Abstract
Losartan is a highly consumed antihypertensive worldwide and commonly found in effluents of municipal wastewater treatment plants. In the environment, losartan can promote harmful effects on organisms. Thus, an option to face this pollutant is the treatment by photochemical advanced oxidation processes. This dataset has two main components: 1) theoretical calculations on reactivity indexes for losartan, and 2) degradation of the pollutant throughout TiO2-photocatalysis and UVC/persulfate (UVC/PS). The first part of the work presents the data about HOMO and LUMO energies, optimized geometry, dipolar moment, HOMO/LUMO energy gap and total density distribution, in addition to ionization energy, electron affinity, chemical potential, hardness, softness and electrophilicity for losartan. Meanwhile, the second one depicts information on the routes involved in the degradation of the pharmaceutical by the oxidation processes, mineralization, toxicity evolution and losartan removal from a complex matrix (synthetic fresh urine). The data reported herein may be utilized for further researches related to elimination of pharmaceuticals in primary pollution sources such as urine. Moreover, this work also provides experimental and theoretical data useful for the understanding of the response of losartan to oxidative and photochemical processes.
Keywords: Antihypertensive elimination, Photochemical advanced oxidation processes, Pollutants degradation, Urine treatment, Water decontamination
Specifications table
| Subject | Environmental chemistry |
| Specific subject area | Advanced oxidation process |
| Type of data | Table Figure |
| How data were acquired | Data were acquired by using HPLC-DAD and Gaussian 09 (software of quantum chemistry), Method: ground state, DFT, B3LYP, Basis: 6-311g ++ (2d, 2p). |
| Data format | Raw Analyzed |
| Parameters for data collection | The experiments were carried out at fixed operational conditions to establish the capability of TiO2-photocatalysis and UVC/Persulfate to degrade a highly consumed antihypertensive. |
| Description of data collection | The degradation at lab-scale of the antihypertensive losartan (LOS) by two photochemical process was performed. Initially, computational calculations on LOS were carried out. Then, the treatment in distilled water was done and the routes of process action were determined by using scavengers. Afterwards, data about mineralization and toxicity evolution were obtained. Finally, the information on matrix effect by LOS degradation in synthetic fresh urine was attained. |
| Data source location | Universidad de Antioquia UdeA, Medellín, Colombia; Universidad Santiago de Cali, Cali, Colombia; Universidad Tecnológica de Pereira, Pereira, Colombia |
| Data accessibility | Mendeley data repository through the following link: https://data.mendeley.com/datasets/7pbnd4vvm5/draft?a=a3dc88ff-086e-4baf-93b6-ab49d900e8cd |
Value of the data
-
•
Data are useful to analyze similarities and differences between TiO2-photocatalysis and UVC/Persulfate for degrading pharmaceuticals such as losartan antihypertensive.
-
•
Data can benefit people researching on elimination of antihypertensives by photochemical advanced oxidation processes in aqueous matrices.
-
•
Data can be utilized for further insights about degradation of pharmaceuticals in a complex matrix, such as hospital effluents.
-
•
Data are valuable for future works on oxidation processes, photochemistry and organic reactions of losartan.
1. Data Description
Dataset presented in this work have two main parts, the first component deals with computational calculations on losartan and the second one contains information about the degradation of the pharmaceutical by two advanced oxidation processes (i.e., TiO2-photocatalysis and UVC/persulfate). These photochemical processes are widely used for degrading organic pollutants in aqueous matrices [1], [2], [3], [4].
It should be mentioned that for double bonds in alkenes and aromatic rings (as contained in losartan structure), frontier orbitals (i.e., highest occupied molecular orbital-HOMO and in the lowest unoccupied molecular orbital-LUMO) can be useful to predict radical attack positions [5]. Then, energies of HOMO and LUMO, in addition to optimized geometry, dipolar moment, HOMO/LUMO energy gap and total density distribution, were theoretically stated, this information is presented in Table 1, Table 2. Meanwhile, Table 3 contains other reactivity indexes (such as ionization energy, electron affinity, chemical potential, hardness, softness and electrophilicity) for losartan.
Table 1.
Computational calculations for losartan.
| Parameter | Results |
|---|---|
| Chemical structure of losartan | 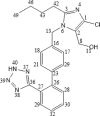 |
| Total energy | 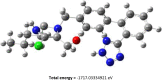 |
| Dipolar moment |  |
| Highest occupied molecular orbital (HOMO) | 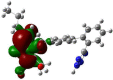 |
| Lowest unoccupied molecular orbital (LUMO) | 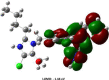 |
| Gap energy (EGAP) | 2.02 eV |
| Total density distribution |  |
Table 2.
Total density distribution for losartan.
| Moiety | Atom | Densities | Atoms numeration |
|---|---|---|---|
| Imidazole | 1 C | -0.013722 |  |
| 2 C | 0.123255 | ||
| 3 C | -2.42648 | ||
| 4 N | 0.053696 | ||
| 5 H | 0.179991 | ||
| 6 N | 0.310516 | ||
| Chlorine | 7 Cl | -0.046173 | |
| Alcohol | 8 C | -0.72382 | |
| 9 H | 0.101355 | ||
| 10 H | 0.143965 | ||
| 11 O | -0.458425 | ||
| 12 H | 0.3764 | ||
| Biphenyl | 13 C | 0.294884 | |
| 14 H | 0.099872 | ||
| 15 H | 0.102713 | ||
| 16 C | 0.548818 | ||
| 17 C | -0.382691 | ||
| 18 C | -0.470243 | ||
| 19 C | -0.512244 | ||
| 20 H | 0.136655 | ||
| 21 C | -0.637257 | ||
| 22 H | 0.150577 | ||
| 23 C | 0.667093 | ||
| 24 H | 0.168494 | ||
| 25 H | 0.175748 | ||
| 26 C | 0.391657 | ||
| 27 C | 0.446463 | ||
| 28 C | -0.177684 | ||
| 29 C | -0.194079 | ||
| 30 C | -0.374148 | ||
| 31 H | 0.169917 | ||
| 32 C | -0.404455 | ||
| 33 H | 0.169726 | ||
| 34 H | 0.160523 | ||
| 35 H | 0.154444 | ||
| Tetrazole | 36 C | -0.005707 | |
| 37 N | -0.290324 | ||
| 38 N | -0.354756 | ||
| 39 N | 0.004876 | ||
| 40 N | 0.12792 | ||
| 41 H | 0.302545 | ||
| Butyl | 42 C | 1.416066 | |
| 43 C | -0.575332 | ||
| 44 H | -0.001771 | ||
| 45 H | 0.034663 | ||
| 46 C | -0.046357 | ||
| 47 H | 0.078481 | ||
| 48 H | 0.05442 | ||
| 49 C | -0.479371 | ||
| 50 H | 0.076031 | ||
| 51 H | 0.069222 | ||
| 52 H | 0.103527 | ||
| 53 H | 0.090485 | ||
| 54 H | 0.090042 |
Table 3.
Reactivity indexes for losartan.
| Ionization energy (eV) | Electron affinity (eV) | Chemical potential (eV) | Global Hardness (eV) | Local softness (eV) | Global index of electrophilicity (eV) |
|---|---|---|---|---|---|
| 2.2005 | 2.1434 | 2.1719 | 0.0571 | 17.513 | 42.062 |
Regarding losartan degradation by the AOPs, in Fig. 1 is shown the antihypertensive evolution during the treatment in distilled water using TiO2-photocatalysis (TiO2 PC). Fig. 1 also presents data on removal by photolysis (UVA), the pollutant degradation in presence of potassium iodide and isopropanol scavengers (TiO2 PC/KI and TiO2 PC/IPA, respectively) and replacing water media by acetonitrile solvent (TiO2 PC/ACN) to provide information about the routes involved in the process [1,2]. In turn, Fig. 2 presents the degradation of losartan by the UVC/PS system, control experiments (action of persulfate-PS or the light-UVC), plus the dataset for experiments when isopropanol (UVC/PS/IPA, which is a scavenger of hydroxyl and sulfate radicals [6]) is added.
Fig. 1.
Degradation of losartan using TiO2-photocatalysis (TiO2 PC). [Losartan]= 43.38 µmol L-1, initial pH: 6.1, [TiO2] = 0.5 g L-1, [KI]= [IPA]= 4.33 mmol L-1 and UVA light power = 75 W.
Fig. 2.
Degradation of losartan by the UVC/PS process. [Losartan]= 43.38 µmol L-1, initial pH: 6.1, [PS] = 500 µmol L-1, [IPA]= 4.33 mmol L-1 and UVC light power = 60 W.
In Fig. 3 is presented the evolution of total organic carbon (TOC) and phytotoxicity under the two processes, for comparative purposes, the TOC removal (Fig. 3A) and toxicity (Fig. 3B) were measured at two normalized times: 1 (when losartan is 100% degraded) and 2 (the double of time required to 100% remove the antihypertensive). Fig. 4. compares the treatment of losartan in distilled water and synthetic fresh urine by TiO2-photocatalysis (Fig. 4A) and UVC/PS (Fig. 4B) processes. Table 4 depicts the synthetic fresh urine composition and Table 5 summarizes the literature search on the interaction/reaction among hydroxyl or sulfate radicals with the urine components, in addition to the pseudo-first order rate constants for losartan degradation by TiO2-photocatalysis and UVC/PS.
Fig. 3.
Extension of advanced oxidation treatments. A. Mineralization of losartan during application of the different processes. B. Toxicity against radish seeds (Raphanus sativus) of treated solution of losartan. Experimental conditions as described in Figs. 1 and 2.
Fig. 4.
Comparison of losartan degradation in distilled water (DW) and simulated fresh urine (Urine). A. TiO2 photocatalysis. B. UVC/PS process. Experimental conditions as described in Figs. 1 and 2.
Table 4.
Composition of synthetic fresh urine ([7]) used for the experiments.
| Compound | Concentration (mol L-1) |
|---|---|
| Urea | 0.2664 |
| CH3COONa | 0.1250 |
| Na2SO4 | 0.01619 |
| NH4Cl | 0.03365 |
| NaH2PO4 | 0.02417 |
| KCl | 0.05634 |
| MgCl2 | 0.003886 |
| CaCl2 | 0.004595 |
| NaOH | 0.00300 |
| pH = 6.1 | |
Table 5.
Rate constants of the reactions between the radical species and the components of fresh urine.
| Reaction | Second order rate constant (k2nd, L mol-1 s-1) | References |
|---|---|---|
| 4.3x109 | [8] | |
| ∼ 2x104 | [9] | |
| 7.0 x107 | [10] | |
| 1.3x1010 | [11] | |
| 7.9x105 | [9] | |
| 6.5x102 | [3] | |
| 3.1×108 | [4] | |
| 6.5x107 | [12] | |
| 3.5×105 | [13] | |
| ) | 5.8x106 | [14] |
| < 7x104 | [13] | |
| Pseudo-first order rate constant (k, min-1) for degradation of losartan by the processes | ||
| TiO2-photocatalysis | 0.004 | In this work |
| UV/PS | 0.029 | In this work |
2. Experimental Design, Materials, and Methods
2.1. Reagents
Acetonitrile, isopropanol, methanol, potassium iodide, potassium persulfate, sodium acetate, sodium chloride, sodium dihydrogen phosphate, sodium hydroxide, sodium sulfate, and urea were provided by Merck. Ammonium chloride, formic acid, calcium chloride and magnesium chloride were provided by PanReac. Titanium dioxide was provided by Evonik. Losartan was purchased from La Santé S.A. The solutions of losartan were prepared using distilled water. In all cases, the initial losartan concentration was 43.38 µmol L-1.
2.2. Reaction systems
A homemade aluminum reflective reactor containing UVC lamps (OSRAM HNS®, 60 W of light power) with main emission at 254 nm was used for the UVC/PS process. Losartan solutions (50 mL) were placed in beakers (100 mL of capacity) under constant stirring. The TiO2-photocatalysis process was carried out in the same reactor but equipped with UVA lamps (Philips BLB, 75 W of light power) having main emission peak at 365 nm. Losartan solutions (50 mL) were also placed in beakers under constant stirring. Additionally, the adsorption/desorption equilibrium on TiO2 catalyst was reached after 30 min in dark.
Aliquots of 0.5 mL were taken periodically from the rectors for kinetics analyses by UHPLC (no more than nine aliquots were considered to avoid modifications of the sample volume higher than 10%). For total organic carbon and toxicity measurements, independent experiments were performed and the whole sample was considered in each case per point of the analyses.
2.3. Analyses
Losartan evolution was determined by means a UHPLC Thermo Scientific Dionex UltiMate 3000 chromatograph equipped with an Acclaim™ 120 RP C18 column (5 µm, 4.6 x150 mm) and a DAD (operated at 230 and 254 nm). The mobile phase was methanol/acetonitrile/formic acid (10 mM and pH 3.0) at 10/44/46 %v/v at 0.6 mL min-1.
Mineralization was established using 10 mL of sample by measuring of total organic carbon (TOC), through a Shimadzu LCSH TOC analyzer (previously calibrated), according to Standard Methods 5310, by combustion with catalytic oxidation at 680 °C using high-purity oxygen gas at a flow rate of 190 mL/min. The apparatus had a non-dispersive infrared detector.
Toxicity against radish seeds (Raphanus sativus) was established by interaction of target solution with the indicator seeds. The solution to be tested (5 mL) was placed in a petri dish; then, ten (10) Raphanus sativus seeds were submerged into the solution. The seeds and solution were in contact during 72 h. Afterward, the length of germinated plants was measured, subsequently a mean value and standard deviation for each tested solution were calculated.
The computational calculations were done by using Gaussian 09 (quantum chemistry software); Method: ground state, DFT, B3LYP; Basis: 6-311g ++ (2d, 2p) [15]. The neutral molecule was considered using the dielectric constant for water.
Acknowledgments
Y. Ávila-Torres thanks Universidad Santiago de Cali for support through the project DGI No. 63661, and MINCIENCIAS (before called COLCIENCIAS) for the fellowship “Jóvenes investigadores por la Paz 2018- J19-18-1, 5381 provided to J. F. Guateque-Londoño”. All the researchers acknowledge to Prof. Hoover Valencia for the acquisition of the theoretical data. Researchers from GIRAB thanks Universidad de Antioquia UdeA for the support provided through “PROGRAMA DE SOSTENIBILIDAD” and the financing from MINCIENCIAS COLOMBIA (before called COLCIENCIAS) through the project No. 111577757323. E. A. Serna-Galvis thanks MINCIENCIAS COLOMBIA (before called COLCIENCIAS) for his PhD fellowship during July 2015-June 2019 (Convocation 647 de 2014).
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships which have, or could be perceived to have, influenced the work reported in this article.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.dib.2020.105692.
Contributor Information
Yenny Ávila-Torres, Email: yennytorres@usc.edu.co.
Ricardo A. Torres-Palma, Email: ricardo.torres@udea.edu.co.
Appendix. Supplementary materials
References
- 1.S.K. Kansal, M. Singh, D. Sud, Studies on photodegradation of two commercial dyes in aqueous phase using different photocatalysts, 141 (2007) 581–590. doi:10.1016/j.jhazmat.2006.07.035. [DOI] [PubMed]
- 2.Chen Y., Yang S., Wang K., Lou L. Role of primary active species and TiO2 surface characteristic in UV-illuminated photodegradation of Acid Orange 7. J. Photochem. Photobiol. A Chem. 2005;172:47–54. doi: 10.1016/j.jphotochem.2004.11.006. [DOI] [Google Scholar]
- 3.Guan Y., Ma J., Li X., Fang J., Chen L. Influence of pH on the Formation of Sulfate and Hydroxyl Radicals in the UV/Peroxymonosulfate System. Environ. Sci. Technol. 2011;45:9308–9314. doi: 10.1021/es2017363. [DOI] [PubMed] [Google Scholar]
- 4.Wojnárovits L., Takács E. Rate constants of sulfate radical anion reactions with organic molecules: A review. Chemosphere. 2018 doi: 10.1016/j.chemosphere.2018.12.156. [DOI] [PubMed] [Google Scholar]
- 5.Pignatello J.J., Oliveros E., Mackay A. Advanced Oxidation Processes for Organic Contaminant Destruction Based on the Fenton Reaction and Related Chemistry. Crit. Rev. Environ. Sci. Technol. 2006;36:1–84. doi: 10.1080/10643380500326564. [DOI] [Google Scholar]
- 6.Systems A. tert-Butanol as an OH-Scavenger. 1992;96:1448–1454. [Google Scholar]
- 7.Amstutz V., Katsaounis A., Kapalka A., Comninellis C., Udert K.M. Effects of carbonate on the electrolytic removal of ammonia and urea from urine with thermally prepared IrO2 electrodes. J. Appl. Electrochem. 2012;42:787–795. doi: 10.1007/s10800-012-0444-y. [DOI] [Google Scholar]
- 8.Lian L., Yao B., Hou S., Fang J., Yan S., Song W. Kinetic Study of Hydroxyl and Sulfate Radical-Mediated Oxidation of Pharmaceuticals in Wastewater Effluents. Environ. Sci. Technol. 2017;51:2954–2962. doi: 10.1021/acs.est.6b05536. [DOI] [PubMed] [Google Scholar]
- 9.Buxton G.V, Greenstock C.L., Helman W.P., Ross A.B. Critical Review of rate constants for reactions of hydrated electron, hydrogen atoms and hydroxyl radicals (⋅OH/⋅O in Aqueous Solution) J. Phys. Chem. Ref. Data. 1988;513 doi: 10.1063/1.555805. [DOI] [Google Scholar]
- 10.Minakata D., Song W., Crittenden J. Reactivity of Aqueous Phase Hydroxyl Radical with Halogenated Carboxylate Anions: Experimental and Theoretical Studies. Environ. Sci. Technol. 2011;45:6057–6065. doi: 10.1021/es200978f. [DOI] [PubMed] [Google Scholar]
- 11.Mincher B.J., Mezyk S.P., Cooper W.J., Cole S.K., Fox R.V, Gardinali P.R. Free-radical chemistry of disinfection byproducts. 3. Degradation mechanisms of chloronitromethane, bromonitromethane, and dichloronitromethane. J Phys Chem A. 2010;114:117–125. doi: 10.1021/jp907305g. [DOI] [PubMed] [Google Scholar]
- 12.Emery R.J., Papadaki M., Freitas dos Santos L.M., Mantzavinos D. Extent of sonochemical degradation and change of toxicity of a pharmaceutical precursor (triphenylphosphine oxide) in water as a function of treatment conditions. Environ. Int. 2005;31:207–211. doi: 10.1016/j.envint.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 13.P. Neta, R.E. Huie, A.B. Ross, Rate Constants for Reactions of Inorganic Radicals in Aqueous Solution, 17 (1988).
- 14.Huie R.E., Clifton C.L. Temperature dependence of the rate constants for reactions of the sulfate radical, SO4-, with anions. Am. Chem. Soc. 1990;94:8561–8567. doi: 10.1021/j100386a015. doi:https://doi.org/ [DOI] [Google Scholar]
- 15.Tomasi J., Mennucci B., Cammi R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005;105:2999–3094. doi: 10.1021/cr9904009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






