Abstract
Background and Aims
Herkogamy, or anther–stigma separation, is known to reduce self-pollen deposition, but little is known about the relative efficacy of different modes or conformations of herkogamy. We assessed the effectiveness of vertical versus lateral herkogamy in preventing or promoting self-pollen deposition in the annual herb Lysimachia arvensis, a plant with lineages that differ in flower colour, and in which flowers first display lateral and then vertical herkogamy. Because mating between the two lineages compromises fitness through the production of low-quality hybrid offspring, we tested the prediction that individuals sampled from sites occupied by both lineages should have flowers that promote autonomous self-pollen deposition and self-fertilization as a result of selection to reduce deleterious reproductive interference.
Methods
We characterized variation in herkogamy within and among 25 pure and mixed populations of L. arvensis in its European range and assessed the effectiveness of lateral versus vertical herkogamy in avoiding self-pollen deposition.
Results
Lateral herkogamy was more effective than vertical herkogamy in limiting self-pollen deposition. In the case of vertical herkogamy, only approach herkogamy was effective. Lineages showed consistent differences in herkogamy traits. In general, angles were smaller for blue than red flowers in most populations, and blue flowers showed approach herkogamy, while red flowers showed predominantly reverse herkogamy. In sympatry, the red lineage showed a reduction of both herkogamy traits while for the blue lineage only lateral herkogamy was reduced.
Conclusions
Our results demonstrate that pollen deposition is affected not only by the degree but also the spatial conformation of herkogamy. They also highlight reduced herkogamy as a potential mechanism for promoting reproductive assurance under pollen limitation, as well as for avoiding reproductive interference between genetically divergent lineages.
Keywords: Anagallis, colour polymorphism, herkogamy, Lysimachia, mating system, reproductive assurance, reproductive isolation, selfing evolution, self-pollen deposition
INTRODUCTION
Mating patterns in plant populations can be strongly influenced by the relative spatial conformation of male and female organs within and among individuals. This applies to the spatial separation of male (staminate) and female (pistillate) flowers in monoecious and dioecious species (known as ‘dicliny’), but particularly to that between the anthers and stigmas of bisexual flowers (‘herkogamy’). Herkogamy limits self-pollination (Brunet and Eckert, 1998; Motten and Stone, 2000; Takebayashi et al., 2006; reviewed in Opedal, 2018), and reduced herkogamy may confer reproductive assurance through a capacity for autonomous self-pollination, which can be advantageous when pollinators are rare or absent (Lloyd, 1992; Eckert et al., 2006). Indeed, the rate of self-fertilization is often high in self-compatible populations in which anthers are close to stigmas, and the experimental exclusion of pollinators has been shown to bring about the rapid evolution of reduced stigma–anther separation (Roels and Kelly, 2011; Brys and Jacquemyn, 2012). Selection on the mating system may thus act directly and quickly through aspects of floral morphology such as herkogamy (Toräng et al., 2017; Opedal et al., 2018).
The ways individuals achieve herkogamy and its effect on self-pollen transfer vary greatly among species. This is particularly striking in heterostylous species, in which anthers are displaced vertically from one another (‘vertical herkogamy’), held either above (‘approach herkogamy’) or below stigmas (‘reverse herkogamy’), or in enantiostylous species, in which anthers are displaced laterally from stigmas to the right or left (‘lateral herkogamy’). Both heterostyly and enantiostyly promote disassortative mating, with pollen transferred less frequently among individuals with the same than with different morphology (Lloyd and Webb, 1992; Barrett et al., 1996, 2000). In species with vertical herkogamy, approach herkogamy is more frequent than reverse (Webb and Lloyd, 1986; Barrett, 2003), although some species show continuous variation in the relative vertical positions of stigmas and anthers, ranging from reverse to approach herkogamy, e.g. Datura stramonium (Motten and Stone, 2000) and Gilia achilleifolia (Takebayashi et al., 2006). Enantiostyly is less common than heterostyly, and monomorphic lateral herkogamy is also relatively rare, e.g. Centaurium species (Brys et al., 2014, 2016). While approach herkogamy appears to be better at reducing self-pollen deposition than reverse herkogamy (Motten and Stone, 2000; Barrett, 2003; Takebayashi et al., 2006), data on self-pollen deposition under reverse herkogamy are not common (Motten and Stone, 2000; Takebayashi et al., 2006). The effectiveness of lateral herkogamy has been rarely reported, but a few species have reported reduced self-pollination in flowers with increased herkogamy, as expected (Brys and Jacquemyn, 2011, 2012). Recently, Toräng et al. (2017) reported a non-standard case of herkogamy in Arabis alpina, whose flowers combine vertical herkogamy and variation in anther orientation. Interestingly, both floral traits in A. alpina covary as a function of pollinator activity, with associated effects on the degree of self-pollen deposition.
The effects of herkogamy can also vary in terms of how it changes over the course of a flower’s life. Such changes can involve not only alterations of the amount of self-pollen deposition over time, but also the mode of self-fertilization and thus the relative costs and benefits of selfing. Three types of autonomous selfing have been recognized: ‘prior selfing’ (selfing before opportunities for outcrossing); ‘competing selfing’ (selfing at the same time as outcrossing, such that self and outcross pollen compete directly); and ‘delayed selfing’ (self-pollination after opportunities for outcrossing have been exhausted) (Lloyd, 1979). These types incur different costs that depend on cross-pollen availability and the impact of inbreeding depression (Lloyd, 1979; Harder and Routley, 2006). Increased selfing may reduce opportunities for pollen export (‘pollen discounting’) as well as ovule availability for outcrossing (‘ovule discounting’; Lloyd, 1992), costs that may be reduced in species with delayed selfing that occurs only after outcrossing has been possible (Lloyd, 1979, 1992; Herlihy and Eckert, 2002; Harder and Routley, 2006; Goodwillie and Weber, 2018). Delayed selfing should be particularly favoured in species with only a single chance to reproduce, such as annuals (Shivanna, 2015). In contrast, under prior and competing selfing, the potential for reproductive success through outcrossing is reduced, and plants incur a cost of pollen and/or ovule discounting (Holsinger et al., 1984; Lloyd, 1992).
While traits such as reduced herkogamy that allow or promote an increased capacity for selfing may evolve in response to selection for reproductive assurance when pollinators or mates are scarce or absent, they can also be the outcome of selection to avoid hybridization with individuals of co-occurring genetically incompatible lineages. In such situations, reduced herkogamy may prevent gene flow between them, thus avoiding potentially deleterious hybridization. For example, an increased capacity for selfing and reproductive divergence between hybridizing populations has been previously reported in closely co-occurring species of Centaurium (Brys et al., 2014), Mimulus (Martin and Willis, 2007; Grossenbacher and Whittall, 2011) and Clerodendrum (Miyake and Inoue, 2003). Variation in floral morphology can thus be a function of both pollinator behaviour and the context of potential interspecific mating and incipient speciation. How such processes might affect selection on different modes of herkogamy has, to our knowledge, never been considered.
The Mediterranean self-compatible annual forb Lysimachia arvensis (Primulaceae) is unusual in presenting both vertical and lateral herkogamy (Jiménez-López et al., 2019a). Flowers show lateral herkogamy when they first open on day 1 as a result of an angular displacement of the stamens from the pistil, but the angle of separation closes on the second and third days, so that anthers end up centrally on the same vertical axis as the pistil and thus potentially showing vertical herkogamy, with anthers above, next to or below the stigma (Jiménez-López et al., 2019a; Fig. 1). The opportunity for self-pollen deposition in L. arvensis may thus vary not only among individuals that differ in the relative lengths of their pistils and/or stamens, but also over time. Both modes of herkogamy in L. arvensis have high heritability, with h2 = 0.843 and 0.635 for lateral and vertical herkogamy, respectively (Jiménez-López et al., 2019a). This variation allows a direct assessment of the distribution of herkogamy within and among populations, based on unmanipulated plants, as well as of its implications for self-pollen deposition. As has been described for Arabis alpina (Toräng et al., 2017), both herkogamy traits in L. arvensis may be selected synergistically through their joint modulation of the capacity for self-pollination.
Fig. 1.
Flowers of the blue and red lineages of Lysimachia arvensis during the first (A, C) and second (B, D) anthesis day.
Lysimachia arvensis also has two different flower colour morphs. Most European populations comprise only plants with red flowers, but plants with blue flowers are found in drier Mediterranean regions, either on their own or mixed with red-flowered plants (Arista et al., 2013). In mixed Mediterranean populations, which are mostly blue-biased, both blue- and red-flowered plants are visited by the same small solitary bees (Ortiz et al., 2015), but pollinators have a clear preference for blue flowers (Ortiz et al., 2015; Jiménez-López et al., 2019b). Red-flowered plants in Mediterranean regions have a higher inbreeding coefficient than blue-flowered plants, or than red-flowered plants in non-Mediterranean regions. This variation corresponds partially with variation in inbreeding depression, with Mediterranean populations showing higher values in red- than in blue-flowered plants (Jiménez-López et al., 2019c). It is not yet known, however, how patterns of herkogamy vary between the two lineages, or what their interactive effects on plant mating might be.
The current taxonomy of L. arvensis recognizes a single species (Pujadas, 1997; Manns and Anderberg, 2009), but individuals with different flower colours likely belong to different incipient biological species. First, they have somewhat different habitat preferences, with the blue-flowered individuals performing better in hot and dry environments and red-flowered individuals preferring wetter sites (Arista et al., 2013). Second, variation in colour in natural populations is largely discrete, with salmon-coloured F1 progeny of parents with different flower colours being uncommon (Jiménez-López et al., 2019b; Supplementary Data Table S1), even in mixed populations, pointing to a degree of prezygotic isolation between the two lineages. Third, although about a quarter of mixed populations show some evidence of sporadic hybridization (Supplementary Data Table S1), experimental crosses indicate that the F2 progeny are either completely sterile or have lower fruit and seed production (F. J. Jiménez-López et al., unpubl. data). Given the clear genetic divergence (Jiménez-López et al., 2019c) and level of apparent isolation between the two colour morphs, we refer to them as the red and blue ‘lineages’ in what follows.
Here, we use the unusual morphological variation in L. arvensis to explore the implications of the mode and degree of herkogamy for both reproductive assurance and the avoidance of reproductive interference between genetically divergent lineages. Specifically, we test the hypothesis that the reduction in herkogamy increases an individual’s capacity for self-fertilization, and we ask whether this capacity is promoted more by lateral or vertical herkogamy. Further, because mating between the two colour morphs reduces the individual reproductive success as a result of the partial sterility of their hybrid progeny, we asked whether patterns of herkogamy in mixed versus pure populations are consistent with selection to avoid inter-lineage pollen transfer (Levin, 1971; Fishman and Wyatt, 1999). Because we noted that pollinator activity is higher on flowers of the blue lineage in mixed populations (Ortiz et al., 2015; Jiménez-López et al., 2019b), we predicted lower levels of herkogamy in individuals of the red lineage in these populations.
MATERIALS AND METHODS
Study populations
We measured flowers in a total of 25 populations of Lysimachia arvensis in both Mediterranean and non-Mediterranean regions of Europe (Supplementary Data Table S2). Populations were visited during the peak of flowering, and each was categorized as belonging to the blue lineage or the red lineage or as a mixed population (red- and blue-flowered plants in the same population). We sampled ten populations from the blue lineage, six from the red lineage and nine mixed populations, with 475 blue- and 430 red-flowered plants sampled in total. Sample sizes varied among populations (from ten to 35 individuals per lineage, mean = 15 for blue-flowered plants and 16 for red-flowered plants) because of variation in population size (Supplementary Data Table S2) and proportion of individuals of each lineage.
Herkogamy traits
We measured traits linked to herkogamy in L. arvensis to assess their role in preventing self-pollination, and to assess variation across populations. All measurements were taken from fresh flowers, starting on their first day of anthesis. We measured the two components of herkogamy in one flower per plant using the software ImageJ, based on photographs taken directly in the field or the glasshouse. To relate herkogamy to self-pollen deposition, individual flowers on plants growing in the glasshouse were photographed in lateral view using a tripod and a graduated scale. To characterize among-population variation in herkogamy for red- and blue-flowered individuals, photographs were taken directly in the field. Here, first-day flowers were cut and photographed in lateral view with a graduated scale. Both herkogamy traits have a high degree of heritability and are expressed similarly under glasshouse and field conditions (Jiménez-López et al., 2019a). Lateral herkogamy was measured as the angle between style and stamens (hereafter ‘style–stamen angle’). We also measured the lengths of stamens and pistils (from the flower base to the centre of anthers or pistils), and we calculated the degree of herkogamy as the difference between pistil and stamen lengths (hereafter ‘stigma–anther displacement’).
Autonomous self-pollen deposition
We measured self-pollen deposition for 59 plants of both the blue and red lineages in a glasshouse in which pollinators were excluded (25 blue-flowered plants from three populations and 34 red-flowered plants from four populations). Plants were chosen with a view to including a wide range of trait variation in our sample. To assess the effect of lateral herkogamy on self-pollen deposition, we sampled a single flower from each plant at the end of its first day of anthesis (first-day flower). To assess the effect of approach or reverse herkogamy on self-pollen deposition, we sampled a second flower from each plant on its second day of anthesis (second-day flower). We stained stigmas from these flowers with aniline blue (Martin, 1959), and counted the number of pollen grains on them under a fluorescence microscope.
Data analysis
To assess herkogamy trait variation among populations and between the two lineages, we performed two-way ANOVAs, with population specified as a random effect and colour as a fixed effect and populations nested within colour. To test whether the two types of herkogamy differed between pure and mixed populations, and whether these differences were associated with colour, we performed a multivariate analysis of variance (MANOVA) using both herkogamy measurements, with colour, population type (pure/mixed) and population size as fixed effects. Subsequently, two-way ANOVAs were carried out for each herkogamy trait to determine whether co-occurrence of lineages affected floral morphology. Here, colour, population type (pure or mixed) and their interaction were used as explanatory variables in the model; population size was excluded from the model, as it was not significant in the previous MANOVA.
To assess the importance of lateral herkogamy in preventing self-pollen deposition, we found that cubic regression provided the best fit to our data, and we report our results accordingly. Self-pollen deposition on stigmas of second-day flowers was estimated as the sum of pollen depositions during the first and second days of anthesis. To ascertain the importance of stigma–anther displacement in preventing self-pollen deposition, we selected second-day flowers whose angle during the previous day was greater than 20°. This selection was based on the results of the cubic regression obtained for first-day flowers, which showed that self-pollen deposition is practically zero when lateral herkogamy is over 20° (see Results section). Our observations indicated a different relationship between self-pollen deposition and herkogamy for flowers displaying approach versus reverse herkogamy. We thus separated the corresponding data into two classes, with measurements of second-day flowers obtained from reverse and approach herkogamy, respectively. While data from reverse herkogamy (displacement <0) did not show any significant relationship between herkogamy and self-pollen deposition, we fitted a significant negative exponential regression between the two variables for flowers with approach herkogamy (displacement >0).
Finally, we determined whether self-pollen deposition among red- and blue-flowered plants growing in pure and mixed populations depended on the two herkogamy traits. Specifically, we inferred ‘predicted self-pollen deposition’ through the use of previously obtained regression curves relating self-pollen deposition to the degree of herkogamy for each herkogamy mode, for both pure and mixed populations. We used generalized linear models to test for differences in predicted self-pollen deposition as a function of the stigma–anther angle and displacement, flower colour and type of population, assuming a Poisson error distribution and a log link function.
RESULTS
Variation in herkogamy traits among populations and lineages
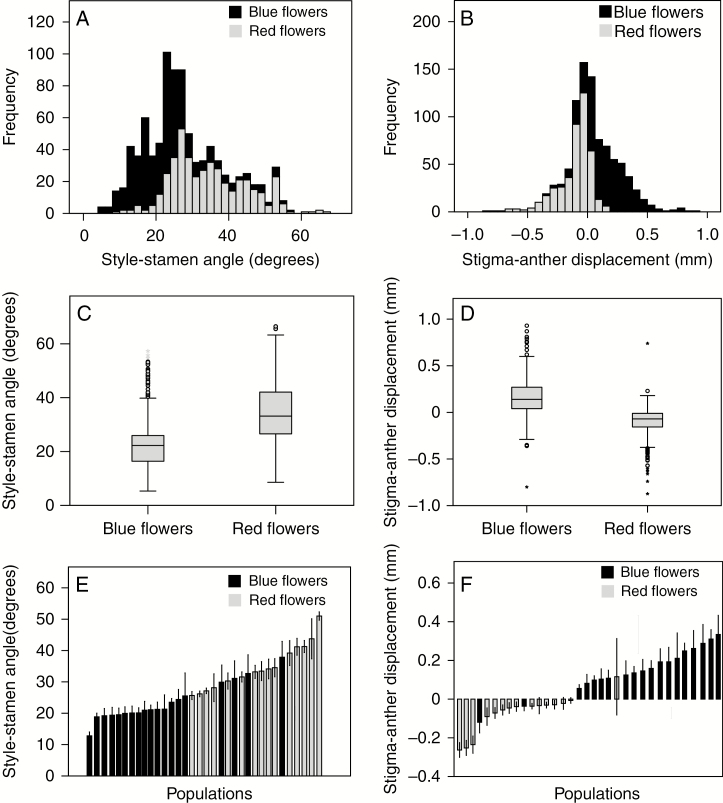
All traits related to herkogamy showed high variation among populations, with the lateral style–stamen angle being the most variable (coefficient of variation 40.7 %; Supplementary Data Table S3). Lateral style–stamen angle varied significantly between the blue and red lineages (F = 513.47, 1 d.f., P < 0.0001) and among populations nested into lineages (F = 22.140, 24 d.f., P < 0.0001; Fig. 2A, C, E). In general, angles were smaller for blue than red flowers in most populations (Fig. 2E). Flowers with angles >20° were found in 59 and 96 % of blue- and red-flowered plants, respectively (Fig. 2A). Vertical stigma–anther displacement also varied significantly between lineages (F = 763.37, 1 d.f., P < 0.0001), and among populations nested into lineages (F = 17.34, 2 d.f., P < 0.0001; Fig. 2B, D, F). In most populations, blue flowers were approach herkogamous, while red flowers were reverse herkogamous or non-herkogamous (Fig. 2F). Indeed, blue flowers showed reverse herkogamy in only two populations, and red flowers showed approach herkogamy in only one population (Fig. 2F). Overall, approach herkogamy was found in 83.8 % of blue flowers, but in only 17.7 % of red ones. Most floral traits were correlated for both colour lineages (Table 1). In blue-flowered plants, lateral style–stamen angle correlated significantly with vertical stigma–anther displacement (R2 = 0.255; Table 1), i.e. the larger the style–stamen angle, the greater the degree of approach herkogamy. In red-flowered plants, however, this correlation was negative, i.e. the larger the style–stamen angle, the greater the degree of reverse herkogamy, though this association was no longer significant after Bonferroni correction (R2 = −0.114; Table 1).
Fig. 2.
Frequency distribution of style–stamen angle in flowers on the first day of anthesis (A) and stigma–anther displacement in flowers on the second day of anthesis (B). Differences between blue and red plants in the style–stamen angle (C) and in stigma–anther displacement (D). Circles represent outliers and asterisks are extreme outliers. Medians, quartiles and ranges of the overall data are shown. (E, F) Means and standard errors of the style–stamen angle (E) and of stigma–anther displacement (F) in each sampled population. Sample sizes: 475 blue flowers from 19 populations and 430 red flowers from 16 populations.
Table 1.
Correlations between all the measured floral traits from the blue (above the diagonal) and red (below the diagonal) lineages of L. arvensis. *P < 0.01 after Bonferroni correction. Means and standard deviations were as follows: (blue flowers) style length, 2.76 ± 0.3 mm; stamen length, 2.6 ± 0.2 mm; style–stamen angle, 22.59 ± 9.3°; stigma–anther displacement; 0.16 ± 0.19 mm; n = 475; (red flowers) style length, 2.31 ± 0.2 mm; stamen length; 2.41 ± 0.2 mm; style–stamen angle, 34.43 ± 10.4°; stigma–anther displacement, −0.1 ± 0.15 mm; n = 430
| Style length | Stamen length | Stigma–anther displacement | Style–stamen angle | |
|---|---|---|---|---|
| Style length | – | 0.755* | 0.551* | 0.220* |
| Stamen length | 0.789* | – | −0.1 | 0.069 |
| Stigma–anther displacement | 0.371* | −0.279* | – | 0.255* |
| Style–stamen angle | −0.385* | −0.322* | −0.114 | – |
MANOVA results showed marked and significant differences in flower morphology between blue- and red-flowered plants in pure versus mixed populations, with a significant interaction between these two factors (Table 2). Flower morphology was not affected by population size, but it was particularly strongly associated with flower colour (Table 2). Interestingly, flowers of both the blue and red lineages had a smaller lateral style–stamen angle in mixed than in pure populations, but stigma–anther displacement only differed in the red lineage. In mixed populations, the stigmas of the red lineage were situated at almost the same level as the anthers, whereas in pure populations they showed marked reverse herkogamy (Table 2, Fig. 3B).
Table 2.
(A) MANOVA between two herkogamy traits: style–stamen angle and stigma–anther displacement as dependent variables and lineage (red or blue), population type (pure or mixed) and their interaction as independent variables. (B) Two-way ANOVAs between each of the two herkogamy traits and lineage, population type and their interaction
| Effect | Wilks’s λ | d.f. | F | P |
|---|---|---|---|---|
| (A) MANOVA | ||||
| Intercept | 0.112 | 2 | 3564.99 | <0.000 |
| Lineage | 0.477 | 2 | 490.71 | <0.000 |
| Population type | 0.963 | 2 | 17.19 | <0.000 |
| Population size | 0.992 | 4 | 1.91 | 0.106 |
| Lineage × population type | 0.966 | 2 | 15.96 | <0.000 |
| (B) Two-way ANOVAs | ||||
| Style–stamen angle | MS | d.f. | F | P |
| Intercept | 734 087.15 | 1 | 7806.53 | <0.000 |
| Lineage | 32 604.8 | 1 | 346.73 | <0.000 |
| Population type | 2894.69 | 1 | 30.78 | <0.000 |
| Lineage × population type | 16.867 | 1 | 0.18 | 0.672 |
| Error | 94.035 | 901 | ||
| Stigma–anther displacement | MS | d.f. | F | P |
| Intercept | 0.531 | 1 | 18.96 | <0.000 |
| Lineage | 16.485 | 1 | 589.02 | <0.000 |
| Population type | 0.589 | 1 | 21.05 | <0.000 |
| Lineage × population type | 1.234 | 1 | 44.10 | <0.000 |
| Error | 0.028 | 901 |
Fig. 3.
Style–stamen angle (A) and stigma–anther displacement (B) of blue and red lineages of L. arvensis in pure and mixed populations. Median, quartiles, maximum and minimum are shown. Different letters (a, b) indicate significant differences for the trait between pure and mixed populations of each lineage. Circles represent outliers and asterisks are extreme outliers.
Association between herkogamy traits and autonomous self-pollination
Self-pollen deposition varied non-linearly with both measures of herkogamy, with lateral herkogamy particularly effective at reducing self-pollen deposition. Specifically, self-pollen deposition decreased from 15 pollen grains for first-day flowers with angles of about 10° to effectively zero for those flowers with angles >20° (Fig. 4A). Vertical stigma–anther displacement for second-day flowers was only effective in preventing self-pollen deposition when the stigma was above the anthers, i.e. in approach-herkogamous flowers (Fig. 4B). In contrast, self-pollen deposition was highly variable for flowers displaying reverse herkogamy, with no dependence on stigma–anther separation (Fig. 4B).
Fig. 4.
Relationship between (A) style–stamen angle and self-pollen deposition in flowers on the first day of anthesis, and (B) stigma–anther displacement and self-pollen deposition in flowers on the second day of anthesis in L. arvensis.
DISCUSSION
Mating effects of lateral versus vertical herkogamy
We found that although both types of herkogamy reduced autonomous self-pollination in L. arvensis, lateral herkogamy was more effective, with no self-pollen deposition observed for flowers with a lateral displacement angle >20°. This result suggests that these flowers thus had little capacity for autonomous self-pollination on their first day of opening. The marked lateral separation between style and anthers in these flowers likely also limits self-pollination even when their pollinators, largely small bees (Ortiz et al., 2015), are collecting pollen. By contrast, on the second day, after movement of the style into a perpendicular position, autonomous self-pollination was more likely, particularly in flowers in which the stigma was at the same level as the anthers, or in reverse-herkogamous flowers.
Although we did not evaluate the effect of herkogamy on the mating system, it is likely that its influence on self-pollen deposition affects selfing rates in wild populations. Covariation between herkogamy and the mating system has been reported for other species with either lateral (Brys and Jacquemyn, 2011) or vertical herkogamy (Motten and Stone, 2000; Takebayashi et al., 2006). More specifically, differences in the roles of approach and reverse herkogamy in controlling self-pollen deposition have been suggested previously (Webb and Lloyd, 1986; Barrett and Shore, 1987; Barrett, 2003). In species with stigmas placed always above the anthers, outcrossing typically increases with herkogamy (Brunet and Eckert, 1998; Herlihy and Eckert, 2007; but see Medrano et al., 2005). However, the role of herkogamy when it ranges from reverse to approach has been less studied and would not necessarily reduce selfing (Kulbaba and Worley, 2008). Our results for L. arvensis are similar to those for Datura stramonium (Motten and Stone, 2000) and Gilia achilleifolia (Takebayashi et al., 2006), in which outcrossing was favoured when the stigma was held above the anthers, but not when it was below them. In L. arvensis, flowers with stigmas at the same level as the anthers or below them received up to 35 pollen grains by autonomous self-pollination. A stigmatic pollen load of two to six grains per ovule has been shown to ensure full seed-set ranges in other species (Cruden, 1977; Shore and Barrett, 1984; Aizen and Harder, 2007), although pollen quality can also limit seed-set (Aizen and Harder, 2007). If the same requirement applies to L. arvensis, in which flowers have between 17 and 30 ovules (Arista et al., 2013; Ortiz et al., 2015), our results suggest that as many as half of the ovules could be fertilized as a result of delayed autonomous selfing facilitated by reverse herkogamy on the second day of anthesis (Lloyd and Schoen, 1992), even though seed-set may not be complete.
Differences in herkogamy between populations and flower colour
The blue and red lineages of L. arvensis differed for both herkogamy traits across the sampled populations. On the one hand, red flowers showed strong lateral herkogamy on the first day, with style–stamen angles generally >20°, but largely either no herkogamy or reverse herkogamy on the second day. These results strongly implicate a strategy of delayed selfing in red-flowered plants (when pollinators are abundant, they are likely to be largely outcrossed on the first day of opening, but they may self-pollinate on their second day). On the other hand, flowers of the blue lineage showed much greater variation in the degree of lateral herkogamy on their first day of opening, with flowers in about half of the studied plants and populations having stigma–stamen angles <20°, therefore being susceptible to some autonomous self-pollination, whereas most blue flowers showed substantial approach herkogamy on the second day of anthesis. While blue-flowered plants with angles of lateral herkogamy >20° thus appear to be incapable of autonomous self-pollination on both days of opening, those with angles <20° (and which display lower approach herkogamy too) likely self-pollinate throughout anthesis, via competing selfing (Lloyd, 1979; Leclerc-Potvin and Ritland, 1994). The mating system of the blue-flowered lineage is thus likely to be more variable than that of the red-flowered lineage.
We do not know the evolutionary basis or history of the floral and likely mating-system differences between the red and blue lineages. However, the observed variation in herkogamy traits, particularly of the blue-flowered lineage of L. arvensis, hints at possible interpopulation differences in the ecological context of mating. For instance, some populations of the blue lineage may be subject to more frequent cross-pollen limitation and thus selection for reproductive assurance, which might not be an important force in other populations. Variation in herkogamy might also reflect spatial variability in the expression of inbreeding depression (e.g. Pujol et al., 2009). Models that incorporate ecological factors, such as cross-pollen availability, gamete discounting (Johnston et al., 2009) or temporal variability in the expression of inbreeding depression (Cheptou and Schoen, 2002), help to account for mixed mating in plants, which is otherwise difficult to explain (Lande and Schemske, 1985; Jarne and Charlesworth, 1993; Holsinger, 1996), and it is possible that such variation plays a role in maintaining mixed mating in L. arvensis.
The lower levels of herkogamy that we found for the red lineage in mixed populations of L. arvensis may reflect either selection for reproductive assurance or selection to avoid mating with the blue morphs in these populations (or both factors). Given that the red lineage of L. arvensis tends to receive fewer pollinator visits than the blue lineage in mixed populations (Ortiz et al., 2015; Jiménez-López et al., 2019b), its lower herkogamy may be a response to stronger selection for reproductive assurance. However, we also found lower lateral herkogamy for individuals of the blue lineage in mixed populations. This finding suggests that the blue lineage may have a greater capacity for selfing in mixed populations, perhaps reflecting selection to avoid the deleterious effects of hybridization with the red lineage. Evolution towards a greater capacity for selfing has been interpreted as a mechanism to avoid reproductive interference between a number of other co-occurring species or divergent lineages in a number studies, e.g. Phlox (Levin, 1985), Arenaria (Fishman and Wyatt, 1999), Ipomoea (Smith and Rausher, 2008), Centaurium (Brys et al., 2014) and Mimulus (Martin and Willis, 2007; Grossenbacher and Whittall, 2011). Such reductions in gene flow between two lineages via enhanced prezygotic isolation should ultimately allow further genetic divergence and the evolution of postzygotic isolation (Coyne and Orr, 2004), and may have been a component contributing to the current pattern of divergence between the red and blue lineages of L. arvensis.
If the observed variation in floral behaviour displayed by the two L. arvensis lineages affects the mating system in the manner we have suggested, we might expect to find associated variation in inbreeding coefficients among populations. This association remains to be studied in detail, but those data that do exist are not entirely consistent with expectations. Of the various combinations of lateral versus vertical herkogamy, only those that involve low lateral and approach herkogamy imply the promotion of predominant prior or competing selfing; this pattern was observed mainly for the blue lineage in mixed populations with the red. Accordingly, the decrease of approach herkogamy in the blue lineage in mixed populations was associated with a significant increase in the predicted self-pollen deposition, and estimates of inbreeding depression in populations of the blue lineage are low (0.19–0.36; Jiménez-López et al., 2019c), as might be expected for a largely selfing lineage (Jiménez-López et al., 2019c). In contrast, the decrease in herkogamy observed for the red lineage did not correspond to greater predicted self-pollen deposition. Moreover, the red lineage showed relatively high coefficients of inbreeding in mixed populations (Jiménez-López et al., 2019c), pointing to likely higher selfing rates in these populations in particular, and consistent with the possibility that selection for reduced interference between lineages has mainly affected the red lineage. Of course, the high values of inbreeding depression estimated for Mediterranean populations of the red lineage (0.61–0.65; Jiménez-López et al., 2019c) should mitigate any advantage of reduced reproductive interference due to selfing in these populations (Delmas et al., 2014).
Conclusions
Our study has uncovered an unusual combination of dynamic lateral and vertical modes of herkogamy in an annual plant with two genetically divergent lineages that have different floral colours and experience different levels of attractiveness to pollinators. The conformation, extent and timing of herkogamy is partially consistent with expectations for populations that vary in their susceptibility to pollinator limitation. At the same time, the extent to which these patterns vary between pure and mixed populations is consistent with selection, on one of the two interacting lineages, to avoid reproductive interference between them via increased self-fertilization. Future work should establish the details of within- versus between-lineage mating in mixed populations, as well as its fitness implications and thus the direction and strength of selection on the floral morphology and behaviour. Certainly, the morphological and genetic divergence between the red and blue lineages of L. arvensis offers scope to test several general hypotheses concerning selection on traits that affect patterns of plant mating within populations and gene flow between diverging lineages.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: mixed populations of Lysimachia arvensis sampled with presence or absence of the hybrid phenotype. Table S2: list of studied populations of Lysimachia arvensis.Table S3: variance components among populations for the four measured traits.
ACKNOWLEDGEMENTS
We thank Regina Berjano for helping in sample collection and Servicios Generales de Herbario e Invernadero de la Universidad de Sevilla for logistical support.
FUNDING
This work was supported by the European Regional Development Fund (ERDF) and grants from the Spanish Ministerio de Economia y Competitividad (MINECO) to M.A. and P.L.O. (CGL2012-33270, CGL2015-63827) and to F.J.J.-L. (BES-2013–062859, EEBB-C-15-00672) and from the Swiss National Science Foundation and the University of Lausanne to J.R.P.
LITERATURE CITED
- Aizen MA, Harder LD. 2007. Expanding the limits of the pollen-limitation concept: effects of pollen quantity and quality. Ecology 88: 271–281. [DOI] [PubMed] [Google Scholar]
- Arista M, Talavera M, Berjano R, Ortiz PL. 2013. Abiotic factors may explain the geographical distribution of flower colour morphs and the maintenance of colour polymorphism in the scarlet pimpernel. Journal of Ecology 101: 1613–1622. [Google Scholar]
- Barrett SCH. 2003. Mating strategies in flowering plants: the outcrossing–selfing paradigm and beyond. Philosophical Transactions of the Royal Society B: Biological Sciences 358: 991–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett SCH, Shore JS. 1987. Variation and evolution of breeding systems in the Turnera ulmifolia L. complex (Turneraceae). Evolution 41: 340–354. [DOI] [PubMed] [Google Scholar]
- Barrett SC, Lloyd DG, Arroyo J. 1996. Stylar polymorphisms and the evolution of heterostyly in Narcissus (Amaryllidaceae). In Lloyd DG, Barret SCH, eds. Floral biology: studies on floral evolution in animal-pollinated plants. Boston, MA: Springer, 339–376. [Google Scholar]
- Barrett SC, Jesson LK, Baker AM. 2000. The evolution and function of stylar polymorphisms in flowering plants. Annals of Botany 85: 253–265. [Google Scholar]
- Brunet J, Eckert CG. 1998. Effects of floral morphology and display on outcrossing in blue columbine, Aquilegia caerulea (Ranunculaceae). Functional Ecology 12: 596–606. [Google Scholar]
- Brys R, Jacquemyn H. 2011. Variation in the functioning of autonomous self-pollination, pollinator services and floral traits in three Centaurium species. Annals of Botany 107: 917–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brys R, Jacquemyn H. 2012. Effects of human‐mediated pollinator impoverishment on floral traits and mating patterns in a short‐lived herb: an experimental approach. Functional Ecology 26: 189–197. [Google Scholar]
- Brys R, Cauwenberghe J, Jacquemyn H. 2016. The importance of autonomous selfing in preventing hybridization in three closely related plant species. Journal of Ecology 104: 601–610. [Google Scholar]
- Brys R, Vanden Broeck A, Mergeay J, Jacquemyn H. 2014. The contribution of mating system variation to reproductive isolation in two closely related Centaurium species (Gentianaceae) with a generalized flower morphology. Evolution 68: 1281–1293. [DOI] [PubMed] [Google Scholar]
- Cheptou PO, Schoen DJ. 2002. The cost of fluctuating inbreeding depression. Evolution 56: 1059–1062. [DOI] [PubMed] [Google Scholar]
- Coyne JA, Orr HA. 2004. Speciation. Sunderland, MA: Sinauer Associates. [Google Scholar]
- Cruden RW. 1977. Pollen-ovule ratios: a conservative indicator of breeding systems in flowering plants. Evolution 31: 32–46. [DOI] [PubMed] [Google Scholar]
- Delmas CE, Cheptou PO, Escaravage N, Pornon A. 2014. High lifetime inbreeding depression counteracts the reproductive assurance benefit of selfing in a mass-flowering shrub. BMC Evolutionary Biology 14: 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert CG, Samis KE, Dart S. 2006. Reproductive assurance and the evolution of uniparental reproduction in flowering plants. In Harder LD, Barrett SCH, eds. Ecology and evolution of flowers. Oxford: Oxford University Press, 183–203. [Google Scholar]
- Fishman L, Wyatt R. 1999. Pollinator-mediated competition, reproductive character displacement, and the evolution of selfing in Arenaria uniflora (Caryophyllaceae). Evolution 53: 1723–1733. [DOI] [PubMed] [Google Scholar]
- Goodwillie C, Weber JJ. 2018. The best of both worlds? A review of delayed selfing in flowering plants. American Journal of Botany 105: 641–655. [DOI] [PubMed] [Google Scholar]
- Grossenbacher DL, Whittall JB. 2011. Increased floral divergence in sympatric monkeyflowers. Evolution 65: 2712–2718. [DOI] [PubMed] [Google Scholar]
- Harder LD, Routley MB. 2006. Pollen and ovule fates and reproductive performance by flowering plants. In Harder LD, Barrett SCH, eds. Ecology and evolution of flowers. Oxford: Oxford University Press, 61–80. [Google Scholar]
- Herlihy CR, Eckert CG. 2002. Genetic cost of reproductive assurance in a self-fertilizing plant. Nature 416: 320–323. [DOI] [PubMed] [Google Scholar]
- Herlihy CR, Eckert CG. 2007. Evolutionary analysis of a key floral trait in Aquilegia canadensis (Ranunculaceae): genetic variation in herkogamy and its effect on the mating system. Evolution 61: 1661–1674. [DOI] [PubMed] [Google Scholar]
- Holsinger KE. 1996. Pollination biology and the evolution of mating systems in flowering plants. Evolutionary Biology 29: 107–149. [Google Scholar]
- Holsinger KE, Feldman MW, Christiansen FB. 1984. The evolution of self-fertilization in plants: a population genetic model. American Naturalist 124: 446–453. [Google Scholar]
- Jarne P, Charlesworth D. 1993. The evolution of the selfing rate in functionally hermaphrodite plants and animals. Annual Review of Ecology, Evolution and Systematics 24: 441–466. [Google Scholar]
- Jiménez-López FJ, Arista M, Talavera M, Pannell JR, Ortiz PL. 2019a Heritabilities of lateral and vertical herkogamy in Lysimachia arvensis. Plant Species Biology 34: 31–37. [Google Scholar]
- Jiménez-López FJ, Matas L, Arista M, Ortiz PL. 2019b Flower colour segregation and flower discrimination under the bee vision model in the polymorphic Lysimachia arvensis. Plant Biosystems. doi: 10.1080/11263504.2019.1651776. [DOI] [Google Scholar]
- Jiménez-López FJ, Ortiz PL, Talavera M, Arista M. 2019c Selfing maintains flower colour polymorphism in L. arvensis despite high inbreeding depression. bioRxiv. doi: 10.1101/761122. [DOI] [Google Scholar]
- Johnston MO, Porcher E, Cheptou PO, et al. 2009. Correlations among fertility components can maintain mixed mating in plants. American Naturalist 173: 1–11. [DOI] [PubMed] [Google Scholar]
- Kulbaba MW, Worley AC. 2008. Floral design in Polemonium brandegei (Polemoniaceae): genetic and phenotypic variation under hawkmoth and hummingbird pollination. International Journal of Plant Sciences 169: 509–522. [Google Scholar]
- Lande R, Schemske DW. 1985. The evolution of self-fertilization and inbreeding depression in plants. I. Genetic models. Evolution 39: 24–40. [DOI] [PubMed] [Google Scholar]
- Leclerc-Potvin C, Ritland K. 1994. Modes of self-fertilization in Mimulus guttatus (Scrophulariaceae): a field experiment. American Journal of Botany 81: 199–205. [Google Scholar]
- Levin D. 1971. The origin of reproductive isolating mechanisms in flowering plants. Taxon 21: 91–113. [Google Scholar]
- Levin DA. 1985. Reproductive character displacement in Phlox. Evolution 39: 1275–1281. [DOI] [PubMed] [Google Scholar]
- Lloyd DG. 1979. Some reproductive factors affecting the selection of self fertilization in plants. American Naturalist 113: 67–79. [Google Scholar]
- Lloyd DG. 1992. Self-and cross-fertilization in plants. II. The selection of self-fertilization. International Journal of Plant Sciences 153: 370–380. [Google Scholar]
- Lloyd DG, Schoen DJ. 1992. Self-and cross-fertilization in plants. I. Functional dimensions. International Journal of Plant Sciences 153: 358–369. [Google Scholar]
- Lloyd DG, Webb CJ. 1992. The evolution of heterostyly. In: Barrett SCH, ed. Evolution and function of heterostyly. Berlin: Springer, 151–178. [Google Scholar]
- Manns U, Anderberg AA. 2009. New combinations and names in Lysimachia (Myrsinaceae) for species of Anagallis, Pelletiera and Trientalis. Willdenowia 39: 49–54. [Google Scholar]
- Martin FW. 1959. Staining and observing pollen tubes in the style by means of fluorescence. Stain Technology 34: 125–128. [DOI] [PubMed] [Google Scholar]
- Martin NH, Willis JH. 2007. Ecological divergence associated with mating system causes nearly complete reproductive isolation between sympatric Mimulus species. Evolution 61: 68–82. [DOI] [PubMed] [Google Scholar]
- Medrano M, Herrera CM, Barrett SC. 2005. Herkogamy and mating patterns in the self-compatible daffodil Narcissus longispathus. Annals of Botany 95: 1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake T, Inoue K. 2003. Character displacement in style length between pollinator-sharing Clerodendrum trichotomum and C. izuinsulare (Verbenaceae). Plant Systematics and Evolution 243: 31–38. [Google Scholar]
- Motten AF, Stone JL. 2000. Heritability of stigma position and the effect of stigma-anther separation on outcrossing in a predominantly self-fertilizing weed, Datura stramonium (Solanaceae). American Journal of Botany 87: 339–347. [PubMed] [Google Scholar]
- Opedal OH. 2018. Herkogamy, a principal functional trait of plant reproductive biology. International Journal of Plant Sciences 179: 677–687. [Google Scholar]
- Ortiz PL, Berjano R, Talavera M, Rodríguez-Zayas L, Arista M. 2015. Flower colour polymorphism in Lysimachia arvensis: how is the red morph maintained in Mediterranean environments? Perspectives in Plant Ecology, Evolution and Systematics 17: 142–150. [Google Scholar]
- Pujadas AJ. 1997. Anagallis. In: Castroviejo S, Aedo C, Laínz M, et al. , eds. Flora iberica, Vol. 5. Madrid: Jardín Botánico de Madrid, 57–62. [Google Scholar]
- Pujol B, Zhou SR, Sanchez Vilas J, Pannell JR. 2009. Reduced inbreeding depression after species range expansion. Proceedings of the National Academy of Sciences of the USA 106: 15379–15383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roels SA, Kelly JK. 2011. Rapid evolution caused by pollinator loss in Mimulus guttatus. Evolution 65: 2541–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivanna KR. 2015. Reproductive assurance through autogamous self-pollination across diverse sexual and breeding systems. Current Science 109: 1255–1263. [Google Scholar]
- Shore JS, Barrett SC. 1984. The effect of pollination intensity and incompatible pollen on seed set in Turnera ulmifolia (Turneraceae). Canadian Journal of Botany 62: 1298–1303. [Google Scholar]
- Smith RA, Rausher MD. 2008. Selection for character displacement is constrained by the genetic architecture of floral traits in the ivyleaf morning glory. Evolution 62: 2829–2841. [DOI] [PubMed] [Google Scholar]
- Takebayashi N, Wolf DE, Delph LF. 2006. Effect of variation in herkogamy on outcrossing within a population of Gilia achilleifolia. Heredity 96: 159–165. [DOI] [PubMed] [Google Scholar]
- Toräng P, Vikström L, Wunder J, Wötzel S, Coupland G, Ågren J. 2017. Evolution of the selfing syndrome: anther orientation and herkogamy together determine reproductive assurance in a self-compatible plant. Evolution 71: 2206–2218. [DOI] [PubMed] [Google Scholar]
- Webb CJ, Lloyd DG. 1986. The avoidance of interference between the presentation of pollen and stigmas in angiosperms. II. Herkogamy. New Zealand Journal of Botany 24: 163–178. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.