Abstract
Background and Aims
Nitrate can stimulate seed germination of many plant species in the absence of light; however, the molecular mechanism of nitrate-promoted seed germination in the dark remains largely unclear and no component of this pathway has been identified yet. Here, we show that a plastid J-domain protein, DJC75/CRRJ, in arabidopsis (Arabidopsis thaliana) is important for nitrate-promoted seed germination in the dark.
Methods
The expression of DJC75 during imbibition in the dark was investigated. The seed germination rate of mutants defective in DJC75 was determined in the presence of nitrate when light cues for seed germination were eliminated by the treatment of imbibed seeds with a pulse of far-red light to inactivate phytochrome B (phyB), or by assaying germination in the dark with seeds harbouring the phyB mutation. The germination rates of mutants defective in CRRL, a J-like protein related to DJC75, and in two chloroplast Hsp70s were also measured in the presence of nitrate in darkness.
Key Results
DJC75 was expressed during seed imbibition in the absence of light. Mutants defective in DJC75 showed seed germination defects in the presence of nitrate when light cues for seed germination were eliminated. Mutants defective in CRRL and in two chloroplast Hsp70s also exhibited similar seed germination defects. Upregulation of gibberellin biosynthetic gene GA3ox1 expression by nitrate in imbibed phyB mutant seeds was diminished when DJC75 was knocked out.
Conclusions
Our data suggest that plastid J-domain protein DJC75 regulates nitrate-promoted seed germination in the dark by upregulation of expression of the gibberellin biosynthetic gene GA3ox1 through an unknown mechanism and that DJC75 may work in concert with chloroplast Hsp70s to regulate nitrate-promoted seed germination. DJC75 is the first pathway component identified for nitrate-promoted seed germination in the dark.
Keywords: Arabidopsis thaliana, chloroplast, NDH complex, nitrate, plastid, J-domain protein, J-like protein, seed germination
INTRODUCTION
Seed germination is a critical step in the life cycle of flowering plants. Since seeds contain limited reserves, seed germination has to be tightly regulated to ensure that germination takes place in a suitable environment where seedlings can reach the sunlight and carry out photosynthesis before seed reserves are exhausted. However, seeds may not be able to germinate even under favourable conditions, which is called seed dormancy (Bentsink and Koornneef, 2008). Dormancy can be gradually relieved by dry storage at ambient temperature, termed after-ripening. Dormancy of the arabidopsis accession Cape Verde Islands (Cvi) is deep and can be maintained for more than 1 year, whereas dormancy of commonly used accessions Columbia (Col) and Landsberg erecta (Ler) is very shallow and can be relieved by a short period of dry storage at room temperature (Basbouss-Serhal et al., 2016; Dekkers et al., 2016; Yazdanpanah et al., 2017). The plant hormones abscisic acid (ABA) and gibberellins (GAs) are involved in seed dormancy and germination with opposite actions (Miransari and Smith, 2014); ABA maintains seed dormancy while GAs promote seed germination. Several environmental factors, such as light, stratification (moist pre-chilling) and nitrate (NO3−), have been shown to promote seed germination in arabidopsis (Bentsink and Koornneef, 2008). These factors stimulate germination by modulating the balance between ABA and GAs (Finkelstein et al., 2008).
The ability of nitrate to stimulate seed germination has been known for more than a century (Lehmann, 1909). Nitrate has been shown to relieve seed dormancy and to stimulate germination of a variety of plants in the light or in darkness (Duermeyer et al., 2018). Under illumination, nitrate can reduce the light requirement for dormancy release of dormant arabidopsis seeds, but nitrate itself failed to release seed dormancy in the absence of light (Batak et al., 2002; Finch-Savage et al., 2007). With regard to non-dormant (after-ripened) seeds, nitrate can stimulate germination in the dark (Shinomura et al., 1994; Batak et al., 2002). Nitrate stimulates seed germination independently of nitrate assimilation and acts as a signal to promote seed germination (Hendricks and Taylorson, 1972; Hilhorst and Karssen, 1989; Alboresi et al., 2005). A few components of the nitrate-promoted seed germination pathway in the light have been characterized (Alboresi et al., 2005; Matakiadis et al., 2009; Yan et al., 2016). Expression of CYP707A2, which encodes ABA 8′-hydroxylase, a key enzyme for ABA catabolism, was shown to be rapidly induced after imbibition in the presence of nitrate and light. The nitrate-induced reduction of ABA content was eliminated in cyp707a2 mutant seeds. Furthermore, nitrate became less efficient at promoting germination of cyp707a2 mutant seeds. These findings indicate that nitrate promotes seed germination under light by upregulating the expression of CYP707A2, thereby lowering ABA content in imbibed seeds (Matakiadis et al., 2009). The transcription factor NIN-like protein 8 (NLP8) has been shown to regulate CYP707A2 expression, and the germination rate of mutants defective in the NLP8 gene was found to be significantly reduced in the presence of nitrate and light (Yan et al., 2016). Binding of NLP8 to the promoter of CYP707A2 to induce CYP707A2 expression was shown to be post-translationally activated by nitrate through an unknown mechanism that decreased ABA content and induced seed germination (Yan et al., 2016).
Nitrate also stimulates seed germination of arabidopsis and some other plant species in the dark (Hendricks and Taylorson, 1972, 1974; Saini et al., 1985; Pons, 1989; Batak et al., 2002). Little is known about how nitrate stimulates seed germination in the absence of light and no component for this pathway has been identified yet. phyB mutant seeds do not germinate in the dark when only micronutrients are present (Shinomura et al., 1994), but addition of nitrate markedly stimulated phyB mutant seed germination in the dark (Batak et al., 2002). This is because the presence of the active form of phytochrome B (phyB-Pfr) produced during seed development on the mother plant acts as a light cue to stimulate the germination of arabidopsis non-dormant seeds in the dark in the absence of nitrate (McCullough and Shropshire, 1970; Shinomura et al., 1994). Here we show that two mutants, djc75-1 and crrl, are defective in nitrate-promoted seed germination in the dark. Both DJC75 (also named DNAJC75, CRRJ and NdhT) and CRRL (also named DNAJD15 and NdhU) have been shown to be subunits of the chloroplast NADH dehydrogenase-like (NDH) complex (Finka et al., 2011; Ifuku et al., 2011; Yamamoto et al., 2011; Pulido and Leister, 2018). DJC75 is a J-domain-containing thylakoid membrane protein (Chiu et al., 2013) and CRRL is a J-like protein related to DJC75 (Pulido and Leister, 2018). We further show that mutants of two chloroplast 70-kDa heat shock proteins (Hsp70s) are also defective in nitrate-promoted seed germination. Our data suggest that DJC75 regulates nitrate-promoted seed germination in the dark by upregulation of expression of the gibberellin (GA) biosynthetic gene GA3ox1 through an unknown mechanism and is a pathway component for nitrate-promoted seed germination in the dark. Additionally, DJC75 may act in concert with chloroplast Hsp70s to regulate nitrate-promoted seed germination.
MATERIALS AND METHODS
Plant growth conditions and seed harvest
Arabidopsis plants were grown in pots in a growth room under continuous light at 22 °C. Plants were irrigated with Hyponex #2 nutrient solution (Hyponex Corp., OH, USA) until flowering. After flowering, plants were irrigated with distilled water. Harvested seeds were stored in paper bags at room temperature for 2 months to release dormancy (Dekkers et al., 2016).
RNA isolation and RT–PCR analysis
RNA was isolated using the Plant Total RNA purification kit (GeneMark) from dormancy-released dry seeds or 1-d imbibed non-dormant seeds on plates with medium containing ½ × Murashige and Skoog (MS) salts (Murashige and Skoog, 1962), 0.4 % agarose, 0.1 % MES and 1 % sucrose pH 5.7. Isolated total RNA was dissolved in DEPC-H2O. RNA samples were then treated with RQ1 RNase-Free DNase (Promega). The first-strand cDNA was synthesized using DNase I-treated RNA, Impro-II Reverse Transcriptase (Promega) and a poly(dT) primer. PCR was performed using 2× PCR master mix (Ampliqon). Primers used were as follows: DJC75 forward primer, 5′-GCCCGGACGAAGGCTGGATCGGAGG-3′; DJC75 reverse primer, 5′-CGATCAAAATATCGAACGTGAGAGC-3′; UBQ10 forward primer, 5′-GGTAGAGAGCTCTGACACCA-3′; UBQ10 reverse primer, 5′-CTTCTTAAGCATAACAGAGACGAG-3′; H3G forward primer, 5′-AACCACTGGAGGAGTCAAGA-3′; H3G reverse primer, 5′-CAATTAAGCACGTTCTCCTCT-3′; XTH5 forward primer, 5′-CATTCCTATTCAGTTCTCTGG-3′; XTH5 reverse primer, 5′-GAACCCATTTGAGACGCTTG-3′; RD29B forward primer, 5′-GCAAGCAGAAGAACCAATCA-3′; RD29B reverse primer, 5′-CTTTGGATGCTCCCTTCTCA-3′; GA3ox1 forward primer, 5′-CCGAAGGTTTCACCATCACT-3′; GA3ox1 reverse primer, 5′-CCCCAAAGGAATGCTACAGA-3′; GA2ox2 forward primer, 5′-AATAACACGGCGGGTCTTCAAATCT-3′; GA2ox2 reverse primer, 5′-TCCTCGATCTCCTTGTATCGGCTAA-3′; CYP707A2 forward primer, 5′-ATGGGGTTGCCTTACATCGGAGA-3′; CYP707A2 reverse primer, 5′-TGGCTTGAACAAGTGAGCTTTGCT-3′; TUB forward primer, 5′-ATCCGTGAAGAGTACCCAGAT-3′; TUB reverse primer, 5′-AAGAACCATGCACTCATCAGC-3′.
Seed germination assay
The germination assays were performed using non-dormant seeds that had been stored for no longer than 4.5 months after harvest. Seeds were incubated with 95 % ethanol for 2 min and then sterilized with solution containing 25 % bleach and 0.25 % SDS for 15 min. After being rinsed four times with sterilized distilled H2O, seeds were sown on the medium indicated. For phyB-off, phyB-on and phyA-on assays in nitrate-free medium, seeds were sown on plates containing ½ × micronutrients of MS salts. For phyB-off and phyB-on assays in the presence of nitrate, seeds were sown on ½ × MS medium containing all micronutrients and macronutrients. For germination assays performed in the dark without any light pretreatment, seeds were sown on medium containing ½ × micronutrients, or ½ × micronutrients with the addition of 9.4 mm KNO3 or with the addition of 9.4 mm KCl as control. All media contained 0.4 % agarose, 0.1 % MES and 1 % sucrose, and pH was adjusted to 5.7 using KOH. The phyB-off, phyB-on and phyA-on assays were performed as described (Oh et al., 2004). For phyB-off assays, seeds after sterilization were illuminated with 5 min of far-red light (0.5 W m−2) to deactivate phyB and were then incubated in darkness at 22 °C for 4 d. For phyB-on assays, seeds were illuminated with 5 min of far-red light (0.5 W m−2), followed by 5 min of red light (5 μmol m−2 s−1) to activate phyB, and then incubated in the dark at 22 °C for 4 d. For phyA-on assays, seeds were first illuminated with 5 min of far-red light (0.5 W m−2) to deactivate phyB, and then incubated in darkness at 22 °C. Two days later, the seeds were illuminated again with 4 h of far-red light (3 W m−2) to activate phyA and then incubated in the dark for 4 d. Germination assays were perfomed at least three times, each time with three replicates of n = 30 seeds for each genotype. Seed germination was defined by radicle emergence. Seeds used were djc75-1 (Salk_111394), djc75-2 (GK-154C09), phyB-5 (CS6213), phyA-201 (CS6219), crrl (GABI_023D06), cphsc70-1 (Salk_140810) and cphsc70-2 (Salk_095715).
Expression pattern analysis
The tissue-specific expression pattern of DJC75 (At4g09350) was retrieved from the public Affimetrix microarray database using Genevestigator (Hruz et al., 2008).
RESULTS
DJC75 expression is induced in seeds during imbibition under both light and dark conditions
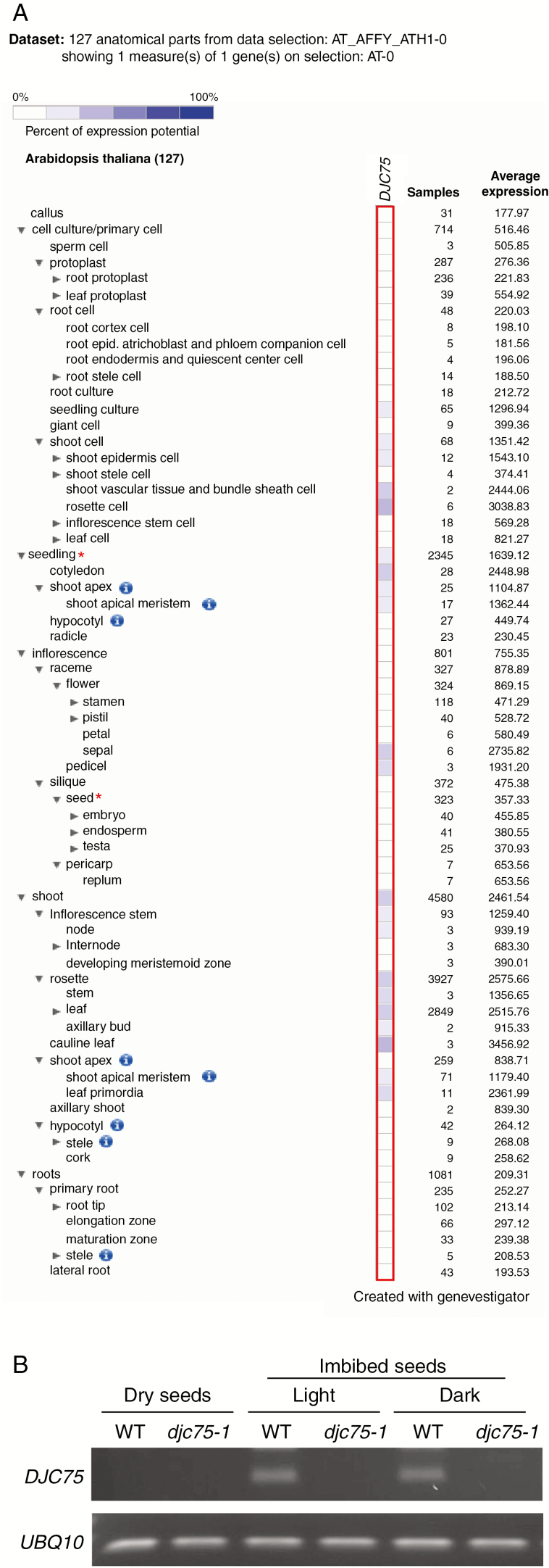
DJC75 has been shown to be a subunit of the NDH complex, which mediates the minor route of photosystem I cyclic electron transport (PSI-CET) in the light (Yamamoto et al., 2011). To investigate whether DJC75 has additional functions, we retrieved the tissue-specific expression pattern of DJC75 from the publicly available mRNA expression database (Hruz et al., 2008). As shown in Fig. 1A, DJC75 is predominantly expressed in green tissues such as leaves, stems, sepals, pedicels and cotyledons, in agreement with the involvement of NDH in PSI-CET in the light (Peltier et al., 2016). We also observed that the level of DJC75 expression was very low in ‘seed’, but a higher level of expression was detected in ‘seedling’ (Fig. 1, red asterisks). This suggests that DJC75 may play a role during seed germination or early seedling development. However, since the expression level shown for ‘seedling’ was an average of samples from both illuminated and non-illuminated seedlings, we reinvestigated DJC75 expression in non-stratified 1-d-imbibed non-dormant seeds under both light and dark conditions by RT–PCR. In wild-type dry seeds, expression of DJC75 was barely detectable, but after 1 d of imbibition DJC75 expression was detected not only in seeds imbibed under light but also in the dark (Fig. 1B). DJC75 was not expressed in the knockout mutant djc75-1 (previously denoted as crrj-1; Yamamoto et al., 2011). Our analysis indicates that during seed germination DJC75 expression is induced even in the absence of light, suggesting that DJC75 may function in seed germination or early seedling development.
Fig. 1.
DJC75 is expressed during seed germination. (A) Tissue expression patterns of DJC75 retrieved from Genevestigator. ‘Seedling’ and ‘seed’ are indicated with red asterisks. (B) RT–PCR analysis of DJC75 in wild type (WT) and the djc75-1 mutant. WT and djc75-1 seeds were imbibed under light and dark conditions for 1 d. UBQ10 was included as a control.
DJC75 is not involved in phyB- or phyA-regulated seed germination
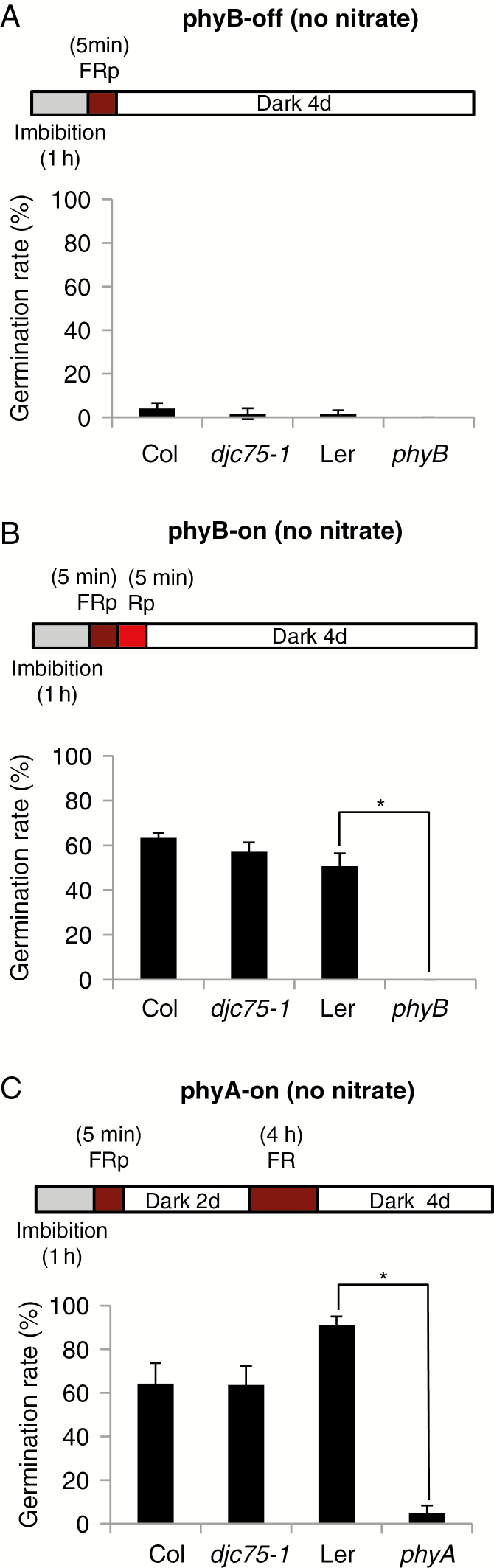
Next, we investigated whether DJC75 plays a role in seed germination. Light promotes arabidopsis seed germination mainly through the action of phyB and phyA (Shinomura, 1997). To investigate whether DJC75 is involved in phyB-regulated seed germination, we performed germination assays using non-stratified seeds and medium lacking nitrate. Germination rates were measured by sowing non-stratified seeds of the djc75-1 mutant and its corresponding wild type (Col) on nitrate-free agar plates and treating the seeds with a pulse of far-red light (FRp) to inactivate phyB (phyB-off), or with FRp followed by a red light pulse (Rp) to activate phyB (phyB-on). Seeds of the phyB mutant and its corresponding wild type (Ler) were included as controls. If DJC75 is involved in phyB-regulated seed germination, the germination rate of djc75-1 mutant seeds would be significantly affected in phyB-on conditions. As shown in Fig. 2A, under the phyB-off condition very few seeds germinated. This is because the action of phyB on seed germination was suppressed. Under the phyB-on condition (Fig. 2B), phyB mutant seeds, but not seeds of its corresponding wild type, failed to germinate. No significant difference in germination rate was observed between the djc75-1 mutant and its wild type, indicating that DJC75 is not involved in phyB-regulated seed germination.
Fig. 2.
DJC75 is not involved in phyB- or phyA-regulated seed germination. Seeds of djc75-1, phyB and phyA mutants and their corresponding wild types (Col for djc75-1 and Ler for phyB and phyA) were sown on nitrate-free medium under phyB-off (A), phyB-on (B) or phyA-on (C) conditions. Means of 4-d germination rates (%) ± s.d. of three biological repeats are plotted. FRp, far-red light pulse; Rp, red light pulse. *P < 0.05 (Student’s t-test).
In addition to phyB, arabidopsis seed germination can also be stimulated through the action of phyA via long-term far-red light illumination (Shinomura, 1997). To test whether DJC75 is involved in phyA-regulated seed germination, we measured the germination rate of non-stratified djc75-1 mutant seeds illuminated with FRp to inactivate phyB followed by long-term far-red illumination to activate phyA (phyA-on) in nitrate-free medium. As shown in Fig. 2C, the germination rate of phyA mutant seeds was very low under these conditions, yet the corresponding wild-type seeds (Ler) geminated normally. The germination rate of djc75-1 mutant seeds was comparable to that of its respective wild type, indicating that the phyA-regulated seed germination pathway is not impaired in the djc75-1 mutant. Together, the data presented in Fig. 2 show that DJC75 is not involved in phyB- or phyA-regulated seed germination in arabidopsis, i.e. DJC75 is not involved in light-regulated seed germination.
DJC75 is involved in nitrate-promoted seed germination in the dark
Next, we examined whether DJC75 is involved in nitrate-promoted seed germination in the dark. First, the germination of non-stratified djc75-1 seeds illuminated with an FRp (phyB-off) to eliminate light cues was assayed in nitrate-containing ½ × MS medium (Murashige and Skoog, 1962). If DJC75 is involved in nitrate-promoted seed germination, we expected to observe a significant difference in germination rate between djc75-1 mutant and wild-type seeds under these conditions. We also included phyB-on conditions in the assay as a light-cue-present control. Under phyB-off conditions, djc75-1 mutant seeds showed a clear germination defect compared with the wild type (Fig. 3A), indicating that DJC75 is involved in nitrate-promoted seed germination in the dark in MS medium. No significant difference was observed between the seed germination rates of wild-type and djc75-1 mutant seeds under the phyB-on condition, confirming that DJC75 is not involved in light-regulated seed germination.
Fig. 3.
DJC75 is important for nitrate-promoted seed germination in the dark. Seeds of wild type and djc75-1 mutant were sown in ½ × MS medium containing nitrate under phyB-off or phyB-on conditions (A). Seeds of two accessions of wild-type (WT), djc75-1, phyB and djc75-1/phyB mutant lines were sown in the media indicated and incubated in the dark (B, C). Means of 4-d germination rates (%) ± s.d. of three biological repeats are plotted. Micro, micronutrients. *P < 0.05 (Student’s t-test).
The seed germination of another T-DNA-inserted mutant, djc75-2, was also examined in ½ × MS medium under phyB-off conditions (Supplementary Data Fig. S1). The djc75-2 mutant is a knockdown mutant; it has a T-DNA insertion in the promoter region 49 bp upstream of the transcription start site. RT–PCR analysis indicated that the djc75-2 mutant had a small amount of DJC75 RNA left (Supplementary Data Fig. S1A, B). The germination kinetics showed that the seed germination rate of djc75-2 mutant on day 2 fell between the rates of wild type and djc75-1, and thereafter its rate was indistinguishable from the wild type (Supplementary Data Fig. S1C). This indicates that although the reduction of DJC75 expression level in the djc75-2 mutant slows germination, the rate of germination of djc75-2 seeds would eventually reach that of wild-type seeds.
Since MS medium contains all necessary macronutrients and micronutrients, we then performed a more stringent germination assay on the djc75-1 mutant using medium supplemented with ½ × MS micronutrients and KNO3 (or addition of KCl instead of KNO3 as a control) to clarify that nitrate is indeed the factor in the MS medium that promotes DJC75-mediated seed germination. In addition, to test the role of DJC75 in germination in the dark, we assayed germination in the dark of non-stratified seeds harbouring the phyB mutation and lacking any light treatment. The phyB mutant seeds did not germinate in the dark in the presence of only micronutrients (Fig. 3B) (Shinomura et al., 1994), and addition of KNO3 (but not KCl) markedly stimulated phyB mutant seed germination in the dark (Fig. 3B, C). We generated a djc75/phyB double mutant to test whether DJC75 plays a role in this nitrate-stimulated germination of phyB mutant seeds. As shown in Fig. 3B, C, the djc75 mutation almost completely abolished nitrate-stimulated germination of phyB mutant seeds, indicating that DJC75 is important for nitrate-promoted seed germination in the dark. The high germination rates in the dark of wild-type Col and Ler and the djc75-1 mutant in all tested media were most likely due to the accumulated active form of phyB (phyB-Pfr) produced during seed development under light on the mother plant (McCullough and Shropshire, 1970).
CRRL is also important for nitrate-promoted seed germination in the dark
DJC75 is encoded by a single gene in arabidopsis. However, a J-like protein, CRRL, is shown to be related to DJC75 but with no conserved tripeptide histidine–proline–aspartic acid (HPD) motif, which is required for Hsp70 interaction (Pulido and Leister, 2018). Both DJC75 and CRRL have been shown to be subunits of the NDH complex (Yamamoto et al., 2011). To investigate whether CRRL also plays a role in nitrate-promoted seed germination, germination assays were performed as described in Fig. 3 to test whether mutation of CRRL results in a germination defect similar to that arising from the djc75 mutation. Similar to our results for the djc75-1 mutant, the crrl mutant also exhibited a germination defect in nitrate-containing medium under phyB-off conditions but not under phyB-on conditions (Fig. 4A). For our germination assays in the dark, the germination rate of crrl/phyB double-mutant seeds was significantly lower than that of the phyB mutant in the medium containing KNO3 (Fig. 4B). These data indicate that CRRL is also important for nitrate-promoted seed germination in the dark. We found that the seed germination rate of crrl/phyB double-mutant seeds was slightly higher on medium including nitrate compared with medium containing KCl, suggesting that germination of these seeds was still marginally stimulated by nitrate (Fig. 4B).
Fig. 4.
CRRL is involved in nitrate-promoted seed germination in the dark. Seeds of wild type (WT) and crrl mutant were sown in ½ × MS medium containing nitrate under phyB-off or phyB-on conditions (A). Seeds of two accessions of wild-type, crrl, phyB and crrl/phyB mutant lines were sown in the media indicated and incubated in the dark (B). Mean 4-d germination rates (%) ± s.d. of three biological repeats are plotted. Micro, micronutrients. *P < 0.05 (Student’s t-test).
cpHsc70-1 and cpHsc70-2 are involved in nitrate-promoted seed germination in the dark
DJC75 contains a J domain, which is required for stimulating ATP hydrolysis by the Hsp70 family of chaperones. J-domain proteins, or J proteins, usually act in concert with Hsp70s (Kampinga and Craig, 2010). There are two chloroplast Hsp70s in arabidopsis, i.e. cpHsc70-1 and cpHsc70-2. Next, we investigated whether the chloroplast Hsp70s are involved in nitrate-promoted seed germination in the dark. Deficiency of both cpHsc70 genes is lethal (Sung et al., 2001), so we performed the germination assays in the dark using non-stratified seeds from single cphsc70-1 or cphsc70-2 mutants, as well as cphsc70-1/phyB and cphsc70-2/phyB double mutants. As shown in Fig. 5A, B, both the cphsc70-1/phyB and cphsc70-2/phyB double mutants exhibited significantly reduced germination rates on nitrate-containing medium relative to that of a phyB single mutant, suggesting that both cpHsc70-1 and cpHsc70-2 are important for nitrate-promoted seed germination in the dark. The germination rates of the cphsc70-1/phyB and cphsc70-2/phyB double mutants on nitrate-containing medium were higher than in medium with KCl. It is possible that each cphsc70 mutation represents partial loss of cpHsc70 function in terms of promoting seed germination through nitrate.
Fig. 5.
Both cpHsc70s are important for nitrate-promoted seed germination in the dark. Seeds of two accessions of wild-type, cphsc70-1 (A) or cphsc70-2 (B), phyB and cphsc70-1/phyB (A) or cphsc70-2/phyB (B) mutant lines were sown in the media indicated and incubated in the dark. Mean 4-d germination rates (%) ± s.d. of three biological repeats are plotted. Micro, micronutrients. *P < 0.05 (Student’s t-test).
Upregulation of GA biosynthetic gene GA3ox1 expression by nitrate in the phyB mutant in the dark is diminished when DJC75 is knocked out
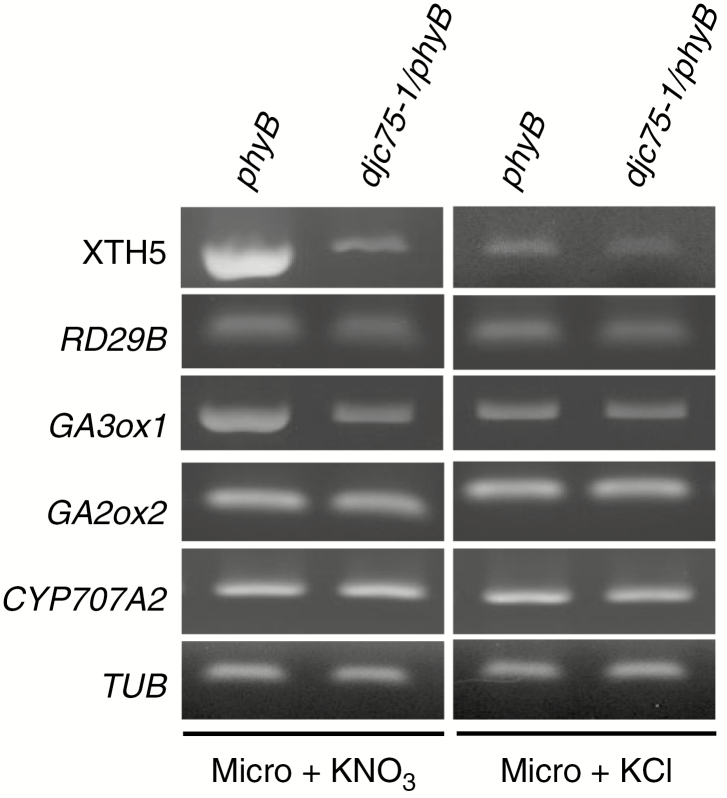
In Fig. 3B, C we show that when the light cues for seed germination were eliminated, mutants harbouring the djc75 mutation were unable to germinate even in the presence of nitrate. To investigate how DJC75 regulates nitrate-promoted seed germination in the absence of light cues, the expression of the GA-responsive gene XTH5 and the ABA-responsive gene RD29B in non-stratified 1-d imbibed seeds of phyB and djc75-1/phyB mutants in the dark was analysed by RT–PCR. As shown in Fig. 6, the expression of XTH5 was elevated by nitrate in the phyB mutant, and this elevation was not observed in djc75-1/phyB mutant seeds. The expression of RD29B otherwise remained relatively unchanged upon nitrate addition. This suggests that DJC75 regulates nitrate-promoted seed germination in the dark through the regulation of GA homeostasis. We next analysed the expression of the GA biosynthetic gene GA3ox1, the GA catabolic gene GA2ox2 and also the ABA catabolic gene CYP707A2. Among the genes analysed, we observed that the expression of GA3ox1 was upregulated by nitrate in imbibed phyB mutant seeds (Fig. 6). These results indicate that DJC75 regulates nitrate-promoted seed germination in the dark by upregulation of the expression of the GA biosynthetic gene GA3ox1 through an unknown mechanism.
Fig. 6.
Upregulation of expression of GA3ox1 by nitrate in phyB mutant in the dark is diminished when the djc75 mutation is introduced. RT–PCR analysis of XTH5, RD29B, GA3ox1, GA2ox2 and CYP707A2 in phyB and djc75-1/phyB mutants. phyB and djc75-1/phyB mutant seeds were imbibed in the dark for 1 d. TUB was included as a control. Micro, micronutrients.
DISCUSSION
The molecular mechanism underlying nitrate-promoted seed germination seems to be different under light and dark conditions. Germination of dormant seeds cannot be stimulated by nitrate in the absence of light, but nitrate can reduce the light requirement for dormancy release in the light (Hilhorst et al., 1986; Finch-Savage et al., 2007). Nitrate can promote the germination of non-dormant seeds of wild-type and phyB mutant seeds in the dark (Shinomura et al., 1994; Batak et al., 2002). By analysing non-dormant seeds, here we have shown that seed germination by a mutant defective in chloroplast DJC75 failed to germinate without light cues even in the presence of nitrate (Fig. 3 ). In addition, upregulation of expression of the GA biosynthetic gene GA3ox1 by nitrate in the dark in non-stratified imbibed phyB mutant seeds was diminished when the djc75 mutation was introduced (Fig. 6). These data suggest that DJC75 plays an unidentified role in the regulation of GA3ox1 expression for nitrate-promoted seed germination in the dark.
It has been suggested that there is more than one nitrate sensor under conditions of light provision, including CHL1 (Konishi and Yanagisawa, 2010; Yan et al., 2016). NLP8 regulates ABA catabolism by inducing expression of an ABA catabolic gene, CYP707A2, so that seed germination can then proceed (Matakiadis et al., 2009; Yan et al., 2016). It is not known how the nitrate signal is transmitted to the transcription factor NLP8. DJC75 is unlikely to be involved in this effect because our data show that the djc75-1 mutant did not exhibit germination defects under conditions of light provision (Fig. 3), and there was no significant difference in the expression of CYP707A2 between phyB and djc75-1/phyB mutant seeds in the presence of nitrate in the dark (Fig. 6).
In addition to seed germination, nitrate also acts as a signal to stimulate primary nitrate responses, such as rapidly induced expression of the nitrate reductase gene NIA1 and the high-affinity nitrate transporter gene NRT2.1 (Medici and Krouk, 2014). Investigations of post-germinative tissues have shown that cytosolic Ca2+ and subgroup III Ca2+-sensor protein kinases operate upstream of the transcription factors NLP6 and NLP7 in the nitrate signalling pathway that regulates primary nitrate responses (Konishi and Yanagisawa, 2010; Liu et al., 2017). It would be interesting to establish whether signalling of subgroup III Ca2+-sensor protein kinases is involved in nitrate-promoted seed germination and whether NLP8 also acts as a master regulator for nitrate-promoted seed germination in the dark.
NDH is a multi-subunit complex located in the thylakoid membrane of chloroplasts, where it functions as a ferredoxin (Fd)-dependent plastoquinone (PQ) reductase that concomitantly pumps protons from the stroma into the lumen for further ATP generation (Shikanai, 2016). In green tissues, NDH mediates the minor PSI-CET pathway under conditions of light (Shikanai, 2007). In contrast, in non-green plastids or chloroplasts in the dark, NDH mediates chlororespiration, which is a non-photochemical reaction that reduces O2 through the oxidation of plastoquinol (PQH2) (Peltier and Cournac, 2002; Peltier et al., 2016). The germination defects under conditions of darkness we observed for the two NDH-defective mutants raised the possibility that NDH-mediated chlororespiration may be involved in nitrate promoted-seed germination in the dark (Figs 3 and 4). However, we cannot exclude that DJC75-mediated nitrate-promoted seed germination in the dark could be independent of NDH activity, i.e. DJC75 may play dual functions.
Arabidopsis cpHsc70-1 and cpHsc70-2 have been shown to play distinct and overlapping roles (Su and Li, 2008, 2010). In Fig. 6, we show that the germination rates of both the cphsc70-1/phyB and cphsc70-2/phyB double mutants promoted by nitrate in the dark were significantly lower than that of the phyB single mutant. Compared with their germination rates in nitrate-free medium, both the cphsc70-1/phyB and cphsc70-2/phyB double mutants still partially maintained their potency for nitrate-promoted germination. This result suggests that cpHsc70-1 and cpHsc70-2 are functionally redundant for nitrate-promoted seed germination. J protein is the co-chaperone of Hsp70s. Though some J proteins act independently of Hsp70 (Sahi et al., 2010), Hsp70 functions are always accomplished through their being recruited by distinct J proteins (Kampinga and Craig, 2010). The conserved tripeptide HPD motif of J proteins is important for stimulating the ATP hydrolysis activity of Hsp70s. It has been shown that the HPD motif of DJC75 is essential for DJC75 function (Yamamoto et al., 2011), indicating that DJC75 may recruit cpHsc70s to perform actions related to nitrate-promoted seed germination in the dark.
Our data indicate that in the presence of light cues, such as being illuminated with a pulse of red light to activate phyB, or occurrence of a high Pfr/Pr ratio of phyB resulting from seed development under continuous light for plant growth, the action of DJC75 in nitrate-promoted seed germination is masked or not required (Fig. 3). Arabidopsis is a facultative long-day plant in which long days promote more rapid floral induction, but flowering still ultimately occurs under short days. Therefore, when seeds are produced under short days in natural environments, the action of DJC75 in nitrate-promoted seed germination in the dark would be more important because the seeds produced under short-day conditions would have a lower Pfr/Pr ratio of phyB than seeds produced under long-day conditions. No matter what photoperiod plants grow in, DJC75-mediated nitrate-promoted seed germination in the dark may act as a survival mechanism to stimulate the germination of more seeds. Our work reveals new directions for studies of plastids in nitrate-promoted seed germination.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figure S1: the djc75-2 mutant is a T-DNA-inserted knockdown mutant with a weak germination defect in nitrate-containing media.
ACKNOWLEDGEMENTS
We thank Dr Shu-Hsing Wu for the gift of phyB and phyA seeds and Dr Hsou-min Li for critical reading of the manuscript. No conflicts of interest are declared.
FUNDING
This work was supported by National Cheng Kung University, Taiwan, and the Ministry of Science and Technology, Taiwan (grant MOST 106-2311-B-006-007 to C.-C.C.).
LITERATURE CITED
- Alboresi A, Gestin C, Leydecker MT, Bedu M, Meyer C, Truong HN. 2005. Nitrate, a signal relieving seed dormancy in Arabidopsis. Plant, Cell & Environment 28: 500–512. [DOI] [PubMed] [Google Scholar]
- Basbouss-Serhal I, Leymarie J, Bailly C. 2016. Fluctuation of Arabidopsis seed dormancy with relative humidity and temperature during dry storage. Journal of Experimental Botany 67: 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batak I, Dević M, Gibal Z, Grubišić D, Poff KL, Konjević R. 2002. The effects of potassium nitrate and NO-donors on phytochrome A- and phytochrome B-specific induced germination of Arabidopsis thaliana seeds. Seed Science Research 12: 253–259. [Google Scholar]
- Bentsink L, Koornneef M. 2008 Seed dormancy and germination. The Arabidopsis Book 6: e0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CC, Chen LJ, Su PH, Li Hm. 2013 Evolution of chloroplast J proteins. PLoS ONE 8: e70384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekkers BJ, Pearce SP, van Bolderen-Veldkamp RP, Holdsworth MJ, Bentsink L. 2016. Dormant and after-ripened Arabidopsis thaliana seeds are distinguished by early transcriptional differences in the imbibed state. Frontiers in Plant Science 7: 1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duermeyer L, Khodapanahi E, Yan D, Krapp A, Rothstein S, Nambara E. 2018. Regulation of seed dormancy and germination by nitrate. Seed Science Research 28: 150–157. [Google Scholar]
- Finch-Savage WE, Cadman CS, Toorop PE, Lynn JR, Hilhorst HW. 2007. Seed dormancy release in Arabidopsis Cvi by dry after-ripening, low temperature, nitrate and light shows common quantitative patterns of gene expression directed by environmentally specific sensing. Plant Journal 51: 60–78. [DOI] [PubMed] [Google Scholar]
- Finka A, Mattoo RU, Goloubinoff P. 2011. Meta-analysis of heat- and chemically upregulated chaperone genes in plant and human cells. Cell Stress Chaperones 16: 15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R, Reeves W, Ariizumi T, Steber C. 2008. Molecular aspects of seed dormancy. Annual Review of Plant Biology 59: 387–415. [DOI] [PubMed] [Google Scholar]
- Hendricks SB, Taylorson RB. 1972 Promotion of seed germination by nitrates and cyanides. Nature 237: 169–170. [Google Scholar]
- Hendricks SB, Taylorson RB. 1974. Promotion of seed germination by nitrate, nitrite, hydroxylamine, and ammonium salts. Plant Physiology 54: 304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilhorst HWM, Karssen CM. 1989. Nitrate reductase independent stimulation of seed germination in Sisymbrium officinale L. (hedge mustard) by light and nitrate. Annals of Botany 63: 131–137. [Google Scholar]
- Hilhorst HWM, Smitt AI, Karssen CM. 1986. Gibberellin-biosynthesis and -sensitivity mediated stimulation of seed germination of Sisymbrium officinale by red light and nitrate. Physiologia Plantarum 67: 285–290. [Google Scholar]
- Hruz T, Laule O, Szabo G, et al. 2008. Genevestigator v3: a reference expression database for the meta-analysis of transcriptomes. Advances in Bioinformatics 2008: 420747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifuku K, Endo T, Shikanai T, Aro EM. 2011. Structure of the chloroplast NADH dehydrogenase-like complex: nomenclature for nuclear-encoded subunits. Plant & Cell Physiology 52: 1560–1568. [DOI] [PubMed] [Google Scholar]
- Kampinga HH, Craig EA. 2010. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nature Reviews Molecular Cell Biology 11: 579–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M, Yanagisawa S. 2010. Identification of a nitrate-responsive cis-element in the Arabidopsis NIR1 promoter defines the presence of multiple cis-regulatory elements for nitrogen response. Plant Journal 63: 269–282. [DOI] [PubMed] [Google Scholar]
- Lehmann E. 1909. Zur Keimungphysiologie und -biologie von Ranunculus sclereatus L. und einigen anderen Samen. Berichte der Deutschen Botanischen Gesellschaft 27: 476–497. [Google Scholar]
- Liu KH, Niu Y, Konishi M, et al. 2017. Discovery of nitrate-CPK-NLP signalling in central nutrient-growth networks. Nature 545: 311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matakiadis T, Alboresi A, Jikumaru Y, et al. 2009. The Arabidopsis abscisic acid catabolic gene CYP707A2 plays a key role in nitrate control of seed dormancy. Plant Physiology 149: 949–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough JM, Shropshire W Jr.. 1970. Physiological predetermination of germination responses in Arabidopsis thaliana (L.) HEYNH. Plant and Cell Physiology 11: 139–148. [Google Scholar]
- Medici A, Krouk G. 2014. The primary nitrate response: a multifaceted signalling pathway. Journal of Experimental Botany 65: 5567–5576. [DOI] [PubMed] [Google Scholar]
- Miransari M, Smith DL. 2014. Plant hormones and seed germination. Environmental and Experimental Botany 99: 110–121. [Google Scholar]
- Murashige T, Skoog F. 1962. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum 15: 473–497. [Google Scholar]
- Oh E, Kim J, Park E, Kim JI, Kang C, Choi G. 2004. PIL5, a phytochrome-interacting basic helix-loop-helix protein, is a key negative regulator of seed germination in Arabidopsis thaliana. Plant Cell 16: 3045–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier G, Cournac L. 2002. Chlororespiration. Annual Review of Plant Biology 53: 523–550. [DOI] [PubMed] [Google Scholar]
- Peltier G, Aro EM, Shikanai T. 2016. NDH-1 and NDH-2 plastoquinone reductases in oxygenic photosynthesis. Annual Review of Plant Biology 67: 55–80. [DOI] [PubMed] [Google Scholar]
- Pons TL. 1989. Breaking of seed dormancy by nitrate as a gap detection mechanism. Annals of Botany 63: 139–143. [Google Scholar]
- Pulido P, Leister D. 2018. Novel DNAJ-related proteins in Arabidopsis thaliana. New Phytologist 217: 480–490. [DOI] [PubMed] [Google Scholar]
- Sahi C, Lee T, Inada M, Pleiss JA, Craig EA. 2010. Cwc23, an essential J protein critical for pre-mRNA splicing with a dispensable J domain. Molecular and Cellular Biology 30: 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini HS, Bassi PK, Spencer MS. 1985. Seed germination in Chenopodium album L: relationships between nitrate and the effects of plant hormones. Plant Physiology 77: 940–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikanai T. 2007. Cyclic electron transport around photosystem I: genetic approaches. Annual Review of Plant Biology 58: 199–217. [DOI] [PubMed] [Google Scholar]
- Shikanai T. 2016. Chloroplast NDH: a different enzyme with a structure similar to that of respiratory NADH dehydrogenase. Biochimica et Biophysica Acta 1857: 1015–1022. [DOI] [PubMed] [Google Scholar]
- Shinomura T. 1997. Phytochrome regulation of seed germination. Journal of Plant Research 110: 151–161. [DOI] [PubMed] [Google Scholar]
- Shinomura T, Nagatani A, Chory J, Furuya M. 1994. The induction of seed germination in Arabidopsis thaliana is regulated principally by phytochrome B and secondarily by phytochrome A. Plant Physiology 104: 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su PH, Li Hm. 2008. Arabidopsis stromal 70-kD heat shock proteins are essential for plant development and important for thermotolerance of germinating seeds. Plant Physiology 146: 1231–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su PH, Li Hm. 2010. Stromal Hsp70 is important for protein translocation into pea and Arabidopsis chloroplasts. Plant Cell 22: 1516–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung DY, Vierling E, Guy CL. 2001. Comprehensive expression profile analysis of the Arabidopsis Hsp70 gene family. Plant Physiology 126: 789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Peng L, Fukao Y, Shikanai T. 2011. An Src homology 3 domain-like fold protein forms a ferredoxin binding site for the chloroplast NADH dehydrogenase-like complex in Arabidopsis. Plant Cell 23: 1480–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D, Easwaran V, Chau V, et al. 2016. NIN-like protein 8 is a master regulator of nitrate-promoted seed germination in Arabidopsis. Nature Communications 7: 13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdanpanah F, Hanson J, Hilhorst HWM, Bentsink L. 2017. Differentially expressed genes during the imbibition of dormant and after-ripened seeds – a reverse genetics approach. BMC Plant Biology 17: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.