Abstract
Background and Aims
The Piedra Chamana fossil forest in northern Peru is an assemblage of angiosperm woods and leaves preserved in volcaniclastic rocks dated to 39 Mya (late Middle Eocene). We analysed the anatomical and morphological features of the fossils to reconstruct the palaeoenvironment during this time of global warmth, taking advantage of the co-occurrence of woods and leaves to compare different proxies and analytical approaches.
Methods
Wood characters analysed include vessel-related functional traits, traits linked to Baileyan trends, and quantitative features such as vessel diameter and density. Diameter-distribution and diameter and position plots are used to represent vessel diameter and arrangement. Leaf margin and area analysis provides additional climate estimates.
Key Results
The fossil woods show many similarities with modern tropical-forest woods and tropical fossil-wood assemblages; closest correspondence within the Neotropics is to semi-deciduous lowland tropical forest with moderate precipitation (~1000–1200 mm). Features unusual for the modern South American tropics are mainly vessel-related characters (semi-ring porosity, grouped vessels, helical vessel thickenings, short vessel elements) linked to water stress or seasonal water availability. Leaf analysis indicates mean annual temperature of 31 °C (n = 19, 100 % entire-margined) and mean annual precipitation of 1290 mm (n = 22, predominantly microphylls and notophylls).
Conclusions
The palaeovegetation was clearly lowland tropical forest with a dry aspect, but anomalous aspects of the wood anatomy are consistent with the high temperatures indicated by the leaves and are probably explained by differences in seasonality and water stress compared to the present-day Neotropics. A close modern analogue may be in very seasonal regions of Asia. Pronounced monsoonal (summer-rain) conditions may relate to a location (palaeolatitude of 13°S) outside the near-equatorial tropics.
Keywords: Fossil forest, petrified wood, northern Peru, Eocene climate, Neotropical forest history
INTRODUCTION
The Piedra Chamana petrified forest is located in the Cordillera Occidental of northern Peru near the small village of Sexi. Fossil woods and leaves, occurring in volcanic rocks of the Huambos Formation (39 Mya; Woodcock et al., 2009; Navarro et al., 2012), add to the sparse fossil plant record of the New World tropics during the Palaeogene and provide information about the forest and palaeoenvironmental history of South America. The fossils (all monocots or eudicots) record the vegetation of lowland South America in the late middle Eocene, before the rise of the present-day Andes and relatively early in the history of the modern angiosperm-dominated forests of the New World. At this time, the Earth’s climate was transitioning from the global warmth of the late Palaeocene/early Eocene to cooler conditions and ice sheet growth beginning at the end of the Eocene but was also variable, with periods of warming (middle Eocene Climate Optimum, from 40.5 to 40 Mya) superimposed on the general cooling trend (Baatsen et al., 2016, 2018).
This study is a contribution toward the development of fossil wood as a source of information about terrestrial palaeoecology and environments. Interpretations are based on characterization of the anatomical features of the woods, particularly vessel-related features, and identification of wood functional types/ecologically significant anatomical features. Detailed descriptions and identifications of the woods are presented elsewhere (Woodcock et al., 2017, 2019). The focus here is on the ecological information expressed in the anatomical and morphological characteristics of the fossils independent of taxonomic considerations. In addition to analysis of the wood anatomy, we also provide climate estimates based on analysis of the leaves.
BACKGROUND
The Piedra Chamana fossil forest is located in central Cajamarca (6°35′S, 79°10′E), near the village of Sexi at an elevation of ~2500 m (Woodcock et al., 2009). The present vegetation at the site is evergreen sclerophyllous forest (Aragon et al., 2006; Aragon and Woodcock, 2010).
The sequence of events that preserved the fossils began ~39 Mya when an erupting volcano buried the forest in volcanic ash to a depth of ~1 m. Subsequently, a volcanic flow that has variously been interpreted as a lahar (Woodcock et al., 2009) or pyroclastic flow (Navarro et al., 2012) moved through the area, breaking off the forest trees and transporting material from upslope that is preserved in the unit overlying the ash fall. This unit was emplaced not long after the ash fall and was a low-energy flow transporting material from nearby upslope locations.
The age determination is based on 40Ar/39Ar dates of 39.35 ± 0.21 Mya on plagioclase from the ash layer and 39.52 ± 0.11 on sanidine in an underlying ignimbrite (Woodcock et al., 2009) and is consistent with earlier dates of 39 ± 1.0 for the basal member of the Huambos Formation (Noble et al., 1990). The fossiliferous rocks include the ashfall deposit containing permineralized angiosperm wood and monocot stems and leaf impression fossils and the overlying volcanic flow containing permineralized woods and stems (Fig. 1). Palaeosols underlying the ash layer are formed on volcaniclastic sediments and show indications of fluvial bedding (poorly sorted beds thinning upsection; Woodcock et al., 2009; Terry et al., 2019) that could relate to an unstable volcanic substrate or a basin-fill setting.
Fig. 1.
(A) Logs in situ in the volcanic flow. (B,C) Leaves excavated from the ashfall deposit (MUSM-PB-200B, -147B). The hole in C may represent the impression of an accretionary lapilli. (D) Palm stems showing persistent leaf bases. (E,F) Monocot leaves (MUSM-PB-229, -107). Scales are in millimetres.
The fossil site includes an extensive volume of plant fossils that have weathered out of the flow deposit or are still encased within it. The fossils in the ashfall can be observed at several locations where this underlying deposit is exposed and trees extend upright through the ash layer in growth position, in many cases appearing rooted in a basal palaeosol. The woods in the overlying unit, which are better preserved than those associated with the ash fall and palaeosols, make up the majority of the specimens described here; however, we have identified taxa common to both and consider the entire assemblage as representing vegetation growing within an area of not more than a few square kilometres. The random dispersal and orientation of leaf fossils in the ashfall layer suggest that the leaves were stripped from trees as a consequence of the ash fall and deposited in the immediate vicinity.
All of the represented taxa are angiosperms (Fig. 1). Woods and leaves of eudicots dominate the assemblage. The largest wood specimen is a trunk 75 cm in diameter and 10 m long, although most of the long specimens (7–11 m) have diameters ≤ 40 cm. The monocot material includes stems, petioles and leaves and shows a considerable amount of morphological diversity, including columnar, rhizomatous and climbing forms. The monocots have not to date been extensively sampled and are undoubtedly present at a higher diversity than presently known. The largest monocot stems measure 22–26 cm in diameter.
The fossil site is in a low region of the Andean chain with highest elevations in the region ~3500 m (Amotape-Huancabamba Biogeographic Zone; Young and Reynel, 1997; Weigend et al., 2005). The rise of the Andes is linked to delamination of the Pacific plate and associated tectonism that produced the unusually high elevations (to nearly 7000 m) characterizing the chain and associated high plateaus (Garzione et al., 2008). Although estimates vary, uplift of the Andean chain did not begin prior to 40 Mya (Poulsen et al., 2010). At the time the fossil forest was growing, elevations along the western, active margin of the continent are estimated at no more than one-third of present values (Gregory-Wodzicki, 2000; Graham et al., 2001; Garzione et al., 2008). South America has drifted north since this time in the Eocene. The palaeolatitude for the fossil forest is estimated at ~13°S (Smith et al., 1994).
MATERIALS AND METHODS
Woods
Preliminary typing of the specimens was carried out in the field using high-magnification hand lenses and small microscopes with the goal of collecting a minimum of two specimens of each type. The total number of wood specimens collected was 113, with 78 of these sectioned in part or in whole. Microscopic analysis of the woods led to identification of the 30 wood types analysed here (Woodcock et al., 2017, 2019). Palaeoenvironmental interpretations made from the woods are based on: (1) analysis of wood functional–anatomical features relating to vessel diameter and arrangement – we include here a new way of representing radial variations in vessel diameter and density; (2) evaluation of the woods with respect to other functional–anatomical traits and traits linked to the Bailyean trends (Wheeler and Baas, 2019); and (3) analysis of quantitative wood characters including vessel diameter and density, estimated specific gravity, vessel-element length and intervessel pit diameter. Although growth rings occur in tropical trees, this feature is not considered because determinations as to presence/absence rely on a variety of anatomical features and can be ambiguous and environmental correlates are not clear-cut (Tarelkin et al., 2016).
Anatomical measurements and descriptions follow IAWA guidelines (IAWA Committee, 1989). Designations relating to porosity rely, to the extent possible, on determinations made by the first author based on wood slides or photographs of transverse sections. Vessel diameter-and-position plots show the variation in size and arrangement of the vessels in the radial direction (i.e. along the axis of growth). To produce these plots, all vessels were measured and their position was plotted within a 0.4-mm-wide area extending radially (Fig. 2). The length of the measurement area was 4 mm except in cases where a larger area was needed to show the radial variation present (as in the semi-ring-porous woods). This is a broader area than that typically observable within the transverse field of view at low power. Vessels in multiples were measured and counted individually. Where vessels deviated from circular in outline, the short and long axes were measured to give an average diameter; this choice was made based on what seemed optimal for representing variability within the wood and the finding that tangential diameter alone did not give as good a graphical representation of the variability. Vessel diameter-distributions provide a statistical representation of the variation in vessel diameter. These distributions were generated from the above measurements or, in the case of woods with low vessel density, were based on measurements of all vessels within a 4-mm-wide area up to a total of 60 vessels. Measurements included all the vessels within a given area because randomization of measurements tends to normalize distributions and can obscure significant aspects of the distribution. Bin size was standardized across specimens for the purposes of comparison, and the Shapiro–Wilk W test (Shapiro and Wilk, 1965) was used to test for significant deviations from normality. Only in the case of a significantly non-normal distribution were aspects of the distribution such as skewness or occurrence of outliers or secondary modes considered meaningful. Checks were made to ensure that patterns were consistent within specimens.
Fig. 2.
Orientation and dimensions of the measurement area for the diameter-and-position plots.
Specific gravity was estimated using the point-count method (Wheeler et al., 2007b). This technique involves superposing a grid on a transverse section and counting the number of points intersecting with the cell walls, which have a standard specific gravity of 1.05; 300 counts were done per specimen. Estimates could not be obtained for all specimens due to preservation.
The characterization of tropical forest types, based on mean annual precipitation (MAP), is as follows: very dry tropical (VDT) forest/woodland/scrub (MAP < 600–900 mm), dry tropical (DT) forest (MAP 900–1700 mm), wet tropical (WT) forest (MAP 1700–3500 mm) and very wet tropical (VWT) forest (MAP > 3500 mm).
Information used in the comparative anatomical analyses (and in the extended discussion of ring porosity) came from a variety of sources: (1) The published literature, including studies of woods from Venezuelan VDT forest [19 spp.; MAP 558 mm, mean annual temperature (MAT) 26 °C, 7 dry months; Lindorf, 1994] and Brazilian forest trees (semi-humid to superhumid regions of Brazil, 491 spp.; Alves and Angyalossy-Alfonso, 2000). (2) Data supplied by Hugo Martinez-Cabrera (Martinez-Cabrera and Cevallos-Ferriz, 2008) for the following floras: Chamela, Mexico, VDT forest (19°30′N, 800 mm, 25 °C, 9 months < 100 mm, 62 spp.); Oculian, Mexico, montane forest (19°N, 1310 mm, 16 °C, 27 spp.); Manaus, Brazil, WT forest (3°S, 2275 mm, 27 °C, 3 months < 100 mm, 25 spp.); Porto Velho, Brazil, WT Forest (8°S, 2360 mm, 25 °C, 3 months < 100 mm, 25 spp.); Tafelberg, Surinam, WT Forest (3°N, 3000 mm, 3 months < 100 mm, 25 spp.); and Los Tuxlas, Mexico, WT forest (18°N, 4600 mm, 23 °C, 1 month < 100 mm, 55 spp.). (3) Wood slide collections available to the first author, including material from Kanepu‘u Hawai‘i, equable VDT forest/shrubland (20°N, 600 mm, 22 °C, 9 months < 100 mm, 16 spp.; D.W.W., unpublished); NW Costa Rica, DT Forest (11°N, 1650 mm, 27 °C, 5 months < 100 mm, 63 spp.; Woodcock, 1994); and Tambopata, Peru, WT Forest (13°S, 2400 mm, 23 °C, 3 months < 100 mm; 74 spp.; Woodcock, et al., 2000). And (4) anatomical information available in online databases (InsideWood, 2004-onwards; Richter and Dallwitz, 2000-onwards). We provide species lists for the wood floras where available (Supplementary Data). Information on distribution of taxa is from sources including InsideWood (2004-onwards), Richter and Dallwitz (2000-onwards), the Global Biodiversity Information Facility (N.d.), Burns and Honkala (1990), and Orwa et al. (2009).
Leaves
The leaves, mainly eudicots but including some monocots, were recovered from the ashfall deposit at one location (UTM 17M 717078 × 9273638, datum WGS84) where the ash was less indurated and the leaves appeared better preserved and were present in greater abundance than elsewhere.
Characters of leaf morphology and architecture were used to differentiate morphotypes, which are equivalent to distinct species but not classified taxonomically. A total of 227 leaf specimens were examined, and 136 of those could be provisionally assigned to 23 morphotypes. The remaining 91 specimens were not adequately preserved for assignment to morphotype. Ten leaf specimens showed anatomical and morphological characters typical of monocots. The site remains undercollected for documenting the full diversity of leaf morphotypes in the assemblage, and additional collecting would undoubtedly produce additional morphotypes. Leaves within each morphotype were scored for size class and the presence or absence of teeth. Limitations in the quality of preservation in some specimens limited the analysis to 22 non-monocot morphotypes for size analysis and 19 for leaf margin analysis. These sample sizes are near the minimum for accurate analysis, which generally requires 20 or more morphotypes showing leaf margins for mean annual temperature estimates and 25 or more morphotypes showing size for precipitation estimates (see Wolfe, 1993). The incomplete (fragmentary) nature of many specimens made it impractical to make precise leaf size measurements; however, in most cases, leaves could be assigned to bins of defined leaf size classes (e.g. microphyll, notophyll and mesophyll; see specific size categories in Wolfe, 1993; Wilf et al., 1998). These were scored using transparency overlays that demarcate leaf sizes, and means of the Raunkiaer–Webb size categories were used to estimate the mean natural log leaf area (following Wilf et al., 1998). Leaf size classes that varied within a single morphotype were scored proportionately (e.g. a morphotype with both microphyll and notophyll leaves received a score of 0.5 for each size class). Larger leaves may be more poorly represented in the assemblage due to undersampling or taphonomic biases, such as physical abrasion or sorting as they were stripped from trees during the ashfall, and may be less easily recognized due to the fragmentary nature of the collection. In many of the fragmentary specimens, only portions of the leaf margins could be observed. Failed tooth preservation due to fragmented or poorly preserved leaves may underestimate the proportion of leaves with teeth, particularly if leaves only have teeth in the distal quarter or half of the lamina (Royer et al., 2005).
The fossils are part of the permanent collections of the Museo de Historia Natural/Universidad Mayor Nacional San Marcos (MUSM) in Lima.
RESULTS AND DISCUSSION
Vessel-related functional–anatomical traits
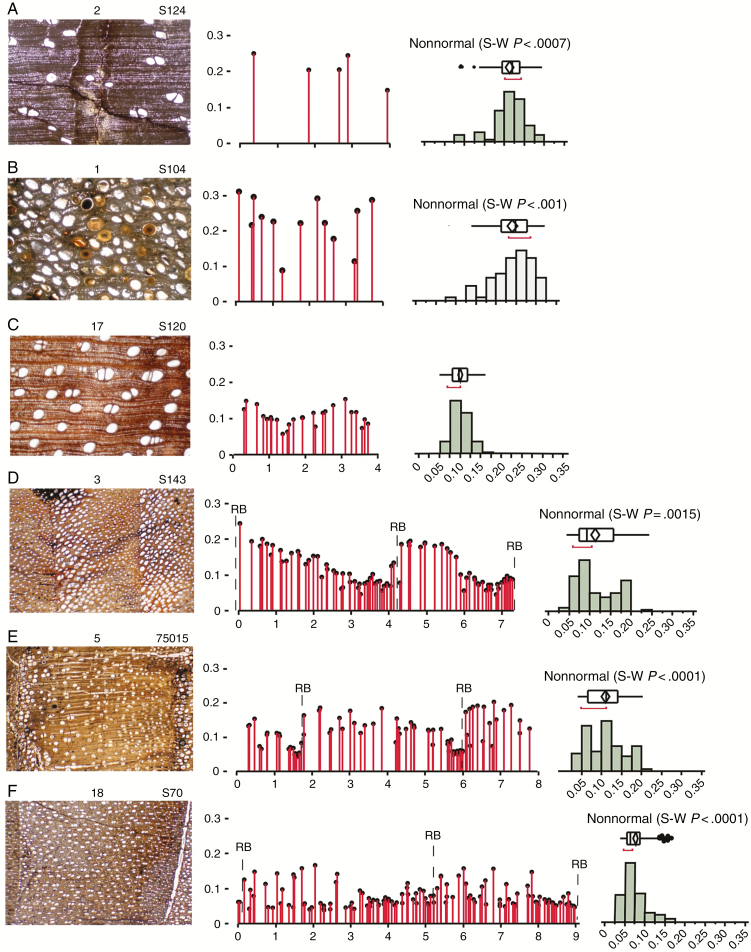
Transverse sections of the 30 wood types (Fig. 3) document the diversity in vessel diameter and arrangement seen in the fossils. We recognize four functional–anatomical traits relating to vessel diameter and arrangement. These are discussed in relation to representative diameter-and-position plots and diameter distributions in Fig. 4. The semi-ring-porous woods have three subtypes and are treated in more detail. Diffuse porosity, the more common condition, could also be considered a functional–anatomical trait but is not considered as such here. Note also that not all of these traits are mutually exclusive.
Fig. 3.
Transverse sections of the fossil woods arranged in decreasing diameter of the largest vessels. The wood taxa are numbered sequentially in the lower left. Abundance and size classes are indicated at the top where these determinations were made. Abundance categories go from most (AC1) to least (AC3) abundant. Numbers at centre left are specimen numbers. Scale bars = 250 µm.
Fig. 4.
Representative transverse sections, vessel diameter-and-position plots, and diameter distributions for some of the wood-anatomical traits discussed in the text. Transverse sections are oriented with the long axis along the radial direction (i.e. rotated 90° relative to the images in Fig. 3); numbers at the top indicate correspondence to the wood types and specimens in Fig. 3. The vessel diameter-and-position plots show the variation in vessel diameter (in millimetres) along the radial axis (scale in millimetres) within the measurement area (RB = ring boundary for woods with rings). Diameter distributions with diameters in millimetres; box plots show median, upper and lower quartiles (ends of boxes), and interquartile range (whiskers); lower bracket shows the most dense 50 % of the data. Normality is tested for using the Shapiro–Wilks test, with significantly non-normal distributions as indicated.
Vessels few, wide. This designation refers to diffuse-porous woods with vessels <5 mm−2 and > 175 µm in diameter (wood types 2, 4 and 7 in Fig. 3; Fig. 4A). Width of the vessels indicates that these are tall canopy or emergent trees based on the scaling of vessel diameter with height/axis length (Olson et al., 2014) In the IAWA database, the combination of vessels that are wide and also sparsely occurring (vessels > 200 µm and < 5 mm−2 in the IAWA Hardwood List; IAWA Committee, 1989) does not occur outside the tropics (in North America north of Mexico, taxa with this characteristic are restricted almost entirely to south Florida). Although this functional trait has been considered to be limited to wet tropical forest trees (Wheeler and Manchester, 2002), our data show that taxa with this characteristic extend into dry and very dry forest associations (Table 1), probably in or near seasonally flooded or floodplain areas where deep-rooted trees experience favourable growth conditions.
Vessels wide (Dia. Cat. IV), closely spaced, and in > 1 diameter class (but not ring porous). One specimen, the widest-vesseled wood in the assemblage, has these characteristics (wood type 1 in Fig. 3, Fig. 4B). This combination of features is typical of woody vines or lianas and generally occurs with abundant apotracheal parenchyma, vasicentric tracheids and a small proportion of fibrous ground tissue (Carlquist, 1988; Wheeler and Manchester, 2002), characters also evident in the fossil.
-
Woods semi-ring porous. Ring- and semi-ring porous woods exhibit variation in vessel diameter across the ring and have correspondingly distinct ring boundaries (see IAWA Committee, 1989, for definitions). For comparison, vessel pattern and diameter distribution for a typical diffuse-porous wood (wood type 17) are shown in Fig. 4C. Six of the woods are semi-ring porous and four others show a tendency toward semi-ring porosity or are intermediate between diffuse and semi-ring porous. Analysis of vessel patterns shows that three subtypes can be recognized:
3a. An even gradation in vessel diameter across the ring and a distribution in which narrow latewood vessels are present in greatest abundance and wider earlywood vessels constitute an outlier or secondary mode (wood types 3 and 14 in Fig. 3; Fig. 4D).
3b. Three populations of vessels present: large-diameter earlywood vessels, medium-diameter vessels distributed over most of the ring (and encompassing the modal value) and small-diameter (grouped) vessels in the very last part of the ring (wood type 5 in Fig. 3; Fig. 4E).
3c. Narrow vessels present in greatest abundance and distributed throughout the ring, with an outlier or secondary mode of wider vessels in the earlywood (wood types 18, 24 and 28 in Fig. 3; Fig. 4F).
Table 1.
Percentage occurrence for wood functional–anatomical traits discussed in the text. Species lists are given in the Supplementary Data
| Peru fossils, n = 30 | Mex MT forest (Oculian), n = 27 | HI VDT forest (Kanepu‘u), n = 16 | Mex VDT forest (Chamela), n = 62 | Ven VDT forest (Mamo), n = 19 | NW CR DT forest, n = 65 | Mex WT forest (Los Tuxlas), n = 55 | Peru WT forest (Tambopata), n = 64 | Brazil WT forest (Manaus, Porto Velho), n = 25,25 | Surinam WT forest, n = 25 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Few, wide vessels | 10 | 0 | 0 | 2 | _ | 9 | 9 | 13 | 4,0 | 4 |
| Radial vessel groupings ≥ 4 common | 20 | __ | 38 | _ | 90 | 14 | _ | 4 | _ | _ |
MT, montane tropical; VDT, very dry tropical; DT, dry tropical; WT, wet tropical; NW CR, NW Costa Rica.
Ring- and semi-ring porosity, considered broadly, occurs almost exclusively in deciduous trees (Boura and De Franceschi, 2007) and is most common in the temperate latitudes, where it occurs in dominant forest trees, smaller trees and shrubs, and woody vines (often in open forests, early successional settings or forest edge locations), and is more prevalent in drier areas (Woodcock, 1989). Prevalence is much lower in the tropics, especially the South American tropics, where occurrence is around 2–3 % of the tree flora (Alves and Angyalossy-Alfonso, 2000; InsideWood, 2004-onwards).
Fossil wood assemblages from the temperate latitudes show an increase in ring- and semi-ring-porous woods over time, beginning with very low levels in the Cretaceous and increasing to just over 40 % of woods in the Pliocene and Recent (Wheeler and Baas, 2019). This trend has been related to the increasing prevalence of seasonal climates since the Cretaceous (Baas and Wheeler, 2011; Wheeler and Baas, 2019). Tropical assemblages, in contrast, have much lower incidence (< 10 %), although there may be an increase in the Eocene (as seen here) and Oligocene (Wheeler and Baas, 2019).
The pattern represented by Subtype 3a (Fig. 4D) is similar to those seen in semi-ring-porous temperate latitude woods (Woodcock, 1994), although the latter typically have largest-diameter vessels concentrated at the growth ring boundary whereas the wood seen here can have the widest vessels a distance back from the boundary. Subtypes 3c and 3d (Fig. 4E, F) have not been previously described and appear to combine a typically, and probably exclusively, tropical pattern (more than one vessel diameter class but vessels not arranged in typical ring-porous fashion) with one (the semi-ring-porous condition) most common in the temperate latitudes; the occurrence of both narrow and wide vessels at the beginning of the growth season suggests water stress and cavitation risk even at the time of maximum water availability. All three subtypes are distinct from woods that are ring porous sensu strictu and have an especially distinct ring structure through some combination of wide vessels occurring along the ring boundary, an abrupt change in vessel size across the ring, different types of vessels in early- and latewood (solitary in earlywood, grouped in latewood), and exceptionally wide (> 250 µm in diameter) earlywood vessels. Carlquist (1988) considered ring- and semi-ring porosity to be an assemblage of phenomena and distinguished ten ring- or semi-ring types. Our results also indicate that significant anatomical diversity is subsumed under the terms.
Although largely a temperate-latitude adaptation, ring porosity is found at the edges of the tropics in the Southwest US deserts at~25–35°N (shrubs/trees with narrow to moderately wide vessels in Dia. Cat. I–III in the InsideWood database) and the mountains of Mexico and Central America to around 15°N (mesic-forest trees with ranges extending south from the eastern United States or Gulf Coast and wide earlywood vessels in Dia. Cat. IV; e.g. Ptelea trifoliata, Carya ovata). Elsewhere in the tropics, taxa with the tendency toward ring-porosity appear to be almost entirely semi-ring porous. The very dry forests/scrublands of the western coast of Mexico (~20°N, MAP < 800 mm) include semi-ring-porous taxa (generally with narrow vessels in Dia. Cat. II). Occurrence is 5 % in Costa Rican dry tropical forest woods, where three species of Cordia show this tendency. Semi-ring-porous taxa are often limited to dry forest or more open (savannah or scrub) vegetation (e.g. species of Dalbergia, small/medium-sized trees with vessels in Dia. Cat. III), but some range more broadly, occurring in dry forest and also semi-evergreen and even evergreen forest at low population densities, usually as pioneers and in more open situations or areas of well-drained soils (e.g. Cedrela odorata, Cordia alliodora, large trees, Dia. Cat. IV). They appear to be absent from the wettest forests (MAP > 3500–4000 mm and a very short/absent dry season). In the Old World tropics, semi-ring-porous taxa are important dominant trees (Tectona grandis, Gmelina arborea, Vessel Dia. Cat. IV; some Lagerstroemia species, Dia. Cat. III–IV) in Asian forests with highly seasonal precipitation (summer-rain monsoon) and more pronounced annual temperature variability than most parts of the tropics (Ashton 2014). (Some species are variably semi-ring porous, tending toward the diffuse-porous condition in the wetter/more equatorial parts of their range, e.g. Cinnamomum; Richter and Dallwitz, 2000-onward.)
4. Vessels in radial multiples or clusters. These two traits, defined in the IAWA coding system as vessels in radial multiples >4 common and vessel clusters common, occur in 6/29 (wood types 3, 23, 24, 25, 27 and 29 in Fig. 3) and 2/29 (wood types 14, 28), respectively, of the non-liane taxa represented in the assemblage. (Radial multiples of 2–3 occurs commonly and is not considered here.) Long radial multiples and vessel clusters do not generally co-occur, but ring- and semi-ring-porous woods often have grouped vessels of one or the other type (as in wood types 3, 14, 24 and 28). A total of 28 % of the Piedra Chamana taxa have grouped vessels, compared with the tropical South American tree flora in which grouped vessels are much less common (9 % radial multiples and 3 % clusters, InsideWood 2004-onwards). Surveys of individual clades and regional wood floras have shown that this character is most prevalent in dry areas (Baas and Carlquist, 1985; Carlquist, 1988), and the degree of vessel grouping has been shown to be positively related to cavitation resistance in Acer species (Lens et al., 2011). In the tropics, the occurrence of radial groupings appears to be high in very dry forest and lower elsewhere (Table 1). The degree of vessel grouping can be quantified and is considered further below.
Baileyan trends and other functional–anatomical traits
Wheeler and Baas (2019) reviewed the occurrence in the fossil record of a range of wood-anatomical functional traits relating mainly to the Baileyan specialization trends (such as the sequence from scalariform to simple vessel perforations). Their analysis shows that character-states considered to be more derived increase since the Cretaceous in temperate-latitude wood assemblages whereas more-derived traits predominate over geological time in tropical assemblages. The Peru fossils generally conform with these results, exhibiting low/zero prevalence of less-derived features – scalariform perforations (0/30), scalariform-opposite intervascular pits (0/30), solitary vessels (3/30), vessel elements ≥ 800 µm (0/30) – and higher values of more-derived traits – septate fibres [12 (2 entirely,10 occasionally)/30, marginal parenchyma (10/30), homocellular rays (7/30), storied rays (5/30)] (Woodcock et al., 2017, 2019).
One exception is helical vessel thickenings (not a character relating to the Baileyan trends but included in the analysis of Wheeler and Baas). This feature, seen in 3/30 of the wood types, is very rare/absent in tropical assemblages and in the modern tropics, where it has been reported in 2–4 % of the modern tropical flora (InsideWood, 2004-onwards). The increase over time to 42 % of the temperate woody flora has been linked to water stress associated with freezing or drought (Lens et al., 2011; Wheeler and Baas, 2019). The lower likelihood of observing helical thickenings in fossil as compared to modern woods (owing to preservation) adds to the significance of this determination. The occurrence of (exclusively) diffuse or diffuse-in-aggregates axial parenchyma (10/30) is also higher than in the tropics/tropical assemblages (5–17 %) but probably reflects the dominance in the assemblage of malvalean taxa (Woodcock et al., 2019), which often have parenchyma of this type. Lastly, average vessel-element length > 800 µm (0/30) shows the low prevalence typical of tropical fossil assemblages but contrasts with the modern tropical flora with 25 % occurrence. This disjunction between the fossil and modern floras has been noted by Wheeler and Baas (2019) and is considered further below.
Quantitative vessel-related variables
Analysis of these variables is complicated by the different types of representations used (e.g. means vs. categorical representations), the availability of comparative data, the often non-normal nature of the distributions, and the fact that distribution end-members are often significant biologically (e.g. woods with few, wide vessels, very high- or low-density woods). There is the additional problem of determining/representing vessel diameter and density in woods with complicated vessel patterning such as the ring- and semi-ring-porous taxa. Some observations can be advanced, however.
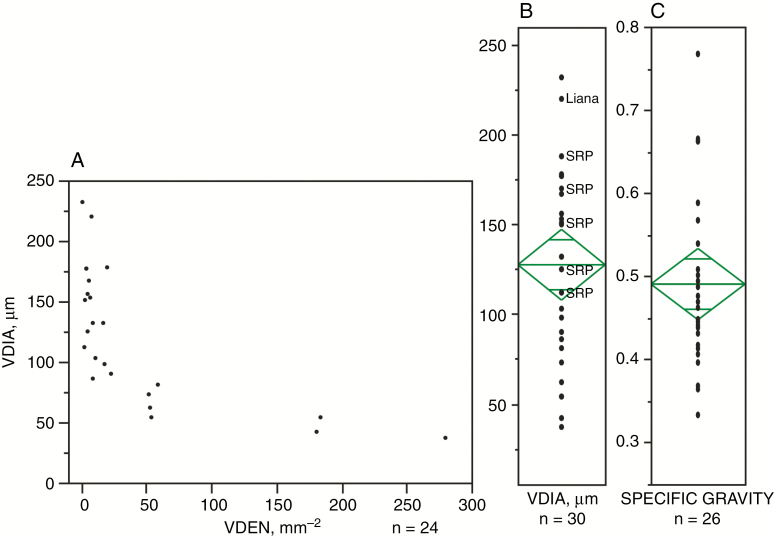
Vessel diameter and density and estimated specific gravity (Fig. 5) are probably most useful in giving an overall picture, or fingerprint, of the vegetation. Thus, the large range in vessel diameter is typical of the tropics and indicates the presence of a range of tree/woody plant heights/axis lengths based on generalized scaling relationships to tree diameter and height (Olson et al., 2014, 2018; but see Pfautsch et al., 2016; Hacke et al., 2017). Vessel density likewise shows a large range typical of diverse tropical forest. These two variables can also be informative when considered together; for example, woods with few, wide vessels are an indicator of good access to water. Estimated specific gravity also shows the similarly large range found in the tropics (and specific gravity ≤ 0.4 is a tropical indicator). The average (0.49 ± 11) is lower than that of Neotropical tree species (0.645, n = 2456; Chave et al., 2006) or the major regional subdivisions of the Neotropical forests (Central American dry and wet forest, southwestern Amazon forests, etc.) (0.602–0.717; Baker et al., 2004; Chave et al., 2006) but well within the range of variability seen in subtypes within these forests, which include early successional and floodplain associations in which trees with wood of lower specific gravity predominate (Woodcock et al., 2000).
Fig. 5.
Vessel diameter (VDIA) and density (VDEN) and estimated specific gravity for the fossil woods. (A) Vessel diameter vs. density omitting the semi-ring-porous woods (n = 24). (B) Distribution of vessel diameter (n = 30); values for the semi-ring-porous woods (SRP) are the average of the wide vessels. (C) Distribution of estimated specific gravity (n = 26); estimates could not be obtained for some specimens because of preservation (Supplementary Data Table S1).
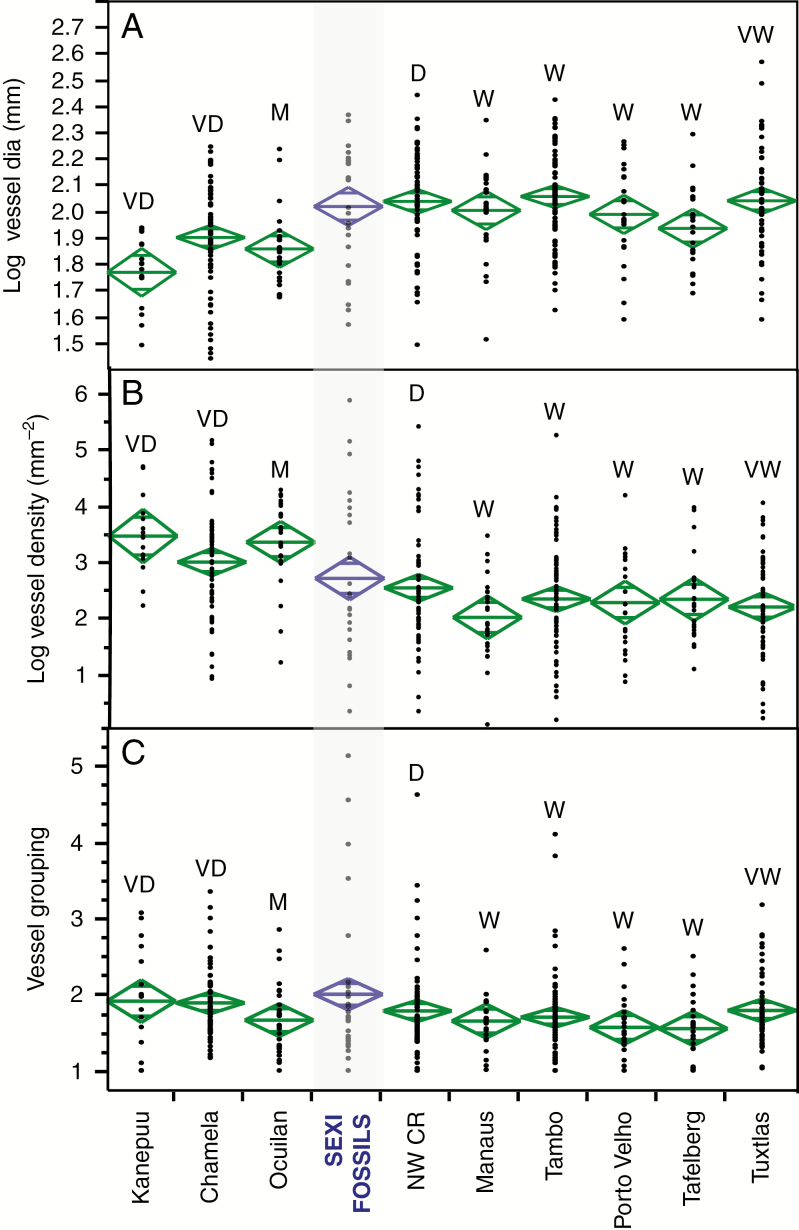
Following from studies that have analysed mean values of these quantitative variables (e.g. Martinez-Cabrera and Cevallos-Ferriz, 2008), we provide comparisons of means for tropical forest types where data are available (Fig. 6). All the variables show a considerable amount of overlap among the different forest types. Average vessel diameter and density and vessel grouping (Fig. 6A, B) show some differentiation between the driest and wettest associations, but the Peru fossils can be clearly differentiated only from very dry forest (Kanepu‘u HI) and only with respect to vessel diameter (Tukey–Kramer all-pairs comparison with alpha set at 0.05). Vessel grouping (Fig. 6C), a measure of the degree to which vessels are grouped (Carlquist, 1988), shows little variation among the various forests, although high values occur only in one or two forest types, as well as the fossil assemblage.
Fig. 6.
Comparison of quantitative vessel-related variables. (A) Log vessel diameter. (B) Log vessel density. (C) Degree of vessel grouping. Diamonds show means and 95 % confidence intervals. VD = very dry; M = montane; D = Dry Tropical Forest, W = Wet Tropical Forest, VW = Very Wet Tropical Forest. Data for Mexico and South America supplied by Hugo Martinez-Cabrera for the indicated species (species lists in Supplementary Data Table S2).
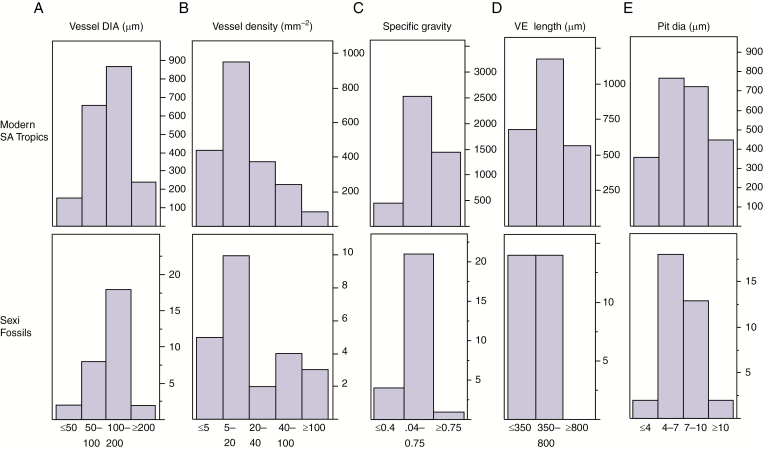
Quantitative wood-anatomical variables are expressed in categorical form for the purposes of description and identification. Here we compare the fossils with modern tropical South American trees using categorical data from the InsideWood database (InsideWood, 2004-onwards). Vessel diameter and density (Fig. 7A, B) show distributions similar to the modern. Estimated specific gravity (Fig. 7C) is shifted toward lower values, as noted above. Vessel element length (Fig. 7D) is also shifted toward lower values, with 0 % occurrence of vessel elements > 850 µm. This disjunction between the modern tropics and tropical assemblages is notable because tropical assemblages in general show few differences from modern tropical woods (Wheeler and Baas, 2019). These authors advance several possible explanations for this result, one of which relates to measurements (taken from macerations for modern woods and longitudinal sections for fossils), but we think there is some validity to the shorter lengths found as the detailed descriptions including comparisons to related modern taxa often show a degree of difference that is unlikely to be attributable to measurement method alone (Woodcock et al., 2017, 2019). Regarding interpretations, Carlquist’s (1988) linking of shorter vessel elements with xeric conditions is supported by some findings (the increase with increasing precipitation in some oak species, Villar-Salvador et al., 1997; the significantly higher values in mature floodplain forest among a range of Amazon forest types, Woodcock et al., 2000); and although vessel-element length is not usually considered among the important variables affecting xylem functioning (Gleason et al., 2016), long vessel elements were found to be more vulnerable to cavitation under water stress in Acer (Lens et al., 2011). Intervessel pit diameter is also somewhat different from the modern. The average of 6.5 ± 1.8 µm is low compared to the world flora and woods from tropical forest areas (India, Southeast Asia, tropical Africa, the Neotropics), where most woods have small to medium-sized pits (Wheeler et al., 2007a). [Phylogenetic influences could come into play here, but the assemblage is dominated by Malvaceae, which tend to have large pits (Woodcock et al., 2017, 2019).] Although smaller pits might be expected to increase cavitation resistence, in Acer a range of other pit-related characteristics (depth of the pit chamber, thickness of the pit membrane, size of the pores within the membrane) are more clearly related to vulnerability to embolism (Lens et al., 2011).
Fig. 7.
Comparisons between the fossils and the modern tropical South American tree flora using categorical data from the InsideWood database. (A) Vessel diameter, (B) vessel density, (C) specific gravity, (D) vessel element length and (E) pit diameter.
Leaf data
The leaves include 23 morphotypes, some fairly fragmentary, among the 136 specimens that were suitable for examination from the total collection of 227 specimens. One morphotype strongly dominates the assemblage, with at least 58 specimens that are clearly assignable to this type. Although assessments are tentative due to the fragmentary nature of many of the specimens and the potential taphonomic biases that may under-represent large leaves, leaf margins and sizes nevertheless provide a basis for estimating MAT and MAP, respectively.
No teeth were observed in any of the specimens and all of the leaves appear to have entire margins except for one or two that are lobed but lack teeth (n = 19). However, some morphotypes are represented by single specimens that do not have the complete margin preserved. The equation used to estimate MAT is from Wilf (1997) where MAT = 28.6P + 2.24 (where P is the proportion of entire-margined morphotypes), indicating an MAT of 30.8 ± 2 °C. The fossil leaves, apparently 100 % entire-margined, are at the upper limit of the calibration datasets.
Leaf sizes of the fossil morphotypes according to the Raunkiaer–Webb system (Webb, 1959; Wilf et al.,1998) are predominantly split between microphylls and notophylls, although two are mesophylls (n = 22). The estimate for precipitation is derived using the equation from Wilf et al. (1998) in which ln(MAP) = 0.548 MlnA + 0.768 (where MlnA is the mean of the natural logarithm of the leaf areas, calculated here in terms of the proportions of the means of individual size categories between and within morphotypes). MlnA for the 22 fossil leaf morphotypes is 7.47, yielding a precipitation estimate of 1290 mm. The proportion of leaves in the larger size categories is low (only 9 % mesophyll) relative to tropical rainforest (Amazon forests with MAP as low as 1200–2000 mm and a 5- to 8-month dry season are dominated by mesophylls; Malhado et al., 2009).
SUMMARY AND CONCLUSIONS
Palaeoenvironmental characterizations based on the woods and leaves from the Piedra Chamana petrified forest are summarized in Table 2. Multiple indicators considered here, as well as other characteristics of the assemblage (diversity of taxa and growth forms, presence of palm/non-palm monocots, presence of mangroves, and geological context), establish that the palaeovegetation was diverse forest growing in a frost-free climate at an elevation near sea level (lowland tropical forest). The fossil woods share many functional–anatomical traits with modern tropical forest woods. They also show the prevalence of features or character-states considered more derived that is typical of modern and fossil tropical woods (Wheeler and Baas, 2019). A range of wood-anatomical traits indicate a lack of correspondence to very dry (MAP < 800–1000 mm) or very wet (MAP > 3000 mm) tropical forest. Functional traits unusual for the tropics or occurring at a higher prevalence than in modern-day tropical forests are mostly vessel-related and are either more typical of drier and more seasonal climates (semi-ring porosity, grouped vessels) or have been hypothesized to relate to dry (less mesic) conditions or water stress (short vessel elements, helical thickenings). The semi-ring-porous taxa also reveal a deciduous component to the flora. Taken together, these considerations indicate precipitation on the low end of the range for dry tropical forest (~1000–1200 mm). MAP estimated from the leaves (1290 mm) is also within the range of dry tropical forest. The high MAT indicated by the leaf analysis (31 °C) may explain the anomalous wood-anatomical characteristics as being a response to high levels of water stress in a very warm climate with strongly seasonal precipitation.
Table 2.
Summary of palaeoenvironmental indicators
| Wood functional–anatomical traits | No. of taxa | % occurrence | Significance |
|---|---|---|---|
| Few, wide vessels | 3/30 | 10 | Not occurring outside the tropics (indicates frost-free climates). Limited to wet tropical forest and deep-rooted trees in environments where water is not limiting for growth. |
| Semi-ring porosity | 6/30 | 25 | More prevalent than in modern South American dry-to-wet tropical forests. Establishes deciduous component to the flora (Boura & Francheschi, 2007). Includes complex patterns indicating a different pattern of water need compared to the present? |
| Vessels in radial multiples or clusters | 8/29 | 28 | Values high for the modern tropical flora, intermediate between very dry and more mesic tropical forest. |
| Helical vessel thickenings | 3/30 | 10 | Rare/absent in the present-day tropics. Linked to water stress and climate seasonality (Wheeler & Baas 2019). |
| Wood quantitative variables | Av. | ||
| Estimated specific gravity | 0.49 ± 0.11 | Values low for tropical forest but within the range of tropical forest types. Indicates low-biomass association. Values < 0.4 are a tropical indicator. | |
| Vessel diameter & density | Large ranges typical of tropical forest; large range in vessel diameter reflecting the range of tree heights expected for tropical forest. | ||
| Vessel diameter & density means comparisons | Fossil assemblage significantly different from driest forest. | ||
| Vessel element length | 339 ± 123 µm | Values low relative to modern tropical flora and lengths ≥ 800 µm not present, consistent with the fossil/modern disjunction described by Wheeler and Baas (2019). Long vessel elements linked to mesic conditions (Carlquist, 1988). | |
| Intervessel pit diameter | 6.5 ± 1.8 µm | Values low relative to modern tropical flora. | |
| Degree of vessel grouping means comparisons | Means comparisons not significant but some taxa show a high degree of vessel grouping relative to modern tropical associations. | ||
| Leaves | |||
| Leaves 100 % entire-margined (n = 23) | Tropical temperature indicator. | ||
| Leaves notophylls to microphylls (megaphylls absent) | Indicates relatively dry conditions; distinct from wet lowland tropical forest with megaphylls predominating. | ||
| Leaf margin & area analysis | |||
| Eq. in Wilf, 1997 | MAT 31°C | ||
| Eq. in Wilf et al., 1998 | MAP 1290 mm |
The occurrence of woods with few, wide vessels, the range of growth-forms represented (canopy and emergent trees, climbers, and understorey elements), and the many fossil trees with long narrow trunks provide a picture of a relatively mesic forest of some stature growing in a lowland location with moderately good water availability, at least for deep-rooted trees. The low specific gravity of the woods is also consistent with a riverine or floodplain type of location. The semi-ring-porous taxa, in contrast, are more probably a component of drier interfluvial or upland vegetation that would have been deciduous or semi-deciduous. [Floristic evidence relating to this interpretation is discussed by Woodcock et al. (2019).] Trees with semi-ring-porous wood are scarce in the New World tropics (2 % of the South American tropical tree taxa; InsideWood database), occurring mainly at the periphery of the Amazon in drier climate areas, and are only slightly more prevalent in Central South Asia and tropical mainland Africa and Islands (5 % and 8 %, respectively; InsideWood, 2004-onwards). In Indian forests, where semi-ring-porous taxa have the highest percentage occurrence (although less diverse than in Africa), they are characteristic elements of drier tropical forests intermediate between perhumid Dipterocarp forest and dry woodland, especially short to tall deciduous forest (forest types as in Ashton, 2014). The latter experience a dry season of 7–9 months and the very high maximum monthly temperatures (32–35 °C) typical of the Indian subcontinent; this is a higher degree of seasonality than currently occurs in the New World tropics and suggests these forests and environments as the closest modern physiognomic/anatomical analogue.
The palaeolatitude of the fossil forest (13°S) places it at the margins of the equatorial wet zone, where latitudinally averaged precipitation falls off steeply both at present and in climate model simulations for the Eocene (Beerling and Woodward, 2001; Shellito et al., 2002); the dry conditions indicated could thus be a reflection of latitudinal controls. In contrast, the Palaeocene Cerrejón flora from Colombia (leaves 50 % mesophyll or larger and 78 % entire-margined) clearly represents wet tropical forest (estimated MAP > 2500 mm; Wing et al., 2009) growing in a near-equatorial location (palaeolatitude of 5°N). Temperature estimates for the Cerrejón flora (≥ 28 °C; Wing et al., 2009) are above the range for modern tropical forests and are supported by faunal evidence (estimated MAT of 28–31 °C; Jaramillo et al., 2010 and supplemental materials). The Piedra Chamana leaves, apparently 100 % entire-margined and at one extreme of the calibration datasets, suggest temperatures at the high end of this range (31 °C), consistent with the very warm, seasonal climate indicated by the wood anatomy.
We show here that analysis of wood-anatomical features of a diverse fossil wood assemblage such as the Piedra Chamana fossils allows for (1) placement to vegetation type(s) with known climatic parameters based on characteristics of the anatomy, in this way yielding quantitative estimates of palaeoclimate variables, and (2) identification of features that are anomalous with respect to modern associations, which can in turn provide additional climate and environmental information and serve as a source of hypotheses about wood–climate relationships.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic. oup.com/aob and consist of the following. Table S1: Estimated specific gravity for the Peru fossils. Table S2: Species lists for the wood floras used in analysis of quantitative wood-anatomical variables.
FUNDING
This work was supported by the American Philosophical Society (grant to D.W.), the National Science Foundation (grant number 0403510 to D.W. and H.M.), and the National Geographic Society (grant CP-103R-17 to D.W.).
ACKNOWLEDGEMENTS
We appreciate the provision of anatomical data by Hugo Cabrera-Martinez, comments of Robyn Burnham on an earlier version of the manuscript, and assistance and support from Ysabel Prado.
LITERATURE CITED
- Alves ES, Angyalossy-Alfonso V. 2000. Ecological trends in the wood anatomy of some Brazilian species. 1. Growth rings and vessels. International Association of Wood Anatomists Journal 21: 3–20. [Google Scholar]
- Aragon-Carrasco S, Rimarchin L, Ayasta J, Woodcock D. 2006. Inventario preliminar de la flora del Distrito de Sexi, Cajamarca [Preliminary inventory of the flora of the District of Sexi, Cajamarca.] Arnaldoa 13: 358–367. [Google Scholar]
- Aragon-Carrasco S, Woodcock DW. 2010. Plant community structure and conservation of a northern Peru sclerophyllous forest. Biotropica 42: 262–270. [Google Scholar]
- Ashton P. 2014. On the forests of tropical Asia: least memory fade. Kew: Royal Botanical Gardens. [Google Scholar]
- Baas P, Carlquist S. 1985. A comparison of ecological wood anatomy of floras of Southern California and Israel. International Association of Wood Anatomists Bulletin.n.s 6: 349–353. [Google Scholar]
- Baas P, Wheeler E. 2011. Wood anatomy and climate change. In: Hodkinson TR, Jones MB, Waldren S, Parnell JA, eds. Climate change ecology and systematics. Cambridge: Cambridge University, 141–155. [Google Scholar]
- Baatsen M, Douwe JJ, Van Hinsbergen JJ, et al. 2016. Reconstructing geographical boundary conditions for palaeoclimate modelling during the Cenozoic. Climate of the Past 12: 1635–1644. [Google Scholar]
- Baatsen M, von der Heydt AS, Huber M, et al. 2018. Equilibrium state and sensitivity of the simulated middle-to-late Eocene climate. Climate of the Past Discussion 2018: 1–49. doi: 10.5194/cp-2018-43 [DOI] [Google Scholar]
- Baker TR, Phillips OL, Malhi Y, et al. 2004. Variation in wood density determines spatial patterns in Amazonian forest biomass. Global Change Biology 10: 545–562. [Google Scholar]
- Beerling DJ, Woodward FI. 2001. Vegetation and the terrestrial carbon cycle: modeling the first 400 million years. Cambridge: Cambridge University Press. [Google Scholar]
- Boura A, De Franceschi D. 2007. Is porous wood structure exclusive of deciduous trees? Comptes Rendus Palevol 6: 385–391. [Google Scholar]
- Burns RM, Honkala BH, tech. coords . 1990. Silvics of North America: 1. Conifers; 2. Hardwoods. Agriculture Handbook, 654. Washington, DC: U.S. Department of Agriculture, Forest Service. [Google Scholar]
- Carlquist S. 1988. Comparative wood anatomy. New York: Springer. [Google Scholar]
- Chave J, Muller-Landau HC, Baker TR, Easdale TA, ter Steege H, Webb CO. 2006. Regional and phylogenetic variation of wood density across 2456 Neotropical tree species. Ecological Applications 16: 2356–2367. [DOI] [PubMed] [Google Scholar]
- Garzione CN, Hoke GD, Libarkin JC, et al. 2008. Rise of the Andes. Science (New York, N.Y.) 320: 1304–1307. [DOI] [PubMed] [Google Scholar]
- Gleason SM, Westoby M, Jansen S, et al. 2016. Weak tradeoff between xylem safety and xylem‐specific hydraulic efficiency across the world’s woody plant species. New Phytologist 209: 123–136. [DOI] [PubMed] [Google Scholar]
- Global Biodiversity Information Facility. N.d. What is GBIF? Available at: https://www.gbif.org/what-is-gbif.
- Graham A, Gregory-Wodzicki KM, Wright KM. 2001. Studies in Neotropical paleobotany. XV. A Mio-Pliocene palynoflora from the Eastern Cordillera, Bolivia: Implications for the uplift history of the Central Andes. American Journal of Botany 88: 1545–1557. [PubMed] [Google Scholar]
- Gregory-Wodzicki KM. 2000. Uplift history of the central and northern Andes: a review. Geological Society of America Bulletin 112: 1091–1105. [Google Scholar]
- Hacke UG, Spicer R, Schreiber SG, Plavcová L. 2017. An ecophysiological and developmental perspective on variation in vessel diameter. Plant, Cell & Environment 40: 831–845. [DOI] [PubMed] [Google Scholar]
- IAWA Committee . 1989. IAWA list of macroscopic features for hardwood identification. IAWA Bulletin 10: 219–332. [Google Scholar]
- InsideWood . 2004-onwards. Available at: http://insidewood.lib.ncsu.edu/search.
- Jaramillo C, Ochoa D, Contreras L, et al. 2010. Effects of rapid global warming at the Paleocene-Eocene boundary on Neotropical vegetation. Science 330: 957–959. [DOI] [PubMed] [Google Scholar]
- Lens F, Sperry JS, Christman MA, Choat B, Rabaey D, Jansen S. 2011. Testing hypotheses that link wood anatomy to cavitation resistance and hydraulic conductivity in the genus Acer. The New Phytologist 190: 709–723. [DOI] [PubMed] [Google Scholar]
- Lindorf H. 1994. Eco-anatomical wood features of species from a very dry tropical forest. International Association of Wood Anatomists Journal 15: 361–376. [Google Scholar]
- Malhado ACM, Malhi Y, Whittaker RJ, et al. 2009. Spatial trends in leaf size of Amazonian rainforest trees. Biogeosciences Discussions 6: 2125–2162. [Google Scholar]
- Martinez-Cabrera HI, Cevallos-Ferriz SRS. 2008. Palaeoecology of the Miocene El Cien Formation (Mexico) as determined from wood anatomical characters. Review of Palaeobotany and Palynology 150: 154–167. [Google Scholar]
- Navarro P, Pajuelo D, Woodcock D, Ordoñez E. 2012. Nuevos aportes sobre la geologia del Bosque Petrificado Piedra Chamana, Sexi—Cajamarca [New reports concerning the geology of the Petrified Forest Piedra Chamana, Sexi—Cajamarca]. In: XVI Congreso Peruano de Geologia short papers, Lima, Sept. 23–26, 2012.
- Noble DC, McKee EH, Mourier T, Megard F. 1990. Cenozoic stratigraphy, magmatic activity, compressive deformation, and uplift in northern Peru. Geological Society of America Bulletin 102: 1105–1113. [Google Scholar]
- Olson ME, Anfodillo T, Rosell JA, et al. 2014. Universal hydraulics of the flowering plants: vessel diameter scales with stem length across angiosperm lineages, habits and climates. Ecology Letters 17: 988–997. [DOI] [PubMed] [Google Scholar]
- Olson ME, Soriano D, Rosell JA, et al. 2018. Plant height and hydraulic vulnerability to drought and cold. Proceedings of the National Academy of Sciences 115: 7551–7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orwa C, Mutua A, Kindt R, Jamnadass R, Simons A. 2009. Agroforestree Database: a tree reference and selection guide version 4.0. Available at: http://www.worldagroforestry.org/af/treedb/.
- Pfautsch S, Harbusch M, Wesolowski A, et al. 2016. Climate determines vascular traits in the ecologically diverse genus Eucalyptus. Ecology Letters 19: 240–248. [DOI] [PubMed] [Google Scholar]
- Poulsen CJ, Ehlers TA, Insel N. 2010. Onset of convective rainfall during gradual late Miocene rise of the Central Andes. Science 328: 490–493. [DOI] [PubMed] [Google Scholar]
- Richter HG, Dallwitz MJ. 2000-onwards. Commercial timbers: descriptions, illustrations, identification, and information retrieval. In English, French, German, Portuguese, and Spanish. Version: 25th June 2009. Available at: http://delta-intkey.com. [Google Scholar]
- Royer DL, Wilf P, Janesko DA, Kowalski EA, Dilcher DL. 2005. Correlations of climate and plant ecology to leaf size and shape: potential proxies for the fossil record. American Journal of Botany 2005: 1141–1151. [DOI] [PubMed] [Google Scholar]
- Shapiro SS, Wilk MB. 1965. An analysis of variance test for normality (complete samples). Biometrika 52: 591–611. [Google Scholar]
- Shellito CJ, Sloan LC, Huber M. 2002. Climate model sensitivity to atmospheric CO2 levels in the Early-Middle Paleogene. Palaeogeography, Palaeoclimatology, Palaeoecology 3028: 1011. [Google Scholar]
- Smith AG, Smith DG, Funnel BM. 1994. Atlas of Mesozoic and Cenozoic coastlines. Cambridge: Cambridge University Press. [Google Scholar]
- Tarelkin Y, Delvauz C, De Ritter M, El Berkani T, de Canniere C, Beekman H. 2016. Growth-ring distinctness and boundary anatomy variability in tropical trees. International Association of Wood Anatomists Journal 37: 260–294. [Google Scholar]
- Terry DO, Woodcock DW, Meyer HW, Allen SE. 2019. Paleopedeology of the Piedra Chamana Fossil Forest, Peru. Paleontology on Public Lands. In: Proceedings of the Eleventh Conference on Fossil Resources, Casper, Wyoming, May 30-June 2, 2019, 25–26. [Google Scholar]
- Villar-Salvador P, Castro-Díez P, Pérez-Rontomé C, Montserrat-Martí G. 1997. Stem xylem features in three Quercus (Fagaceae) species along a climatic gradient in NE Spain. Trees 12: 90–6. [Google Scholar]
- Webb LJ. 1959. A physiognomic classification of Australian rain forests. Journal of Ecology 47: 551–570. [Google Scholar]
- Weigend M, Rodriguez EF, Arana C. 2005. The relict forests of Northwest Peru and Southwest Ecuador. Revista Peruana de Biología 12: 185–194. [Google Scholar]
- Wheeler EA, Baas P. 2019. Wood evolution: Baileyan trends and functional traits in the fossil record. International Association of Wood Anatomists Journal 40: 488–529. [Google Scholar]
- Wheeler EA, Baas P, Rodgers S. 2007a. Variations in dicot wood anatomy: a global analysis based on the InsideWood database. International Association of Wood Anatomists Journal 28: 229–258. [Google Scholar]
- Wheeler EA, Manchester SR. 2002. Woods of the Eocene Nut Beds Flora, Clarno Formation, Oregon USA. International Association of Wood Anatomists Journal, Supplement 3:188. [Google Scholar]
- Wheeler EA, Wiemann MC, Fleagle JG. 2007b. Woods from the Miocene Bakate, Ethiopia: anatomical characteristics, estimates of original specific gravity and ecological characteristics. Review of Palaeobotany and Palynology 146: 193–207. [Google Scholar]
- Wilf P. 1997. When are leaves good paleothermometers? A new case for leaf margin analysis. Paleobiology 23: 373–390. [Google Scholar]
- Wilf P, Wing SL, Greenwood DR, Greenwood CL. 1998. Using fossil leaves as paleoprecipitation indicators: an Eocene example. Geology 26: 203–206. [Google Scholar]
- Wing SL, Herrera F.Caramillo CA, Gomez-Navarro Wilf CP, Labandeira CP. 2009. Late Paleocene fossils from the Cerrejon Formation, Colombia, are the earliest record of Neotropical rainforest. Proceedings of the National Academy of Sciences USA 106: 18627–18632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe JA. 1993. A method of obtaining climatic parameters from leaf assemblages. U.S. Geological Survey Bulletin 2040: 1–71. [Google Scholar]
- Woodcock D. 1989. Significance of ring porosity in analysis of a Sangamon flora. Palaeogeography, Palaeoclimatology, Paleoecology 73: 197–204. [Google Scholar]
- Woodcock D. 1994. Occurrence of woods with a gradation in vessel size across a ring. International Association of Wood Anatomists Bulletin 15: 377–385. [Google Scholar]
- Woodcock D, Dos Santos G, Reynel C. 2000. Wood characteristics of Amazon forest types. International Association of Wood Anatomists Journal 21: 277–292. [Google Scholar]
- Woodcock DW, Meyer H, Dunbar N, McIntosh W, Prado I, Morales G. 2009. Geologic and taphonomic context of El Bosque Petrificado Piedra Chamana (Cajamarca, Peru). Geological Society of America Bulletin 121: 1172–1178. [Google Scholar]
- Woodcock DW, Meyer HW, Prado Y. 2017. The Piedra Chamana fossil woods (Eocene, Peru). International Association of Wood Anatomists Journal 38: 313–365. [Google Scholar]
- Woodcock DW, Meyer HW, Prado Y. 2019. The Piedra Chamana fossil woods (Eocene, Peru), II. International Association of Wood Anatomists Journal 40: 551–595. [Google Scholar]
- Young KR, C Reynel C0.. 1997. Huancabamba Region, Perú and Ecuador. In: Davis SD, Heywood VH, Herrera-MacBryde O, Villalobos J, Hamilton AC, eds. Centers of plant diversity, a guide and strategy for their conservation Vol 3. The Americas. Cambridge: IUCN Publications Unit, 465–469. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.