Abstract
Purpose
Periorbital myxedema is one the most challenging symptoms for patients with Graves' eye disease (GED). The treatment of this condition is complex and often unsatisfactory. This case demonstrates the use of intralesional hyaluronidase to treat cosmetically concerning periorbital myxedema.
Observations
Follow up showed no clinically significant recurrence of myxedema over one year.
Conclusion
Intralesional hyaluronidase represented an effective and safe treatment of periorbital myxedema in a patient with Graves' eye disease.
Keywords: Periorbital myxedema, Graves' disease, Graves' eye disease, Thyroid eye disease, Hyaluronidase
Abbreviations used
- GD
Graves' disease
- GED
Graves' eye disease
- GO
Graves' orbitopathy
- HA
Hyaluronic acid
- ILH
Intralesional hyaluronidase
1. Introduction
Orbitopathy with periorbital edema is seen to some degree in 25% of all patients with Graves' disease (GD).1 Graves' orbitopathy (GO) involves extraocular muscle enlargement with orbital fat expansion.2 GO has an active inflammatory phase and a chronic fibrotic phase associated with the deposition of glycosaminoglycans, including hyaluronic acid.3 Until the FDA approval of teprotumumab, there were no treatments for Graves' eye disease that targeted the underlying pathogenic mechanisms. Management requires a team approach and relies on restoring the euthyroid state, treating the active disease with a variety of procedures, prescribing immunomodulators, and modifying risk factors.1,3
We report on the successful treatment of cosmetically concerning periorbital myxedema with dermopathy using intralesional hyaluronidase in a patient with GED. Hyaluronidase is available in human recombinant and non-human preparations.4 It is FDA approved for a variety of indications and is widely used in ophthalmology as an adjunct to local anesthesia and to correct hyaluronic acid (HA) filler complications.4 Hyaluronidase has been used with success to treat severe pretibial myxedema.5
2. Case report
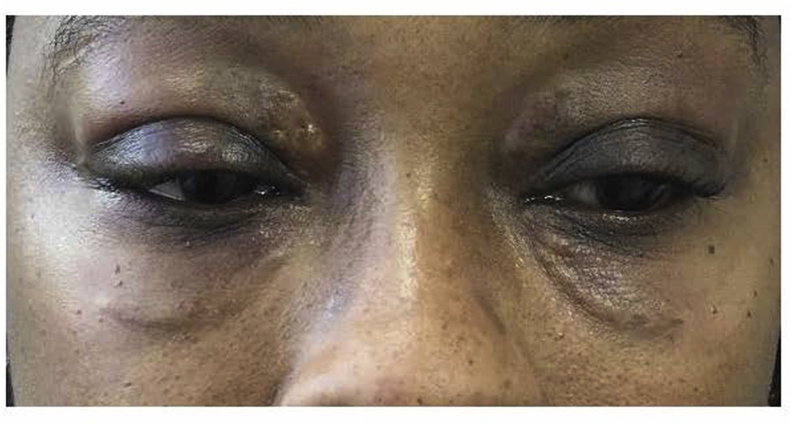
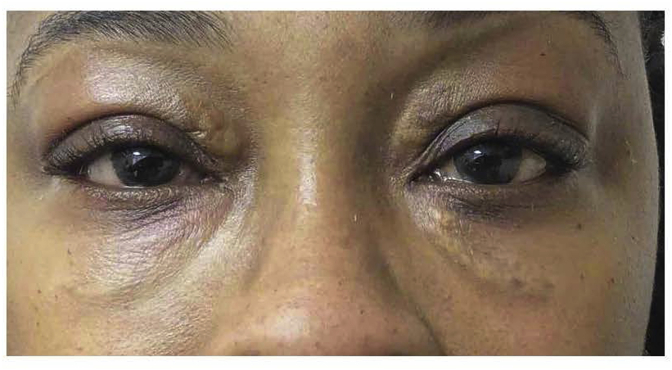
A 45-year-old woman with a 10-year history of GD, initially treated with radioactive iodine and now maintained in a euthyroid state with levothyroxine, presents with gradual upper and lower eyelid swelling over the same period (Fig. 1). A diagnosis of periorbital myxedema with dermatopathy was made. We have had extensive experience using hyaluronidase to treat complications arising from hyaluronic acid fillers and after a discussion of the risks and possible benefits, the patient was offered a trial of intralesional injection with hyaluronidase (ILH). We used an established protocol of 10 units of hyaluronidase per 0.1 cc of presumed hyaluronic acid based on observation of the amount of skin distortion compared to similar distortions we have seen with lumps from HA fillers.4, 6 The patient was treated with a total of 120 units of hyaluronidase (Vitrase; hyaluronidase Ovine 200 USP Units/ml, Bausch and Lomb) mixed 1:1 with xylocaine with epinephrine (1:100,000) injected into the dermis and subcutis of each lesion. The dose rationale was based on the standard use of hyaluronidase used for treating the undesired effects of hyaluronidase fillers. The hyaluronidase was injected in the subcutis covering the entire area involved with approximately .1 cc per each involved centimeter. Some immediate improvement was noted, and the patient reported no adverse effects except swelling and mild bruising. The injections yielded dramatic improvement over the next 7 days and the improvements persisted for one year after the injection (Fig. 2). She will pursue further treatment with ILH in the future if needed.
Fig. 1.
Patient at presentation with persistent periorbital myxedema.
Fig. 2.
Patient at 7 months following one treatment with intralesional hyaluronidase 30 units to each tear trough/lower lid and 30 units each to medial upper lid.
3. Discussion
Late stage periorbital myxedema is seen most commonly in GED. It is difficult to treat and may exacerbate or not improve with the treatment of the underlying condition.1 GO can be the most serious feature of GD. In addition to the ocular debilitation that accompanies GO, the cosmetic appearance is very concerning for patients. ILH gave our patient persistent and excellent cosmetic improvement with a simple in office procedure. Ophthalmologists are very familiar with the use of hyaluronidase. Hyaluronidase is very easy to administer and has few adverse effects. Swelling, erythema and mild itching occur in 25% of patients.4, 6 Anaphylaxis and angioedema are rare side effects especially with human derived hyaluronidase.4, 6 Patients can be observed in the office after injection or a pretreatment skin test may be done as recommended by the manufacturer.4, 6 Pretreatment skin tests are not done routinely in the dermatologic setting.5 This case demonstrates that ILH is a possible safe and effective treatment for refractory cases of periorbital myxedema. More studies need to be done to determine the proper schedule for injections, dosing and duration of effect.
Funding
No funding or grant support.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
The following authors have no financial disclosures: ALL, SS, KMW.
Acknowledgements
None.
References
- 1.Bartalena L., Fatourechi V. Extrathyroidal manifestations of Graves' disease: a 2014 update. J Endocrinol Invest. 2014;37(8):691–700. doi: 10.1007/s40618-014-0097-2. [DOI] [PubMed] [Google Scholar]
- 2.Xin Z., Hua L., Yang Y.L. A pathway analysis based on genome-wide DNA methylation of Chinese patients with Graves' orbitopathy. BioMed Res Int. 2019;2019:1–10. doi: 10.1155/2019/9565794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kazim M., Goldberg R.A., Smith T.J. Insights into the pathogenesis of thyroid-associated orbitopathy: evolving rationale for therapy. Arch Ophthalmol. 2002;120(3):380–386. doi: 10.1001/archopht.120.3.380. [DOI] [PubMed] [Google Scholar]
- 4.Bailey S., Fagien S., Rohrich R. Changing role of hyaluronidase in plastic surgery. Plast Reconstr Surg. 2014;133(2):127e–132e. doi: 10.1097/prs.0b013e3182a4c282. [DOI] [PubMed] [Google Scholar]
- 5.Hoesly P.M., Tolaymat L.M., Sluzevich J.C., Keeling J.H. Pretibial myxedema successfully treated with intralesional hyaluronidase. JAAD Case Rep. 2018;4(9):874–876. doi: 10.1016/j.jdcr.2018.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shumate G.T., Chopra R., Jones D., Messina D.J., Hee C.K. In vivo degradation of crosslinked hyaluronic acid fillers by exogenous hyaluronidases. Dermatol Surg. 2018;44(8):1075–1083. doi: 10.1097/dss.0000000000001525. [DOI] [PubMed] [Google Scholar]