miguel García-Hidalgo and Michele Colangelo for their invaluable help processing branches in the laboratory, and the FEBIMED research group for their input to data analysis.
LITERATURE CITED
- Alder NN, Pockman WT, Sperry JS, Nuismer S. 1997. Use of centrifugal force in the study of xylem cavitation. Journal of Experimental Botany 48: 665–674. [Google Scholar]
- Alla A, Camarero JJ. 2012. Contrasting responses of radial growth and wood anatomy to climate in a Mediterranean ring-porous oak: implications for its future persistence or why the variance matters more than the mean. European Journal of Forest Research 131: 1537–1550. [Google Scholar]
- Allen CD, Breshears DD, McDowell NG. 2015. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 6: 129. [Google Scholar]
- Amigo J, Rodríguez-Guitián MA. 2011. Bioclimatic and phytosociological diagnosis of the species of the Nothofagus genus (Nothofagaceae) in South America. International Journal of Geobotanical Research 1: 1–20. [Google Scholar]
- Baas P. 1973. The wood anatomical range in Ilex (Aquifoliaceae) and its ecological and phylogenetic significance. Blumea 21: 193–258. [Google Scholar]
- Borghetti M, Gentilesca T, Leonardi S, van Noije T, Rita A. 2016. Long-term temporal relationships between environmental conditions and xylem functional traits: a meta-analysis across a range of woody species along climatic and nitrogen deposition gradients. Tree Physiology 37: 4–17. [DOI] [PubMed] [Google Scholar]
- Brodersen CR, McElrone AJ, Choat B, Lee EF, Shackel KA, Matthews MA. 2013. In vivo visualizations of drought-induced embolism spread in Vitis vinifera. Plant Physiology 161: 1820–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlquist S, Hoekman DA. 1985. Ecological wood anatomy of the woody southern Californian flora. IAWA Bulletin 6: 319–347. [Google Scholar]
- Carrer M, von Arx G, Castagneri D, Petit G. 2015. Distilling environmental information from time series of conduit size: the standardization issue and its relation to allometric and hydraulic constraints. Tree Physiology 35: 27–33. [DOI] [PubMed] [Google Scholar]
- Castagneri D, Fonti P, von Arx G, Carrer M. 2017. How does climate influence xylem morphogenesis over the growing season? Insights from long-term intra-ring anatomy in Picea abies. Annals of Botany 119: 1011–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly C, Halbleib M, Smith JI, et al. 2008. Physiographically sensitive mapping of temperature and precipitation across the conterminous United States. International Journal of Climatology 28: 2031–2064. [Google Scholar]
- Davis SD, Sperry JS, Hacke UG. 1999. The relationship between xylem conduit diameter and cavitation caused by freezing. American Journal of Botany 86: 1367–1372. [PubMed] [Google Scholar]
- Dettmann S, Pérez CA, Thomas FM. 2013. Xylem anatomy and calculated hydraulic conductance of four Nothofagus species with contrasting distribution in South-Central Chile. Trees – Structure and Function 27: 685–696. [Google Scholar]
- Du H, Liu J, Li MH, et al. 2018. Warming-induced upward migration of the alpine treeline in the Changbai Mountains, northeast China. Global Change Biology 24: 1256–1266. [DOI] [PubMed] [Google Scholar]
- Fajardo A, Piper FI. 2011. Intraspecific trait variation and covariation in a widespread tree species (Nothofagus pumilio) in southern Chile. The New Phytologist 189: 259–271. [DOI] [PubMed] [Google Scholar]
- Fajardo A. 2018. Insights into intraspecific wood density variation and its relationship to growth, height and elevation in a treeline species. Plant Biology (Stuttgart, Germany) 20: 456–464. [DOI] [PubMed] [Google Scholar]
- Fajardo A, Gazol A, Mayr C, Camarero JJ. 2019a Recent decadal growth reverts warming-triggered growth enhancement in contrasting climates in the southern Andes treeline. Journal of Biogeography 46: 1367–1379. [Google Scholar]
- Fajardo A, Martínez-Pérez C, Cervantes-Alcayde MA, Olson ME. 2019b Stem length, not climate, controls vessel diameter in two tree species across a sharp precipitation gradient. New Phytologist 225: 2347–2355. [DOI] [PubMed] [Google Scholar]
- Fisher JB, Goldstein G, Jones TJ, Cordell S. 2007. Wood vessel diameter is related to elevation and genotype in the Hawaiian tree Metrosideros polymorpha (Myrtaceae). American Journal of Botany 94: 709–715. [DOI] [PubMed] [Google Scholar]
- Fonti P, von Arx G, García-González I, et al. 2010. Studying global change through investigation of the plastic responses of xylem anatomy in tree rings. The New Phytologist 185: 42–53. [DOI] [PubMed] [Google Scholar]
- Fritts HC. 1976. Tree Rings and Climate. Caldwell: The Blackburn Press. [Google Scholar]
- García-Cervigón AI, Olano JM, von Arx G, Fajardo A. 2018. Xylem adjusts to maintain efficiency across a steep precipitation gradient in two coexisting generalist species. Annals of Botany 122: 461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Forner N, Biel C, Savé R, Martínez-Vilalta J. 2017. Isohydric species are not necessarily more carbon limited than anisohydric species during drought. Tree Physiology 37: 441–455. [DOI] [PubMed] [Google Scholar]
- Gärtner H, Lucchinetti S, Schweingruber FH. 2015. A new sledge microtome to combine wood anatomy and tree-ring ecology. IAWA Journal 36: 452–459. [Google Scholar]
- Gaussen H, Bagnouls F. 1953. Dry season and xerothermic index. Bulletin de la Société d’Histoire Naturelle de Toulouse 88: 193–240. [Google Scholar]
- Gea-Izquierdo G, Battipaglia G, Gärtner H, Cherubini P. 2013. Xylem adjustment in Erica arborea to temperature and moisture availability in contrasting climates. IAWA Journal 34: 109–126. [Google Scholar]
- Gleason SM, Westoby M, Jansen S, et al. 2016. Weak tradeoff between xylem safety and xylem-specific hydraulic efficiency across the world’s woody plant species. The New Phytologist 209: 123–136. [DOI] [PubMed] [Google Scholar]
- Guijarro JA. 2016. climatol: Climate tools (series homogenization and derived products). R package version 3.0. Available at: https://CRAN.R-project.org/package=climatol [Google Scholar]
- Hacke UG, Sperry JS. 2001. Functional and ecological xylem anatomy. Perspectives in Plant Ecology, Evolution and Systematics 4: 97–115. [Google Scholar]
- Hacke UG, Sperry JS, Pockman WT, Davis SD, McCulloh KA. 2001. Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 126: 457–461. [DOI] [PubMed] [Google Scholar]
- Hacke UG, Sperry JS, Wheeler JK, Castro L. 2006. Scaling of angiosperm xylem structure with safety and efficiency. Tree Physiology 26: 689–701. [DOI] [PubMed] [Google Scholar]
- Hacke UG, Spicer R, Schreiber SG, Plavcová L. 2017. An ecophysiological and developmental perspective on variation in vessel diameter. Plant, Cell & Environment 40: 831–845. [DOI] [PubMed] [Google Scholar]
- Hamann A, Wang T, Spittlehouse DL, Murdock TQ. 2013. A comprehensive, high-resolution database of historical and projected climate surfaces for western North America. Bulletin of the American Meteorological Society 94: 1307–1309. [Google Scholar]
- Harsch MA, Hulme PE, McGlone MS, Duncan RP. 2009. Are treelines advancing? A global meta-analysis of treeline response to climate warming. Ecology Letters 12: 1040–1049. [DOI] [PubMed] [Google Scholar]
- Herrera CM, Medrano M, Bazaga P. 2015. Continuous within-plant variation as a source of intraspecific functional diversity: Patterns, magnitude, and genetic correlates of leaf variability in Helleborus foetidus (Ranunculaceae). American Journal of Botany 102: 225–232. [DOI] [PubMed] [Google Scholar]
- Herrera CM. 2017. The ecology of subindividual variability in plants: patterns, processes and prospects. Web Ecology 17: 51–64. [Google Scholar]
- Jaeger B. 2017. r2glmm: computes R squared for mixed (multilevel) models. R package version 0.1.2. Available at: https://CRAN.R-project.org/package=r2glmm. [Google Scholar]
- Jump AS, Mátyás C, Peñuelas J. 2009. The altitude-for-latitude disparity in the range retractions of woody species. Trends in Ecology & Evolution 24: 694–701. [DOI] [PubMed] [Google Scholar]
- Kelly AE, Goulden ML. 2008. Rapid shifts in plant distribution with recent climate change. Proceedings of the National Academy of Sciences of the United States of America 105: 11823–11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körner C. 2003. Alpine Plant Life. Berlin: Springer. [Google Scholar]
- Lara A, Villalba R, Wolodarsky-Franke A, Aravena JC, Luckman BJ, Cuq E. 2005. Spatial and temporal variation in Nothofagus pumilio growth at tree line along its latitudinal range (35°40’–55° S) in the Chilean Andes. Journal of Biogeography 32: 879–893. [Google Scholar]
- Lechthaler S, Turnbull TL, Gelmini Y, et al. 2019. A standardization method to disentangle environmental information from axial trends of xylem anatomical traits. Tree Physiology 39: 495–502. [DOI] [PubMed] [Google Scholar]
- Lloret F, Escudero A, Iriondo JM, Martínez-Vilalta J, Valladares F. 2012. Extreme climatic events and vegetation: the role of stabilizing processes. Global Change Biology 18: 797–805. [Google Scholar]
- López BC, Sabaté S, Gracia CA, Rodríguez R. 2005. Wood anatomy, description of annual rings, and responses to ENSO events of Prosopis pallida HBK, a wide-spread woody plant of arid and semi-arid lands of Latin America. Journal of Arid Environments 61: 541–554. [Google Scholar]
- Maechler M. 2016. diptest: Hartigan’s dip test statistic for unimodality – corrected. R package version 0.75–7. Available at: https://CRAN.R-project.org/package=diptest. [Google Scholar]
- Maherali H, Pockman WT, Jackson RB. 2004. Adaptive variation in the vulnerability of woody plants to xylem cavitation. Ecology 85: 2184–2199. [Google Scholar]
- Martínez-Vilalta J, García-Forner N. 2016. Water potential regulation, stomatal behavior and hydraulic transport under drought: deconstructing the iso/anisohydric concept. Plant, Cell and Environment 40: 962–976. [DOI] [PubMed] [Google Scholar]
- McDowell N, Pockman WT, Allen CD, et al. 2008. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? The New Phytologist 178: 719–739. [DOI] [PubMed] [Google Scholar]
- Medeiros JS, Pockman WT. 2014. Freezing regime and trade-offs with water transport efficiency generate variation in xylem structure across diploid populations of Larrea sp. (Zygophyllaceae). American Journal of Botany 101: 598–607. [DOI] [PubMed] [Google Scholar]
- Meinzer FC, Grantz DA, Goldstein G, Saliendra NZ. 1990. Leaf water relations and maintenance of gas exchange in coffee cultivars grown in drying soil. Plant Physiology 94: 1781–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencuccini M. 2003. The ecological significance of long-distance water transport: short-term regulation, long-term acclimation and the hydraulic costs of stature across plant life forms. Plant, Cell and Environment 26: 163–182. [Google Scholar]
- Meyer D, Dimitriadou E, Hornik K, Weingessel A, Leisch F. 2017. e1071: Misc functions of the Department of Statistics, Probability Theory Group (formerly: E1071), TU Wien. R package version 1.6–8. Available at: https://CRAN.R-project.org/package=e1071. [Google Scholar]
- Nakagawa S, Schielzeth H. 2013. A general and simple method for obtaining R2 from generalized linear mixed effect models. Methods in Ecology and Evolution 4: 133–142. [Google Scholar]
- Nonweiler TRF. 1975. Flow of biological fluids through non-ideal capillaries. In: Zimmermann MH, Milburn JA, eds. Encyclopaedia of plant physiology, New Series, Vol 1. Transport in plants. I. Phloem transport. Berlin: Springer, 474–477. [Google Scholar]
- Noshiro S, Baas P. 1998. Systematic wood anatomy of Cornaceae and allies. IAWA Journal 19: 43–97. [Google Scholar]
- Olano JM, Almería I, Eugenio M, von Arx G. 2013. Under pressure: how a Mediterranean high-mountain forb coordinates growth and hydraulic xylem anatomy in response to temperature and water constraints. Functional Ecology 27: 1295–1303. [Google Scholar]
- Olano JM, González-Muñoz N, Arzac A, et al. 2017. Sex determines xylem anatomy in a dioecious conifer: hydraulic consequences in a drier world. Tree Physiology 37: 1493–1502. [DOI] [PubMed] [Google Scholar]
- Olson ME, Anfodillo T, Rosell JA, et al. 2014. Universal hydraulics of the flowering plants: vessel diameter scales with stem length across angiosperm lineages, habits and climates. Ecology Letters 17: 988–997. [DOI] [PubMed] [Google Scholar]
- Oksanen J, Blanchet FG, Friendly M, et al. 2019. vegan: Community Ecology Package. R package version 2.5–4. Available at: https://CRAN.R-project.org/package=vegan [Google Scholar]
- Pacheco A, Camarero JJ, Carrer M. 2016. Linking wood anatomy and xylogenesis allows pinpointing of climate and drought influences on growth of coexisting conifers in continental Mediterranean climate. Tree Physiology 36: 502–512. [DOI] [PubMed] [Google Scholar]
- Paradis E, Schliep K. 2019. ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics (Oxford, England) 35: 526–528. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team 2019. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1–140. Available at: https://CRAN.R-project.org/package=nlme. [Google Scholar]
- R Core Team 2019. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; Available at: https://www.R-project.org/. [Google Scholar]
- Rita A, Cherubini P, Leonardi S, Todaro L, Borghetti M. 2015. Functional adjustments of xylem anatomy to climatic variability: insights from long-term Ilex aquifolium tree-ring series. Tree Physiology 35: 817–828. [DOI] [PubMed] [Google Scholar]
- Robert EMR, Koedam N, Beeckman H, Schmitz N. 2009. A safe hydraulic architecture as wood anatomical explanation for the difference in distribution of the mangroves Avicennia and Rhizophora. Functional Ecology 23: 649–657. [Google Scholar]
- Scholz A, Rabaey D, Stein A, Cochard H, Smets E, Jansen S. 2013. The evolution and function of vessel and pit characters with respect to cavitation resistance across 10 Prunus species. Tree Physiology 33: 684–694. [DOI] [PubMed] [Google Scholar]
- Schreiber SG, Hacke UG, Hamann A. 2015. Variation of xylem vessel diameters across a climate gradient: insight from a reciprocal transplant experiment with a widespread boreal tree. Functional Ecology 29: 1392–1401. [Google Scholar]
- Scott DW. 1992. Multivariate density estimation. Theory, practice and visualization. New York: Wiley. [Google Scholar]
- Sigdel SR, Wang Y, Camarero JJ, Zhu H, Liang E, Peñuelas J. 2018. Moisture-mediated responsiveness of treeline shifts to global warming in the Himalayas. Global Change Biology 24: 5549–5559. [DOI] [PubMed] [Google Scholar]
- Silverman BW. 1986. Density estimation. London: Chapman and Hall. [Google Scholar]
- Sperry JS. 2003. Evolution of water transport and xylem structure. International Journal of Plant Sciences 164: S115–S127. [Google Scholar]
- Trifilò P, Barbera PM, Raimondo F, Nardini A, Lo Gullo MA. 2014. Coping with drought-induced xylem cavitation: coordination of embolism repair and ionic effects in three Mediterranean evergreens. Tree Physiology 34: 109–122. [DOI] [PubMed] [Google Scholar]
- Tyree MT, Zimmermann MH. 2002. Xylem structure and the ascent of sap. Berlin: Springer-Verlag. [Google Scholar]
- van der Graaff NA, Baas P. 1974. Wood anatomical variation in relation to latitude and altitude. Blumea 22: 101–121. [Google Scholar]
- van der Oever L, Baas P, Zandee M. 1981. Comparative wood anatomy of Symplocos and latitude and altitude of provenance. IAWA Bulletin 2: 3–24. [Google Scholar]
- Villar-Salvador P, Castro-Díez P, Pérez-Rontomé C, Montserrat-Martí G. 1997. Stem xylem features in three Quercus (Fagaceae) species along a climatic gradient in NE Spain. Trees – Structure and Function 12: 90–96. [Google Scholar]
- von Arx G, Dietz H. 2005. Automated image analysis of annual rings in the roots of perennial forbs. International Journal of Plant Sciences 166: 723–732. [Google Scholar]
- von Arx G, Kueffer C, Fonti P. 2013. Quantifying plasticity in vessel grouping – added value from the image analysis tool ROXAS. IAWA Journal 34: 433–445. [Google Scholar]
- von Arx G, Crivellaro A, Prendin AL, Čufar K, Carrer M. 2016. Quantitative wood anatomy-practical guidelines. Frontiers in Plant Science 7: 781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanne AE, Westoby M, Falster DS, et al. 2010. Angiosperm wood structure: Global patterns in vessel anatomy and their relation to wood density and potential conductivity. American Journal of Botany 97: 207–215. [DOI] [PubMed] [Google Scholar]
- Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. New York: Springer. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.