Abstract
Objectives
The current demographic information from China reports that 10%-19% of patients hospitalized with coronavirus disease (COVID-19) were diabetic. Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) are considered first-line agents in patients with diabetes because of their nephroprotective effects, but administration of these drugs leads to upregulation of angiotensin-converting enzyme 2 (ACE2), which is responsible for the viral entry of severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2). Data are lacking to determine what pulmonary effects ACEIs or ARBs may have in patients with diabetes, which could be relevant in the management of patients infected with SARS-CoV-2. This study aims to assess the prevalence of pulmonary adverse drug effects (ADEs) in patients with diabetes who were taking ACEI or ARBs to provide guidance as to how these medications could affect outcomes in acute respiratory illnesses such as SARS-CoV-2 infection.
Methods
1DATA, a unique data platform resulting from collaboration across veterinary and human health care, used an intelligent medicine recommender system (1DrugAssist) developed using several national and international databases to evaluate all ADEs reported to the Food and Drug Administration for patients with diabetes taking ACEIs or ARBs.
Results
Mining of this data elucidated the proportion of a cluster of pulmonary ADEs associated with specific medications in these classes, which may aid health care professionals in understanding how these medications could worsen or predispose patients with diabetes to infections affecting the respiratory system, specifically COVID-19. Based on this data mining process, captopril was found to have a statistically significantly higher incidence of pulmonary ADEs compared with other ACEIs (P = 0.005) as well as ARBs (P = 0.012), though other specific drugs also had important pulmonary ADEs associated with their use.
Conclusion
These analyses suggest that pharmacists and clinicians will need to consider the specific medication’s adverse event profile, particularly captopril, on how it may affect infections and other acute disease states that alter pulmonary function, such as COVID-19.
In late 2019, an outbreak of pneumonia, later found to be caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), occurred in Wuhan, China. Coronavirus disease (COVID-19) patient symptomatology includes fever, dyspnea, myalgia, and pneumonia, but can also progress to acute respiratory distress syndrome (ARDS), acute cardiac injury, as well as acute kidney injury and death.1 A study evaluating 191 patients with COVID-19 found that 48% of patients had comorbid conditions, including 19% with diabetes.2 There was a statistically significant difference (P = 0.001) in mortality between patients with comorbid conditions, including diabetes, compared with those without.2 It is known that long term hyperglycemia has deleterious effects on many organ systems—most notably the eyes, kidneys, nerves, and heart. However, less research has described the pathophysiologic effects diabetes may have on the respiratory system. In light of the recent COVID-19 outbreak, more research is needed to understand the effects that diabetes (and its medications) may have on the respiratory system and how that could affect the management of diseases such as COVID-19.
The renin-angiotensin system (RAS) is implicated in the pathophysiology of numerous disease states including diabetic nephropathy and hypertension. Drugs affecting this system have been explored to manage nephropathy occurring in patients with diabetes, and angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) are the ones currently recommended.3 , 4 Currently available ACEIs differ based on potency, pharmacokinetics (especially tissue distribution), and whether the molecule is a prodrug. ACEIs are also delineated into 3 structural classes based on the functional group responsible for binding to ACE.5 , 6 Although ACEIs and ARBs are generally considered to have similar adverse event profiles, evaluation of postmarketing ADEs may shed light on minute differences that could have important clinical impacts.
RAS has been a subject of discussion in the wake of the SARS-CoV-2 pandemic because ACE2, a membrane-bound receptor involved in RAS, has been found to be the host-cell receptor responsible for viral entry. This was also true for SARS-CoV, which lead to an outbreak in 2002. Administration of ACEIs and ARBs, as well as thiazolidinediones and ibuprofen, leads to the upregulated expression of ACE2.7 The upregulation of ACE2 receptors theoretically puts patients at higher risk of infection with SARS-CoV-2 because more target host receptors would be available for cellular virus entry. Conversely, ACE2 presence was found to be protective in lung tissue of animal models due to the conversion of angiotensin II to angiotensin (1-7), which has vasodilatory properties. Animal models have shown an increase in ACE concentration can result in pulmonary fibrosis, asthma, and ARDS. The effects of ACE-inhibiting medications, which will lower the activity of ACE (and therefore the concentration of angiotensin II), would theoretically be protective against patients developing ARDS.7 This has led to the hypothesis that ACEIs or ARBs may be detrimental in early SARS-CoV-2 infection but paradoxically protective in later stages. RAS is exceedingly complex, and conflicting data are available regarding the contribution of ACEIs and ARBs on the mortality and morbidity of COVID-19 patients.8
Objective(s)
In this study, we aimed to assess if each ACEI or ARB had particularly serious pulmonary adverse event profiles, which could either place patients with diabetes at increased risk for SARS-CoV-2 infection due to diminished lung function or may affect their management.
Methods
Data sources and data mining
The 1DATA partnership between the University of Missouri-Kansas City and Kansas State University has led to the development of a platform to share human and animal health care data.9 In this partnership, public databases were integrated into the 1DATA database (www.1DATA.life), and, in this study, were used to assess the incidence of ADEs related to ACEIs and ARBs in patients with diabetes.10
The data used in this study were curated from multiple publicly available data sources for patients with diabetes including the Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS), which houses all ADEs reported to the FDA by pharmaceutical companies, health care providers, and consumers. The data, including the diabetes dataset, are updated quarterly by the FDA and currently includes reports submitted from the first quarter of 2004 to the last quarter of 2019. This dataset mainly focuses on drugs and their ADEs but also includes other pertinent data such as disease information, drug information, adverse drug event data, demographic information, as well as outcome data.
Internationally, ADE terminologies are reported by a similar process to the Medical Dictionary for Regulatory Activities (MedDRA). In this database, ADE terminologies are hierarchically structured to regulate information for medical products on a global level. The data structure of these terms is organized in accordance with MedDRA terminology as well as the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use, an international safety reporting guidance database. We used the MedDRA hierarchy for regulatory information of medical products in diabetes including low-level terms (LLTs) as well as high-level terms (HLTs). LLTs provide very descriptive information that is grouped into preferred terms and the HLTs, which provide information on anatomy, pathology, physiology, etiology, or function. HLTs are then clustered into system organ classes, which are grouped based on etiology, manifestation site, or purpose. Here we used the 23.0 or earlier version of MedDRA10
Data mining algorithms were used for diabetes datasets to identify postmarketing ADEs reported more frequently than expected by comparing those frequencies with information on all drugs and events in the database using the proportional reporting ratio (PRR), as explained below.11 Databases are reported by a combination of active ingredients, generic names, or brand names. Hence, the drug names were mapped to drug parents using the DrugBank (Alberta Innovates–Health Solutions, The Metabolomics Innovation Center).12 Finally, ADEs associated with medications in the ACEI and ARB classes administered to patients with diabetes were recorded.
Statistical analysis
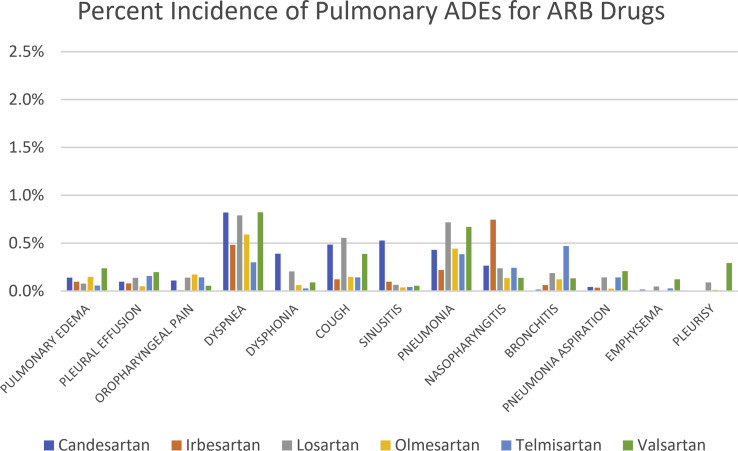
Statistical analysis was performed using Statistical Analysis Software (SAS) (SAS University Edition version 9.4, Cary, NC). First, data based on the frequency of each ADE related to respiratory, thoracic, and mediastinal disorders or infections were parsed in the MedDRA and FAERS databases. Specific ADEs collected were pulmonary edema, pleural effusion, oropharyngeal pain, dyspnea, dysphonia, cough, sinusitis, pneumonia, nasopharyngitis, bronchitis, pneumonia aspiration, emphysema, and pleurisy (Figure 1 ). We then employed a method proposed and implemented by the FDA for analyzing ADE disproportionality in pharmacovigilance data by observed-expected ratios.11 This method, the PRR, provides a statistical summary for the commonality of an ADE for a specific drug as compared with the entire database for drugs in the same or other classes.11
Figure 1.
Relative percentages of reported pulmonary adverse events of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers from the Food and Drug Administration Adverse Event Reporting System and Medical Dictionary for Regulatory Activities databases organized by drug. Abbreviations used: ACEIs, angiotensin-converting enzyme inhibitors; ADEs, adverse drug effects; ARBs, angiotensin II receptor blockers.
We addressed confounding factors including patient demographics and drugs that are underreported in voluntary reporting systems including the FAERS since conditional slicing and subsetting can confine the use of quantitative signal detection methods such as PRR. For this purpose, we were able to correct the analysis after applying logistic regression for the known covariates of age, weight, and sex, and combine this approach with PRR to improve analyses of drug effects using the diabetes datasets. We found that these factors do not play a substantial role in the analysis of the data. We found that the most important ADEs (pulmonary edema, dyspnea, dysphonia, cough, bronchitis, and pleurisy), which may be very relevant to SARS-CoV-2 infection, are not statistically significantly affected by any of these covariates (age, sex, and weight). As a result, we assumed that:
However, the following ADEs exhibited some sex or age effects (ADE: effect): (1) pleural effusion: sex; (2) oropharyngeal pain: sex; (3) sinusitis: sex; (4) pneumonia: age; (5) nasopharyngitis: sex; (6) pneumonia aspiration: sex; and (7) emphysema: age.
This helped us to estimate a PRR for a specific drug-ADE combination by calculating the following equation:
| (1) |
where gives the total number of a specific event for a given drug in . Here and represent the number of all events and drugs in the drug class. denotes the drug class excluding the specific drug . Also, shows the total events for the given drug . When the distribution of PRR samples are all positive, we then applied a log transformation to data and then found the confidence interval13 using the following equation:
where
Friedman test results
Sample differences among the 3 groups, captopril, ACEIs, and ARBs, were assessed for a pairwise analysis with the assumption that data were not normally distributed using the nonparametric Friedman test for 2 independent unequal-sized data using SAS. Friedman test was also applied to perform multiple comparison tests (P values for statistical significance < 0.05). For the nonparametric Friedman test of statistical significance, 4 pairwise and multiple comparisons were performed based on the ARBs and ACEIs excluding captopril, hence denoted as ACEI-1 (angiotensin-converting enzyme inhibitors, excluding captopril). Tests performed included ACEI-1 versus ARB drugs, ACEI-1 drugs versus captopril alone, captopril versus ARB drugs, and captopril versus all ACEI-1 and ARB drugs.
Results
We had no a priori hypothesis regarding which of the ACEIs or ARBs would be distinct in terms of their ADE profile. After analysis, captopril alone showed a clear signal distinct from other ACEIs and ARBs. Therefore, we proceeded with some specific, pairwise analysis of captopril to see if any other distinctions were found. Thirteen different pulmonary ADEs were selected to assess the related variation due to adverse event differences. Percent incidence of reported pulmonary ADEs for each drug can be found in Figure 1. These values represent the number of reported adverse events for that specific drug and ADE as compared with all (pulmonary and nonpulmonary) ADEs reported for that drug. Results of the Friedman test showed that all 4 comparative analyses were statistically significant except the ACEI-1 drugs versus ARB drugs comparison (P = 0.07), suggesting that ACEIs (excluding captopril) and ARBs may have similar pulmonary ADE profiles in patients with diabetes (Table 1 ). The Friedman test results also showed that captopril had statistically significant increases in pulmonary ADEs in patients with diabetes as compared with other ACEIs (P = 0.005) as well as compared with ARBs (P = 0.012). For multiple comparisons among all the groups using this test, captopril versus all ACEI-1 drugs versus ARB drugs, a P value of 0.004 was seen indicating statistically significant differences in pulmonary ADE occurrences for the 2 drug groups compared with captopril. Our results highlight a statistically significant difference of pulmonary ADEs for captopril, an ACEI, but also noted additional pulmonary ADEs of concern with other ACEIs and ARBs as well (Supplementary Figures 1 and 2).
Table 1.
Results from the nonparametric Friedman test of statistical significance for 4 pairwise comparisons
| Comparison | P value |
|---|---|
| Captopril versus ACEI-1 | 0.005a |
| Captopril versus ARBs | 0.01a |
| ACEI-1 versus ARBs | 0.07 |
| Captopril versus all ACEI-1 drugs versus ARB drugs | 0.004a |
Abbreviations used: ARBs, angiotensin II receptor blockers; ACEI-1, angiotensin-converting enzyme inhibitors excluding captopril.
Denotes statistical significance (P < 0.05).
To meet PRR reporting requirements, 3 criteria must be satisfied: (1) more than 3 reported incidences, (2) a PRR greater than 2, and (3) a PRR that is greater than the lower 95% CI boundary, with the lower CI itself being over 1. After applying these criteria, captopril had reportable incidences for most of the reported pulmonary ADEs in patients with diabetes. Other drugs, including ARBs, met the criteria for some pulmonary ADEs (Supplementary Table 1) but did not show the same trends across multiple ADEs as depicted with captopril.
Discussion
Evaluation of the collated databases revealed that captopril, the first ACEI approved back in 1981, has a higher incidence of pulmonary ADEs in patients with diabetes as compared with other ACEI drugs (P = 0.005) as well as a statistically significant difference in pulmonary events compared with ARBs (P = 0.012) (Table 1).
Captopril’s high incidence of pulmonary ADEs highlights the fact that the drugs belonging in one class are not identical and that its pharmacokinetics and pharmacodynamics can affect the patients’ health especially during acute processes like COVID-19. This is especially important as current observational studies of COVID-19 patients tend to group drugs within a class and are not analyzing the potential differences within each class. ACEIs can be broadly classified into 3 structural classes: sulfhydryl-, dicarboxyl-, and phosphorous-containing molecules. Notably, captopril is the only currently available ACEI belonging to the sulfhydryl-containing class and may explain the higher incidence of ADEs observed.5 The binding of ACEIs to ACE has shown notable differences when modeled via Autodock Vina14 and maybe a source of variation in reported ADEs, though this remains under examination. Areas of future research, structure-activity relationship, and binding affinity may also explain the pulmonary ADE differences between captopril and other ACEIs and ARBs. For example, captopril has an inhibitory constant (Ki) of 1.7 nM, a measurement used to describe the potency of an inhibitor to its target, as compared with enalapril’s Ki of 0.2 nM.5 Finally, we have focused this discussion on how these pulmonary ADEs may affect COVID-19 morbidity and mortality; however, it is useful to consider how these RAS antagonists, when given to diabetics, may affect the management of other pulmonary diseases (e.g., COPD, pneumonia).
Health care providers have been left with many questions when treating patients with COVID-19, including how ACEIs or ARBs may affect their clinical course.15 Results from this study may be helpful when prescribing or continuing ACEIs or ARBs for patients with diabetes and infections or illnesses that may affect pulmonary function, such as COVID-19. This research highlights the important caveat that generalizations cannot always be safely made to a medication class, and health care providers should be well aware of nuances between each drug and how it may contribute to the clinical course of infections, particularly during the COVID-19 pandemic. The complexity of considering each drug that the patient is taking and how it affects both chronic and acute illnesses emphasizes the benefit a pharmacist—the medication expert—has on a patient’s care team and ultimately the patient’s health outcomes.
Forthcoming 1DATA consortium studies include analysis of electronic medical records to evaluate the prevalence of ACEIs or ARBs in patients with COVID-19, and to elucidate if patients on specific medications were more likely to have a difficult clinical course. In addition, evaluation of the binding affinity and structure-activity relationship of captopril may help explain why it is responsible for a disproportional amount of pulmonary ADEs in patients with diabetes.
Limitations
A limitation of this analysis relates to it being a retrospective analysis of curated ADE databases from spontaneous reporting systems. Nuances in reporting ADEs between FAERS and MedDRA databases could have resulted in the exclusion or inclusion of data that may have affected our datasets.
Conclusion
Statistical analysis suggests that the ACEI, captopril, has a statistically significantly different ADE profile for pulmonary-related events than ARBs as well as other ACEIs. However, there are considerable inter- and intraclass variations across other individual RAS drugs, suggesting that these also merit attention.
Biographies
Emma G. Stafford, PharmD, Teaching Assistant Professor, 1DATA Consortium, Division of Pharmacology and Pharmaceutical Sciences, University of Missouri-Kansas City School of Pharmacy, Kansas City, MO
JimE.Riviere, DVM, PhD, Distinguished Professor Emeritus, 1DATA Consortium, Kansas State University, Manhattan, KS and North Carolina State University, Raleigh, NC
Xuan Xu, PhD, Research Associate and Postdoctoral Fellow, 1DATA Consortium, Department of Mathematics, Institute of Computational Comparative Medicine, Kansas State University, Manhattan, KS
Jessica Kawakami, PhD, Postdoctoral Fellow, 1DATA Consortium, University of Missouri-Kansas City School of Biological and Chemical Sciences, Kansas City, MO
Gerald J. Wyckoff, PhD, Professor, Interim Chair, 1DATA Consortium, Molecular Biology and Biochemistry, School of Biological and Chemical Sciences Division of Pharmacology and Pharmaceutical Sciences, University of Missouri-Kansas City School of Pharmacy, Kansas, MO
Majid Jaberi-Douraki, PhD, Associate Professor, 1DATA Consortium, Department of Mathematics, Institute of Computational Comparative Medicine, Kansas State University, Manhattan, KS
Footnotes
Disclosure: The authors declare no relevant conflicts of interest or financial relationships.
Funding: Gerald J. Wyckoff and Majid Jaberi-Douraki accepted funding from BioNexus KC for this project.
Appendix
Supplementary Figure 1.
Relative percentages, organized by symptoms, of pulmonary adverse drug effects for angiotensin-converting enzyme inhibitor drugs. Abbreviations used: ACEI, angiotensin-converting enzyme inhibitor; ADEs, adverse drug effects.
Supplementary Figure 2.
Relative percentages, organized by symptoms, of pulmonary adverse drug effects for ARB drugs. Abbreviations used: ADEs, adverse drug effects; ARB, angiotensin II receptor blocker.
Supplementary Table 1.
Adverse drug effects meeting the criteria for reporting
| Pulmonary ADE | Benazepril | Captopril | Enalapril | Fosinopril | Lisinopril | Perindopril | Quinapril | Ramipril | Candesartan | Irbesartan | Losartan | Olmesartan | Telmisartan | Valsartan |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PULMONARY EDEMA | 0 | 3 | 1 | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 2 |
| PLEURAL EFFUSION | 0 | 3 | 2 | 3 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| OROPHARYNGEAL PAIN | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 2 | 2 | 1 | 1 |
| DYSPNEA | 1 | 3 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 |
| DYSPHONIA | 0 | 0 | 1 | 0 | 1 | 3 | 0 | 0 | 3 | 0 | 3 | 1 | 0 | 1 |
| COUGH | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| SINUSITIS | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 3 | 3 | 1 | 1 | 0 | 0 | 1 |
| PNEUMONIA | 1 | 3 | 2 | 0 | 1 | 3 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 2 |
| NASOPHARYNGITIS | 0 | 0 | 1 | 0 | 1 | 3 | 0 | 1 | 1 | 3 | 1 | 1 | 1 | 1 |
| BRONCHITIS | 0 | 3 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 3 | 1 |
| PNEUMONIA ASPIRATION | 0 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 2 |
| EMPHYSEMA | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| PLEURISY | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 3 |
Note: Numbers in the table indicate how many of the following criteriaa are met: (1) at least 3 incidences, (2) a proportional reporting ratio greater than 2, and (3) a proportional reporting ratio that is more than the lower 95% CI boundary with the lower CI being greater than 1.
Source: U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research and Center for Biologics Evaluation and Research. Guidance for Industry Good Pharmacovigilance Assessment Guidance for Industry Practices and Pharmacoepidemiologic Assessment. Available at: http://www.fda.gov/cber/guidelines.htm. Accessed April 14, 2020.
References
- 1.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study [published correction appears in Lancet. 2020 Mar 28;395(10229):1038] Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.James P.A., Oparil S., Carter B.L. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth joint national committee (JNC 8) [published correction appears in JAMA. 2014 May 7;311(17):1809] JAMA. 2014;311(5):507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association Standards of medical care in diabetes-2020 abridged for primary care providers. Clin Diabetes. 2020;38(1):10–38. doi: 10.2337/cd20-as01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hilal-Danden R. Chapter 26: Renin and angiotensin. In: Brunton L.L., Hilal-Dandan R., Knollman B.C., editors. Goodman and Gilman’s: The Pharmacological Basis of Therapeutics. 13th ed. McGraw-Hill; New York: 2020. [Google Scholar]
- 6.Furberg C.D., Pitt B. Are all angiotensin-converting enzyme inhibitors interchangeable? J Am Coll Cardiol. 2001;37(5):1456–1460. doi: 10.1016/s0735-1097(01)01161-5. [DOI] [PubMed] [Google Scholar]
- 7.Patel A.B., Verma A. COVID-19 and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: what is the evidence? JAMA. 2020;323(18):1769–1770. doi: 10.1001/jama.2020.4812. [DOI] [PubMed] [Google Scholar]
- 8.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4):e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Staley J., Mazloom R., Lowe P. Novel data sharing agreement to accelerate big data translational research projects in the one health sphere. Top Companion Anim Med. 2019;37:100367. doi: 10.1016/j.tcam.2019.100367. [DOI] [PubMed] [Google Scholar]
- 10.Xu X., Mazloom R., Goligerdian A. Making sense of pharmacovigilance and drug adverse event reporting: comparative similarity association analysis using AI machine learning algorithms in dogs and cats. Top Companion Anim Med. 2019;37 doi: 10.1016/j.tcam.2019.100366. [DOI] [PubMed] [Google Scholar]
- 11.U.S. Food and Drug Administration Tissue guidances. http://www.fda.gov/cber/guidelines.htm Available at:
- 12.Wishart D.S., Knox C., Guo A.C. DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006;34(Database issue):D668–D672. doi: 10.1093/nar/gkj067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Böhm R. Primer on disproportionality analysis. Openvigil. openvigil.sourceforge.net/doc/DPA.pdf Available at.
- 14.Trott O., Olson A.J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31(2):455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brett A.S., Rind D.M. ACE inhibitors and ARBs during the COVID-19 pandemic. NEJM J Watch. https://www.jwatch.org/na51345/2020/04/09/ace-inhibitors-and-arbs-during-covid-19-pandemic Available at. Accessed May 5, 2020.