Abstract
A characteristic of bacterial zoonoses, diseases caused by bacteria that can be transmitted to humans from animals, is a propensity to re-emerge. Several studies demonstrate their ongoing transmission in Nigeria, the most populous country in Africa. However, as local epidemiological data on bacterial zoonoses are inadequate the extent and impact of these infectious diseases is under-reported. Consequently, they are not a targeted priority of national public health policies. This limited recognition is despite indications of their possible roles in the widespread prevalence of non-malarial undifferentiated fever in Nigeria. While a number of animal reservoirs and arthropod vectors have been identified in the transmission routes of these diseases, an escalation of cases of undiagnosed febrile illness highlights the urgent need for a comprehensive assessment of other potential reservoirs, vectors and transmission cycles that may increase the local risk of infection with bacterial zoonoses. Animal health interventions have been proposed as a cost-effective strategy. Here, we present a broad overview of bacterial zoonotic infections of humans in Nigeria in the context of evolving epidemiological patterns. Further, we propose that facilitating the operation of a community-based One Health program is essential to providing the comprehensive epidemiological information that is required to improve prioritization of bacterial zoonoses. This would provide a driver for much needed investment in relevant public health interventions in Africa's most populous country.
Keywords: Public health, Infectious disease, Medical microbiology, Microbiology, Epidemiology, Bacteriology, Diagnostics, Bacterium, Zoonosis, Vector, Reservoir, Transmission, Fever, Nigeria, One health
Public Health; Infectious Disease; Medical Microbiology; Microbiology; Epidemiology; Bacteriology; Diagnostics; Bacterium; Zoonosis; Vector; Reservoir; transmission; fever; Nigeria; One Health
1. Introduction
Infections of domestic and wild animals that are transmitted directly or by indirect means to humans are a major cause of morbidity and mortality worldwide, including in Nigeria, the nation with the largest population and economy in Africa [1]. Approximately 60% of the 1,500 or more infectious microorganisms that are known human pathogens are recognized as zoonotic, i.e. they normally exist in animals but they can also infect people [2, 3]. While around 73% of currently emerging and re-emerging pathogenic agents cause zoonoses [2, 3], even more common infectious diseases of great importance to global public health, such as HIV/AIDS and malaria, are known to have emerged from zoonotic origins [2]. It has thus been speculated that present and future generations may face the highest risk of exposure to zoonotic infections in human history, requiring us to address the impact this may have on life expectancy and quality of health [2, 3].
Bacterial zoonoses such as bubonic plague and bovine tuberculosis exacted an extremely heavy toll on humankind in the medieval period, a time prior to the introduction of acceptable hygiene practices and the advent of vaccines and antibiotics. In this light, the present growing emergence and spread of bacterial zoonoses has generated worldwide concern, with animals commonly identified as reservoir hosts shown to harbour a great diversity of potential pathogens [3]. It is estimated that the livelihood of more than 600 million people globally is livestock-dependent. These communities represent up to 70% of the marginalized and poor population that is most exposed to the risk of zoonoses but which is typically isolated from delivery of adequate healthcare [4].
Bacterial zoonoses represent a class of often-neglected human infections that may be responsible for a significant proportion of febrile illnesses without localizing features, especially in malaria-endemic areas of sub-Sahara Africa where frequently they may be misdiagnosed as the more prevalent protozoan infections [5]. Although there are no published estimates of incidence of fever of unknown origin (FUO) in Nigeria the condition remains a challenging medical problem and reports have shown indications of common occurrence and subsequent misdiagnoses in aetiological determination [6, 7]. Notwithstanding the prevalence of FUO, potentially important fever-causing pathogens have not been investigated rigorously in many low- and middle-income nations, especially in rural locations, despite the apparent exposure of humans to animal reservoirs and vectors of zoonotic infections [8]. A comprehensive understanding of preventable and treatable infectious causes of severe febrile illness is crucial to realizing a degree of disease control in developing countries and to improving outcomes in resident healthcare-seeking patients [8].
This review discusses neglected zoonotic bacterial pathogens in Nigeria, West Africa. The focus is on common emerging and re-emerging zoonoses, drawing on notable developments in the field from recent global research, highlighting novel findings from those Nigerian studies conducted to date and identifying key aspects of their epidemiology that are not yet described in local investigations. We particularly highlight important bacterial zoonoses for which insights into transmission have not advanced in Nigeria as rapidly as elsewhere in the world. Most of these zoonotic diseases have also been identified as potentially significant contributors to cases of febrile illness in other parts of malaria-endemic sub-Sahara Africa [5]. Embracing the One Health concept to combat bacterial zoonoses in Nigeria fosters a collaborative, multisectoral and transdisciplinary approach to human and animal health management and interventions, the principles of which can be applied in similar low-income contexts elsewhere.
2. Main text
2.1. Emerging and re-emerging bacterial zoonoses of notable public health importance in Nigeria
Since they are often associated with a high case fatality rate, zoonoses dominate Nigeria's register of infectious diseases for which emergency notification of incidence is required [9]. Notifiable zoonoses in Nigeria include anthrax, plague, Lassa fever, Ebola virus disease, rabies, trypanosomiasis, salmonellosis, tuberculosis, influenza and yellow fever, most of which are considered endemic in the country [1, 9, 10]. Sporadic cases of other zoonotic infections such as leptospirosis, scabies, pentastomiasis and African histoplasmosis are additionally thought to occur [1]. Cryptosporidiosis and food-borne Campylobacter and Escherichia coli O157:H7 infections also constitute nationally important zoonoses [1]. Throughout the developing world, neglected zoonotic diseases are considered endemic, with an inherent capacity to result in localized outbreaks and larger epidemics [11].
Bacterial zoonotic infections have a remarkable potential to re-emerge after being considered as eradicated or under control, and thus they pose serious and continual threats to public health [3]. Sometimes a bacterial zoonosis may also emerge newly in an area where it was previously unknown. For instance, the isolation of the Gram-negative bacterial zoonotic agent Candidatus Neoehrlichia mikurensis from 4% of the dog tick pools collected from four states in Nigeria is its first known description in Africa [12]. The emergence and re-emergence of bacterial zoonoses have been attributed to a combination of climatic, ecological, agricultural and socio-economic factors that create an uncertain public health situation [1]. The scenario presented is of an increasing risk of bacterial zoonotic infections to public health in Nigeria. However, here – as across a large geographical footprint of the developing world [8] – fewer studies are conducted to investigate this class of zoonosis than most other infectious agents.
2.1.1. Bartonellosis
Bartonellae are facultative intracellular, fastidious, bacteria that infect both erythrocytes and endothelial cells, employing a silent strategy to prevent their rapid clearance by the host's immune system [13]. More than 30 species of this group of Gram-negative bacteria are currently known, many of which were described only recently. This includes a growing number of Bartonella species recognized as human pathogens responsible for a variety of clinical manifestations [14].
Species of Bartonella such as B. elizabethae and B. grahamii, known to cause human diseases in Peru and Thailand [13, 15], were identified in 26% of commensal rodents in a study conducted in Jos, the capital city of Plateau State, Nigeria [16]. Another investigation detected DNA of Bartonella spp. in Nigeria similar to those revealed in Ghana and Kenya in 51.4% of bat blood samples screened [7]. An apparently new species of the bacterium, named B. rousetti, was reported recently in Ondo State, Nigeria, with indications of ongoing human infection in Idanre communities [14]. The infection prevalence among inhabitants was approximately 4%. To date, this is the only known report of possible human infection with bartonellosis in Nigeria.
Immunosuppression arising from varied causes such as cancer treatment, pregnancy, organ transplant, diabetes, alcoholism and even infancy is identified as an important risk factor for humans to contract bartonellosis and other bacterial zoonotic infections [3].
2.1.2. Brucellosis
Brucella spp. are Gram-negative, facultative coccobacilli. Brucellosis remains a major neglected zoonotic disease of low-income nations. Although assessments of existing data suggest ongoing transmission of human brucellosis in Nigeria, information on the causative Brucella species is not sufficiently clear from the limited bacteriological studies [11, 17]. To date, reports of human infections are restricted to serological detection of human antibodies (Table 1), and this may not provide clear representation of the distribution of human infection by B. abortus or B. melitensis [17]. Zoonotic infection with Brucella is associated with handling of wild and domestic animals, providing opportunity for direct transmission to occur [17]. Consumption of raw or unpasteurized milk also constitutes an important transmission route for Brucella spp. [17].
Table 1.
Reports of human infection with important bacterial zoonoses in Nigeria.
| Zoonosis | Year of Report | Location | Study Subjects | Species/Serovars | Prevalence |
|---|---|---|---|---|---|
| Bartonellosis | 2018 [14] | Idanre | Community residents | Bartonella rousetti | 3.9% (8/204) |
| Brucellosis | 1974 [106] ∗ | Unavailable | |||
| 1976 [107] ∗ | Western Nigeria | Dairy farmers, slaughter men, general population | Brucella abortus | >55.0% | |
| 1977 [108] | Northern and Western Nigeria | Blood donors, ante-natal patients, abattoir workers, veterinarians, dairy farmers, herdsmen | B. abortus | 59.2% (1186/2010) | |
| 1979 [109] | Ibarapa | Herdsmen, abattoir workers, high school students | B. abortus | 35.6% (103/289) | |
| 1980 [110] | Ibadan | Livestock farmers | B. abortus | 5.9% | |
| 1987 [111] | Minna and Abeokuta | B. abortus | 9.0% | ||
| B. melitensis | 11.7% | ||||
| 1993 [112] ∗ | Maiduguri | Unavailable | |||
| 1996 [113] | Calabar | 18.3% | |||
| 2001 [114] | Maiduguri | Patients | B. abortus | 5.2% (26/500) | |
| 2006 [115] | Ibadan | Butchers, abattoir workers, herdsmen | Brucella spp. | 33.3% (7/21) | |
| 2008 [116] | Sokoto | Prison inmates | B. abortus, B. melitensis | 7.1% (2/28) | |
| 2010 [117] | Jos | Abattoir workers | Brucella spp. | 5.0% (5/101) | |
| 2014 [118] | Makurdi | Patients | B. abortus, B. melitensis | 7.6% (79/1040) | |
| Leptospirosis | 1991 [20] | Plateau | Volunteers |
Leptospira pomona, L. canicola, L. grippotyphosa, L. hardjo, L. pyrogenes, L. icterohaemorrhagiae, L. autumnalis |
18.0% (128/710) |
| 1993 [21] | Enugu | Coal miners, butchers, abattoir workers, local farmers, hospital laboratory personnel |
L. canicola, L. pomona, L. icterohaemorrhagiae, L. hardjo, L. pyrogenes, L. autumnalis, L. grippotyphosa |
13.5% (89/661) | |
| 2001 [22] | Nigeria (countrywide) | 20.4% | |||
| 2013 [19] | Abuja | Kennel workers | L. interrogans | 50.0% (10/20) | |
| Q fever | 1959 [119] | Ibadan | Blood donors | Rickettsia burnetii | 3.7% (12/323) |
| 1987 [111] | Minna and Abeokuta | Patients | Coxiella burnetii | 63.3% | |
| 1990 [120] | Sokoto | Patients | C. burnetii | 44.0% (33/75) | |
| Rickettsiosis | 1947 [121] | Jos | Patients | 89.1% (82/92) | |
| 1978 [122] ∗ | Enugu | Patients | Unavailable | ||
| 1987 [111] | Minna and Abeokuta | Patients | Rickettsia conorii | 18.6% | |
| 2008 [123] | Jos | Inmates | R. typhi | 71.1% | |
| Salmonellosis | 1958 [124] ∗ | Ibadan | Patients | NTS | Unavailable |
| 1959 [125] | Ibadan | Blood donors, patients | 74 NTS | 9.5% (200/2117) | |
| 1960 [126] ∗ | Ibadan, Lagos and Jos | Patients, villagers | Salmonella enterica serotypes: S. Dublin, S. Enteritidis, S. Typhimurium, S. Chailey, S. Stanleyville, S. Stockholm, S. Aminatu, S. Ank, S. Agama, S. Kaapstad, S. Aequotoria, S. Nigeria, S. Oranienburg, S. Virchow, S. Utah, S. Wangata, S. Oxford, S. Rubislaw, S. Tel-Elkebir, S. Brazil, S. Infantis, S. Dakar, S. Offa, S. Marseille, S. Loenga | Unavailable | |
| 1983 [127] | Lagos | Patients | S. Typhimurium | 1.2% (12/994) | |
| S. Enteritidis | 0.3% (3/994) | ||||
| S. Oranienburg | 0.91% (9/994) | ||||
| 1988 [128] | Abeokuta |
S. Mbandaka, S. Gold Coast, S. Durham, S. Antsalova, S. Wippra, S. Agama, S. Isangi |
4.2% (9/216) | ||
| 1994 [129] | Lagos | Paediatric patients | S. Typhimurium | 1.3% (4/315) | |
| 1995 [130] | Southeast Nigeria | Patients |
S. Typhimurium, S. Enteritidis, S. Hardar, S. Virchow, S. Bredeney, non-typeable strains |
7.4% (60/809) | |
| 2000 [131] | Lagos | Patients | S. Arizonae | 2.5% (16/635) | |
| 2002 [132] | Nsukka | Patients | 11.0% (55/500) | ||
| 2005 [133] ∗ | Zaria | Patients | NTS | Unavailable | |
| 2007 [134] | Lagos | Patients |
S. Enteritidis, S. Arizonae |
10.2% (45/441) | |
| 2008 [135] | Lagos | Patients | Blood (S. Typhimurium, S. Enteritidis) | 3.0% (6/201) | |
| Stool (S. Typhimurium) | 6.3% (3/48) | ||||
| 2008 [136] | Lagos | Food vendors | S. Enteritidis | 7.6% (4/53) | |
| 2009 [137] | Ibadan | Paediatric patients | S. Typhimurium | 0.08% (1/1210) | |
| 2010 [45] | Ibadan | Patients |
S. Enteritidis, S. Dublin, S. Typhimurium, S. Jukestown, S. Monschaui, S. Oritamerin, S. Apapa |
3.2% (32/991) | |
| 2010 [138] | Lagos | Patients | 4.3% (6/140) | ||
| 2011 [91] | Abuja | Paediatric patients | NTS | 0.8% (8/969) | |
| 2012 [139] | Lagos | Patients | NTS | 64.5% (54919/85187) | |
| 2013 [103] | Maiduguri | Patients |
S. Hadar, S. Eko, S. Enteritidis, S. Give, S. Uganda, S. Amager, S. Verviers, Salmonella 47:mt:- |
5.5% (27/490) | |
| 2014 [140] | Lagos | Patients | S. Enteritidis | 1.9% (2/105) | |
| 2015 [141] | Kano and Abuja | Paediatric patients | NTS | 0.9% (94/10133) | |
| 2017 [142] | Ibadan | Patients | S. Colindale, S. Agama, S. Bredney, S. Butantan, S. Chandans, S. Corvallis, S. Dakar, S. Gatehead, S. Give, S. Kentucky, S. Kibusi, S. Liverpool, S. Nigeria, S. Oranienburg, S. Poona, S. Typhimurium, S. Rubislaw, S. Urbana, Salmonella 4,5,12:i:-, Salmonella 43:d:-, Salmonella 6,7,d | 7.6% (30/394) | |
| 2019 [143] | Lagos | Food handlers |
S. Limete, S. Portland, S. Huettwillen, S. Mowanjum, S. Typhimurium, S. Takoradi, S. Chagoua |
1.96% (7/358) | |
| Yersiniosis | 1982 [100] | Zaria | Patients | Yersinia enterocolitica | 21.3% (10/47) |
| 1983 [127] | Lagos | Patients | Y. enterocolitica | 1.4% (14/994) | |
| 1983 [144] | Lagos | Patients | Y. enterocolitica | 0.55% (6/1082) | |
| 1986 [145] | Lagos and Jos | Patients | Y. enterocolitica | 9.9% (99/1000) | |
| 1987 [146] | Minna and Abeokuta | Patients | Yersinia spp. | 16.4% | |
| 1987 [147] | Ile-Ife | Pupils | Y. enterocolitica | 4.3% (32/752) | |
| 1990 [148] | Calabar | Patients | Y. enterocolitica | 1.0% | |
| 1992 [149] | Lagos | Patients | Yersinia spp. | 1.4% | |
| 1992 [150] | Ibadan | Patients | Y. enterocolitica | 3.8% (8/210) | |
| 1993 [151] | Enugu | Patients | Y. enterocolitica | 1.4% (9/638) | |
| 1994 [129] | Lagos | Paediatric patients | Y. enterocolitica | 0.6% (2/315) | |
| 1996 [152] ∗ | Lagos | Patients | Y. enterocolitica | <0.6% | |
| 1997 [153] | Edo, Lagos and Cross River | Patients | Y. enterocolitica | 1.9% (45/2400) | |
| 2009 [55] | Plateau | Patients |
Y. enterocolitica, Y. pseudotuberculosis |
5.8% (29/500) |
NTS = Non-typhoidal Salmonella spp.
Limited accessible data.
2.1.3. Leptospirosis
In a study of risk factors for Leptospira infection published in 2015 a trend towards disease outbreak in Nigeria was reported for this zoonotic pathogen, a Gram-negative, obligate aerobe spirochaete [18]. Leptospirosis is an important bacterial zoonosis with a significant level of sporadic occurrences, especially in northern Nigeria where animal husbandry is common [1]. A national average incidence of six clinical cases of leptospirosis is reported annually, which is considered to be an underestimate [19]. In Plateau and Enugu States, human infection rates of 18.0% and 13.5%, respectively, were detected in epidemiological surveys reported between 1991 and 1993 [20,21], while a more recent countrywide investigation in 2001 reported a prevalence of 20.4% [22]. Studies over the past two decades and more latterly in different regions of Nigeria have detected by serology testing and urine examination various serovars of the pathogenic Leptospira interrogans, such as Hardjo and Canicola, which have been implicated as the cause of human disease in other parts of the world [18, 20, 21, 22, 23].
Infection with Leptospira has been associated with handling of wild and domestic animals, by which direct transmission may occur through the skin [24]. Contact with pets and livestock in northern Nigeria poses an increased risk of Leptospira infection, which may be acquired via abraded skin during incautious handling of infected animals and their fluids or tissues [18, 19]. However, the contrary nature of observations from other countries suggests that there is no significant association between a person contracting leptospirosis and their close contact with livestock or other animals, implying a complex pattern of region- and setting-specific factors in infection risk [25]. As investigations of leptospirosis in Nigeria are few in number [25], clustered mainly to the north and conducted among typical risk groups [18, 19], it is uncertain if such heterogeneity in findings exists across all the different regions and varied settings nationally.
Other indirect sources of Leptospira infection include soil and water contaminated with urine or other body fluids from infected animals via which Leptospira interrogans may enter the human body through mucous membranes of the eyes and nose during activities such as bathing [18, 26]. Food-borne transmission may also be possible but current evidence suggests that this is rare [27]. Heavy rainfall and flooding are also associated with outbreaks of leptospirosis around the world, in areas prone to overcrowding, inadequate waste disposal and poor hygiene [26, 28]. Such conditions are prevalent in Nigeria among the socioeconomically disadvantaged populations living in shanty towns, slums and relief camps [29].
2.1.4. Q fever
Q fever in humans is caused by infection with Coxiella burnetii, a Gram-negative coccobacillus that is widely regarded as a potential agent of bioterrorism [30]. Since the first description of the disease in slaughterhouse workers in Brisbane, Australia, in 1937, Q fever has become one of the bacterial zoonotic infections with a rising global distribution, especially in developing countries like Nigeria [3, 8]. C. burnetii has been reported at high serological prevalence in West African nations and has also been detected in veterinary studies in Nigeria, where 27% of the livestock and 0.1–14% of the ticks screened in Oyo State were infected [31]; cattle, goats and sheep are most commonly infected and can serve as reservoirs for the bacterium. Several indications of local human infection have also been documented in the country (Table 1).
Consumption of raw or unpasteurized milk is a noted means of infection with Coxiella burnetii [3]. Shedding of the bacterium was reported in milk samples from cattle in Zaria, Nigeria, in studies conducted between 1983 and 1984 [32, 33, 34]. Associated with dairy farms, Q fever is a devastating zoonotic disease, especially within goat herds, and poses a threat even in industrialized nations such as the Netherlands, where it accounted for outbreaks from 2007 to 2010 [3,35]. In Nigeria, among Fulani pastoralists who commonly practice dairy farming [36, 37, 38], the possible risk to livestock of C. burnetii infection should be assessed.
The most important transmission route associated with C. burnetii infection in humans is inhalation of aerosolized organisms [39]. Aerosols carrying C. burnetii may arise from its shedding by infected animals, usually during parturition or lactation, and are inhaled from vaginal mucus, placental fluid, urine and milk, as well as from contaminated environmental dust [34, 35]. Acquisition of Q fever via inhalation of contaminated aerosols or ingestion of raw milk or fresh dairy products has been reported in humans [39, 40], yet has not been described in Nigeria. A national assessment of the current risk of C. burnetii infection from environmental aerosols and dairy products may be justified, especially as around 90% of the milk produced in rural areas is consumed raw [41]. Q fever is also known to pose the risk to immunodeficient individuals of a chronic disease condition [34].
2.1.5. Rickettsiosis
Rickettsiae are obligate intracellular pathogens that have a wide global distribution and which commonly belong to the typhus group (TG) or spotted fever group (SFG). Studies have reported this group of Gram-negative coccobacilli as potential agents of zoonoses in Nigeria, detected by PCR and sequencing in 8.8% of dogs and 10.5% of their ectoparasites in Plateau, Kwara, Kaduna and Rivers States [12, 42]. Rickettsia massiliae, R. conorii israelensis and R. africae-like species have been identified as agents of bacterial zoonoses with possible ongoing transmission in the country.
Rickettsia africae, the causative agent of African tick-bite fever in humans, has been detected in several species of ticks, the arthropod vectors, from Nigeria and other West African countries [12]. The aetiological agent of Mediterranean spotted fever is R. conorii israelensis, the presence of which in Nigeria was first reported in 2013 [12]. R. felis, a prominent emerging rickettsial pathogen that is common in Senegal and Kenya [43], has a largely unknown epidemiology in the rest of Africa, including Nigeria [12, 31, 42, 43].
2.1.6. Salmonellosis
Non-typhoidal salmonellosis is one of the most important enteric zoonoses globally [44]. Salmonella enterica serovars Typhimurium and Enteritidis are reportedly the leading causes of sporadic outbreaks of human salmonellosis in Africa, a trend that is observed in Ibadan, the capital and most populous city of Oyo State, Nigeria, where these serovars collectively accounted for 62.5% of non-typhoidal Salmonella (NTS) infections in examined patients [45, 46, 47]. Other less common serovars of the Gram-negative, flagellate, facultatively aerobic bacillus have a more geographically restricted distribution.
The rare S. enterica Hidudiffy was the predominant serovar in 95% of the 41 serotyped isolates in a study conducted in northern Nigeria but was absent in a similar investigation in the southern region of the country [45, 46, 47]. This is believed to be attributable to such factors as variation in climate or farming systems between the two regions and may need verification [45]. Other rare serovars that have been detected in Nigeria include S. enterica Apapa, Mouschaui and Vinohrady [45, 46, 47, 48]. In a recent large-scale surveillance program in Nigeria, S. enterica Eko was identified as a major serovar – constituting 17.5% of 149 positive samples – among the 17 different serotypes that are potentially being transmitted locally in north-eastern parts of the country [47].
Zoonotic agents like Salmonella may be acquired from contact with animals, their dung and droppings, as well as via indirect transmission by ingesting contaminated food [3]. This bacterium is a frequent cause of human food-borne bacterial gastroenteritis in both developing and developed regions of the world. Research performed on food products from markets in the Nigerian states of Sokoto, Plateau and Oyo associated Salmonella spp. with common transmission via contamination of poultry meat and eggs [3, 44, 49, 50, 51]. A recent investigation in the north-east of the country also identified vegetables as a potential source of salmonellosis transmission, corroborating reports of the increasing global frequency of Salmonella outbreaks [48]. Salmonella is also a leading cause of bacteraemia in at-risk populations including immunosuppressed individuals and infants across sub-Saharan Africa [3, 46].
2.1.7. Yersiniosis/Plague
Plague is an important re-emergent bacterial zoonosis caused by Yersinia pestis, one of the most extensively studied pathogenic bacteria, with current case reports restricted to Africa [52]. Although earlier modelling of the geographical distribution of plague in Africa using 1970–2007 human plague data suggested that Nigeria has had no report of plague, subsequent examination of historical records from 1877 to 2008 indicated that plague has occurred in the country [52, 53]. The disease takes three main forms: pneumonic, generally contracted by inhalation of aerosolized organisms; septicaemic and bubonic, which are each spread via flea bites, contact with an infected animal, their dung and droppings, or through ingestion of contaminated food [3, 54].
Infection with Y. pseudotuberculosis in humans is rare, and the report of Y. pseudotuberculosis in Nigeria is possibly the first report of this Gram-negative, facultatively anaerobic coccobacillus in the tropics [55]. Gastrointestinal yersiniosis, due to infection with the pathogenic Y. enterocolitica, was also documented in 5.8% of patients examined in the same study, conducted in Plateau State [55]. Bioserotypes 1B/O:8, 2/O:5,27, 2/O:9, 3/O:3 and 4/O:3 constitute the pathogenic strains of Y. enterocolitica that are associated most commonly with human yersiniosis outbreaks [56]. Similar strains have been reported from human gastrointestinal infections recorded in different parts of the country [55].
Y. enterocolitica is a ubiquitous food-borne microorganism [56]. Together with Y. pseudotuberculosis, it is known to occur predominantly in temperate zones [55]. However, aspects of the epidemiology of food-borne yersiniosis in developing countries, such as contamination routes in food, remain poorly understood and require clarification [56].
2.2. Animal reservoirs and the role of arthropods in transmission
Livestock and animal reservoirs are recognized to contribute substantially to the continued transmission of bacterial zoonoses. This involves passive transmission through bites and scratches, as well as contamination of the environment [26, 30, 57]. In addition, active transmission by vectors is implicated in bacterial zoonotic infections [3]. The widespread distribution of zoonotic pathogens in arthropods as well as domestic and wild animals represents a large reservoir of these disease-causing agents (Table 2). As a result, there is a perpetually high risk of bacteria circulating between infected and susceptible animals, which carries the potential for spread to human hosts [41].
Table 2.
Transmission routes and availability of vaccines for important bacterial zoonoses in Nigeria.
| Zoonosis | Direct/Indirect Transmission Routes | Vectors | Reservoirs | Local Availability of Vaccines |
|---|---|---|---|---|
| Bartonellosis | ∗Scratches or bites of cats and other animals |
∗Bat flies [7] ∗Human, cat and dog fleas [54, 76, 78] Ectoparasites of rats and mice [16] Sand flies, ticks [3, 12, 16] |
∗Cats [58, 59] ∗Bats [7] Rats and mice [13, 16] |
Animal: unknown Human: unknown |
| Brucellosis | Handling of animals [17] Raw or unpasteurized milk [17] |
Livestock and wild animals [17] |
Animal: no known government policy since discontinuation in 1954 [17] Human: unknown |
|
| Leptospirosis |
∗Contact with animals or tissue and body fluid of animals via broken skin [18] Food-borne [18, 26] Contaminated water and soil [18, 26] |
∗Ticks [26, 74] | Wild and domestic animals [24] ∗Rats, other rodents [54] |
Animal: bacterins with limited success [154] Human: unknown |
| Q fever | Raw or unpasteurized milk [3] ∗Inhalation of aerosolized organisms [39] |
Ticks [39, 40] ∗Filth flies [30] |
Livestock [34, 40] |
Animal: unknown Human: unknown |
| Rickettsiosis |
∗Fleas [54, 75] Ticks [31] ∗Ae.albopictus [81] ∗An.gambiae [83] |
Rodents [54] |
Animal: unknown Human: unavailable [155] |
|
| Salmonellosis | Food-borne [3] Contact with animals Contaminated water [3] |
Livestock, camels [47] ∗Household and captive reptiles [45] Amphibians [45] |
Animal: available for limited serovars in poultry [156] Human: under development [157] |
|
| Yersiniosis/Plague |
∗Food-borne [3] Contact with animals Inhalation of aerosolized organisms [54] |
∗Human and cat fleas [52, 54] Other fleas [54, 75] |
Rodents [13, 54] Livestock [55] |
Animal: unknown Human: unknown |
Notable emerging and/or under-studied transmission routes in Nigeria.
2.2.1. Animal reservoirs
Proximity to animals by virtue of a person's lifestyle or type of occupation is generally associated with the re-emergence and escalated risk of infection with bacterial zoonoses. This exposes – among others – farmers, nomads, hunters, veterinarians, pet dealers, wildlife workers, families with pets, butchers and abattoir workers to an increased likelihood of infection [3, 9]. Consequently, bacterial zoonoses are often regarded as occupational diseases in Nigeria [9, 18], where close contact with animals as well as exposure to their tissues and body fluids have been implicated as major risk factors for infection. A prime example is that of leptospirosis contracted during livestock slaughter and processing in abattoirs [18].
It is estimated that approximately 20% of animal bites or scratches become infected, which is an important transmission route to humans for Bartonella henselae, the causative agent of cat-scratch disease. Cats are under-studied as potential reservoirs of locally acquired Bartonella infection in Nigeria despite their relative popularity as companion animals and the frequent occurrence of strays [58, 59], reportedly even in hospital premises in the north of the country [60]. However, bats have been identified as reservoirs of Bartonella in Nigeria in a first finding reported in 2014, where four species of the bacterium were isolated in 15.5% of the Micropterus spp., Eidolon helvum, Epomophorus spp., Rhinolophus spp. and Chaerephon nigeriae bats sampled in the north-eastern city of Bauchi [7]. This is similar to findings elsewhere in Africa, from Kenya, Ghana and Algeria [61, 62, 63].
Bats are believed to serve as hosts of numerous pathogenic bacteria but most studies in Nigeria have chosen to focus on their being a source of infection of zoonotic viral agents. Accordingly, the primary aim of the recent bat surveillance by the Nigerian Health Ministry and partner agencies was to identify new and existing viruses in the flying mammals [7, 64]. Bats are known to live in proximity to humans in many Nigerian communities, where they are used for food, rituals and cultural practices, and thus they may play yet undiscovered roles in the transmission of the Bartonella pathogen and other zoonotic bacterial agents [7, 14].
Bartonella is also associated with rodents [13]. These small mammals are common natural Bartonella reservoirs and close associations with humans due to overcrowded conditions in rural communities enhance infection transmission [15, 16]. Rodents are also reservoirs of other zoonotic bacteria like Yersinia pestis and Rickettsia typhi, the causative agent of murine typhus [54]. Y. pestis has been detected in over 50 species of wild rodents in natural plague foci in Africa and across the globe [13, 54]. Rodents, notably rats, are also widely recognized as notorious reservoirs of Leptospira interrogans, usually spreading leptospirosis through their infected urine [28]. A range of wild and domestic animals that serve as reservoirs of Leptospira spp. have been studied extensively for leptospirosis in Nigeria. Dogs, cattle, pigs, sheep and goats are commonly known worldwide to shed the bacterium in their urine, thereby contaminating the environment for several months or years after infection [23, 24, 28]. However, very few attempts have been made to investigate the contribution of rodents to the epidemiology of the disease in Nigeria, with the last known report as long ago as 1990 [28,65].
Livestock are important reservoirs of zoonotic infections like brucellosis and Q fever, for which ruminants provide a major source of human infections [17, 34, 40]. A study in Oyo State indicated that cows may be reservoirs of Q fever in Nigeria [31]. Livestock are also sources of food-borne zoonotic pathogens like Y. pseudotuberculosis and Y. enterocolitica, for which pigs, sheep, goats and cattle were identified as principal reservoirs of the pathogenic serotypes of human infection in Nigeria [55]. Also, genomic analysis of Salmonella enterica Eko isolates, agents of salmonellosis, collected from different sources in Nigeria showed an association of camels with NTS outbreaks [47]. This implicates camels, along with cattle, as a primary source of local human infections.
In developing countries, where the sources and transmission routes of salmonellosis are poorly understood, common food-producing animals are regarded as the primary reservoirs of Salmonella. This includes chickens, which studies in Nigeria, like elsewhere, identified as predominant reservoirs of a wide range of Salmonella serovars including Hadar and others known for their ability to colonize poultry [46, 48]. However, Salmonella transmission has also been associated with household reptiles like wall geckos and captive reptiles in Nigeria and across the world, suggesting the potentially emerging role of reptiles as reservoirs of the disease [45]. The first reported case of human infection with S. enterica Apapa was linked to a pet lizard as the source of infection [66]. Infections with this and other similar, rare serovars Jukestown, Mouschaui and Oritamerin were detected in Ibadan, where these bacteria were absent from chickens screened for salmonellosis. This suggests transmission by unverified infection sources such as household lizards that are common in the area, as previously these rare serovars have been linked to amphibians and reptiles [45]. Molecular studies may be required to identify the sources associated with human infection, in order to establish key environmental reservoirs of infection.
2.2.2. Ticks
Tick-borne pathogens are recognized as important aetiological agents of human and animal diseases [16]. Considered second only to mosquitoes as carriers of infectious agents [16, 67], ticks were originally identified in the 1930s as potential vectors of Q fever following isolation of the first strain of Coxiella burnetii from a Rocky Mountain wood tick, Dermacentor andersoni [40]. Due to the frequent detection of C. burnetii in field-sampled ticks and experimental demonstration of vector competence in some tick species, it is currently considered that ticks act as transmitters of C. burnetii [40].
The tropical bont tick, Amblyomma variegatum, has a proficient capacity to support transmission of C. burnetii, and it has been identified as a reservoir of C. burnetii and Rickettsia africae in Nigeria and Uganda [39, 68]. A. variegatum is the largest and most abundant tick species in Nigeria, posing a significant threat of Q fever transmission in Plateau, Nassarawa and Oyo States where C. burnetii has been isolated from collected ticks [39]. There is a need, however, to interpret carefully the detection of C. burnetii in ticks until such time as a method for direct differentiation of C. burnetii and Coxiella-like bacteria is developed [40]. Nonetheless, having followed the currently recommended protocols for C. burnetii screening, these findings are highly suggestive of a reservoir role for ticks in the transmission of Q fever in Nigeria.
Tick-borne rickettsiosis is also common in West Africa. Rickettsia conorii israelensis has been reported in Senegal [69] and was first detected in Nigeria in the brown dog tick, Rhipicephalus sanguineus [12]. R. africae was identified in cattle ticks, including those of the Rhipicephalus and Hyalomma genera, from a study that screened fed and questing ticks in Oyo State [31]. This rickettsial species is frequently associated with Amblyomma ticks and less often with other potential vectors such as Rhipicephalus annulatus [70]. However, R. africae was detected in feeding ticks only, which may have been acquired in a blood meal from an infected host [31, 70, 71, 72]. Thus, from these reports it is unclear which tick species are possible vectors of R. africae in Nigeria and there is a need for further elucidation of their vector competence. Another study in Nigeria in which R. africae was absent from dogs and brown dog ticks may point to a specific association of this zoonotic bacterium with cattle and game ticks [12], as reported previously in South Africa [73]. The presence of Rickettsia massilae and R. rickettsii in unfed ticks but not in their cattle hosts suggests that ticks are both a major vector and reservoir for this infectious agent in Nigeria [31].
Similarly, in a study in Plateau State, Nigeria, Bartonella spp. DNA was detected in R. sanguineus but was absent from rodent hosts [16]. This may indicate that the brown dog tick, whose role in Bartonella transmission has not been proven previously, may be a potential local reservoir. Comparison of the prevalence of bacterial pathogens in Nigerian feeding and questing ticks reiterates that their detection in the former does not establish vector competence, that is the ability of the tick to acquire, maintain and transmit a given species of bacterium [31]. For instance, a higher prevalence of C. burnetii was observed in feeding Rhipicephalus evertsi ticks than in questing ticks collected from cattle in Oyo State, Nigeria. This stresses the need for experimental demonstration of vector competence in ticks that are able to maintain transstadial transmission of important zoonotic bacterial pathogens [31].
Although the in vitro vector competence of tick species and the isolation of Leptospira spp. from them were both demonstrated several decades ago, it is a common misconception that the association of arthropods with leptospirosis transmission is unsubstantiated [24, 74]. However, Leptospira DNA was detected for the first time in ticks of the Ixodes genus collected from a flooded area of Poland, Eastern Europe [74]. This may indicate a transition of Leptospira, aided by environmental changes, to adapt to new hosts and maintain transmission through them, as was noted previously for L. interrogans Hardjo serovar [26]. Confirmation of this finding requires further investigation in other regions of the world where ticks are known to contribute to transmission of zoonotic bacteria. However, while there are areas of Nigeria that are prone to flooding, Ixodes ticks of medical and veterinary importance are not known to be present in these geographical zones [16].
2.2.3. Fleas and other ectoparasites
The last decade has witnessed emerging reports on fleas, popularly known for transmitting the plague-causing Yersinia pestis, as potential contributors to the transmission of zoonotic agents like Rickettsia typhi, R. felis and Bartonella henselae [54, 75]. Thus, there is a need to investigate this haematophagous insect vector in Nigeria, a geographical area where information on the possible role of fleas in transmission of zoonoses is scanty. Performed in 2011, the first known screening of rodents and their ectoparasites for Bartonella in Nigeria detected species of the bacterium in 28% of the fleas, ticks, earwigs, and other arthropods found; some isolates represent known strains while others are of uncertain identity and require further characterization and evaluation for possible pathogenic status [16]. The number of known vectors of Bartonella also continues to increase, with reports of other haematophagous arthropods such as sand flies, lice and mites as transmitters of bartonelloses between mammalian hosts, including humans [3, 12]. This calls for investigations in localities where the endemicity of these emerging vectors may overlap with the habitats of known reservoirs of Bartonella spp. in Nigeria.
Meanwhile, the human flea, Pulex irritans, which has been found to infest pets in Nigeria [76], and the cat flea, Ctenocephalides felis, have been associated with the recently re-emerged Bartonella quintana infection in some parts of the developed world [54]. Cat fleas and some other flea species are also regarded as major reservoirs and biological vectors of R. felis, capable of horizontal and vertical transmission of this pathogen. However, contrary reports from Senegal suggest that cat fleas do not contribute to local transmission of R. felis despite the high prevalence of human infections [54, 77]. Cat fleas, found on both cats and dogs, are also responsible for the transmission of B. henselae [3, 54, 78].
Dog fleas like Ctenocephalides canis have been associated with B. henselae elsewhere globally [79]. Although these ectoparasites have equally been reported at high prevalence in Nigeria, where pet dogs are common and share close bonds with humans, the potential roles of dog fleas in the local transmission of zoonotic infection is poorly understood in the country [76, 80]. It is also believed that any species of flea is capable of transmitting Y. pestis under appropriate conditions, and that P. irritans may play an important role in human-to-human transmission of the plague [54]. Often regarded as a mere nuisance due to earlier reports of vector incompetence for Y. pestis, cat fleas were suspected to account for a plague outbreak in Uganda [54]. Yet, while the human flea and other flea species are known to be found on pets in Nigeria, there is currently no record associating fleas with local transmission of Y. pestis [52].
2.2.4. Emerging role of mosquitoes and other flies
Recent findings indicate that mosquitoes may have emerged as vectors of Rickettsia spp. in West Africa. R. felis was detected in the Asian tiger mosquito, Aedes albopictus, in Gabon, a country to the south of Nigeria, at infection loads similar to those observed in the cat flea, which is currently regarded as the primary vector [81].
A species invasive to Nigeria, since its discovery in Delta State Ae. albopictus now dominates over the native yellow fever mosquito, Ae. aegypti. Recent analysis reveals a wide distribution across the south of the country [82]. Ae. albopictus was originally identified in Nigeria in 1991 and first reported in Gabon in 2006. While its role in transmitting arboviral infections is a major focus of investigation in Nigeria its possible association with R. felis does not appear to be a research priority [82]. In Senegal, a West African country where Ae. albopictus is not known to occur, the major African malaria vector, Anopheles gambiae, presents a risk of rickettsial infection as R. felis and a new Rickettsia sp. were detected in An. gambiae and An. melas [83]. This zoonotic bacterial agent was also detected in Anopheles vectors in Gabon and Cote d’Ivoire.
Similar observations suggesting a possible role for mosquitoes in transmission of rickettsiae were documented in China, where R. felis and a species closely related to the new Rickettsia species reported in Senegal and Cote d’Ivoire were also detected in An. sinensis and Culex pipiens [84, 85]. The conclusions drawn from these studies add to the growing evidence implicating mosquitoes from a range of genera in the transmission of SFG rickettsiae infections in different parts of the world where they are endemic. It is particularly noteworthy that in China the rickettsial organisms were also detected in the plant-feeding male Cx. pipiens, revealing a capacity for this mosquito species to maintain rickettsial infection by vertical transovarian transmission.
These findings are strong indications that mosquito transmission may play a part in the high risk of infection with R. felis that was reported in Senegal where transmission via fleas is absent [43, 77]. However, the potential role of mosquito species in the transmission of rickettsial zoonoses in Nigeria are not yet understood or described. This can also be said of other arthropods, especially filth flies such as the housefly, Musca domestica, which have been identified as potential mechanical vectors capable of transmitting zoonotic infectious agents like Coxiella burnetii in other parts of the world [30, 86]. Blood-feeding bat flies have also been proposed as possible contributors to local transmission of bartonellosis in Nigeria [7].
2.3. Human health implications, surveillance and public health interventions
2.3.1. Known challenges
The prevalence of infection in surveillance indicators such as arthropods and other animal hosts is commonly used to assess the risk of the local transmission of pathogens to humans. Hence, within a defined geographical area, the distribution and abundance of ticks and other haematophagous vectors is considered to determine the epidemiology of vector-borne infections [16, 87]. Studies in Nigeria have shown possible ongoing transmission of emerging and re-emerging bacterial zoonoses with which a number of potential animal reservoirs and vectors have been associated (Table 2), while limited assessments of infection risk have also been undertaken [41]. These investigations represent the few efforts at surveillance of bacterial zoonoses in the country. Human infection data also constitute an important surveillance indicator of infection risks [87]. However, these are essentially lacking in Nigeria for bacterial zoonoses such as bartonellosis, brucellosis and Q fever for which little is known about rates of infection (Table 1). Further research is required to provide comprehensive information on clinical cases and prevailing infection rates [17, 41]. Serological evidence of human brucellosis has been reported but no details of Brucella isolation from patients in Nigeria are available [17].
Estimates of vector-borne infectious diseases burden in low- and middle-income countries tend to focus on malaria and dengue while bacterial zoonoses are largely overlooked [8]. This is despite reports from Nigeria that warrant the assessment of disease burden in humans of Coxiella burnetii, Rickettsia conorii israelensis and other pathogenic zoonotic bacteria, plus consideration of the public health implications associated with their various animal reservoirs and vectors in the country [16, 31]. In Nigeria, brucellosis is frequently misdiagnosed as malaria [17], a common occurrence in the case reporting of bacterial zoonoses in Africa and other malaria-endemic regions [88]. A recent study in northern Tanzania showed that clinical cases of bacterial zoonoses such as leptospirosis, brucellosis, rickettsioses and Q fever may often be excluded or misdiagnosed as the more prevalent malaria in areas of its endemicity [5]. Following a steady decline in malaria burden, there has been a gradually increasing awareness of bacterial zoonoses as major causes of FUO in sub-Sahara Africa [5, 89, 90]. In Abuja, Nigeria's centrally located capital city, where the historical over-diagnosis of malaria is suspected, non-typhoidal Salmonella infection was reported in the aetiological diagnoses of febrile cases [91]. In contrast, for an FUO case in south-east Nigeria important aetiological agents may have been overlooked and presumptive malaria treatment was adopted, a common practice in this area that has also been reported for the case management of febrile paediatric patients [92, 93].
Access to diagnostic tools is a conspicuous challenge. The multiple causes of fever are difficult to distinguish clinically and hence many cases of FUO in low-resource contexts may be attributed to either lack of access to or limited provision of suitable medical microbiology laboratory services [8]. In the case of brucellosis, for instance, serological diagnostic tools are typically sophisticated as they were developed in high-income countries long after the disease was eradicated. As a consequence, these are better suited to well-equipped laboratories that are extremely scarce in resource-limited settings, especially for point-of-care needs [17]. It was reported that Nigerian abattoir workers who eventually received a correct diagnosis of Brucella infection frequently complained of continued treatment for malaria despite their condition failing to improve [17]. This is a good example of the actuality that patients in Africa infected with bacterial zoonoses are likely to be discharged from hospital without receiving a correct diagnosis and thus the appropriate specific treatment.
It is also evident that clinicians often lack information on the local epidemiology of causes of severe febrile illness. As a result, internationally set management guidelines and disease control programs currently have insufficient data to set local priorities for prevention [8]. Focus on a wide range of potential causes of FUO, especially zoonotic infections, has been recommended for patient management and disease control in resource-limited settings [5]. It is expected that better national surveillance and reporting will improve disease control strategies, whereby clinicians play a pivotal role in the effective management of zoonoses with enhanced differential diagnoses [3].
Notable antimicrobial resistance of public health concern in food-borne zoonoses such as salmonellosis has been reported in Nigeria [45, 94], but the indiscriminate use of antibiotics as animal growth promoters remains a common practice and calls for stringent regulations to be imposed. Isolates of Salmonella enterica Typhimurium similar to the multidrug-resistant ST313 clone of sub-Sahara Africa, as well as other strains resistant to antimicrobials such as tetracycline and sulfamethoxazole, were identified in Ibadan [45]. This was attributed to the non-selective use of antibiotics at therapeutic or subtherapeutic levels, a practice that was observed in cattle examined from the same locality in which residual doses of oxytetracycline and penicillin-G were detected [95]. Of related concern, recent investigation in south-west Nigeria showed an increasing trend in the use of fluoroquinolones, tetracyclines, beta-lactams/aminoglycosides and macrolides for questionable purposes ranging from animal disease management to livestock growth promotion [94]. Meanwhile, other countries led by the USA have prohibited the use of quinolones as growth promoters in poultry feed due to the public health implications of similarities of pharmaceuticals used to treat human and animal infections [3, 94]. In Nigeria, there is no such strict legislation governing antibiotic residues in animal tissues, while there is inadequate emphasis on regulation of veterinary drugs for human and animal health safety as the responsibility of the local food and drug administration agency [96].
2.3.2. A One Health approach
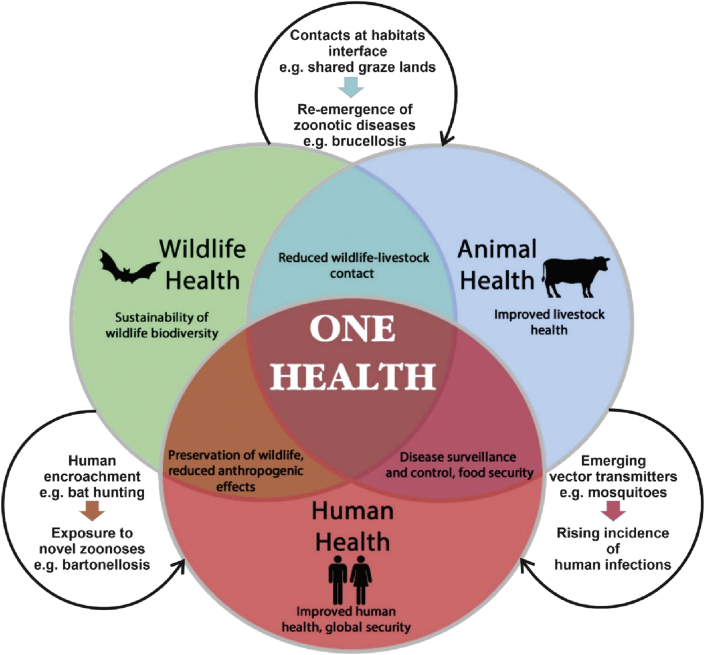
Although approaches to public health interventions for zoonotic infections in Nigeria have been proposed, a case for disease prioritization and consequently, investment and funding, will depend on evaluation of both human and animal health impacts [11, 41]. Early reports identified the importance of the joint endeavours of medical and veterinary professionals to the control of zoonoses affecting public health and animal welfare in Nigeria [1]. Subsequently, the role of key stakeholders in the Ministries of Agriculture, Health and Information to enhance the health of both human and animal populations has been emphasized [9, 41]. The complex interrelations and interdependence of human, animal and environmental health has given rise to the ‘One Health’ concept (Figure 1). This advocates for the collaborative efforts of professionals from multiple disciplines, such as clinicians, veterinarians and environmentalists, working at state, national and international levels in order to achieve better public health outcomes [2]. While there is increasing global support for nations to adopt the One Health approach, in Nigeria there is a failure to recognize interdisciplinary commonalities, especially between clinicians and veterinarians. This remains a significant challenge to progress in tackling zoonotic infections in the country despite rising awareness of misdiagnosed undifferentiated fever [2, 41]. In 2009, the US Centers for Disease Control and Prevention launched the Animal-Human Interface Project (AHIP) in Nigeria, providing consultations and technical training to facilitate the progress of in-country One Health [64]. Several collaborative projects were fostered by the AHIP along with partner agencies in Nigeria to investigate a range of zoonotic infections; however, the predominant attention was focused on viral diseases. This is possibly a reflection of the assertion that viral zoonoses are capable of influencing greater political and economic responses, as exemplified by the recent Ebola outbreaks in Nigeria and DR Congo [97, 98] as well as the current COVID-19 global pandemic.
Figure 1.
Schematic representation of the One Health concept.
Vector-borne diseases are a major target of the One Health approach. Together with other endemic infectious diseases these have increased in global incidence during the past two decades. A large number of vector-borne infections have emerged in regions where they were hitherto unknown [3]. In regard to Nigeria, this heightened public health threat emphasizes a need for up-to-date and authentic information on bacterial zoonoses, especially for vector-borne zoonotic agents that have been shown in recent times to emerge in novel vectors, such as Rickettsia felis in mosquitoes [76]. A correlation exists between the epidemiology of R. felis infection and malaria in sub-Sahara Africa [71]. This indicates an increased risk of rickettsial zoonoses in this region compared to others. Mosquito species identified as potential vectors of R. felis in other West African nations as well as in China, and which may be responsible for its transmission in Senegal [83], are known to be endemic to Nigeria [77, 81, 82, 83, 84, 85]. Although human rickettsial infections have yet to be confirmed in Nigeria, as a precautionary measure they are highlighted as a source of potential disease for incoming travelers [99]. Another pathogenic agent of bacterial zoonosis, Yersinia pseudotuberculosis, was described recently for the first time in Nigeria [55], which indicates an extension of its geographical range into the country.
In the One Health strategy, a major recommendation for food-borne zoonoses is farm-level intervention (Figure 1). This includes routine vaccination, immune stimulants and probiotic feed additives for animal health management as well as other initiatives including animal welfare policies and measures to curb antimicrobial resistance [3]. However, successful adoption of such interventions in Nigeria is hampered by setbacks such as problems of sustainability due to the dismantling of disease control programs [41]. Moreover, culling of livestock with subsequent compensation of farmers, a strategy that has proved beneficial to zoonosis control in the developed world, has been ineffective in Nigeria. This is because farmers, notably Fulani pastoralists, are not willing to cooperate when infectious disease control involves deliberate loss of their herds [41]. Yet, changing lifestyles among settled Fulani communities, including healthcare-seeking attitudes and practices, may lead possibly to increased receptiveness of this influential group to comply with animal health interventions in the future [37].
2.3.3. Under-reporting abets under-recognition
The risk of infection with bacterial zoonoses is particularly high in rural communities and among those in close contact with wild animals or livestock. Unfortunately, however, zoonoses affecting primarily subsistence farmers and low-income residents of non-urban localities are not assigned a defined control or prevention program in Nigeria's national healthcare scheme [11, 41]. Under-reporting of case numbers and under-estimation of the human impact of a disease are recognized as factors in it being devalued for health policy prioritization and investment for intervention [11].
In sub-Sahara Africa, where hospitals and healthcare clinics typically are not readily accessible to the most affected people, accurate statistics on morbidity and mortality resulting from bacterial zoonoses are difficult to obtain [11]. Bartonella spp. have been associated with endocarditis and neuroretinitis in other parts of the world, such as Peru [13], but there is no known link to human disease for the identical strains which have been isolated in Nigeria [12]. In Senegal, where levels of detection of C. burnetii in reservoir cattle are lower than those in Nigeria, seroprevalence rates in humans are reportedly as high as 21.4%–51% [34], suggesting that these may be even surpassed in Nigeria [31]. However, this possibility needs to be substantiated through future investigations. An exception to the systemic trend to under-report is food-borne yersiniosis caused by the pathogenic Y. enterolitica. This is diagnosed among Nigerian communities at levels similar to those reported by developed nations, in all situations being associated primarily with chronic gastrointestinal illness and glomerulopathy [56, 100].
A case of human leptospirosis in Plateau State and a localized outbreak among dogs and their handlers in a kennel in Abuja have been reported [19, 20], yet no known survey of leptospirosis as a cause of febrile illness has been conducted in the country [28]. In 2006, zoonotic infections including leptospirosis, brucellosis and salmonellosis were identified as under-recognized and under-reported in Nigeria [101]. The true extent of the health impacts of these zoonoses, particularly in the human population, was unknown. Moving forward more than a decade, the situation is still largely unchanged for most of these diseases, exemplified by brucellosis [17]. Consequently, under-appreciation of pathogenic agents like zoonotic bacteria in Nigeria contributes to the lack of an integrated approach to avert morbidity and mortality from febrile illnesses. This relative neglect contrasts starkly with pneumonia and diarrhoea, for instance, each of which is the focus of a well-coordinated global effort across most of the range of its causative pathogens [4, 5].
Understanding the case distribution as well as health impacts of zoonotic diseases is an important step in planning and implementing effective control and prevention measures [102]. In developing countries, coordinated epidemiological systems for national surveillance of zoonotic infections are generally inadequate despite indications of a substantial burden of disease [46, 49]. A large-scale study was carried out in 2009–2011 to provide the first comprehensive surveillance of salmonellosis in human and non-human samples in Nigeria but this was limited to only one state in the north-east [103], so is unlikely to be representative of the entire country since some serovars of NTS are commonly known to have a geographically restricted distribution [47]. Reports on human brucellosis have concentrated on the classic at-risk population and thereby neglected other potentially susceptible groups [17]. As a consequence, around a century since its original identification in Nigeria, very little is understood of Brucella regarding accurate evaluation of its zoonotic potential and thus to inform the roll-out of relevant control measures. A vaccination program was discontinued in 1954, yet brucellosis remains a national health concern [17]. Using global burden of human disease methodology, Nigerian policymakers depend on unreliable metrics obtained mostly from scant data from other regions, thereby contributing to a systemic under-valuation of zoonotic diseases nationally [11]. The resultant lack of resource commitment to health issues in Nigeria and other developing countries presents an ongoing challenge to interdisciplinary One Health collaborations and partnerships that aim to harness evaluation and analysis of health status to enhance public health surveillance and disease control [41].
2.3.4. Cost-effective strategies
Cost-effectiveness of interventions has been proposed as an alternative criterion to overall burden of disease to determine priority-setting for investment in disease control [104]. The presence of multiple reservoir hosts was highlighted as a major hurdle to the eradication of zoonoses in Nigeria. Hence, interventions targeted extensively at animal reservoirs and accompanied by promotion of public awareness have been suggested as cost-effective strategies to control bacterial and non-bacterial zoonoses in the country [9, 41]. Investing resources into the surveillance and treatment of animal populations rarely enjoys robust and influential support in most of the developing world despite the rewards of improved human and animal health in promoting food security and eradicating poverty [11]. The costs of intervention for zoonotic diseases can seem excessive when balanced against the public health benefits alone. Vaccines have either not been developed or are unavailable locally in Nigeria (Table 2). It is believed that evidence of disease burdens imposed on communities by zoonotic diseases combined with a demonstration of the cost-effectiveness of integrated control can strengthen the case for a One Health approach to endemic zoonotic disease control [11]. There are strong indications that investment in animal health interventions and veterinary care will facilitate protection of humans against exposure to transmission of zoonotic infections from animal hosts [41, 105].
The full benefits of a multidisciplinary analysis covering health and economic factors across all sectors will easily outweigh costs and enable the health sector to present arguments to policymakers based on the rate of return on investment rather than on the impact on disability life-adjusted years [11]. It is therefore recommended that interventions against zoonotic infections in Nigeria should be viewed as investments in human capital and form an integral part of poverty reduction plans [11].
3. Conclusions
In order to improve human and animal health security in Nigeria, there is a need for cost-effective strategies that will increase the prioritization of bacterial zoonoses in health policies and encourage investments in health interventions. Current epidemiological data and surveillance of bacterial zoonoses in the country are inadequate, a circumstance that obstructs the progress of One Health development. This constitutes a setback to collaborative engagements between relevant disciplines and qualified professionals such as veterinarians and clinicians. While various species and strains of common and rare pathogenic zoonotic bacteria have been identified in vectors and animal reservoirs in Nigeria, for most of these there is very limited information available on their potential impact on human health. Recent research indicating the emergence of some zoonotic bacterial pathogens in hitherto unknown vectors and in new geographical locations calls for re-assessment of their previously recorded national distribution or officially listed non-occurrence. There is a clear and significant knowledge gap in regard to bacterial zoonoses in Nigeria, the closing of which is crucial to the success of future control and preventive public health interventions. The implementation of such measures will likely lead to a reduction in local incidence rates of patients with undifferentiated fever.
Declarations
Author contribution statement
Both authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Coker A.O., Isokpehi R.D., Thomas B.N., Fagbenro-Beyioku A.F., Omilabu S.A. Zoonotic infections in Nigeria: overview from a medical perspective. Acta Trop. 2000;76(1):59–63. doi: 10.1016/s0001-706x(00)00091-7. [DOI] [PubMed] [Google Scholar]
- 2.Yakubu Y., Junaidu A.U., Magaji A.A., Salihu M.D., Mahmuda A., Shehu S. One Health — the fate of public health in Nigeria. Asian J. Med. Sci. 2011;3(1):47–49. [Google Scholar]
- 3.Cantas L., Suer K. Review: the important bacterial zoonoses in “One Health” concept. Front. Public Health. 2014;2:144. doi: 10.3389/fpubh.2014.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ngutor K.S. A multidisciplinary approach in the control of zoonoses in Nigeria. J. Vet. Adv. 2012;2(12):557–567. [Google Scholar]
- 5.Crump J.A., Morrissey A.B., Nicholson W.L., Massung R.F., Stoddard R.A., Galloway R.L. Etiology of severe non-malaria febrile illness in Northern Tanzania: a prospective cohort study. PLoS Negl. Trop. Dis. 2013;7(7):e2324. doi: 10.1371/journal.pntd.0002324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oladosu O.O., Oyibo W.A. Overdiagnosis and overtreatment of malaria in children that presented with fever in Lagos, Nigeria. ISRN Infect. Dis. 2013;2013:914675. [Google Scholar]
- 7.Kamani J., Baneth G., Mitchell M., Mumcuoglu K.Y., Gutiérrez R., Harrus S. Bartonella species in bats (Chiroptera) and bat flies (Nycteribiidae) from Nigeria, West Africa. Vector Borne Zoonotic Dis. 2014;14(9):625–632. doi: 10.1089/vbz.2013.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prasad N., Murdoch D.R., Reyburn H., Crump J.A. Etiology of severe febrile illness in low- and middle-income countries: a systematic review. PLoS One. 2015;10(6):e0127962. doi: 10.1371/journal.pone.0127962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Musa O.I., Akanbi A.A., II, Bolarinwa O.A. Epidemiology and control of zoonotic infections in Nigeria. Afr. Sci. 2007;8(2):109–114. [Google Scholar]
- 10.Isere E.E., Fatiregun A.A., Ajay I.O. An overview of disease surveillance and notification system in Nigeria and the roles of clinicians in disease outbreak prevention and control. Niger. Med. J. 2015;56(3):161–168. doi: 10.4103/0300-1652.160347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welburn S.C., Beange I., Ducrotoy M.J., Okello A.L. The neglected zoonoses — the case for integrated control and advocacy. Clin. Microbiol. Infect. 2015;21(5):433–443. doi: 10.1016/j.cmi.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Kamani J., Baneth G., Mumcuoglu K.Y., Waziri N.E., Eyal O., Guthmann Y. Molecular detection and characterization of tick-borne pathogens in dogs and ticks from Nigeria. PLoS Negl. Trop. Dis. 2013;7(3):e2108. doi: 10.1371/journal.pntd.0002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutiérrez R., Krasnov B., Morick D., Gottlieb Y., Khokhlova I.S., Harrus S. Bartonella infection in rodents and their flea ectoparasites: an overview. Vector Borne Zoonotic Dis. 2015;15(1):27–39. doi: 10.1089/vbz.2014.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bai Y., Osinubi M.O.V., Osikowicz L., McKee C., Vora N.M., Rizzo M.R. Human exposure to novel Bartonella species from contact with fruit bats. Emerg. Infect. Dis. 2018;24(12):2317–2323. doi: 10.3201/eid2412.181204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin-Alonso A., Soto M., Foronda P., Aguilar E., Bonnet G., Pacheco R. Bartonella spp. and Yersinia pestis reservoirs, Cusco, Peru. Emerg. Infect. Dis. 2014;20(6):1069–1070. doi: 10.3201/eid2006.131194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamani J., Morick D., Mumcuoglu K.Y., Harrus S. Prevalence and diversity of Bartonella species in commensal rodents and ectoparasites from Nigeria, West Africa. PLoS Negl. Trop. Dis. 2013;7(5):e2246. doi: 10.1371/journal.pntd.0002246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ducrotoy M.J., Bertu W.J., Ocholi R.A., Gusi A.M., Bryssinckx W., Welburn S. Brucellosis as an emerging threat in developing economies: lessons from Nigeria. PLoS Negl. Trop. Dis. 2014;8(7) doi: 10.1371/journal.pntd.0003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abiayi E.A., Inabo H.I., Jatau E.D., Makinde A.A., Sar T.T., Ugbe D.A. Knowledge, attitudes, risk factors and practices (KARP) that favor Leptospira infection among abattoir workers in North Central Nigeria. Asian J. Epidemiol. 2015;8(4):104–113. [Google Scholar]
- 19.Awosanya E.J., Nguku P., Oyemakinde A., Omobowale O. Factors associated with probable cluster of leptospirosis among kennel workers in Abuja, Nigeria. Pan Afr. Med. J. 2013;16(1):144. doi: 10.11604/pamj.2013.16.144.3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ezeh A.O., Adesiyun A.A., Addo P.B., Ellis W.A., Makinde A.A., Bello C.S. Serological and cultural examination for human leptospirosis in Plateau State, Nigeria. Cent. Afr. J. Med. 1991;37(1):11–15. [PubMed] [Google Scholar]
- 21.Onyemelukwe N.F. A serological survey for leptospirosis in the Enugu area of eastern Nigeria among people at occupational risk. J. Trop. Med. Hyg. 1993;96(5):301–304. [PubMed] [Google Scholar]
- 22.Agunloye C.A., Alabi F.O., Odemuyiwa S.O., Olaleye O.D. Leptospirosis in Nigerians: a seroepidemiological survey. Indian Vet. J. 2001;78(5):371–375. [Google Scholar]
- 23.Brown K., Prescott J. Leptospirosis in the family dog: a public health perspective. Can. Med. Assoc. J. 2008;178(4):399–401. doi: 10.1503/cmaj.071097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luiz-Adrian J.A. Leptospirosis: identification and treatment in companion animals. Pac. Agric. Nat. Resour. 2008;15(1):1–4. [Google Scholar]
- 25.Mwachui M.A., Crump L., Hartskeerl R., Zinsstag J., Hattendorf J. Environmental and behavioural determinants of leptospirosis transmission: a systematic review. PLoS Negl. Trop. Dis. 2015;9(9) doi: 10.1371/journal.pntd.0003843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terpstra W.J. Historical perspectives in leptospirosis. Indian J. Med. Microbiol. 2006;24(4):316–320. doi: 10.4103/0255-0857.29407. [DOI] [PubMed] [Google Scholar]
- 27.Gkogka E., Reij M.W., Havelaar A.H., Zwietering M.H., Gorris L.G. Risk-based estimate of effect of foodborne diseases on public health, Greece. Emerg. Infect. Dis. 2011;17(9):1581–1590. doi: 10.3201/eid1709.101766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Vries S.G., Visser B.J., Nagel I.M., Goris M.G.A., Hartskeerl R.A., Grobusch M.P. Leptospirosis in sub-Saharan Africa: a systematic review. Int. J. Infect. Dis. 2014;28(1):47–64. doi: 10.1016/j.ijid.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 29.Ito E.E., Egwunyenga A.O. Schistosomiasis: the aftermath of 2012 floods in Delta State, southern Nigeria. Int. Med. J. 2015;22(4):218–223. [Google Scholar]
- 30.Nelder M.P., Lloyd J.E., Loftis A.D., Reeves W.K. Coxiella burnetii in wild-caught filth flies. Emerg. Infect. Dis. 2008;14(6):1002–1004. doi: 10.3201/eid1406.071691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reye A.L., Arinola O.G., Hübschen J.M., Muller C.P. Pathogen prevalence in ticks collected from the vegetation and livestock in Nigeria. Appl. Environ. Microbiol. 2012;78(8):2562–2568. doi: 10.1128/AEM.06686-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adesiyun A.A., Jagun A.G., Tekdek L.B. Coxiella burnetii antibodies in some Nigerian dairy cows and their suckling calves. Int. J. Zoonoses. 1984;11(2):155–160. [PubMed] [Google Scholar]
- 33.Adesiyun A.A., Jagun A.G., Kwaga J.K., Tekdek L.B. Shedding of Coxiella burnetii in milk by Nigerian dairy and dual purposes cows. Int. J. Zoonoses. 1985;12(1):1–5. [PubMed] [Google Scholar]
- 34.Vanderburg S., Rubach M.P., Halliday J.E.B., Cleaveland S., Reddy E.A., Crump J.A. Epidemiology of Coxiella burnetii infection in Africa: a OneHealth systematic review. PLoS Negl. Trop. Dis. 2014;8(4) doi: 10.1371/journal.pntd.0002787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abbasi S., Farzan R., Momtaz H. Molecular detection of Coxiella burnetii in goat bulk milk samples in some provinces of Iran. Afr. J. Biotechnol. 2011;10(80):18513–18515. [Google Scholar]
- 36.Köster H.W., de Wolff J., Ikpo K.P. International Fertilizer Development Center, Muscle Shoals; USA: 2012. Dairy Development Programme in Nigeria Baseline Report. Accelerating Agribusiness in Africa (AAA) Baseline Project. [Google Scholar]
- 37.Omotayo A.M., Dipeolu M.A., Ekpo U.F. John Archers; Ibadan, Nigeria: 2005. Health Consequences of Lifestyle Changes Among Settled Fulani Pastoralists in South Western Nigeria. [Google Scholar]
- 38.Saleh M.K., Atala T.K., Omokore D.F., Ahmed B., Ali F.S., Kajang G.Y. Performance of improved dairy cattle technologies among farmers in Northern Nigeria. J. Agric. Ext. 2016;20(1):1–12. [Google Scholar]
- 39.Ogo N.I., Fernández de Mera I.G., Galindo R.C., Okubanjo O.O., Inuwa H.M., Agbede R.I.S. Genetic characterization of Coxiella burnetii in Amblyomma variegatum ticks from North-central Nigeria: public health importance. Vet. World. 2013;6(10):818–822. [Google Scholar]
- 40.Elsa J., Duron O., Séverine B., González-Acuña D., Sidi-Boumedine K. Molecular methods routinely used to detect Coxiella burnetii in ticks cross-react with Coxiella-like bacteria. Infect. Ecol. Epidemiol. 2015;5:29230. doi: 10.3402/iee.v5.29230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ehizibolo D.O., Ehizibolo P.O., Ehizibolo E.E., Sugun M.Y., Idachaba S.E. The control of neglected zoonotic diseases in Nigeria through animal intervention. Afr. J. Biomed. Res. 2011;14(1):81–88. [Google Scholar]
- 42.Lorusso V., Wijnveld M., Majekodunmi A.O., Dongkum C., Fajinmi A., Dogo A.G. Tick-borne pathogens of zoonotic and veterinary importance in Nigerian cattle. Parasite. Vector. 2016;9:217. doi: 10.1186/s13071-016-1504-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mediannikov O., Socolovschi C., Edouard S., Fenollar F., Mouffok N., Bassene H. Common epidemiology of Rickettsia felis infection and malaria. Africa. Emerg. Infect. Dis. 2013;19(11):1775–1783. doi: 10.3201/eid1911.130361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olatoye O., Ogunsemoyin O. Prevalence and antibiotics resistance of Campylobacter jejuni in retail chickens in Oyo State, Nigeria. Food Sci. Qual. Manag. 2016;48(1):7–11. [Google Scholar]
- 45.Fashae K., Ogunsola F., Aarestrup F.M., Hendriksen R.S. Antimicrobial susceptibility and serovars of Salmonella from chickens and humans in Ibadan, Nigeria. J. Infect. Dev. Ctries. 2010;4(8):484–494. doi: 10.3855/jidc.909. [DOI] [PubMed] [Google Scholar]
- 46.Kagambèga A., Lienemann T., Aulu L., Traoré A.S., Barro N. Prevalence and characterization of Salmonella enterica from the feces of cattle, poultry, swine and hedgehogs in Burkina Faso and their comparison to human Salmonella isolates. BMC Microbiol. 2013;13:253. doi: 10.1186/1471-2180-13-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leekitcharoenphon P., Raufu I., Nielsen M.T., Rosenqvist Lund B.S., Ameh J.A., Ambali A.G., Sørensen G. Investigating Salmonella Eko from various sources in Nigeria by whole genome sequencing to identify the source of human infections. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0156212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raufu I.A., Zongur L., Lawan F.A., Bello H.S., Adamu M.S. Prevalence and antimicrobial profiles of Salmonella serovars from vegetables in Maiduguri, North eastern Nigeria. Sokoto J. Vet. Sci. 2014;12(1):23–28. [Google Scholar]
- 49.Coker A.O., Isokpehi R.D., Thomas B.N., Amisu K.O., Obi C.L. Human campylobacteriosis in developing countries. Emerg. Infect. Dis. 2002;8(3):237–244. doi: 10.3201/eid0803.010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salihu M.D., Junaidu A.U., Abubakar M.B., Magaji A.A., Mohammed L.G. Isolation and characterization of Campylobacter species from camel (Camelus dromedarius) in Sokoto state, Northwestern Nigeria. Int. J. Anim. Vet. Adv. 2009;1(1):25–27. [Google Scholar]
- 51.Mai H.M., Zahraddeen D., Qadeers M.A., Bawa I.A., Echeonwu I.E. Investigation of some species of Salmonella in table eggs sold at different markets in Jos South, Plateau State, Nigeria. Global Adv. Res. J. Microbiol. 2013;2(11):234–238. [Google Scholar]
- 52.Neerinckx S.B., Peterson A.T., Gulinck H., Deckers J., Leirs H. Geographic distribution and ecological niche of plague in sub-Saharan Africa. Int. J. Health Geogr. 2008;7:54. doi: 10.1186/1476-072X-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neerinckx S., Bertherat E., Leirs H. Human plague occurrences in Africa: an overview from 1877 to 2008. Trans. R. Soc. Trop. Med. Hyg. 2010;104:97–103. doi: 10.1016/j.trstmh.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 54.Bitam I., Dittmar K., Parola P., Whiting M.F., Raoult D. Fleas and flea-borne diseases. Int. J. Infect. Dis. 2010;14(8):e667–e676. doi: 10.1016/j.ijid.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 55.J Okwori A.E., Martínez P.O., Fredriksson-Ahomaa M., Agina S.E., Korkeala H. Pathogenic Yersinia enterocolitica 2/O:9 and Yersinia pseudotuberculosis 1/O:1 strains isolated from human and non-human sources in the Plateau State of Nigeria. Food Microbiol. 2009;26(8):872–875. doi: 10.1016/j.fm.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 56.Rahman A., Bonny T.S., Stonsaovapak S., Ananchaipattana C. Yersinia enterocolitica: epidemiological studies and outbreaks. J. Pathog. 2011;2011:239391. doi: 10.4061/2011/239391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rolain J.M., Gouriet F., Brouqui P., Larrey D., Janbon F., Vene S. Concomitant or consecutive infection with Coxiella burnetii and tickborne diseases. Clin. Infect. Dis. 2005;40(1):82–88. doi: 10.1086/426440. [DOI] [PubMed] [Google Scholar]
- 58.Sowemimo O.A. Prevalence and intensity of gastrointestinal parasites of domestic cats in Ode – Irele and Oyo communities, Southwest Nigeria. J. Parasitol. Vector Biol. 2012;4(1):7–13. [Google Scholar]
- 59.Maryam B.K.Y., Gana B.A., Ahmed G.Y., Abubakar W.M., Istifanus S. A survey of blood types of the domestic cat (Felis catus) in Maiduguri, North-eastern Nigeria. J. Veterinar. Sci. Technol. 2014;5(1):152. [Google Scholar]
- 60.Raji A.A., Magaji A.A., Bello M.B., Lawal M.D., Mamuda A., Yahaya M.S. Prevalence of gastrointestinal parasites of stray cats: a case study of two hospitals in Sokoto Metropolis, Sokoto, Nigeria. J. Bacteriol. Parasitol. 2013;4(4):175. [Google Scholar]
- 61.Kosoy M., Bai Y., Lynch T., Kuzmin I.V., Niezgoda M., Franka R. Bartonella spp. in bats, Kenya. Emerg. Infect. Dis. 2010;16(1):1875–1881. doi: 10.3201/eid1612.100601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Billeter S.A., Hayman D.T.S., Peel A.J., Baker K., Wood J.L., Cunningham A. Bartonella species in bat flies (Diptera: Nycteribiidae) from western Africa. Parasitology. 2012;139(3):324–329. doi: 10.1017/S0031182011002113. [DOI] [PubMed] [Google Scholar]
- 63.Leulmi H., Aouadi A., Bitam I., Bessas A., Benakhla A., Raoult D. Detection of Bartonella tamiae, Coxiella burnetii and rickettsiae in arthropods and tissues from wild and domestic animals in northeastern Algeria. Parasite. Vector. 2016;9:27. doi: 10.1186/s13071-016-1316-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Centers for Disease Control and Prevention . National Center for Emerging and Zoonotic Infectious Diseases: One Health Office; 2014. One Health and Zoonoses Activities at 17 Select International Locations.https://www.cdc.gov/ncezid/dhcpp/pdfs/annual-report.pdf [Google Scholar]
- 65.Ezeh A.O., Addo P.B., Adesiyun A.A., Makinde A.A., Bello C.S.S. Serological and bacteriological examination of rodents for leptospirosis in Plateau State, Nigeria. Bull. Anim. Health Prod. Afr. 1990;38:359–360. [Google Scholar]
- 66.Cooke F.J., De Pinna E., Maguire C., Guha S., Pickard D.J., Farrington M. First report of human infection with Salmonella enterica serovar Apapa resulting from exposure to a pet lizard. J. Clin. Microbiol. 2009;47(8):2672–2674. doi: 10.1128/JCM.02475-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parola P., Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin. Infect. Dis. 2001;32(6):897–928. doi: 10.1086/319347. [DOI] [PubMed] [Google Scholar]
- 68.Lorusso V., Gruszka K.A., Majekodunmi A., Igweh A., Welburn S.C., Picozzi K. Rickettsia africae in Amblyomma variegatum ticks, Uganda and Nigeria. Emerg. Infect. Dis. 2013;19(10):1705–1707. doi: 10.3201/eid1910.130389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ratmanov P., Mediannikov O., Raoult D. Vectorborne diseases in West Africa: geographic distribution and geospatial characteristics. Trans. R. Soc. Trop. Med. Hyg. 2013;107(5):273–284. doi: 10.1093/trstmh/trt020. [DOI] [PubMed] [Google Scholar]
- 70.Yssouf A., Socolovschi C., Kernif T., Temmam S., Lagadec E., Tortosa P. First molecular detection of Rickettsia africae in ticks from the Union of the Comoros. Parasite. Vector. 2014;7:444. doi: 10.1186/1756-3305-7-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mediannikov O., Diatta G., Zolia Y., Balde M.C., Kohar H., Trape J.F. Tick-borne rickettsiae in Guinea and Liberia. Ticks Tick. Borne Dis. 2012;3(1):43–48. doi: 10.1016/j.ttbdis.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 72.Portillo A., Pérez-Martínez L., Santibáñez S., Blanco J.R., Ibarra V., Oteo J.A. Detection of Rickettsia africae in Rhipicephalus (Boophilus) decoloratus ticks from the Republic of Botswana, South Africa. Am. J. Trop. Med. Hyg. 2007;77(2):376–377. [PubMed] [Google Scholar]
- 73.Frean J., Blumberg L., Ogunbanjo G.A. Tick bite fever in South Africa. South Afr. Fam. Pract. 2008;50(2):33–35. [Google Scholar]
- 74.Wójcik-Fatla A., Zajac V., Cisak E., Sroka J., Sawczyn A., Dutkiewicz J. Leptospirosis as a tick-borne disease? Detection of Leptospira spp. in Ixodes ricinus ticks in eastern Poland. Ann. Agric. Environ. Med. 2012;19(4):656–659. [PubMed] [Google Scholar]
- 75.Dobler G., Pfeffer M. Fleas as parasites of the family Canidae. Parasite. Vector. 2011;4:139. doi: 10.1186/1756-3305-4-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ugbomoiko U.S., Ariza L., Heukelbach J. Parasites of importance for human health in Nigerian dogs: high prevalence and limited knowledge of pet owners. BMC Vet. Res. 2008;4:49. doi: 10.1186/1746-6148-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Diemea C., Bechaha Y., Socolovschia C., Audolya G., Berengera J., Faye O. Transmission potential of Rickettsia felis infection by Anopheles gambiae mosquitoes. Proc. Natl. Acad. Sci. U.S.A. 2015;112(26):8088–8093. doi: 10.1073/pnas.1413835112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Centers for Disease Control and Prevention . 2014. Cat-Scratch Disease (Bartonella henselae Infection). Healthy Pets, Healthy People.https://www.cdc.gov/healthypets/diseases/cat-scratch.html [Google Scholar]
- 79.Ishida C., Tsuneoka H., Iino H., Murakami K., Inokuma H., Ohnishi T. Bartonella henselae infection in domestic cat and dog fleas. Kansenshogaku Zasshi. 2001;75(2):133–136. doi: 10.11150/kansenshogakuzasshi1970.75.133. [DOI] [PubMed] [Google Scholar]
- 80.Chukwu C.C. Prevalence of fleas on dogs in Anambra state of Nigeria. Int. J. Zoonoses. 1985;12(3):192–195. [PubMed] [Google Scholar]
- 81.Socolovschi C., Pagés F., Raoult D. Rickettsia felis in Aedes albopictus mosquitoes, Libreville, Gabon. Emerg. Infect. Dis. 2012;18(10):1687–1689. doi: 10.3201/eid1810.120178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Adeleke M.A., Sam-Wobo S.O., Garza-Hernandez J.A., Oluwole A.S., Mafiana C.F., Reyes-Villanueva F. Twenty-three years after the first record of Aedes albopictus in Nigeria: its current distribution and potential epidemiological implications. Afr. Entomol. 2015;23(2):348–355. [Google Scholar]
- 83.Socolovschi C., Pagés F., Ndiath M.O., Ratmanov P., Raoult D. Rickettsia species in African Anopheles mosquitoes. PLoS One. 2012;7(10):e48254. doi: 10.1371/journal.pone.0048254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang J., Lu G., Kelly P., Zhang Z., Wei L., Yu D., Kayizha S., Wang C. First report of Rickettsia felis in China. BMC Infect. Dis. 2014;14:682. doi: 10.1186/s12879-014-0682-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang J., John Kelly P., Lu G., Cruz-Martinez L., Wang C. Rickettsia in mosquitoes, Yangzhou, China. Emerg. Microb. Infect. 2016;5(10):e108. doi: 10.1038/emi.2016.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nichols G.L. Fly transmission of Campylobacter. Emerg. Infect. Dis. 2005;11(3):361–364. doi: 10.3201/eid1103.040460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bustamante D.M., Lord C.C. Sources of error in the estimation of mosquito infection rates used to assess risk of arbovirus transmission. Am. J. Trop. Med. Hyg. 2010;82(6):1172–1184. doi: 10.4269/ajtmh.2010.09-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Omitola O.O., Mogaji H.O., Taylor-Robinson A.W. Managing febrile illness in malaria-endemic areas: developing novel diagnostics using host immunological signatures as surrogate markers of infection. Curr. Immunol. Rev. 2019;15(2):202–206. [Google Scholar]
- 89.Bertherat E., Renaut A., Nabias R., Dubreuil G., Georges-Courbot M.C. Leptospirosis and Ebola virus infection in five gold-panning villages in northeastern Gabon. Am. J. Trop. Med. Hyg. 1999;60(4):610–615. doi: 10.4269/ajtmh.1999.60.610. [DOI] [PubMed] [Google Scholar]
- 90.Steinmann P., Bonfoh B., Péter O., Schelling E., Traoré M., Zinsstag J. Seroprevalence of Q fever in febrile individuals in Mali. Trop. Med. Int. Health. 2005;10(6):612–617. doi: 10.1111/j.1365-3156.2005.01420.x. [DOI] [PubMed] [Google Scholar]
- 91.Obaro S., Lawson L., Essen U., Ibrahim K., Brooks K., Otuneye A. Community acquired bacteremia in young children from Central Nigeria — a pilot study. BMC Infect. Dis. 2011;11:137. doi: 10.1186/1471-2334-11-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Onodugo O., Iroezindu M. Fever of unknown origin in a Nigerian doctor: an unusual case. Internet J. Trop. Med. 2013;9(1):1–4. [Google Scholar]
- 93.Okoro C.I., Chukwuocha U.M., Nwakwuo G.C., Ukaga C.N. Presumptive diagnosis and treatment of malaria in febrile children in parts of South Eastern Nigeria. J. Infect. Dis. Ther. 2015;3(5):240. [Google Scholar]
- 94.Adesokan H.K., Akanbi I.O., Akanbi I.M., Obaweda R.A. Pattern of antimicrobial usage in livestock animals in south-western Nigeria: the need for alternative plans. Onderstepoort J. Vet. Res. 2015;82(1):816. doi: 10.4102/ojvr.v82i1.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Adesokan H.K., Agada C.A., Adetunji V.O., Akanbi I.M. Oxytetracycline and penicillin-G residues in cattle slaughtered in south-western Nigeria: implications for livestock disease management and public health. J. South Afr. Vet. Assoc. 2013;84(1):E1–E5. doi: 10.4102/jsava.v84i1.945. [DOI] [PubMed] [Google Scholar]
- 96.Omeiza G.K., Ajayi I.E., Ode O.J. Assessment of antimicrobial drug residues in beef in Abuja, the Federal capital territory, Nigeria. Vet. Ital. 2012;48(3):283–289. [PubMed] [Google Scholar]
- 97.Obilade T.T. The political economy of the Ebola virus disease (EVD); taking individual and community ownership in the prevention and control of EVD. Healthcare. 2015;3(1):36–49. doi: 10.3390/healthcare3010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Omoleke S.A., Mohammed I., Saidu Y. Ebola viral disease in West Africa: a threat to global health, economy and political stability. J. Publ. Health Afr. 2016;7(1):534. doi: 10.4081/jphia.2016.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Centers for Disease Control and Prevention . Division of Global Migration and Quarantine; 2016. Travelers' Health: Health Information for Travelers to Nigeria. Clinician View.http://wwwnc.cdc.gov/travel/destinations/clinician/none/nigeria [Google Scholar]
- 100.Awunor-Renner C., Lawande R.V. Yersinia and chronic glomerulopathy in the savannah region of Nigeria. Br. Med. J. 1982;285(6353):1464. doi: 10.1136/bmj.285.6353.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Adesiji Y.O., Fagbami A.H. Epidemiology of bacterial zoonoses in Nigeria. Niger. J. Health Biomed. Sci. 2006;5(1):98–104. [Google Scholar]
- 102.Smith D.L., Dushoff J., McKenzie F.E. The risk of a mosquito-borne infection in a heterogeneous environment. PLoS Biol. 2004;2(11):e368. doi: 10.1371/journal.pbio.0020368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Raufu I., Bortolaia V., Svendsen C.A., Ameh J.A., Ambali A.G., Aarestrup F.M. The first attempt of an active integrated laboratory-based Salmonella surveillance programme in the north-eastern region of Nigeria. J. Appl. Microbiol. 2013;115(4):1059–1067. doi: 10.1111/jam.12304. [DOI] [PubMed] [Google Scholar]
- 104.Canning D. Priority setting and the ‘neglected’ tropical diseases. Trans. R. Soc. Trop. Med. Hyg. 2006;100(6):499–504. doi: 10.1016/j.trstmh.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 105.Elelu N., Aiyedun J.O., Mohammed I.G., Oludairo O.O., Odetokun I.A., Mohammed K.M. Neglected zoonotic diseases in Nigeria: role of the public health veterinarian. Pan Afr. Med. J. 2019;32(1):36. doi: 10.11604/pamj.2019.32.36.15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Falade S. Brucella agglutinating antibodies in the sera of persons dwelling in Ibadan and the surrounding districts. J. Niger. Vet. Med. Assoc. 1974;3:21–23. [Google Scholar]
- 107.Alausa O.K., Awoseyi A. Brucellosis: the situation in western Nigeria. Trop. Geogr. Med. 1976;28(1):54–59. [PubMed] [Google Scholar]
- 108.Alausa O.K. Brucellosis: epidemiology and practical problems of control in Nigeria. Publ. Health. 1977;91(3):141–146. doi: 10.1016/s0033-3506(77)80018-8. [DOI] [PubMed] [Google Scholar]
- 109.Alausa O.K. The investigation and control of a large-scale community outbreak of brucellosis in Nigeria. Publ. Health. 1979;93(3):185–193. doi: 10.1016/s0033-3506(79)80124-9. [DOI] [PubMed] [Google Scholar]
- 110.Alausa O.K. Incidence and seasonal prevalence among an occupationally-exposed population to brucellosis. Trop. Geogr. Med. 1980;32(1):12–15. [PubMed] [Google Scholar]
- 111.Sixl W., Rosegger H., Schneeweiss H., Withalm H., Schuhmann G. Serological investigations in Nigeria for anthropozoonoses in human sera: brucellosis, echinococcosis, toxoplasmosis, chlamydial diseases, listeriosis, rickettsiosis (Coxiella burnetii and Rickettsia conori) J. Hyg. Epidemiol. Microbiol. Immunol. 1987;31(4 Suppl):493–495. [PubMed] [Google Scholar]
- 112.Brisibe F., Nawathe D.R., Bot C.J. Serological prevalence of brucellosis in sheep goats and human beings in Maiduguri Metropolis. Trop. Vet. 1993;11:27–33. [Google Scholar]
- 113.Useh M.F., Udo S.M., Oghomu C.J. Sero-epidemiology and perception of human brucellosis in Calabar, Nigeria. Cent. Afr. J. Med. 1996;42(6):184–185. [PubMed] [Google Scholar]
- 114.Baba M.M., Sarkindared S.E., Brisibe F. Serological evidence of brucellosis among predisposed patients with pyrexia of unknown origin in the North Eastern Nigeria. Cent. Eur. J. Publ. Health. 2001;9(3):158–161. [PubMed] [Google Scholar]
- 115.Cadmus S.I.B., Ijagbone I.F., Oputa H.E., Adenosak H.L., Stack J.A. Serological survey of brucellosis in livestock animals and workers in Ibadan, Nigeria. Afr. J. Biomed. Res. 2006;9(3):163–168. [Google Scholar]
- 116.Junaidu A.U., Oboegbulem S.I., Salihu M.D. Seroprevalence of brucellosis in prison farm in Sokoto, Nigeria. Asian J. Epidemiol. 2008;1(1):24–28. [Google Scholar]
- 117.Gusi A.M., Bertu W.J., Mwankwon E.S., Hassan M., Ocholi R.A., Bot C.J. Prevalence of Brucella antibodies in animals and butchers at Jos abattoir, Nigeria. Vom. J. Vet. Sci. 2010;7(1):30–34. [Google Scholar]
- 118.Ofukwu A.R., Yohanna C.A., Abuh H.A. Brucella infection among hospital patients in Makurdi, North Central Nigeria. Med. Online. 2007 http://www.priory.com/med/brucella.htm [Google Scholar]
- 119.Collard P., Udeozo I.O.K. Serological evidence of the existence of Q-fever in Ibadan. West Afr. Med. J. 1959;8(8):137–141. [PubMed] [Google Scholar]
- 120.Blondeau J., Yates L., Martin R., Marrie T., Ukoli P., Thomas A. Q fever in Sokoto, Nigeria. Ann. N. Y. Acad. Sci. 1990;590(1):281–282. doi: 10.1111/j.1749-6632.1990.tb42233.x. [DOI] [PubMed] [Google Scholar]
- 121.Findlay G.M., Elmes B.G.T. Typhus in northern Nigeria. II. Laboratory investigations. Trans. R. Soc. Trop. Med. Hyg. 1947;41(3):339–352. doi: 10.1016/s0035-9203(47)90094-1. [DOI] [PubMed] [Google Scholar]
- 122.Emejuaiwe S.O., Njoku-Obi A.N. Serological evidence for the presence of rickettsial infections in parts of Nigeria. Niger. Med. J. 1978;8(6):514–517. [PubMed] [Google Scholar]
- 123.Lohy N., Stella O., Vendagor N., Mathias K., Chukwu O. Serological evidence of infection with Rickettsia typhi among inmates in Jos Prison, Nigeria. Highl. Med. Res. J. 2008;6(1):1–2. [Google Scholar]
- 124.Collard P., Sen R. Salmonella types isolated in Ibadan, Nigeria. Trans. R. Soc. Trop. Med. Hyg. 1958;52(3):283–287. doi: 10.1016/0035-9203(58)90089-0. [DOI] [PubMed] [Google Scholar]
- 125.Collard P., Sen R., Montefiore D. The distribution of Salmonella agglutinins in sera of healthy adults at Ibadan. J. Hyg. 1959;57(4):427–434. doi: 10.1017/s0022172400020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Collard P., Sen R. Serotypes of Salmonella at Ibadan, Nigeria, with special note of the new serotypes isolated in Nigeria. J. Infect. Dis. 1960;106(5-6):270–275. doi: 10.1093/infdis/106.3.270. [DOI] [PubMed] [Google Scholar]
- 127.Dosunmu-Ogunbi O., Coker A.O., Agbonlahor D.E., Solanke S.O., Uzoma K.C. Local pattern of acute enteric bacterial infections in man — Lagos, Nigeria. Dev. Biol. Stand. 1983;53(3):277–283. [PubMed] [Google Scholar]
- 128.Mascher F., Reinthaler F.F., Sixl W. Serotyping of Salmonella strains in south-west Nigeria: epidemiological aspects. Geogr. Med. 1988;18(1):171–174. [PubMed] [Google Scholar]
- 129.Ogunsanya T.I., Rotimi V.O., Adenuga A. A study of the aetiological agents of childhood diarrhoea in Lagos, Nigeria. J. Med. Microbiol. 1994;40(1):10–14. doi: 10.1099/00222615-40-1-10. [DOI] [PubMed] [Google Scholar]
- 130.Oboegbulam S.I., Oguike J.U., Gugnani H.C. Microbiological studies on cases diagnosed as typhoid/enteric fever in south-east Nigeria. J. Commun. Dis. 1995;27(2):97–100. [PubMed] [Google Scholar]
- 131.Akinyemi K.O., Coker A.O., Olukoya D.K., Oyefolu A.O., Amorighoye E.P., Omonigbehin E.O. Prevalence of multi-drug resistant Salmonella typhi among clinically diagnosed typhoid fever patients in Lagos, Nigeria. Z. Naturforsch. C. J. Biosci. 2000;55(5-6):489–493. doi: 10.1515/znc-2000-5-630. [DOI] [PubMed] [Google Scholar]
- 132.Nzeako B., Okafor N. Bacterial enteropathogens and factors associated with seasonal episodes of gastroenteritis in Nsukka, Nigeria. Br. J. Biomed. Sci. 2002;59(2):76–79. doi: 10.1080/09674845.2002.11783638. [DOI] [PubMed] [Google Scholar]
- 133.Ibrahim Y.K.E., Adedare T.A., Ehinmidu J.O. Antibiotic sensitivity profiles of Salmonella organisms isolated from presumptive typhoid patients in Zaria, northern Nigeria. Afr. J. Med. Med. Sci. 2005;34(2):109–114. [PubMed] [Google Scholar]
- 134.Akinyemi K.O., Bamiro B.S., Coker A.O. Salmonellosis in Lagos, Nigeria: incidence of Plasmodium falciparum-associated co-infection, patterns of antimicrobial resistance, and emergence of reduced susceptibility to fluoroquinolones. J. Health Popul. Nutr. 2007;25(3):351–358. [PMC free article] [PubMed] [Google Scholar]
- 135.Adeleye A., Smith S., Akanmu S., Bamiro S., Sobande O., Igbinosum E. Chromosomally mediated antibiotic resistance in non-typhoidal Salmonellae isolated from HIV patients in Lagos. W. Indian Med. J. 2008;57(5):519–520. [PubMed] [Google Scholar]
- 136.Smith S.I., Alao F., Goodluck H.T., Fowora M., Bamidele M., Omonigbehin E. Prevalence of Salmonella typhi among food handlers from bukkas in Nigeria. Br. J. Biomed. Sci. 2008;65(3):158–160. doi: 10.1080/09674845.2008.11978119. [DOI] [PubMed] [Google Scholar]
- 137.Falade A.G., Lagunju I.A., Bakare R.A., Odekanmi A.A., Adegbola R.A. Invasive pneumococcal disease in children aged < 5 years admitted to 3 urban hospitals in Ibadan, Nigeria. Clin. Infect. Dis. 2009;48(Suppl. 2):S190–S196. doi: 10.1086/596500. [DOI] [PubMed] [Google Scholar]
- 138.Adeyemi A.I., Sulaiman A.A., Solomon B.B., Chinedu O.A., Victor I.A. Bacterial bloodstream infections in HIV-infected adults attending a Lagos teaching hospital. J. Health Popul. Nutr. 2010;28(4):318–326. doi: 10.3329/jhpn.v28i4.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Akinyemi K.O., Oshundare Y.O., Oyeyinka O.G., Coker A.O. A retrospective study of community-acquired Salmonella infections in patients attending public hospitals in Lagos, Nigeria. J. Infect. Dev. Ctries. 2012;5(6):387–395. doi: 10.3855/jidc.2120. [DOI] [PubMed] [Google Scholar]
- 140.Adagbada A.O., Coker A.O., Smith S.I., Adesida S.A. The prevalence and plasmid profile of non-typhoidal salmonellosis in children in Lagos metropolis, South-western Nigeria. Pan Afr. Med. J. 2014;19:359. doi: 10.11604/pamj.2014.19.359.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Obaro S.K., Hassan-Hanga F., Olateju E.K., Umoru D., Lawson L., Olanipekun G. Salmonella bacteremia among children in central and northwest Nigeria, 2008-2015. Clin. Infect. Dis. 2015;61(Suppl. 4):S325–S331. doi: 10.1093/cid/civ745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fashae K., Leekitcharoenphon P., Hendriksen R.S. Phenotypic and genotypic comparison of salmonellae from diarrhoeic and healthy humans and cattle, Nigeria. Zoonoses Public Health. 2017;65(1):e185–e195. doi: 10.1111/zph.12427. [DOI] [PubMed] [Google Scholar]
- 143.Ajayi A., Smith S.I., Kalpy J.C., Bode-Sojobi I.O., René Y.K., Adeleye A.I. Molecular Diversity and antibiotic resistance gene profile of Salmonella enterica serovars isolated from humans and food animals in Lagos, Nigeria. Acta Microbiol. Immunol. Hung. 2019;66(4):509–527. doi: 10.1556/030.66.2019.034. [DOI] [PubMed] [Google Scholar]
- 144.Agbonlahor D.E., Odugbemi T.O., Dosunmu-Ogunbi O. Isolation of species of Yersinia from patients with gastroenteritis in Nigeria. J. Med. Microbiol. 1983;16(1):93–96. doi: 10.1099/00222615-16-1-93. [DOI] [PubMed] [Google Scholar]
- 145.Agbonlahor D.E., Odugbemi T.O., Dosunmu-Ogunbi O. Prevalence of antibodies to human serogroups of Yersinia enterocolitica in blood donors in Nigeria. Cent. Afr. J. Med. 1986;32(9):217–220. [PubMed] [Google Scholar]
- 146.Sixl W., Schneeweiss H., Withalm H., Schuhmann G., Rosegger H. Serological testing of human blood samples for infectious diseases in the Abeokuta and the Minna Hospitals/Nigeria. J. Hyg. Epidemiol. Microbiol. Immunol. 1987;31(4 Suppl):490–492. [PubMed] [Google Scholar]
- 147.Olusanya O., Olusanya O.I. Campylobacter jejuni and Yersinia enterocolitica in urban and rural Nigerian school children. J. Diarrhoeal Dis. Res. 1987;5(2):102–106. [PubMed] [Google Scholar]
- 148.Eko F.O., Utsalo S.J. Comparative study of the prevalence and clinical profiles of diarrheas due to Aeromonas and other enteric pathogens. J. Hyg. Epidemiol. Microbiol. Immunol. 1990;34(2):183–189. [PubMed] [Google Scholar]
- 149.Esumeh F., Odugbemi T. Screening for Listeria monocytogenes and other related species from faecal specimens at the Lagos University Teaching Hospital. Lagos. Afr. J. Med. Med. Sci. 1992;21(2):47–50. [PubMed] [Google Scholar]
- 150.Ikheloa J.O., Aruna M.B., Ayoade G.O. Biotypes and sensitivity screening Yersinia enterocolitica as an infective agent in man and swine in Nigeria. Rev. Elev. Med. Vet. Pays Trop. 1992;45(2):135–137. [PubMed] [Google Scholar]
- 151.Onyemelukwe N.F. Yersinia enterocolitica as an aetiological agent of childhood diarrhoea in Enugu, Nigeria. Cent. Afr. J. Med. 1993;39(9):192–195. [PubMed] [Google Scholar]
- 152.Kesah C.N., Coker A.O., Alabi S.A., Olukoya D.K. Prevalence, antimicrobial properties and beta-lactamase production of haemolytic enterobacteria in patients with diarrhoea and urinary tract infections in Lagos, Nigeria. Cent. Afr. J. Med. 1996;42(5):147–150. [PubMed] [Google Scholar]
- 153.Obi C.L., Coker A.O., Epoke J., Ndip R.N. Enteric bacterial pathogens in stools of residents of urban and rural regions in Nigeria: a comparison of patients with and without diarrhoea and controls without diarrhoea. J. Diarrhoeal Dis. Res. 1997;15(4):241–247. [PubMed] [Google Scholar]
- 154.Sansi J.A.A. Challenges and options for developing leptospirosis vaccine effectiveness in Nigeria. Asian J. Biomed. Pharmaceut. Sci. 2018;8:54. [conference abstract] [Google Scholar]
- 155.International Association for Medical Assistance to Travellers, Nigeria General Health Risks: African Tick-Bite Fever. https://www.iamat.org/country/nigeria/risk/african-tick-bite-fever Last updated 16 April 2020.
- 156.Fagbamila I.O., Barco L., Mancin M., Kwaga J., Ngulukun S.S., Zavagnin P., A.A. Salmonella serovars and their distribution in Nigerian commercial chicken layer farms. PLoS One. 2017;12(3):e0173097. doi: 10.1371/journal.pone.0173097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Haselbeck A.H., Panzner U., Im J., Baker S., Meyer C.G., Marks F. Current perspectives on invasive nontyphoidal Salmonella disease. Curr. Opin. Infect. Dis. 2017;30(5):498–503. doi: 10.1097/QCO.0000000000000398. [DOI] [PMC free article] [PubMed] [Google Scholar]