Summary
An unprecedented desaturation method via redox-neutral hydrogen transfer process has been disclosed under mild conditions for the selective formation of terminal alkene with alkyl diazo compounds and aza-o-QMs. The control experiments and DFT calculations suggest that the visible light was introduced as a key parameter to enhance the reactivity via a radical process in the formation of closed-shell cyclopropane intermediate, followed by a ring opening and redox-neutral hydrogen transfer process to give the desaturated product. The high regioselectivity in this transformation is enabled by the internal amino species as an ancillary group (AG) in the final olefin formation step. This method provides a missing link in the expeditious preparation of synthetically useful 2-allyl anilines with broad substrate generality. Further applications of these generated products in N-heterocycle construction, including 5- and 6-membered rings with structural diversity, have been tactfully explored, which highlight the potential in methodology development and drug discovery.
Subject Areas: Organic Chemistry, Materials Chemistry, Chemical Synthesis
Graphical Abstract

Highlights
-
•
Highly site and regioselective synthesis enabled by ancillary group
-
•
Desaturation via redox-neutral inert hydrogen transfer process
-
•
Missing link in the synthesis of 2-allyl anilines with board substrate scope
-
•
Methodology development and diversity synthesis based on 2-allyl anilines
Organic Chemistry; Materials Chemistry; Chemical Synthesis
Introduction
Alkene is one of the common and key chemical stocks, which is prevalent in natural (Moosophon et al., 2009, Rukachaisirikul et al., 2012) and synthetic molecules (Kolb et al., 1994, Singh et al., 2012, Poplata et al., 2016) with a wide spectrum of applications. Its practical and selective synthesis has drawn broad attentions in synthetic chemistry (Negishi et al., 2008). In this context, carbonyl olefination, alkene metathesis, and coupling reactions constitute the most general and widely used methods for the selective construction of C=C bonds (Blakemore, 2002, Seechurn et al., 2012). Mechanistically, in the formation of alkenes via reactive intermediates, such as metal carbenes (Doyle et al., 1998, Zheng et al., 2015), organometal species (Bras and Muzart, 2011, Barluenga et al., 2007, Barluenga and Valdés, 2011, Xia et al., 2017), and cationic and radical intermediates (Mohrig, 2013), the β-H elimination step is regarded as the key process for the selectivity control (Figure 1A) (Seechurn et al., 2012). However, selective β-H elimination for the generation of terminal alkenes is still a great challenge in this content. Arguably, the formation of internal alkenes is usually favored via these conventional intermediates (see Note S1 for extended bibliography).
Figure 1.
Strategies for the Desaturation
(A) General approaches to alkenes via β-H elimination/shift.
(B) Site-controlled desaturation.
(C) Our proposal: ancillary group (AG)-assisted desaturation via novel and relative stable intermediate.
(D) This work: formation of terminal alkenes via selective hydrogen transfer with the assistance of AG.
Recently, methods for the site-controlled desaturation via activating the inert C(sp3)-H bonds with the assistance of the embedded directing group (DG) or the tethered radical initiator (RI) have achieved great breakthrough (Figure 1B) (Cekovic et al., 1979, Bigi et al., 2011, Voica et al., 2012, Chen and Baran, 2009, Chuentragool et al., 2018, Parasram et al., 2017, Chen and Dong, 2019, Chen et al., 2018a, Cheng et al., 2018b). Representative advances have been reported by Cekovic (Cekovic et al., 1979), White (Bigi et al., 2011), Baran (Voica et al., 2012, Chen and Baran, 2009), Gevorgyan (Chuentragool et al., 2018, Parasram et al., 2017), and others (Chen and Dong, 2019, Chen et al., 2018a, Cheng et al., 2018b). Nevertheless, the selectivity control in the following β-H elimination step is still a big challenge in some cases, although the initial radical intermediate formation step has been enabled selectively. Inspired by these advances, we reasoned that, if the intermediate could be temporarily stabilized before the H-elimination, thus, by avoiding the general β-H-elimination process, an alternative pathway, a delayed and selective β′-H elimination, might be enabled to form the terminal alkenes with the assistance of a neighboring functional group (or named as ancillary group, AG) (Figure 1C).
As a continuation of our interest in the transformations of diazo compounds (Kang et al., 2019, Zeng et al., 2019, Zhang et al., 2019a), we envisioned that intermediate A (Figure 1D in dashed box), which is formed via the electrophilic addition of diazo compound to aza-o-QM (Lee et al., 2014, Yang et al., 2012, Wang et al., 2016, Yang and Gao, 2018), is relatively stable and herein could act as a proper candidate for delaying the followed H-elimination based on previous observations (Ma et al., 2016, Dong et al., 2016, Zheng et al., 2017). In this protocol, the amino group was designed as the AG for the selective desaturation (Figure 1D in dashed box). At the stage, challenges are initially evaluated (Figure 1D in black box): (1) side reactions could take place from the in situ generated alkyl diazocompounds and the aza-o-QMs individually or in combination, which would disturb the formation of the intermediate A; (2) the formation of energetically favorable internal alkene; (3) the AG acts as the nucleophile, thus leading to the 5-membered N-heterocycle via intramolecular addition.
Results and Discussion
We began our investigation by using hydrazone 2a and N-(ortho-chloromethyl)arylamide 3a as the model substrates. After the reaction mixture was stirred under natural light at 0°C to room temperature (rt) in 1,2-dichloromethane (DCE) for 10 h, all 2a and 3a were consumed. To our delight, the desired product 4a was isolated in 43% yield together with the annulation product 13 in 15% yield (Table 1, entry 1). To indentify the internal alkene 4a′, the crude reaction mixture was submitted to the proton NMR analysis and only trace of the internal alkene was observed (terminal:internal >20:1, see Figure S110). Besides, other observed side products may come from the decomposition of diazocompound 1a and the dimerization of 3a via N-alkylation reaction (Zhan et al., 2015). Then the reaction was conducted in the dark to decrease the decomposition of 1a. However, the 2-allyl aniline 4a was only yielded in 35% (entry 2), which indicates that the light might have a positive effect on this reaction, and the annulation product 13 was isolated in 18% yield. To verify the assumption, compact fluorescent light (CFL) was introduced, and the yield of 4a was improved to 59%. Meanwhile, the yield of annulation product 13 was 13%, which is not significantly different under natural light or in the dark (entries 1 to 3). After that, we tried to reduce the reaction's temperature (from −20°C or −40°C to rt) to avoid the side reactions of 3a (entries 4 to 5). Much to our delight, the by-product 13 was inhibited obviously (i.e., 8% yield in entry 4, and <5% yield in entries 5–8), and the best results have been obtained in terms of yield when the reaction was conducted under CFL at −40°C to rt (entry 5, 80% yield, see also Method A in SI). Investigation of different inorganic bases could not enhance the yields (entries 6 and 7). To simplify the operation, we also conducted a one-pot method, using iodosobenzene as the oxidant instead of manganese dioxide, and the desired product 4a could be isolated in 66% yield (entry 8, see also Method B in SI).
Table 1.
Reaction Optimization
 | |||
|---|---|---|---|
| Entry | Variation from the Standard Conditionsa | Yield (%)b |
|
| 4a | 13 | ||
| 1 | Under natural light, 0°C to rt | 43 | 15 |
| 2 | In the dark, 0°C to rt | 35 | 18 |
| 3 | Under CFL, 0°C to rt | 59 | 13 |
| 4 | Under CFL, −20°C to rt | 70 | 8 |
| 5 | Under CFL, −40°C to rt | 80 | <5 |
| 6 | Na2CO3 as the base, under CFL, −40°C to rt | 73 | <5 |
| 7 | t-BuOLi as the base, under CFL, −40°C to rt | 54 | <5 |
| 8c | PhIO instead of MnO2, Na2CO3 as the base, in one pot | 66 | <5 |
Reaction conditions: 2a (0.45 mmol), MnO2 (8.0 equiv. based on 2a), MgSO4 (60.0 mg), and DCE (3.0 mL) was pre-stirred for 45 min, then the reaction mixture was filtered and injected to the oven-dried tube, which was equipped with 3a (0.15 mmol), Cs2CO3 (2.0 equiv.), and the mixture was carried out at −40°C to room temperature under argon atmosphere for 10 h with the irradiation of visible light (8 W CFL).
Isolated yield based on 3a.
Reaction conditions: 2a (0.30 mmol), PhIO (0.30 mmol), 3a (0.15 mmol), Na2CO3 (0.30 mmol), 4Å MS (50 mg), and DCE (2.0 mL) was carried out in one pot at −40°C to rt. under argon atmosphere for 10 h with the irradiation of visible light (8 W CFL).
With the optimized reaction conditions in hand (see also Method A in SI), we turned our attention to exploration of the scope of applicable hydrazones, and the results have been listed in Scheme 1. Hydrazones containing both electron-donating and electron-withdrawing groups on the aryl ring gave the desired products in high yields (4a-4i). Notably, the iodide substituent is tolerated well, which usually would not survive in the transition-metal-catalyzed transformations, generating the desired product 4g in 76% yield. Moreover, both the alkynyl and alkenyl hydrazones are tolerated under current reaction conditions and produced the terminal-alkene products in >80% yield (4j and 4k). The 2-naphthyl and 2-thienyl hydrazones worked well, leading to the corresponding products 4l and 4m in 76% and 78% yields, respectively. The desaturated products 4n and 4o were generated smoothly in 83% and 78% yields from ethyl and cyclic hydrazones, and two isomers were detected in the case with 4n (see Figures S111 and 112). Furthermore, the representative examples of L-menthol, 1,2,3,4-diacetone galactose, and cholesterol derivatives (4p-4r) smoothly delivered the corresponding products in good yield, which highlighted the applicability of this method for the late-stage modification of complex molecules. In addition, the reaction performed well on a gram scale (5.0 mmol) with synthetic useful yields (note c, 60% yield). We also demonstrated the results with the fungible one-pot method in a few examples. Generally, the reactions went smoothly albeit in slightly lower yields compared with the optimal method (note b, see also Method B in SI). The structure of product 4f was confirmed by X-ray analysis (see Table S1).
Scheme 1.
Reaction Scope
aReaction conditions: 2 (0.45 mmol), MnO2 (8.0 equiv. based on 2), MgSO4 (60.0 mg), and DCE (3.0 mL) was pre-stirred for 45 min, then the reaction mixture was filtered and the filtrate was injected to the oven-dried tube containing a magnetic stirring bar, 3 (0.15 mmol), and Cs2CO3 (2.0 equiv.) at −40°C under argon atmosphere. Then the reaction mixture was stirred under these conditions with the irradiation of visible light (8 W CFL) for 10 h, and the reaction temperature warmed up to rt slowly in this period of time. Yields are given in isolated yields.
bReaction conditions: conditions in Table 1, entry 8 was used. 2 (0.30 mmol), PhIO (0.30 mmol), 3 (0.15 mmol), Na2CO3 (0.30 mmol), and 4Å MS (50 mg) in DCE (2.0 mL). Yields are given in isolated yields.
cThe reaction was conducted on a 5.0-mmol scale.
dThe reaction was conducted in the absence of base.
Subsequently, a series of N-(ortho-chloromethyl) aryl amides 3 were explored. Arylamides with substituent at the benzylic position, including methyl and phenyl groups, underwent the transformation smoothly to give the corresponding products in high yields (5a and 5b). Good tolerance for both electron-donating and electron-withdrawing groups on the different position of phenyl ring of 3 was observed, and the desired products were afforded in 76%–84% yields (5c–5i). Substituents on the sulfonyl part had little impact on the reaction, and corresponding products were isolated in high yields (5j and 5k). In addition, substrate with N-CO2Bn group instead of N-Ts was examined, and the reaction proceeded smoothly to give 5l in 65% yield. Moreover, the azadiene was also tolerated well in the transformation in the absence of the base, affording the product 5m in 67% yield. Notably, the by-products (internal alkenes or annulation product) were not detected in all above cases.
Encouraged by the above results, we further explored this formal coupling reaction with carbonyl diazo compounds 6, which are readily available and stable, as the starting materials in the absence of oxidant (Scheme 2, see also Method C in SI). Herein, minor optimization with Na2CO3 as the base rather than Cs2CO3 was applied in the absence of visible light (8 W CFL), and no obvious difference was observed when the reactions were conducted under the visible light or dark. A variety of diazoamides were first investigated, and both electron-donating and electron-withdrawing groups on the aryl ring of 6 generally gave the desired 2-allyl aniline products 7a–7d in good yields (76%–84%). The sterically hindered ortho-methyl-substituted substrate 6e afforded the product 7e in moderate yield. The N-benzyl-protected diazoamide (6f) performed well and gave 7f in 69% yield. In addition, the α-methyl diazoacetates and diazophosphonate were also tolerated under these conditions, and the corresponding alkenes were isolated in moderate to high yields (7g–7j).
Scheme 2.
Scope of α-Methyl Diazo Compounds 6
Reaction conditions: 6 (0.15 mmol), 3 (0.20 mmol), Na2CO3 (1.2 equiv., 25.4 mg) in DCE (2.0 mL) at 0°C under argon atmosphere for 8 h, and the reaction temperature warmed up to room temperature in this period of time.
Next, we turned our attention to other types of diazo compounds that do not have the α-methyl group, and the cyclized products 9 were obtained (Figure 2, see also Method D in SI). The α-phenyl diazoacetate 8a generated the formal [4 + 1] product 9a in 55% yield, and the residual of unreacted diazoacetate 8a was recovered in 40% (Figure 2A). We also examined the ethyl diazoacetate 8b (EDA) and diazoacetamide 8c, and both delivered the isomerized products 9b (see Figure S113) and 9c in 62% and 70% yields, respectively (Figure 2B). Besides, when diphenyl diazo compound 8d was subjected to the optimized conditions, the annulation product 9d was isolated in 45% yield contaminated with the internal olefin 9d′ in 50% yield (Figure 2C). Both the structures of 9a and 9d′ were confirmed by X-ray (Figure 2D, see also Table S2).
Figure 2.
Cyclization Transformations of 3a with Other Types of Diazo Compounds without the α-Methyl Group (A) With α-phenyl diazoacetate. (B) With ethyl diazoacetate (EDA) and diazoacetamide. (C) With diphenyl diazo compound. (D) X-ray diffraction of compounds 9a and 9d′.
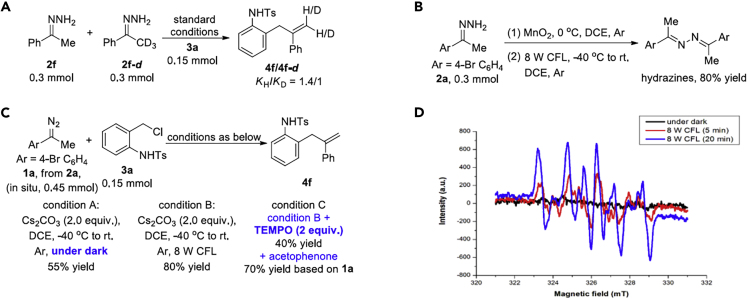
Control experiments were subsequently conducted to verify the proposed reaction mechanism (see also Methods for mechanistic studies in SI). First, the intramolecular isotope labeling experiments show that the reaction undergoes a hydrogen transfer process (see Figures S114 and S115). The intermolecular kinetic isotope effect (KIE) experiment (kH/kD = 1.4:1) shows that the hydrogen transfer process is not the rate-limiting step (Figure 3A, see also Figure S114). Then 2a was exposed under the 8-W CFL, and the hydrazine product was generated in 80% yield, which suggested the possibility of the existence of free carbene (Figure 3B) (Sha and Wei, 2013). The parallel reactions were conducted, and the results are shown in Figure 3C. Variable from optimized conditions in Table 1, we carried out the template reaction in the dark, and the bishomoallylic amine 4f was given in 55% yield (Figure 3C, condition A), whereas 4f was obtained in 80% yield under optimal conditions in the presence of visible light (Figure 3C, condition B). Then preliminary radical-inhibition test was conducted. Partial inhibition was observed when TEMPO was added, and the yield of 4f dropped to 40% yield (Figure 3C, condition C), which might be contributed via the non-radical pathway. Meanwhile, 70% yield of acetophenone (yield based on 1a) was observed after the reaction, due to the oxidization of the free carbene by TEMPO. In addition, electron paramagnetic resonance (EPR) experiments were carried out (Figure 3D, see also Figure S116). No obvious signal of the diradical spin adduct was observed when diazo compound (1a) was treated with 3a in the presence of 5,5-dimethyl-1-pyrroline N-oxide (DMPO) at room temperature in dark. On the other hand, the EPR signal of the carbon-diradical spin adduct emerged after the reaction mixture was irradiated by 8-W CFL lamps for 5 min, which obviously strengthened in the following 20 min (Figure 3D, blue line). These experiments indicated that the visible light facilitates the reaction pathway via the radical process. On basis of the literature reports (Boratyński et al., 2010, Gallagher et al., 2019, Hommelsheim et al., 2019, Jurberg and Davies, 2018, Snyder and Dougherty, 1989, Zhang et al., 2019b) and the EPR signal, we deduced that the radical process may be involved under the visible light conditions.
Figure 3.
Control Experiments and EPR Analysis
(A) Kinetic isotope effect (KIE) experiment.
(B) Control experiment in the absence of aza-o-QMs.
(C) The parallel reactions under dark or in the presence of TEMPO.
(D) Electron paramagnetic resonance (EPR) experiments.
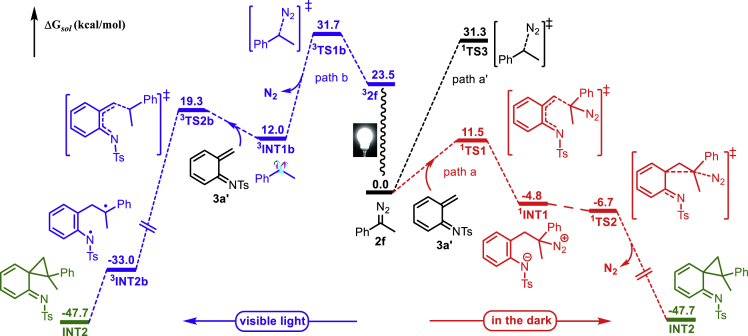
Computational studies were carried out to gain a mechanistic insight on this selective formation of the 2-allyl aniline 4f (see also Computational Methods in SI). The diazo substrate 2f could undergo intermolecular electrophilic attack to the terminal alkenyl carbon of the in situ generated intermediate 3a′ to form an adduct 1INT1 (Figure 4, the red line, path a). The optimized transition state (TS) of this step is shown as 1TS1. The predicted energy barrier is 11.5 kcal/mol relative to separated 2f and 3a'. The formed 1INT1 is very ready to release N2 via 1TS2 to yield the corresponding cyclopropane derivative INT2. Computational study results indicate that it is very facile for INT1 to convert to INT2 via 1TS2 along with the extrusion of N2 in a concerted manner. The direct N2 dissociation from 2f (via 1TS3) to afford a free carbene intermediate (path a’), however, has a much higher energy barrier, which is unlikely to occur compared with the competitive intermolecular electrophilic addition process. Alternatively, a possible reaction route leading to INT2 in the presence of visible light was also considered (Figure 4, the blue line, path b). Irradiated by visible light, the diazo compound 2f might be excited to triplet state (32f). Subsequently, the dissociation of N2 via 3TS1b could follow to yield the triplet carbene intermediate 3INT1b. Afterward, the formed 3INT1b could attack to the terminal alkenyl carbon of the in situ-generated intermediate 3a′ to form the intermediate INT2 via 3TS2b. The predicted energy barrier of this step is only 7.3 kcal/mol. Thus, it is also feasible for the formation of INT2 in the presence of light.
Figure 4.
Energy Profile (in kcal/mol) for the Formation of INT2
It should be noted that the formed INT2 might not be stable under the driving force of the aromatization and the ring strain release of the three-membered ring. Next, we evaluated two competitive processes, desaturation versus ring expansion, which were observed above. The hydrogen transfer from the methyl group attached to the three-membered ring to the N atom of the imine moiety can generate the unsaturated amine product 4f (path a, Figure 5) and the [1,3] C-migration can lead to the cyclized product 9 (path b, Figure 5). The located TS for path a is shown as TS4, in which the C3 … H bond distance is lengthened to 1.22 Å, whereas the H … N distance is shortened to 1.52 Å. Meanwhile, the C1 … C2 distance is lengthened to 2.29 Å (Figure 5A). The calculated ΔGǂ of the hydrogen transfer step is 8.0 kcal/mol relative to INT2, and the formed product 4f is exothermic by 19.2 kcal/mol. The optimized TS of the [1,3] C-migration to form product 9 is shown as TS5, in which the C1 … C2 distance is lengthened to 2.44 Å, whereas the C2 … N distance is shortened to 2.80 Å (Figure 5B). The predicted energy barrier of path b is 11.4 kcal/mol, which is 3.4 kcal/mol higher in energy than that of path a. Therefore, computational results suggest that it is more feasible for INT2 to form product 4f via the redox-neutral hydrogen transfer pathway (it is consistent with the KIE experiment, see Figure 3A). In the cases of substrates without the adjacent C-H bond, the annulation product 9 could be formed via the [1,3] C-migration pathway. The carbocation intermediate is very unlikely to form as a key intermediate (Suneja and Schneider, 2018, Pandit et al., 2019), which cannot be located as a local minimum computationally, owing to the presence of highly nucleophilic negatively charged C1 and N atoms. Thus, this alternative mechanistic pathway is discarded. However, other possibility could not be totally ruled out so far.
Figure 5.
Energy Profiles (in kcal/mol) for the Formation of 4f and 9 from INT2 (A) Optimized transition state of TS4. (B) Optimized transition state of TS5.
Recently, elegant cycloannulation reactions of o-QMs and diazo ketones/esters were reported by Schneider (Suneja and Schneider, 2018) and Ryu (Pandit et al., 2019), independently. And corresponding cyclopropane derivatives are the key intermediates in these transformations. However, the manner of ring opening is different from ours. Besides, redox-neutral hydrogen transfer processes instead of intramolecular rearrangement (Li et al., 2019, Gandamana et al., 2018, Mavroskoufis et al., 2020, Mori et al., 2018, Kaiser et al., 2019, Tian et al., 2019, Haibach and Seidel, 2014) thereafter took place in our protocol to form the desaturation products (see Note S2 for extended discussion). Moreover, the desaturation product of 2-allyl anilines are prized building blocks for the divergent synthesis of heterocycles through various transformations (Lu et al., 2018, Lin et al., 2017, Wdowik and Chemler, 2017, Jiang et al., 2017, Chemler, 2013, Du et al., 2015, Miyazaki et al., 2014, Pan et al., 2014, Yu et al., 2017). Yet the current methods for the synthesis of these motifs usually took a long synthetic route and with limited substrate scope (Du et al., 2015, Miyazaki et al., 2014, Pan et al., 2014, Yu et al., 2017) (see Note S3 for extended discussion). This method provides a missing link in the synthesis of 2-allyl anilines with high control of the terminal selectivity and broad substrate generality under mild conditions.
To show the synthetic utility of this method, a variety of 5- and 6-membered N-heterocycles have be expeditiously synthesized with these obtained 2-allyl aniline products (Figure 6, see also Methods for further transformations in SI). Catalytic 5-exo-cyclization, which selectively promoted the formation of new C-N bonds in conjunction with vicinal C-CN (Pan et al., 2014), C-Br (Yu et al., 2017), C-O, C-H, or C-S bonds, delivered the corresponding functionalized indolines 10–14 in high yields. In addition, the 6-endo-cyclization products 15 and 16 were smoothly obtained in 70% and 82% yields under catalytic vicinal chlorination and reductive conditions, respectively. Owing to the limitation of diazo compounds, the di-alkyl-substituted alkenes were not directly delivered currently. However, they can be realized by the late-state modifications, i.e., the corresponding 2-allyl aniline 17 could be readily generated from 7a with lithium aluminum hydride (LAH) in 70% yield, which further demonstrate the utility of our methodology. Comparing with the results of 16 and 17, we reasoned that the product 16 may come from the annulation under the alkaline condition then reduction. To verify this assumption, the reaction of 7f was conducted only under the basic condition, and the corresponding annulation product was observed. After screening the bases, we found that DBU is the best one for this annulation. This discovery was applied in the efficient synthesis of 18 (from the generated 2-allyl aniline 7j), which could be further used for the synthesis of a leukemia inhibitor in three steps with high yields (Figure 7) (Chen et al., 2016).
Figure 6.
Versatile Transformations with Obtained 2-Allyl Anilines
(A) BrCN, Et3N, Et2O, 0°C, 3 h, then B(C6F5)3 (20 mol%), PhMe, 100°C, 48 h.
(B) 10 mol% BINAP(S), NBS, toluene/CH2Cl2, -78°C, 50 h.
(C) m-CPBA, CH2Cl2, rt, 36 h.
(D) NbCl5 (2.5 mol %), AgNTf2 (5 mol %), DCE, 80°C, 6 h.
(E) CuCl (20 mol%), B2Pin2 (20 mol%), NFSI, CH3CN, 45°C, 15 h.
(F) CaCl2 (0.50 mmol), Pd(OAc)2 (0.01 mmol) and H2O2 (30% aq wt.), HOAc, rt, 30 h.
(G) LiAlH4, THF, 0°C to rt, 3 h.
(H) LiAlH4, THF, 0°C to rt, 3 h.
Figure 7.
Formal Synthesis of a Leukemia Inhibitor
Conclusion
In summary, we have developed a novel visible-light-enhanced desaturation method for highly site-/regio-controlled synthesis of 2-allylic anilines. Mechanically, two reaction pathways, including a direct electronic addition of diazo compound and light-enhancing induced radical processes, may simultaneously contribute to the formation of closed-shell cyclopropane intermediate, followed by a ring opening and redox-neutral hydrogen transfer process to give the desaturated product. This desaturated approach provides a facile access to a wide range of 2-allyl aniline derivatives under mild conditions with good tolerance of functional groups. Notably, further applications in N-heterocycle construction, including 5- and 6-membered-ring with a variety of functional groups, have been explored as well, which show potential in diversity synthesis and drug discovery. Novel desaturation method could be envisioned with this AG-assisted hydrogen transfer strategy.
Limitations of the Study
Owing to the limitation of diazo compounds, the di-alkyl substituted alkenes were not directly delivered currently.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Xinfang Xu (xinfangxu@suda.edu.cn).
Materials Availability
All unique/stable reagents generated in this study are available from the Lead Contact with a completed Materials Transfer Agreement.
Data and Code Availability
The crystallography data have been deposited at the Cambridge Crystallographic Data Center (CCDC) under accession number CCDC: 1579217 (4f); 1579222 (9a); 1579218 (9d′) and can be obtained free of charge from www.ccdc.cam.ac.uk/getstructures. Original/source data for Schemes 1 and 2 together with Figures 2, 3, 6, and 7 in the paper is available at https://doi.org/10.17632/k23x374cz3.1.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This project was support by National Natural Science Foundation of China (21971262 to X.X.; 21973068 to X.B.), and the Program for Guangdong Introducing Innovative and Entrepreneurial Teams (No. 2016ZT06Y337 to W.H.) is greatly acknowledged.
Author Contributions
X.X. supervised the project. Y.Z., K.H., and S.D. conducted the experimental works. L.Q. performed X-ray analysis. P.D., D.G., L.K., and X.B. performed the computational studies. Y.Z., J.H., W.H., X.B., and X.X. co-wrote the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: June 26, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101168.
Contributor Information
Wenhao Hu, Email: huwh9@mail.sysu.edu.cn.
Xiaoguang Bao, Email: xgbao@suda.edu.cn.
Xinfang Xu, Email: xinfangxu@suda.edu.cn.
Supplemental Information
References
- Barluenga J., Valdés C. Tosylhydrazones: new uses for classic reagents in palladium-catalyzed cross-coupling and metal-free reactions. Angew. Chem. Int. Ed. 2011;50:7486–7500. doi: 10.1002/anie.201007961. [DOI] [PubMed] [Google Scholar]
- Barluenga J., Moriel P., Valdés C., Aznar F. N-Tosylhydrazones as reagents for cross-coupling reactions: a route to polysubstituted olefins. Angew. Chem. Int. Ed. 2007;46:5587–5590. doi: 10.1002/anie.200701815. [DOI] [PubMed] [Google Scholar]
- Bigi M.A., Reed S.A., White M.C. Diverting non-haem iron catalyzed aliphatic C–H hydroxylations towards desaturations. Nat. Chem. 2011;3:216–222. doi: 10.1038/nchem.967. [DOI] [PubMed] [Google Scholar]
- Blakemore P.R. The modified Julia olefination: alkene synthesis via the condensation of metallated heteroarylalkylsulfones with carbonyl compounds. J. Chem. Soc. Perkin Trans. 2002;1:2563–2585. [Google Scholar]
- Boratyński P.J., Pink M., Rajca S., Rajca A. Isolation of the triplet ground state aminyl diradical. Angew. Chem. Int. Ed. 2010;49:5459–5462. doi: 10.1002/anie.201002811. [DOI] [PubMed] [Google Scholar]
- Bras J.L., Muzart J. Intermolecular dehydrogenative Heck reactions. Chem. Rev. 2011;111:1170–1214. doi: 10.1021/cr100209d. [DOI] [PubMed] [Google Scholar]
- Cekovic Z., Dimitrijevic L., Djokic G., Srnic T. Remote functionalization by ferrous ion–cupric ion induced decomposition of alkyl hydroperoxides. Tetrahedron. 1979;35:2021–2026. [Google Scholar]
- Chemler S.R. Copper's contribution to amination catalysis. Science. 2013;341:624–626. doi: 10.1126/science.1237175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Baran P.S. Total synthesis of eudesmane terpenes by site-selective C–H oxidations. Nature. 2009;459:824–828. doi: 10.1038/nature08043. [DOI] [PubMed] [Google Scholar]
- Chen M., Dong G. Copper-catalyzed desaturation of lactones, lactams, and ketones under pH-neutral conditions. J. Am. Chem. Soc. 2019;141:14889–14897. doi: 10.1021/jacs.9b07932. [DOI] [PubMed] [Google Scholar]
- Chen L., Wilder P.T., Drennen B., Tran J., Roth B.M., Chesko K., Shapiroac P., Fletcher S. Structure-based design of 3-carboxy-substituted 1,2,3,4-tetrahydroquinolines as inhibitors of myeloid cell leukemia-1 (Mcl-1) Org. Biomol. Chem. 2016;14:5505–5510. doi: 10.1039/c5ob02063h. [DOI] [PubMed] [Google Scholar]
- Chen M., Rago A.J., Dong G. Platinum-catalyzed desaturation of lactams, ketones, and lactones. Angew. Chem. Int. Ed. 2018;57:16205–16209. doi: 10.1002/anie.201811197. [DOI] [PubMed] [Google Scholar]
- Cheng W.-M., Shang R., Fu Y. Irradiation-induced palladium-catalyzed decarboxylative desaturation enabled by a dual ligand system. Nat. Commun. 2018;9:5215–5223. doi: 10.1038/s41467-018-07694-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuentragool P., Parasram M., Shi Y., Gevorgyan V. General, mild, and selective method for desaturation of aliphatic amines. J. Am. Chem. Soc. 2018;140:2465–2468. doi: 10.1021/jacs.8b00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong K., Yan B., Chang S., Chi Y., Qiu L., Xu X. Transition-metal-free fluoroarylation of diazoacetamides: a complementary approach to 3-fluorooxindoles. J. Org. Chem. 2016;81:6887–6892. doi: 10.1021/acs.joc.6b01286. [DOI] [PubMed] [Google Scholar]
- Doyle M.P., McKervey M.A., Ye T. John Wiley & Sons; 1998. Modern Catalytic Methods for Organic Synthesis with Diazo Compounds. [Google Scholar]
- Du W., Gu Q., Li Z., Yang D. Palladium(II)-catalyzed intramolecular tandem aminoalkylation via divergent C(sp3)–H functionalization. J. Am. Chem. Soc. 2015;137:1130–1135. doi: 10.1021/ja5102739. [DOI] [PubMed] [Google Scholar]
- Gallagher N., Zhang H., Junghoefer T., Giangrisostomi E., Ovsyannikov R., Pink M., Rajca S., Casu M.B., Rajca A. Thermally and magnetically robust triplet ground state diradical. J. Am. Chem. Soc. 2019;141:4764–4774. doi: 10.1021/jacs.9b00558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandamana D.A., Wang B., Tejo C., Bolte B., Gagosz F., Chiba S. Alkyl ethers as traceless hydride donors in Brønsted acid catalyzed intramolecular hydrogen atom transfer. Angew. Chem. Int. Ed. 2018;57:6181–6185. doi: 10.1002/anie.201801953. [DOI] [PubMed] [Google Scholar]
- Haibach M.C., Seidel D. C-H bond functionalization through intramolecular hydride transfer. Angew. Chem. Int. Ed. 2014;53:5010–5036. doi: 10.1002/anie.201306489. [DOI] [PubMed] [Google Scholar]
- Hommelsheim R., Guo Y., Yang Z., Empel C., Koenigs R.M. Blue-light-induced carbene-transfer reactions of diazoalkanes. Angew. Chem. Int. Ed. 2019;58:1203–1207. doi: 10.1002/anie.201811991. [DOI] [PubMed] [Google Scholar]
- Jiang H.-J., Liu K., Yu J., Zhang L., Gong L.-Z. Switchable stereoselectivity in bromoaminocyclizationof olefins: using Brønsted acids of anionic chiral cobalt(III) complexes. Angew. Chem. Int. Ed. 2017;56:11931–11935. doi: 10.1002/anie.201705066. [DOI] [PubMed] [Google Scholar]
- Jurberg I.D., Davies H.M.L. Blue light-promoted photolysis of aryldiazoacetates. Chem. Sci. 2018;9:5112–5118. doi: 10.1039/c8sc01165f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser D., Tona V., Gonçalves C.R., Shaaban S., Oppedisano A., Maulide N. A general acid-mediated hydroaminomethylation of unactivated alkenes and alkynes. Angew. Chem. Int. Ed. 2019;58:14639–14643. doi: 10.1002/anie.201906910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Z., Wang Y., Zhang D., Wu R., Xu X., Hu W. Asymmetric counter-anion-directed aminomethylation: synthesis of chiral β-amino acids via trapping of an enol intermediate. J. Am. Chem. Soc. 2019;141:1473–1478. doi: 10.1021/jacs.8b12832. [DOI] [PubMed] [Google Scholar]
- Kolb H.C., VanNieuwenhze M.S., Sharpless K.B. Catalytic asymmetric dihydroxylation. Chem. Rev. 1994;94:2483–2547. [Google Scholar]
- Lee A., Younai A., Price C.K., Izquierdo J., Mishra R.K., Scheidt K.A. Enantioselective annulations for dihydroquinolones by in situ generation of azolium enolates. J. Am. Chem. Soc. 2014;136:10589–10592. doi: 10.1021/ja505880r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Preinfalk A., Maulide N. Enantioselective redox-neutral coupling of aldehydes and alkenes by an iron-catalyzed “catch−release” tethering approach. J. Am. Chem. Soc. 2019;141:143–147. doi: 10.1021/jacs.8b12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J.-S., Wang F.-L., Dong X.-Y., He W.-W., Yuan Y., Chen S., Liu X.-Y. Catalytic asymmetric radical aminoperfluoroalkylation and aminodifluoromethylation of alkenes to versatile enantioenriched-fluoroalkyl amines. Nat. Commun. 2017;8:14841–14852. doi: 10.1038/ncomms14841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Nakatsuji H., Okumura Y., Yao L., Ishihara K. Enantioselective halo-oxy- and halo-azacyclizations induced by chiral amidophosphate catalysts and halo-Lewis acids. J. Am. Chem. Soc. 2018;140:6039–6043. doi: 10.1021/jacs.8b02607. [DOI] [PubMed] [Google Scholar]
- Ma C., Xing D., Hu W. Catalyst-free halogenation of α-diazocarbonyl compounds with N-halosuccinimides: synthesis of 3-halooxindoles or vinyl halides. Org. Lett. 2016;18:3134–3137. doi: 10.1021/acs.orglett.6b01346. [DOI] [PubMed] [Google Scholar]
- Mavroskoufis A., Rajes K., Golz P., Agrawal A., Ruβ V., Göze J.P., Hopkinson M.N. N-Heterocyclic carbene catalyzed photoenolization/Diels–Alder reaction of acid fluorides. Angew. Chem. Int. Ed. 2020;59:3190–3194. doi: 10.1002/anie.201914456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki Y., Ohta N., Semba K., Nakao Y. Intramolecular aminocyanation of alkenes by cooperative palladium/boron catalysis. J. Am. Chem. Soc. 2014;136:3732–3735. doi: 10.1021/ja4122632. [DOI] [PubMed] [Google Scholar]
- Mohrig J.R. Stereochemistry of 1,2-elimination and proton-transfer reactions: toward a unified understanding. Acc. Chem. Res. 2013;46:1407–1416. doi: 10.1021/ar300258d. [DOI] [PubMed] [Google Scholar]
- Moosophon P., Kanokmedhakul S., Kanokmedhakul K., Soytong K. Prenylxanthones and a bicyclo[3.3.1]nona-2,6-diene derivative from the fungus emericella rugulosa. J. Nat. Prod. 2009;72:1442–1446. doi: 10.1021/np800805f. [DOI] [PubMed] [Google Scholar]
- Mori K., Isogai R., Kamei Y., Yamanaka M., Akiyama T. Chiral magnesium bisphosphate-catalyzed asymmetric double C(sp3)−H bond functionalization based on sequential hydride shift/cyclization process. J. Am. Chem. Soc. 2018;140:6203–6207. doi: 10.1021/jacs.8b02761. [DOI] [PubMed] [Google Scholar]
- Negishi E.-I., Huang Z., Wang G., Mohan S., Wang C., Hattori H. Recent advances in efficient and selective synthesis of di-, tri-, and tetrasubstituted alkenes via Pd-catalyzed alkenylation-carbonyl olefination synergy. Acc. Chem. Res. 2008;41:1474–1485. doi: 10.1021/ar800038e. [DOI] [PubMed] [Google Scholar]
- Pan Z., Pound S.M., Rondla N.R., Douglas C.J. Intramolecular aminocyanation of alkenes by N-CN bond cleavage. Angew. Chem. Int. Ed. 2014;53:5170–5174. doi: 10.1002/anie.201310983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit R.P., Kim S.T., Ryu D.H. Asymmetric synthesis of enantioenriched 2-aryl-2,3-dihydrobenzofurans by a Lewis acid catalyzed cyclopropanation/intramolecular rearrangement sequence. Angew. Chem. Int. Ed. 2019;58:13427–13432. doi: 10.1002/anie.201906954. [DOI] [PubMed] [Google Scholar]
- Parasram M., Chuentragool P., Wang Y., Shi Y., Gevorgyan V. General, auxiliary-enabled photoinduced Pd-catalyzed remote desaturation of aliphatic alcohols. J. Am. Chem. Soc. 2017;139:14857–14860. doi: 10.1021/jacs.7b08459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poplata S., Tröter A., Zou Y.-Q., Bach T. Recent advances in the synthesis of cyclobutanes by olefin [2 + 2] photocycloaddition reactions. Chem. Rev. 2016;116:9748–9815. doi: 10.1021/acs.chemrev.5b00723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rukachaisirikul V., Rodglin A., Sukpondma Y., Phongpaichit S., Buatong J., Sakayaroj J. Phthalide and isocoumarin derivatives produced by an acremonium sp. isolated from a mangrove rhizophora apiculate. J. Nat. Prod. 2012;75:853–858. doi: 10.1021/np200885e. [DOI] [PubMed] [Google Scholar]
- Seechurn C.C.C.J., Kitching M.O., Colacot T.J., Snieckus V. Palladium-catalyzed cross-coupling: a historical contextual perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 2012;51:5062–5085. doi: 10.1002/anie.201107017. [DOI] [PubMed] [Google Scholar]
- Sha Q., Wei Y. Base and solvent mediated decomposition of tosylhydrazones: highly selective synthesis of N-alkyl substituted hydrazones, dialkylidenehydrazines, and oximes. Tetrahedron. 2013;69:3829–3835. [Google Scholar]
- Singh C., Hassam M., Verma V.P., Singh A.S., Naikade N.K., Puri S.K., Maulik P.R., Kant R. Bile acid-based 1,2,4-trioxanes: synthesis and antimalarial assessment. J. Med. Chem. 2012;55:10662–10673. doi: 10.1021/jm301323k. [DOI] [PubMed] [Google Scholar]
- Snyder G.J., Dougherty D.A. 2,4-Dimethylene-1,3-cyclobutanediyl, the non-kekule isomer of benzene: synthesis, EPR, and electronic spectroscopy. J. Am. Chem. Soc. 1989;111:3927–3942. [Google Scholar]
- Suneja A., Schneider C. Phosphoric acid catalyzed [4 + 1]-cycloannulation reaction of ortho-Quinone methides and diazoketones: catalytic, enantioselective access toward cis-2,3-dihydrobenzofurans. Org. Lett. 2018;20:7576–7580. doi: 10.1021/acs.orglett.8b03311. [DOI] [PubMed] [Google Scholar]
- Tian J.-J., Zeng N.-N., Liu N., Tu X.-S., Wang X.-C. Intramolecular cyclizations of vinyl-substituted N,N-dialkyl arylamines enabled by borane-assisted hydride transfer. ACS Catal. 2019;9:295–300. [Google Scholar]
- Voica A.-F., Mendoza A., Gutekunst W.R., Fraga J.O., Baran P.S. Guided desaturation of unactivated aliphatics. Nat. Chem. 2012;4:629–635. doi: 10.1038/nchem.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Li S., Blümel M., Philipps A.R., Wang A., Puttreddy R., Rissanen K., Enders D. Asymmetric synthesis of spirobenzazepinones with atroposelectivity and spiro-1,2-diazepinones by NHC-catalyzed [3+4] annulation reactions. Angew. Chem. Int. Ed. 2016;55:11110–11114. doi: 10.1002/anie.201604819. [DOI] [PubMed] [Google Scholar]
- Wdowik T., Chemler S.R. Direct synthesis of 2-formylpyrrolidines, 2-pyrrolidinones and 2-dihydrofuranones via aerobic copper-catalyzed aminooxygenation and dioxygenation of 4-pentenylsulfonamides and 4-pentenylalcohols. J. Am. Chem. Soc. 2017;139:9515–9518. doi: 10.1021/jacs.7b05680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y., Qiu D., Wang J. Transition-metal-catalyzed cross-couplings through carbene migratory insertion. Chem. Rev. 2017;117:13810–13889. doi: 10.1021/acs.chemrev.7b00382. [DOI] [PubMed] [Google Scholar]
- Yang B., Gao S. Recent advances in the application of Diels–Alder reactions involving o-quinodimethanes, aza-o-quinone methides and o-quinone methides in natural product total synthesis. Chem. Soc. Rev. 2018;47:7926–7953. doi: 10.1039/c8cs00274f. [DOI] [PubMed] [Google Scholar]
- Yang Q., Xiao C., Lu L., An J., Tan F., Li B., Xiao W. Synthesis of indoles through highly efficient cascade reactions of sulfur ylides and N-(ortho-chloromethyl)aryl amides. Angew. Chem. Int. Ed. 2012;51:9137–9140. doi: 10.1002/anie.201203657. [DOI] [PubMed] [Google Scholar]
- Yu S.-N., Li Y.-L., Deng J. Enantioselective synthesis of 2-bromomethyl indolines via BINAP(S)-catalyzed bromoaminocyclization of allyl aniline. Adv. Synth. Catal. 2017;359:2499–2508. [Google Scholar]
- Zeng Q., Dong K., Pei C., Dong S., Hu W., Qiu L., Xu X. Divergent construction of macrocyclic alkynes via catalytic metal carbene C(sp2)−H insertion and the Buchner reaction. ACS Catal. 2019;9:10773–10779. [Google Scholar]
- Zhan G., Shi M.-L., He Q., Du W., Chen Y.-C. [4 + 3] Cycloadditions with bromo-substituted Morita−Baylis−Hillman adducts of isatins and N-(ortho-chloromethyl)aryl amides. Org. Lett. 2015;17:4750–4753. doi: 10.1021/acs.orglett.5b02279. [DOI] [PubMed] [Google Scholar]
- Zhang C., Hong K., Dong S., Pei C., Zhang X., He C., Hu W., Xu X. Gold(I)-Catalyzed aromatization: expeditious synthesis of polyfunctionalized naphthalenes. iScience. 2019;21:499–508. doi: 10.1016/j.isci.2019.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Yadagiri D., Gevorgyan V. Light-induced metal-free transformations of unactivated pyridotriazoles. Chem. Sci. 2019;10:8399–8404. doi: 10.1039/c9sc02448d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Mao J., Weng Y., Zhang X., Xu X. Cyclopentadiene construction via Rh-catalyzed carbene/alkyne metathesis terminated with intramolecular formal [3 + 2] cycloaddition. Org. Lett. 2015;17:5638–5641. doi: 10.1021/acs.orglett.5b02912. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Bao M., Qiu L., Xu X. Thermally induced reaction of diazoamides with isatins: a complementary approach to the 3,3’-bioxindole derivatives. Tetrahedron Lett. 2017;58:3390–3393. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The crystallography data have been deposited at the Cambridge Crystallographic Data Center (CCDC) under accession number CCDC: 1579217 (4f); 1579222 (9a); 1579218 (9d′) and can be obtained free of charge from www.ccdc.cam.ac.uk/getstructures. Original/source data for Schemes 1 and 2 together with Figures 2, 3, 6, and 7 in the paper is available at https://doi.org/10.17632/k23x374cz3.1.