Abstract
Introduction: During the coronavirus disease 2019 (COVID-19) outbreak, novel approaches to diabetes care have been employed. Care in both the inpatient and outpatient setting has transformed considerably. Driven by the need to reduce the use of personal protective equipment and exposure for patients and providers alike, we transitioned inpatient diabetes management services to largely “virtual” or remotely provided care at our hospital.
Methods: Implementation of a diabetes co-management service under the direction of the University of North Carolina division of endocrinology was initiated in July 2019. In response to the COVID-19 pandemic, the diabetes service was largely transitioned to a virtual care model in March 2020. Automatic consults for COVID-19 patients were implemented. Glycemic outcomes from before and after transition to virtual care were evaluated.
Results: Data over a 15-week period suggest that using virtual care for diabetes management in the hospital is feasible and can provide similar outcomes to traditional face-to-face care.
Conclusion: Automatic consults for COVID-19 patients ensure that patients with serious illness receive specialized diabetes care. Transitioning to virtual care models does not limit the glycemic outcomes of inpatient diabetes care and should be employed to reduce patient and provider exposure in the setting of COVID-19. These findings may have implications for reducing nosocomial infection in less challenging times and might address shortage of health care providers, especially in the remote areas.
Keywords: COVID-19, Telemedicine, Telehealth, Virtual care, Diabetes mellitus, Inpatient medicine, Hospital medicine
Introduction
Inpatient diabetes management and desirable levels of glucose control have been an area of controversy over the past two decades. It has been generally considered a best practice to target blood glucose levels of 140–180 mg/dL since publication of the NICE SUGAR Trial.1 Some literature supports tighter control for specific populations, such as in the setting of cardiothoracic surgery.2 Many diabetes organizations have issued guidelines for the intensity of glucose control in the inpatient setting. The American Diabetes Association (ADA) recommends a general target of 140–180 mg/dL with flexibility and special considerations for certain populations.3 This makes delivering diabetes care to large hospital populations with varied expertise of providers challenging. Some recent studies have suggested that improved glycemic control may reduce hospital-acquired infections and decrease length of stay, particularly when implemented in the context of a diabetes management service.4 For these reasons, the ADA recommends specialized inpatient diabetes management services when possible.3
Intensification of the coronavirus disease 2019 (COVID-19) outbreak caused by the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to new challenges related to diabetes care in the hospital. The Center for Disease Control has recognized diabetes as a significant risk factor for severe COVID-19 disease and mortality. Patients with COVID-19 and diabetes mellitus (DM) in the United States from February 12 to March 28, 2020 accounted for 11% of total cases, >24% of hospitalizations, and >32% of intensive care unit (ICU) admissions.5 More than 35% of COVID-19 mortality in Italy was associated with diabetes.6 Patients with DM and COVID-19 appear to have more difficulty in controlling diabetes.7 Reports suggest that diabetic ketoacidosis (DKA) is prevalent even in patients with type 2 diabetes. Severe insulin resistance has been seen, which requires a different approach to these patients. Some guidance is now available,7 however more data are needed to fully understand how to manage these patients effectively. Clearly, hyperglycemia and ketosis are important risk factors for health outcomes when COVID-19 is associated with DM.
In addition to difficulty in managing hyperglycemia, diabetes care during the COVID-19 pandemic also poses other challenges. Personal protective equipment (PPE) stewardship along with efforts to minimize patient and provider exposure to potential asymptomatic carriers of SARS-CoV-2 poses challenges for traditional face-to-face care. This led many inpatient management services to transition to “virtual care” and in some institutions continuous glucose monitoring (CGM) has been used for inpatient diabetes care by using telehealth. Even though CGM has not been approved by the Food and Drug Administration (FDA) for inpatient diabetes care, the FDA has authorized its use during this COVID-19 pandemic in an attempt to collect new data and facilitate better diabetes outcomes for the inpatient setting.
On July 1, 2019, the University of North Carolina (UNC) division of endocrinology launched a diabetes management service through a diabetes care team (DCT). The goal was to improve glycemic control and support various services with automatic consults for hyperglycemia and co-management through insulin order placement. Before this, the division of endocrinology had largely worked as a traditional consult service, making recommendations to primary services without writing orders. Most diabetes consults were reserved for complicated diabetes care, such as insulin pump management. The DCT started with one advanced practice provider (APP), three rotating attending endocrinologists, and five rotating endocrine fellows. A second APP was added in January 2020. Inpatient service teams for which the DCT was initially deployed were vascular surgery, cardiothoracic surgery, burn surgery, and the heart failure service.
During the week of March 13, 2020, everything changed for the DCT. The threat of COVID-19 had increased in North Carolina and some of the inpatient consult services were changing to virtual care models to reduce PPE use and decrease potential exposures. The division of endocrinology followed suit. Initially, we limited physical exams and shifted inpatient duties to providers younger than 65 years of age. We also limited potential provider exposures by rounding away from the patients' bedside. On March 22, 2020, the service went completely virtual. We aimed at limiting PPE use as well as limiting patient and provider exposures, but we also wanted to offer expertise in diabetes management to all patients infected with COVID-19 and all patients under investigation (PUI) for COVID-19 with automatic consults to the diabetes management service. After multiple weeks of implementation, we reviewed how implementing virtual care has affected glycemic control at the UNC Medical Center in Chapel Hill, NC. We report our experience with inpatient diabetes management through virtual care during the COVID-19 crisis and compare it with data before the pandemic as well as with data both before and after transition to virtual care.
Methods
The DCT changed to a virtual care model and stopped all face-to-face patient contact on March 22, 2020. Patients were called each morning before 9AM and interviewed by phone whenever possible. If the patient was not able to be reached by their phone in their hospital room, a family member was called. When patients and their families were unable to be contacted, the primary nurse for the patient was interviewed each morning. This was performed by two APPs who typically work Monday through Friday. In addition, attending endocrinologists and endocrinology fellows occasionally performed telehealth virtual visits with these patients. Patients were identified by using an Epic report for the four participating primary services (vascular surgery, cardiothoracic surgery, burn surgery, and medicine heart failure). A report was run each morning by the APPs that identified anyone on these four services with a finger stick blood glucose (FSBG) or serum blood glucose test less than 70 mg/dL or greater than 180 mg/dL. COVID-19 patients and PUIs were similarly identified for automatic DCT consultation based on orders for “COVID-19 contact precautions,” a unique order in our Epic electronic medical record (EMR); glycemic reports on these patients flagging those with FSBG or serum glucose <70 or >180 mg/dL, a hemoglobin A1c (HbA1c) >6.4%, or a diagnosis of diabetes. Automatic consults for these COVID-19 and PUI patients were started on April 13, 2020. In addition, the DCT APPs have been collecting information on COVID-19 patients, including: HbA1c before admission, floor versus intensive care unit status, pre-admission diabetes treatment, and highest total daily dose of insulin required during admission.
Communication with primary teams while converting to virtual care was essential. The DCT often communicated solely through the written note in EMR before the COVID-19 outbreak with primary inpatient providers. However, as a virtual care telehealth service, we implemented guidelines to provide verbal and written communication with the primary team each day. In addition to telephone visits, we utilized electronic consults for patients unable to communicate with us. For these consults, we continued to advise the primary hospital team over the phone. We developed Epic smart phrases to standardize note templates to enhance clarity of written communication with the primary teams.
For oversight and collaboration, we continued diabetes rounds between the APPs, endocrine attending, and the endocrine fellow. To limit exposure, we had mandated that team members work from home when possible. To ensure adequate communication and to optimize teaching during rounds, we used Cisco Webex for diabetes rounds. Each provider took turns sharing the EMR through Webex and presenting their patients to the team. All team members could then scrutinize glucose trends from home and provide collaborative assessments and plans. Five weeks into the new process, we were able to evaluate our strategy with FSBG data.
Before the COVID-19 outbreak, we had a data warehouse created to evaluate FSBG data during our initial implementation of the DCT. Data are pulled by using Epic into a data warehouse and include all point-of-care FSBG tests for the hospital and serum blood glucose levels. These data include all patients on non-pediatric floors. In addition, data are linked to individual services, including those that utilize the DCT.
Data were uploaded from the data warehouse to Microsoft Excel. Trends were compared both before and after the implementation of the DCT from May 2019 to January 2020. Data were then compared from January 2020 to April 2020 in 5-week intervals. The first two intervals were the 10 weeks before implementing virtual care, and the last 5-week interval was the time in which virtual care was implemented.
Results
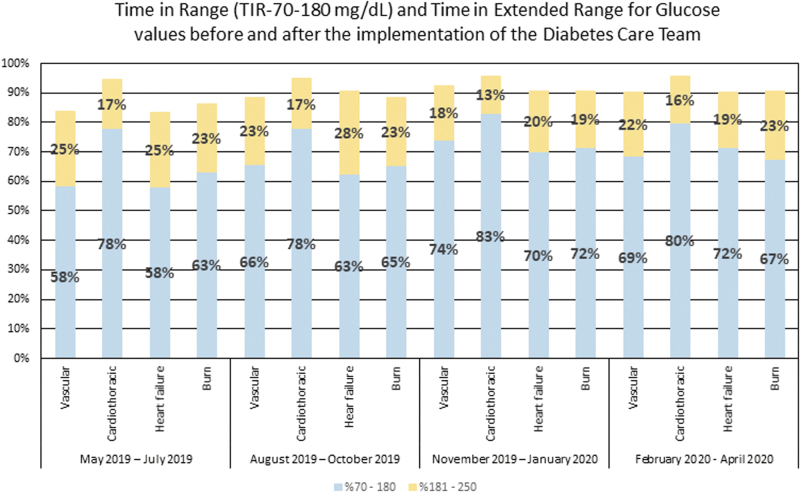
Glycemic improvement was achieved for all services by using the automatic consults after the initial implementation of the DCT in July 2019. Every service had more blood glucose readings between 70 and 180 mg/dL that are now commonly referred to as time-in-range (TIR) after using the DCT than they had earlier (Fig. 1). Moreover, all services also increased in the number of blood glucose levels between 70 and 250 mg/dL (Fig. 1). This led to increased health care system interest in the DCT and the addition of another APP to the team in January 2020.
FIG. 1.
Glycemic TIR (70–180 mg/dL) and time in extended range (181–250 mg/dL) for vascular surgery, cardiothoracic surgery, medicine heart failure, and burn surgery from May 2019 to April 2020. DCT implemented on July 1, 2019. DCT, diabetes care team; TIR, time in range.
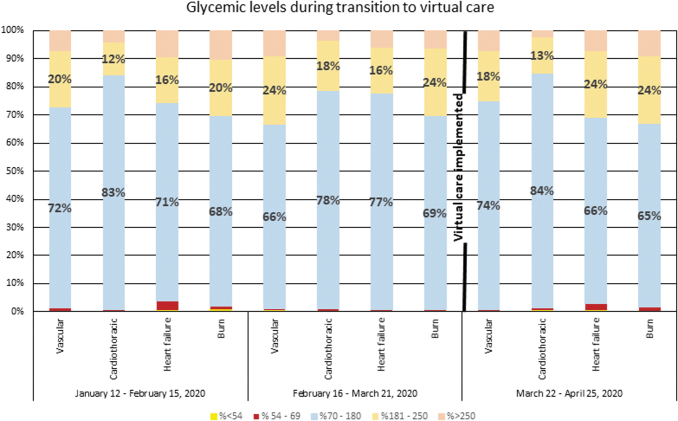
We were able to compare the previous glycemic trends among DCT patients from before to after the transition to virtual care. The number of point-of-care FSBG tests had decreased for all services using the DCT from January 2020 to April 2020, likely as a result of a reduction in elective procedures that were discontinued as COVID-19 admissions slowly increased. Table 1 shows the number of FSBG tests from January 12 to April 25, 2020. Figure 2 shows the data for glycemic levels and highlights the FSBG TIR (70–180 mg/dL) and an expanded TIR (70–250 mg/dL) in 5-week intervals before and during virtual care. The proportion of FSBG results in the two ranges varied from week to week before and after implementing virtual care but overall show fair consistency. As an example, for the vascular surgery service, 95% of the FSBG results were between 70 and 250 mg/dL during the week of April 2019 while utilizing virtual care. Before that, in the preceding 10 weeks, the best week had been essentially identical. Rates of hypoglycemia also appeared to be roughly the same both before and after implementing virtual care (Fig. 2) with overall low rates. The highest rate was 3.8% on the heart failure service that occurred before implanting virtual care.
Table 1.
Number of Finger Stick Blood Glucose Tests from January 12 to April 25, 2020
| Total FSBG tests vascular surgery | Total FSBG tests cardiothoracic surgery | Total FSBG tests medicine heart failure | Total FSBG tests burn surgery | |
|---|---|---|---|---|
| January 12–February 15, 2020 | 917 | 1570 | 943 | 1482 |
| February 16–March 21, 2020 | 907 | 2226 | 588 | 1605 |
| March 22–April 25, 2020 | 542 | 1381 | 400 | 835 |
FSBG, finger stick blood glucose.
FIG. 2.
Glycemic levels for teams utilizing the DCT during the transition from face-to-face care to virtual care. Virtual care implemented on March 22, 2020. TIR (70–180 mg/dL) and expanded TIR (181–250 mg/dL) highlighted earlier with no significant change noted from before and after implementation of virtual care.
As of April 30, 2020, the DCT provided diabetes management for the 10 patients with diabetes who were COVID-19 positive among the 40 patients with COVID-19 precautions (COVID-19 positive or PUI) admitted to our hospital. Of the 10 patients, 8 were managed without insulin in the outpatient setting before admission. Despite this, the average maximal total daily dose of insulin was 73.5 units. This included a 65-year-old patient treated with metformin and SGLT2-inhibitor in the outpatient setting with an HbA1c of 8.2% requiring 261 units of insulin during 24 h in the ICU. Another 82-year-old patient was treated with sulfonylurea only as an outpatient requiring only 2 units of insulin per day with an HbA1c of 7.2% and he was treated on the floor. Another ICU patient who was 28 years old required 66 units of insulin in the ICU and had an HbA1c of 13.5%. High variability in insulin requirements was noted during our limited experience with these patients during the current pandemic.
Discussion and Conclusions
We had two goals during the COVID-19 pandemic at UNC. The first and primary goal was to reduce PPE use and reduce patient and provider exposure. We accomplished this by transitioning to a virtual care system. The second goal was to provide effective diabetes care through virtual means. Glycemic control has not been affected by transition to virtual care. This has important implications for future inpatient diabetes care. Can we provide virtual care to diabetes inpatients at other hospitals in our health care system, several of which do not have diabetes specialty care providers in their community? Should virtual care become the standard for inpatients with contact precautions in general? The bigger question that still remains to be answered is whether reimbursement by the insurers will continue in future (post COVID-19) to be similar to the current COVID-19 pandemic period.
In addition to transitioning how we delivered care, we wanted to make ourselves available to inpatients affected by COVID-19, both to ensure optimal diabetes care in the hospital and, more importantly, to facilitate speedy transitions of care from the ICU to the floor and from the floor to the outpatient setting. Our APPs provide ongoing diabetes care to COVID-19 patients after discharge until they are able to establish care with their usual outpatient providers. Fortunately, UNC had fewer cases than predicted in April 2020, and we were able to plan our diabetes care thoughtfully. Though our experience with COVID-19 has been limited, hospitalizations in our state are still increasing. Automatic consults to patients with COVID-19 and PUI have allowed the primary hospitalist and ICU teams caring for these patients to focus on the multimorbidity that these patients experience, and transitions of care have not been held up for glycemic control issues.
Variability in insulin requirements among patients with COVID-19 has been remarkable. We recently received authorization from the hospital administration to use CGM for diabetes management and believe that this will reduce PPE requirements even further and provider and patient exposures as well as further facilitate glycemic control by using cloud-based telehealth. There have been recent reports of using telehealth successfully in new-onset patients with type 1 diabetes and keeping patients away from the hospital admissions even in the presence of DKA.8,9
The trajectory of the COVID-19 pandemic seems to be slow and prolonged in North Carolina, with most new cases coming from congregate living and workplace clusters. This suggests that the need to preserve PPE will extend for many months. Virtual care will need to continue. We have shown (through limited data) that we can effectively provide care by using a virtual model. Rates of hyperglycemia and hypoglycemia did not significantly differ after transitioning to virtual care. There are obvious limitations to our data, given the short time since the transition to virtual care occurred. The sample size is small and we did not use CGM in our model even though it was authorized recently by the FDA for inpatient settings during COVID-19. Applying these learnings may benefit inpatients with hyperglycemia in dispersed communities, especially in remote places where specialized diabetes care may not be available. Telehealth also may have the potential to reduce the risk of nosocomial infections in the future.
Acknowledgments
The authors thank Elizabeth Harris, MD, Stephen DeCherney, MD, MPH, Deepa Kirk, MD, Donald Caraccio, MD, Sadia Ejaz, MD, Paul Guido, MD, Klara Klein, MD, PhD, Chinelo Okigbo, MD, PhD, Krishna Chagarlamudi, Angela Overman, and John Falk along with the other providers, nurses, and staff involved with the DCT at the UNC Medical Center.
Author Disclosure Statement
M.S.J., A.L.G., B.E.A., S.B.K., and M.M.C. have no disclosures or conflicts of interest. J.B.B.'s contracted consulting fees and travel support for contracted activities are paid to the UNC by Adocia, AstraZeneca, Dance Biopharm, Eli Lilly, MannKind, NovaTarg, Novo Nordisk, Senseonics, vTv Therapeutics, and Zafgen as well as grant support from NovaTarg, Novo Nordisk, Sanofi, Tolerion and vTv Therapeutics. He is also a consultant to Cirius Therapeutics, Inc., CSL Behring, Mellitus Health, Neurimmune AG, Pendulum Therapeutics, and Stability Health. He holds stock/options in Mellitus Health, Pendulum Therapeutics, PhaseBio, and Stability Health.
Funding Information
J.B.B.'s efforts on this project were supported by grants from the National Institutes of Health (UL1TR002489, P30DK124723) and institutional funds. M.S.J., A.L.G., B.E.A., S.B.K., and M.M.C.'s efforts were supported from institutional funds.
References
- 1. Finfer S, Chittock DR, Su SY, et al. : Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009;360:1283–1297 [DOI] [PubMed] [Google Scholar]
- 2. Van den Berghe G, Wilmer A, Hermans G, et al. : Intensive insulin therapy in the medical ICU. N Engl J Med 2006;354:449–461 [DOI] [PubMed] [Google Scholar]
- 3. American Diabetes Association 15: Diabetes care in the hospital: Standards of medical care in diabetes-2020. Diabetes Care 2020;43(Suppl 1):S193–S202 [DOI] [PubMed] [Google Scholar]
- 4. Kyi M, Colman PG, Wraight PR, et al. : Early intervention for diabetes in medical and surgical inpatients decreases hyperglycemia and hospital-acquired infections: A cluster randomized trial. Diabetes Care 2019;42:832–840 [DOI] [PubMed] [Google Scholar]
- 5. CDC COVID-19 Response Team: Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019—United States, February 12–March 28, 2020. MMWR Morb Mortal Wkly Rep 2020;69:382–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Onder G, Rezza G, Brusaferro S: Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA 2020. [Epub ahead of print]; DOI: 10.1001/jama.2020.4683 [DOI] [PubMed] [Google Scholar]
- 7. Bornstein SR, Rubino F, Khunti K, et al. : Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol 2020. DOI: 10.1016/S2213-8587(20)30152-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garg SK, Rodbard D, Hirsch IB, Forlenza GP: Managing new-onset type 1 diabetes during the COVID-19 pandemic: Challenges and opportunities. Diabetes Technol Ther 2020;22:431–439 [DOI] [PubMed] [Google Scholar]
- 9. Peters AL, Garg SK: The silver lining to COVID-19: Avoiding diabetic ketoacidosis admissions with telehealth. Diabetes Technol Ther 2020;22:449–453 [DOI] [PubMed] [Google Scholar]