Abstract
Patient: Female, 53-year-old
Final Diagnosis: Diabetic KetoAcidosis (DKA)
Symptoms: Gum bleeding
Medication: Steroids
Clinical Procedure: —
Specialty: Metabolic Disorders and Diabetics
Objective:
Unusual clinical course
Background:
Steroids are used as anti-inflammatory agents, administered for a variety of medical conditions, either as short-or long-term treatment. Steroid use is associated with many adverse effects, including hyperglycemia, but ketoacidosis is rare.
Case Report:
We present the case of a 53-year-old woman who developed diabetic ketoacidosis after administration of methylprednisolone during treatment of immune thrombocytopenic purpura. She did not have diabetes or a family history of diabetes. Steroid-induced hyperglycemia with insulin resistance, lipolysis, and ketogenesis occurred and were likely to have precipitated the ketoacidosis. Blood glucose, blood gases, and urine test results were diagnostic for ketoacidosis.
Conclusions:
The risk of ketoacidosis and hyperglycemia should be considered in the course of steroid therapy, even without a diagnosis of diabetes, especially in patients who have risk factors for diabetes mellitus including obesity and long-term use of steroids, so that early identification of diabetic ketoacidosis can prevent further morbidity and mortality in chronic patients.
MeSH Keywords: Diabetic Ketoacidosis; Glucocorticoids; Hyperglycemia; Purpura, Thrombocytopenic, Idiopathic; Steroids
Background
Steroids are considered the main treatment for many inflammatory, immunologic, allergic, and malignant diseases, and they have numerous adverse effects on many organ systems, ranging from mild (e.g., skin thinning and weight gain) to serious life-threatening conditions (e.g., serious infection, diabetes ketoacidosis [DKA]). A major adverse effect is hyperglycemia, which can worsen pre-existing diabetes or precipitate new diabetes (steroid-induced diabetes) [1, 2] through multifactorial mechanisms, including increased levels of hepatic glucocorticoids, alteration of receptor function, glucose uptake inhibition in adipose tissue, and abnormal carbohydrate metabolism, which lead to insulin resistance [1–4]. Steroid-induced diabetes has been reported to occur in 1.5–27% of these patients. The huge variation in incidence may be due to differences in the studied populations, dose, and duration of steroid use, and even the diabetes definition used [5–7]. The diabetogenic effect of steroids is usually affected by dose-volume, duration of therapy, structure, and type of preparation [8,9], older age (>65 years), high HbA1c (>6.0%), and low eGFR (<40 ml/min/1.73 m2) [10]. Longer steroids use (usually more than 21 days at a dose equivalent to > 20 mg of prednisolone per day) increases risk of adverse effects, and patients with short-term use of steroids usually have no adverse effects or have only mild adverse effects [11].
Diabetes ketoacidosis (DKA) is an acute complication of hyper-glycemia, with high rates of morbidity and mortality. It is typically characterized by serum glucose > 250 mg/dL, PH <7.3, serum bicarbonate (HCO3) level <18 mEq/L, and elevated serum ketone level (ketonemia) and in urine (ketonuria), elevated anion gap >10, and dehydration [12,13]. It is commonly associated with type 1 diabetes mellitus (T1DM) and it is less common in type 2 diabetes mellitus (T2DM). It is more likely to affect people with T2DM who are experiencing extreme stress conditions like serious infection, myocardial infarctions, trauma, or other emergencies, as well as those taking medications like atypical antipsychotics, glucagon, and steroids [12]. To the best of our knowledge, there are few reported cases of steroid-induced DKA [14], and the present case report is intended to fill this gap in the literature and to improve understanding and management of this condition.
Immune thrombocytopenia (ITP) is an acquired autoimmune disorder characterized by immune-mediated destruction of platelets in asymptomatic adults. It typically presents with isolated new-onset thrombocytopenia. It is defined as platelet count <100×109/L due to destruction of platelets and in the absence of other causes of thrombocytopenia. There are 2 types – primary (accounting for 80% of cases) without underlying causes, and secondary. Diagnosis of ITP is made only after other causes of thrombocytopenia are ruled out. There are no specific tests for ITP, and bone marrow biopsy sometimes provides only limited information [15]. Hyperthyroidism has been reported among some patients with ITP (8–14%) [16,17], and few cases have been reported as associated with T1DM [16,17] with co-existing systemic autoimmune disease. In a study of elderly ITP patients (age >60 years), 22.2% (P 0.61) were diabatic [18]. First-line therapies are corticosteroids (e.g., methylprednisolone 30 mg/kg/day IV for 5–7 days). Up to 85% of patients respond within 5 days, and maintenance oral corticosteroids are required [19], along with IV immunoglobulins (IVIG) 1 g/kg/day IV for 1–2 days [20]. There is a report of 10 patients with adverse events who had received multiple cycles of pulsed high-dose dexamethasone (as first-line alternative therapy for initial management of ITP), with severe vomiting, transient hypertension, and steroid-induced diabetes [21] and 43.8% had to discontinue the steroid as ITP management due to adverse effects [20]. We know of no case report of steroid-induced DKA in ITP patients.
Case Report
A 53-year-old woman with immune thrombocytopenia (ITP) presented to our Emergency Department with gum bleeding occurring over the past 5 days. She had been discharged from the hospital 3 months ago after ITP flare on prednisolone tapering dose, but she discontinued the medication 1 month ago due to oral thrush. This time she presented to us with insidious minimal self-limiting gum bleeding and epistaxis for the last 5 days. She also described gradually progressive polyuria, mainly nocturia, and polydipsia for 1 month. She reported she had gained about 3 kg body weight in the last 3 months. She reported no melena, vomiting, cough, headache, change in level of consciousness, dizziness, chest pain, ecchymosis, localized weakness, fits, or hematuria/dysuria, and had no history of trauma or falling.
Past medical and drug history: ITP has been diagnosed 11 months ago in our hospital after we excluded all other possible causes. She had been admitted 2 times before, and this time she was admitted with active-controlled bleeding with severe thrombocytopenia. The last admission 3 months back was similar to the present admission, with a platelet count of 12×109/L. Initial steroids was given and she was discharged on 4th day with platelet count 25×109/L and no complications during the hospital coarse; 2 readings of RBS were within normal limit, but HbA1c not done at that time. The discharge medication was a tapering dose of prednisolone over 6 weeks and continue with a low dose until the next visit in 12 weeks, but she stopped taking this 5 weeks before the presentation because she had oral thrush and she missed the follow-up in the hematology clinic. She was not taking any medication for the last 4 weeks. She was not known to have diabetes, hypertension, or cardiac disease. She gave a history of check-up visits at an eye clinic 6 months ago, at which a full eye examination including fundoscopy was done and showed no evidence of acute or chronic problems (no retinopathy).
Family History: No significant family history of diabetes.
In the ER, she was fully conscious, oriented, not distressed, and appeared dehydrated. Her BMI was 35 kg/m2, with blood pressure 129/64 mmHg, pulse 110 beat/minute, respiration rate 22 rates/min, O2 saturation 95% on ambient air, and temperature 36.9°C. Her oral cavity was moist, with no signs of active oozing, bleeding, or thrush. The chest was clear with equal air entry bilaterally. The abdomen was soft, lax, and without tenderness. No edema, swelling, redness, or tenderness were noted in the lower limbs.
Her laboratory data at that time were: WBCs 9×109/L, Hb 12.6 g/L, platelets 1×109/L, peripheral blood film showed sever thrombocytopenia, INR 1.05, PT 12 Secs, aPTT 30.2 Secs, Sodium 135 mmol/L, potassium 4.2 mmol/L, chloride 92 mmol/L, calcium 8.9 mg/dL, RBS 551 mg/dL, phosphorus 4.5 mg/dL, magnesium 1.65 mg/dL, creatinine 1.26 mg/dL, urea 34.24 mg/dL, uric acid 6.1 mg/dL, total bilirubin 0.5 mg/dL, direct bilirubin 0.32 mg/dL, albumin 3.7 g/dL, AST 17 U/L, ALT 6 U/L, pH 7.48, pCO2 35, and HCO3 26.5. Urine analysis showed the following: glucose ++++, ketones +, and pus cells 2–5. The lipid profile was: cholesterol 158 mg/dL, HDL 36 mg/dL, LDL 98 mg/dL, triglyceride 118 mg/dL, and HbA1c 14.7. The thyroid profile was: TSH 0.21 mU/L, FT4 14.6 mU/L, FT3 3.24 mU/L, HBV NON-React, HCV NON-React, and HIV NON-React. An ECG showed sinus tachycardia, normal axis deviation, and no ischemic changes. A CT brain was normal study.
She was admitted to the regular ward as a case of ITP flare with newly diagnosed of T2DM, and a plan for glucocorticoids, IVIG, and platelets transfusion for the ITP and insulin basal and sliding scale subcutaneous based on RBS reading for the diabetes.
She received a total of 6 units of platelets transfusion, IV immunoglobulin (IVIG), and methylprednisolone 1 g IV daily for 5 days.
On day 1 she was evaluated by the primary team and was found to be anxious, vomiting, experiencing abdominal pain, confused, and looking ill. A random blood sugar was 465 mg/dL and venous blood gas was pH 7.26, pCO2 16, and HCO3 7.2. The platelet count was 7×109/L. Troponin was normal and an ECG showed sinus tachycardia. A CT brain was repeated and showed no evidence of bleeding. Up to this time she had received the first dose of methylprednisolone. The Endocrine Team was consulted, and bolus IV fluids were started. Urine analysis showed glucose ++++ and ketones ++. In 1-hour the VBG was repeated, showing pH 7.1, HC3 3.2, anion gap (AG) 27, and RBS 570. The DKA hospital protocol was initiated and the patient was moved to the ICU.
On day 3, while she was in the ICU, she was found to be clinically stable, with improved conscious level and no active problems. She had pH 7.31, pCO2 31.5, HCO3 16.5, and anion gap (AG) 10, so her DKA was resolved and she was moved back to the ward. Her platelet count was 32×109/L.
On day 6, she was discharged, as she was clinically improved, with no more bleeding since admission, and with platelet count 69×109/L (Figure 1) and RBS 185 mg/dL, pH 7.4, pCO2 36, and HCO3 22.7.
Figure 1.
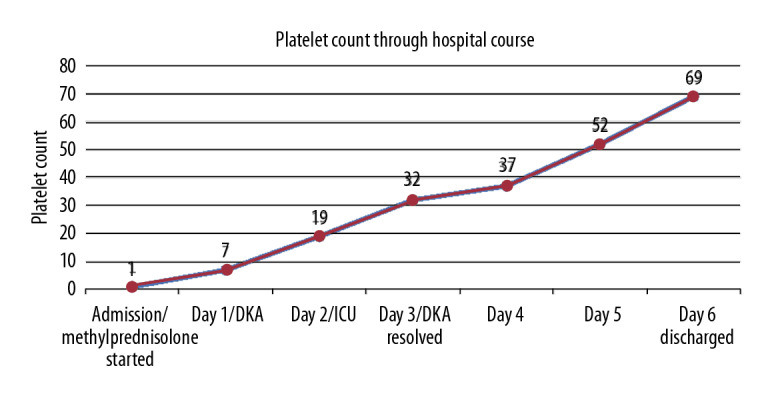
Platelet count results according to the hospital course.
The discharge prescription was insulin glargine 26 units sub-cutaneous once daily, insulin glulisine 12 units subcutaneous 3 times before meals, and metformin 500 mg TID to start with 500 mg daily for the first week and increase weekly to reach 1.5 gm once a day. She was followed up by Hematology, Endocrine, and the Diabetes Center.
The patient was on close follow-up with the endocrinologist, and was evaluated 3 months after discharge. At that time, she was clinically stable, with improved general condition. Her platelet count was 132×109/L and HbA1c was 9.3, although she was not strictly compliant with her medication. She was on the same management plan, but with more intense lifestyle modification and continued close follow-up. At 6-month post-discharge follow-up, the patient stated she had improved adherence to medication, with better glucose readings at home, weight reduction of 6 kg, and new BMI=27 kg/m2 and HbA1c=6.9. A new plan was made to hold the insulin regimen and to continue metformin. During this time the patient was stable, with no active bleeding, no steroids, and no hospital admission, with baseline platelet count 100×109/L.
Discussion
We present the case of a 53-year-old woman who presented with persistent primary ITP that probably developed 3–12 months before diagnosis. She responded to therapy, with no bleeding and platelet count >30×109/L. She had at least doubling of baseline platelet count measured on 2 occasions >7 days [15] and no evidence of previous history of chronic diabetes before using the steroids, as she was asymptomatic 2 months ago), and 2 readings of RBS within normal range (3 months ago). The eye examination results were normal (6 months ago). The absence of retinopathy in such a patient with steroid-induced diabetes suggests there had not been long periods of hyperglycemia [22]. Unfortunately, there were no previous HbA1c results for this patient for comparison, which could be considered a limitation. Steroid-induced hyperglycemia is common in diabetic and non-diabetic patients. More and more reported cases of steroid-induced diabetes have odds ratios of 1.5–2.5, and this ratio can reach 10.34, with a hydrocortisone equivalent of > 120 mg/day. High dosage and longer duration of steroids are strong predictors of diabetes. Also, other risk factors should be considered, such as age, over-weight, and sedentary lifestyle [23]. The occurrence of DKA after receiving steroids therapy is less common.
The effects of systemic steroids on glucose metabolism are multifactorial and still not completely understood. The main mechanisms include increased glucose production via hepatic gluconeogenesis and glycogenolysis, and more insulin resistance by reducing peripheral uptake at adipose and muscle level via inhibition of GLUT4 translocation [24,25]. The acute effects of steroids are more distinct on glucose tolerance than is long-term use, and there is more risk of acute events of hyperglycemia like DKA [25]. Also, the pre-exposure status of patients with impaired glucose tolerance, especially those with high-risk factors of diabetes, is more pronounced to new-onset steroid-induced diabetes in such patients [3].
Our patient had been on low-dose corticosteroid therapy as she was persistently ITP. During those times she developed steroid adverse effects (oral thrush, most likely candida infection) and stopped taking the medication. She started to have symptoms of hyperglycemia and was incidentally discovered in the ER to have diabetes with acute ITP. She was stable and non-critical, with uncontrolled hyperglycemia, so she was started on basal and sliding-scale insulin. Although she received a single dose of pulsed steroids, she developed acute DKA. Symptoms of hyperglycemia and elevated HbA1c means that the patient had previously experienced long periods of hyperglycemia for at least 3 months. Although she did not have a significant family history of diabetes, confounding factors of obesity, genetics, and environmental factors may have played a role, along with the previous long-term use of steroids at her initial presentation of diabetes, which might have been triggered by the steroid therapy.
Poor pre-discharge counseling about possible adverse effects of steroids in a patient receiving steroids for the first time, in addition to poor follow-up from the primary physician while a patient is on long-term steroids as an outpatient, are important factors that contribute to adverse effects of steroids, demonstrating the need for more patient-centered care. Early detection and rapid intense management are crucial in acute DKA, especially in such a patient who needs close observation, as the rapid deterioration in this case happened in a matter of hours to days.
Conclusions
Steroids-induced hyperglycemia is quite common. Our case report emphasizes that DKA can be triggered by steroid therapy and extra attention is needed when steroids are initiated for ITP or chronic disorders. Patients with high-risk factors of diabetes, including obesity, family history of diabetes, chronic use of steroids, prediabetes, and other factors, should be even more concerning. Early detection and treatment can prevent further morbidity and mortality due to DKA.
Footnotes
Conflict of interests
None.
Department and Institution where work was done
Department of Internal Medicine, King Abdulaziz Specialist Hospital, Taif, Saudi Arabia.
References:
- 1.Trence DL. Management of patients on chronic glucocorticoid therapy: An endocrine perspective. Prim Care. 2003;30(3):593–605. doi: 10.1016/s0095-4543(03)00038-1. [DOI] [PubMed] [Google Scholar]
- 2.Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96(1):23–43. doi: 10.1016/s0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- 3.McMahon M, Gerich J, Rizza R. Effects of glucocorticoids on carbohydrate metabolism. Diabetes Metab Rev. 1988;4(1):17–30. doi: 10.1002/dmr.5610040105. [DOI] [PubMed] [Google Scholar]
- 4.Olefsky JM, Kimmerling G. Effects of glucocorticoids on carbohydrate metabolism. Am J Med Sci. 1976;271(2):202–10. doi: 10.1097/00000441-197603000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Marchetti P. New-onset diabetes after liver transplantation: From pathogenesis to management. Liver Transpl. 2005;11(6):612–20. doi: 10.1002/lt.20439. [DOI] [PubMed] [Google Scholar]
- 6.Bloom RD, Crutchlow MF. New-onset diabetes mellitus in the kidney recipient: Diagnosis and management strategies. Clin J Am Soc Nephrol. 2008;3(Suppl. 2):S38–48. doi: 10.2215/CJN.02650707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valderhaug TG, Hjelmesaeth J, Rollag H, et al. Reduced incidence of new-onset posttransplantation diabetes mellitus during the last decade. Transplantation. 2007;84(9):1125–30. doi: 10.1097/01.tp.0000287191.45032.38. [DOI] [PubMed] [Google Scholar]
- 8.Vondra K, Hampl R. [Glucocorticoids and diabetes mellitus] Vnitr Lek. 2006;52(5):493–97. [in Czech] [PubMed] [Google Scholar]
- 9.Simmons LR, Molyneaux L, Yue DK, Chua EL. Steroid-induced diabetes: Is it just unmasking of type 2 diabetes? ISRN Endocrinol. 2012;2012:910905. doi: 10.5402/2012/910905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katsuyama T, Sada KE, Namba S, et al. Risk factors for the development of glucocorticoid-induced diabetes mellitus. Diabetes Res Clin Pract. 2015;108(2):273–79. doi: 10.1016/j.diabres.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Buchman AL. Side effects of corticosteroid therapy. J Clin Gastroenterol. 2001;33(4):289–94. doi: 10.1097/00004836-200110000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Westerberg DP. Diabetic ketoacidosis: Evaluation and treatment. Am Fam Physician. 2013;87(5):337–46. [PubMed] [Google Scholar]
- 13.Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335–43. doi: 10.2337/dc09-9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tiwari A, Al-Robeh H, Sharma H, et al. Steroid-induced diabetic ketoacidosis in a patient with type 2 diabetes mellitus. AACE Clinical Case Reports. 2018;4(2):e131–e33. [Google Scholar]
- 15.Neunert C, Lim W, Crowther M, et al. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117(16):4190–207. doi: 10.1182/blood-2010-08-302984. [DOI] [PubMed] [Google Scholar]
- 16.Kelton JG, Vrbensky JR, Arnold DM. How do we diagnose immune thrombocytopenia in 2018? Hematology Am Soc Hematol Educ Program. 2018;2018(1):561–67. doi: 10.1182/asheducation-2018.1.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamaguchi K, Ookubo Y, Matsuda H, et al. Idiopathic thrombocytopenic purpura subsequent to Graves’ disease and insulin-dependent diabetes mellitus. Diabetes Res Clin Pract. 1987;3(4):233–37. doi: 10.1016/s0168-8227(87)80045-1. [DOI] [PubMed] [Google Scholar]
- 18.Prusek K, Deja G, Jarosz-Chobot P. [Association of idiopathic thrombocytopenic purpura and type 1 diabetes mellitus – a case report] Pediatr Endocrinol Diabetes Metab. 2010;16(3):220–22. [in Polish] [PubMed] [Google Scholar]
- 19.Palandri F, Santoro C, Carpenedo M, et al. Management of elderly patients with immune thrombocytopenia: Real-world evidence from 451 patients older than 60 years. Thromb Res. 2020;185:88–95. doi: 10.1016/j.thromres.2019.11.026. [DOI] [PubMed] [Google Scholar]
- 20.Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115(2):168–86. doi: 10.1182/blood-2009-06-225565. [DOI] [PubMed] [Google Scholar]
- 21.Cooper N, Ghanima W. Immune thrombocytopenia. N Engl J Med. 2019;381(10):945–55. doi: 10.1056/NEJMcp1810479. [DOI] [PubMed] [Google Scholar]
- 22.Din B, Wang X, Shi Y, Li Y. Long-term effect of high-dose dexamethasone with or without low-dose dexamethasone maintenance in untreated immune thrombocytopenia. Acta Haematol. 2015;133(1):124–28. doi: 10.1159/000362529. [DOI] [PubMed] [Google Scholar]
- 23.Clore JN, Thurby-Hay L. Glucocorticoid-induced hyperglycemia. Endocr Pract. 2009;15(5):469–74. doi: 10.4158/EP08331.RAR. [DOI] [PubMed] [Google Scholar]
- 24.Gurwitz JH, Bohn RL, Glynn RJ, et al. Glucocorticoids and the risk for initiation of hypoglycemic therapy. Arch Intern Med. 1994;154(1):97–101. [PubMed] [Google Scholar]
- 25.Perez A, Jansen-Chaparro S, Saigi I, et al. Glucocorticoid-induced hyperglycemia. J Diabetes. 2014;6:9–20. doi: 10.1111/1753-0407.12090. [DOI] [PubMed] [Google Scholar]


