Abstract
HIV-1 compartmentalization in the central nervous system (CNS) and its contribution to neurological disease have been well documented. Previous studies were conducted among people infected with subtypes B or C where CNS compartmentalization has been observed when comparing viral sequences in the blood to virus in cerebrospinal fluid (CSF). However, little is known about CNS compartmentalization in other HIV-1 subtypes. Using a deep sequencing approach with Primer ID, we conducted a cross-sectional study among Nigerian and Malawian HIV-1 cohorts with or without fungal Cryptococcus infection diagnosed as cryptococcal meningitis (CM) to determine the extent of CSF/CNS compartmentalization with CM. Paired plasma and CSF samples from 45 participants were also analyzed for cytokine/chemokine levels. Viral populations comparing virus in the blood and the CSF ranged from compartmentalized to equilibrated, including minor or partial compartmentalization or clonal amplification of a single viral sequence. The frequency of compartmentalized viral populations in the blood and CSF was similar between the CM− and CM+ participants. We confirmed the potential to see compartmentalization with subtype C infection and have also documented CNS compartmentalization of an HIV-1 subtype G infection. Cytokine profiles indicated a proinflammatory environment, especially within the CSF/CNS. However, sCD163 was suppressed in the CSF in the presence of CM, perhaps due to elevated levels of IL-4, which were also a feature of the cytokine profile, showing a distinct cytokine profile with CM.
Keywords: CNS compartmentalization, cryptococcal meningitis, cytokine, HIV-1
Introduction
Infection with HIV-1 leads over time to a loss of CD4+ T cells and an associated state of immunodeficiency (AIDS). The immunodeficiency presents itself in the form of opportunistic infections (OIs). In general, viral pathogenesis is associated with the ability of the virus to persistently replicate within the host in spite of a measurable but ultimately ineffective host immune response.1
HIV-1 replication is associated with the accumulation of genetic diversity. At one extreme, this diversity is well mixed between different sites of viral replication within the various lymphoid tissues that harbor the majority of CD4+ T cells. At the other extreme, there is restricted mixing where founder effects and local replication lead to genetically distinct populations within an infected host. One place that can harbor genetically distinct populations of virus is within the central nervous system (CNS).2–4 The generally low levels of CD4+ T cells within the CNS puts selective pressure on locally replicating viruses to evolve to use the low density of CD4 present on microglia and macrophages within the CNS.4–7 This adaptation is seen as more efficient entry of the virus into cells with a low density of CD4, an evolutionary variant of the virus often called macrophage-tropic.8,9 Thus, the isolated replication of HIV-1 within the CNS can be characterized by a genetically distinct population and in some cases a change in viral entry phenotype.
The CNS is an immune-restricted site in that immune cells of the adaptive immune arm must migrate into this compartment as part of an inflammatory response.10 With advancing immunodeficiency, the CNS becomes one of the sites susceptible to OIs. The compartmentalization of HIV-1 in the CNS, which reflects local viral replication, may be a manifestation of the reduced immune surveillance in this compartment that comes with increasing immunodeficiency. A common OI of the CNS is the fungus Cryptococcus, leading to cryptococcal meningitis (CM). Such an infection is considered an AIDS-defining illness and it remains a leading cause of death in HIV-positive persons in resource-limited settings.
Cryptococcus infection is often systemic, usually beginning with the inhalation of the fungal spores into the lungs and subsequent dissemination to other organs, especially the CNS.11–13 Improved understanding of virus compartmentalization, and the implication for HIV-1 pathogenesis of coinfection with OIs in sub-Saharan Africa, where non-B HIV-1 subtypes predominate, remain important questions.
In this study, we have examined the effect of CM on HIV-1 populations. We carried out an initial survey of HIV-1 compartmentalization in the CNS, as assessed by comparing viral populations in the blood and the cerebrospinal fluid (CSF), followed by evaluation of cytokine levels in the blood and CSF in two HIV-positive cohorts. In one cohort, recruited in Nigeria, we compared viral populations and cytokine levels in the blood and CSF in participants with suspected cases of CM that led to a clinically indicated collection of CSF for diagnostic purposes. In a second cohort, recruited in Malawi, we compared virus and cytokine levels in the blood and CSF of participants with a confirmed case of CM. The prevalence of cryptococcal antigenemia and the incidence of CM are high in much of Sub-Saharan Africa, with the levels higher in Nigeria than in Malawi (reviewed in Rajasingham et al.11).
We used a deep sequencing approach to detect compartmentalization in HIV-1 subtypes AG, G, and C with or without CM coinfection and evaluated the inflammatory state of the CSF associated with CM infection. We found no difference in the frequency of HIV-1 compartmentalization in the presence or absence of CM. In addition, we observed an inflammatory state in the CSF/CNS with CM infection but with the unexpected suppression of sCD163.
Materials and Methods
Study design
A cross-sectional study was conducted among two cohorts of HIV-positive participants attending antiretroviral therapy (ART) clinics in Nigeria and Malawi. The Nigeria cohort includes 13 (M-6, F-7) consenting participants with suspected CM, and consequently with a clinical indication for lumbar puncture (LP), enrolled between July 2015 and March 2016 at Jos University Teaching Hospital, Nigeria. Subsequent testing for cryptococcal antigen showed that only 2 of the 13 did have a Cryptococcus infection, allowing the samples from the negative participants to be used as a comparator group as CM negative. The Malawi cohort includes 32 (M-18, F-14) HIV-positive participants with culture-confirmed CM coinfection, and enrolled in the UNC Project in Malawi as part of the “Advancing Cryptococcal meningitis Treatment for Africa” (ACTA) Study.14,15 The samples were collected between April and November 2016. HIV-1 subtype C predominates in Malawi, while subtypes AG and G among others circulate in Nigeria.16,17
Sample transportation and processing
Collected CSF and blood specimens were transported to the laboratory immediately in a cold box with frozen ice packs. In the laboratory, each aliquot of CSF was centrifuged at 500 × g for 10 min. Subsequently, the supernatant was transferred into two sterile screw-cap tubes and immediately stored at −86°C until analysis. Also, each blood-filled tube was subjected to a low-speed centrifugation at 500 × g for 5 min to enhance separation of plasma. Thereafter, recovered blood plasma was transferred into two new sterile cryovials and stored at −86°C until analysis.
Cytokine evaluation
Blood plasma and CSF of the participants were subjected to cytokines evaluation using Luminex® bead-based multiplex assay (R&D Systems, Minneapolis, MN). Specifically, TNF-α, MMP-9, CXCL10, CCL2, IL-4, IL-17A, CD25, IFN-gamma, IL-13, CD14, and sCD163 concentrations in the plasma and CSF of the participants were determined. All assays were performed following the manufacturer's instructions.
Blood plasma and CSF HIV-1 RNA quantitation
Blood plasma and CSF HIV-1 RNA concentrations were determined for each participant by the UNC Center for AIDS Research HSL Laboratory using the Abbott m2000 integrase-based real-time polymerase chain reaction (PCR) assay. A small aliquot was used in the analysis requiring it to be diluted to a specific starting volume, reducing the sensitivity of the test to 320 copies/mL.
RNA extraction, complementary DNA synthesis, and MiSeq library preparation
HIV-1 RNA was isolated from each blood plasma and CSF sample as previously described.4 Briefly, 1 mL of each specimen was pelleted by centrifugation at 22,000 rpm for 2 h (45,000 × g). Thereafter, viral RNA isolation was carried out using the QIAamp viral RNA Mini Kit (Qiagen). Between 1,000 and 10,000 HIV-1 RNA copies from each sample were then used as template for complementary DNA (cDNA) synthesis.
The HIV-1 RNA was reverse transcribed using 600 U of Superscript III Reverse transcriptase (Invitrogen), with each cDNA reaction mixture containing 0.5 mM deoxynucleoside triphosphates (dNTPs), 0.25 μM BV3R Uni primer (5′-GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTNNNNNNNNNCAGTCCATTTTGCTCTACTAATGTTACAATGTGC-3′), RNA template, 5 μM DTT (dithiothreitol), and 120 U of RNaseOut (Invitrogen) in a total volume of 60 μL. A mixture of the primer, dNTPs, and viral RNA was heated at 65°C for 3–5 min, and then cooled on ice for 1 min. The remaining components of the reaction were subsequently added, incubated at 50°C for 1 h, then 55°C for 1 h, and finally at 70°C for 15 min to inactivate the enzyme. Thereafter, 1 μL Rnase H (Invitrogen) was added to each reaction tube, and incubated at 37°C for 20 min.
Synthesized cDNA was purified using Agencourt RNAClean XP beads (Beckman Coulter), eluted in DNase-free water, and subjected to first-round PCR using KAPA2G Robust system (Roche). The reaction mixture contained 0.2 mM dNTPs, 0.5 μM each of V1F primer (5′-GCCTCCCTCGCGCCATCAGAGATGTGTATAAGAGACAGNNNNTTATGGGATCAAAGCCTAAAGCCATGTGTA-3′) ADPT_2a primer (5′-GTGACTGGAGTTCAGACGTGTGCTC-3′), 2.5 U KAPA Robust polymerase, and cDNA template in a total volume of 50 μL. The reaction was done at thermal cycling conditions of 95°C for 1 min, then, 25 cycles of 95°C for 15 s, 58°C for 1 min, and 72°C for 30 s. The final steps were at 72°C for 3 min, then hold at 4°C.
First-round PCR products were purified using Agencourt AMPure XP beads (Beckman Coulter), eluted in DNase-free water, and subsequently subjected to second-round PCR using the KAPA High-Fidelity System (Invitrogen). The reaction mixture contained 0.2 mM dNTPs, 0.5 μM ADPT_P1 (Universal Adapter, 5′-AATGATACGGCGACCACCGAGATCTACACGCCTCCCTCGCGCCATCAGAGATGTG-3′), 2.5 μM KAPA High-Fidelity polymerase, 0.5 μM Indexed adapter (5′-CAAGCAGAAGACGGCATACGAGATNNNNNNGTGACTGGAGTTCAGACGTGTGCTC-3′, where the N's are a set of 24 barcodes to allow multiplexing of the libraries), DNA template (PCR 1 product), and DNase-free water in a total volume of 25 μL. The reaction was done at the following thermal cycling conditions: 95°C for 2 min, then, 35 cycles of 98°C for 20 s, 63°C for 15 s, and 72°C for 30 s, with the final steps being 72°C for 3 min, and finally hold at 4°C. Gel purification and quantitation of the amplicons were done with the QIAquick® Gel Extraction Kit and the Invitrogen Qubit dsDNA BR Assay Kit, respectively. Extracted DNA was finally pooled into a library, cleaned, and submitted for deep sequencing.
Deep sequencing
Sequencing was done using the Illumina MiSeq platform with the 300 base paired-end read format, and results interpreted using the template consensus sequence (TCS) pipeline 1.3.8 (https://github.com/SwanstromLab/PID) as previously discussed.18 All of the VI/V3 sequences obtained in this study have been submitted to the GenBank Sequence Read Archive (SRA) with accession number PRJNA602884.
Single-genome amplification assay
Single-genome amplification (SGA) of the full-length HIV-1 env gene was done as previously described.4 Briefly, HIV-1 RNA was isolated from selected blood plasma and CSF samples using the QIAamp Viral RNA Mini Kit (Qiagen), and eluted in 60 μL. The HIV-1 RNA was reverse transcribed using Superscript III Reverse transcriptase (Invitrogen). Each reaction mixture contained 5 μL dNTPs (from a 10 mM stock), 1.25 μL EnvN primer (5′-CTGCCAATCAGGGAAGTAGCCTTGTGT-3′), and 20 μL RNA template in a total volume of 65 μL. The mixture was heated at 65°C for 5 min, and then cooled on ice for 1 min. Thereafter, the remaining components consisting of a mixture of 20 μL buffer, 5 μL DTT, 5 μL RNaseOut, and 5 μL Superscript III were added. The cDNA synthesis reaction was done using the following cycling conditions: 50°C for 1 h, 55°C for 1 h, then, at 70°C for 15 min to inactivate the enzyme. Thereafter, 1 μL RNase H was added to each reaction tube, and incubated at 37°C for 20 min.
The cDNA was endpoint diluted and subjected to two rounds of nested PCR using Platinum Taq High-Fidelity polymerase (Invitrogen) in accordance with the manufacturer's direction. Primers cEnvA (CACCGGCTTAGGCATCTCCTATACCAGGAAGAA) and cEnvN (CTGCCAATCAGGGAAGTAGCCTTGTGT) were used for first-round (SGA1) PCR, while cEnvA (CACCGGCTTAGGCATCTCCTATACCAGGAAGAA) and cEnVM (TAGCCCTTCCAGTCCCCCCTTTTCTTTT), were used for the second-round (SGA2) PCR. SGA2 PCR products were subsequently run in 1% agarose gel to determine the appropriate dilution to run a repeat SGA1 and SGA2 PCR protocol such that <30% of the reactions were positive, ensuring most amplicons came from a single cDNA template. The new SGA2 PCR products were subsequently analyzed on 1% agarose gel, and the observed amplicons submitted for sequencing.
DNA sequencing
With a set of eight primers, the full-length env region was sequenced from each SGA2 PCR product (amplicon) using BigDye Terminator chemistry and the protocols recommended by the manufacturer (Applied Biosystems, Foster City, CA). Only chromatograms with single peaks, indicating amplification from one cDNA template, and sequences without positions of mixed bases or frameshift mutations that often result in premature stop codons were included in the analysis. A total of 276 full-length env gene sequences obtained in this study have been deposited in GenBank under the accession numbers MN944109-MN944384.
Phylogenetic analysis of the env gene sequences
Sequences were analyzed using combination of the Sequencher program, version 5.1 (Gene Codes, Ann Arbor, MI), Multiple Sequence Comparison by Log-Expectation (MUSCLE) (EBI web tool), and HIV database tools. Phylogenetic trees were generated using the neighbor-joining method (MEGA 6.0).
Results
Study population
For this cross-sectional study, we obtained blood and CSF samples from 45 HIV-positive participants, 13 participants with suspected CM (seen at the Jos University Teaching Hospital, Nigeria), and 32 cases of culture-confirmed CM (from the UNC Project in Malawi as part of the ACTA study testing several treatment regimens in participants diagnosed with CM14,15). IRB approval was obtained from each home country (Nigeria, Malawi, and United States). Two (A01 and A12) of the 13 participants with suspected CM infection were subsequently confirmed CM positive by antigen testing. The study population was then grouped based on the presence or absence of CM coinfection. Thus, going forward we describe virus populations and cytokine levels in 2 HIV-positive cohorts of 11 CM− (from Nigeria) and 34 CM+ (2 from Nigeria and 32 from Malawi) participants. Available demographic and laboratory data for the participants are shown in Table 1.
Table 1.
Summary of Demographic and Laboratory Data for HIV-1-Infected Cohorts from Nigeria and Malawi
| Gender | On therapy | CD4 cells/μL of blood | HIV RNA copies/mL |
HIV RNA copies/mL |
Next-generation sequences generated |
SGA sequences generated |
CM antigen in CSF | CM confirmation method | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasma | CSF | Plasma | CSF | Plasma | CSF | ||||||
| A02 | Male | Yes | 311 | 27,456 | 3,656 | 47 | 55 | 29 | 26 | No | CrAg test |
| A03 | Female | Yes | 251 | 320 | 320 | — | — | — | — | No | CrAg test |
| A04 | Female | Yes | 51 | 162,544 | 2,104 | 33 | 120 | 24 | 15 | No | CrAg test |
| A05 | Female | Yes | 125 | 5,648 | 4,608 | 33 | 327 | 2 | 22 | No | CrAg test |
| A06 | Female | Yes | 355 | 320 | 320 | — | — | — | — | No | CrAg test |
| A07 | Male | Yes | 245 | 320 | 320 | — | — | — | — | No | CrAg test |
| A08 | Male | No | NA | 3,235,600 | 720 | 779 | 0 | — | — | No | CrAg test |
| A09 | Female | Yes | 84 | 320 | 320 | — | — | — | — | No | CrAg test |
| A10 | Female | Yes | 349 | 439,512 | 554,088 | 883 | 2,320 | 19 | 41 | No | CrAg test |
| A11 | Female | Yes | 406 | 320 | 320 | — | — | — | — | No | CrAg test |
| A13 | Male | Yes | 130 | 1,073,000 | 5,107,488 | 334 | 35,114 | 38 | 36 | No | CrAg test |
| A01 | Male | Yes | 17 | 122,088 | 320 | 110 | — | — | — | Yes | CrAg test |
| A12 | Male | Yes | 16 | 11,480 | 6,344 | 192 | 969 | 6 | 18 | Yes | CrAg test |
| ACTA3153 | Female | Yes | 9 | 10,048 | 1,368 | — | — | ND | ND | Yes | Culture |
| ACTA3154 | Male | Yes | 9 | 320 | 320 | — | — | ND | ND | Yes | Culture |
| ACTA3155 | Male | Yes | 294 | 320 | 320 | — | — | ND | ND | Yes | Culture |
| ACTA3156 | Female | No | 32 | 35,528 | 320 | — | — | ND | ND | Yes | Culture |
| ACTA3157 | Male | No | 15 | 94,392 | 320 | — | — | ND | ND | Yes | Culture |
| ACTA3159 | Male | No | 43 | 680,442 | 14,640 | 905 | — | ND | ND | Yes | Culture |
| ACTA3160 | Male | Yes | 5 | 7,976 | 320 | — | — | ND | ND | Yes | Culture |
| ACTA3162 | Male | Yes | 35 | 70,408 | 320 | 97 | — | ND | ND | Yes | Culture |
| ACTA3163 | Female | No | 18 | 157,120 | 320 | 41 | — | ND | ND | Yes | Culture |
| ACTA3164 | Male | Yes | 26 | 320 | 1,328 | — | — | ND | ND | Yes | Culture |
| ACTA3165 | Female | Yes | 3 | 20,616 | 320 | 4 | — | ND | ND | Yes | Culture |
| ACTA3166 | Female | No | 7 | 181,920 | 1,008 | 75 | — | ND | ND | Yes | Culture |
| ACTA3167 | Male | Yes | 65 | 320 | 320 | — | — | ND | ND | Yes | Culture |
| ACTA3168 | Female | Yes | 121 | 320 | 520 | — | — | ND | ND | Yes | Culture |
| ACTA3169 | Female | Yes | 44 | 320 | 4,224 | — | — | ND | ND | Yes | Culture |
| ACTA3170 | Male | Yes | 19 | 43,496 | 10,184 | 596 | — | ND | ND | Yes | Culture |
| ACTA3171 | Male | No | 28 | 521,920 | 43,200 | 109 | 5 | ND | ND | Yes | Culture |
| ACTA3172 | Female | Yes | 48 | 11,072 | 1,672 | — | — | ND | ND | Yes | Culture |
| ACTA3173 | Male | Yes | 2 | 57,512 | 11,312 | 6 | 1 | ND | ND | Yes | Culture |
| ACTA3174 | Male | Yes | 174 | 400 | 320 | 1 | — | ND | ND | Yes | Culture |
| ACTA3175 | Female | Yes | 6 | 45,670 | 43,583 | 93 | 4 | ND | ND | Yes | Culture |
| ACTA3176 | Male | Yes | 255 | 320 | 320 | — | — | ND | ND | Yes | Culture |
| ACTA3177 | Male | Yes | 294 | 460 | 320 | 3 | — | ND | ND | Yes | Culture |
| ACTA3178 | Female | No | 40 | 22,410 | 4,387 | 63 | 15 | ND | ND | Yes | Culture |
| ACTA3179 | Female | No | 31 | 31,612 | 27,492 | 1,245 | 589 | ND | ND | Yes | Culture |
| ACTA3180 | Female | Yes | 154 | 320 | 320 | — | — | ND | ND | Yes | Culture |
| ACTA3181 | Male | Yes | 189 | 356 | 2,563 | 19 | 64 | ND | ND | Yes | Culture |
| ACTA3182 | Female | No | 2 | 2,198 | 854 | 496 | 693 | ND | ND | Yes | Culture |
| ACTA3183 | Male | Yes | 72 | 7,146 | 1,656 | 526 | 49 | ND | ND | Yes | Culture |
| ACTA3184 | Female | Yes | 85 | 320 | 320 | — | — | ND | ND | Yes | Culture |
| ACTA3185 | Male | No | 26 | 79,432 | 12,495 | 787 | 6,416 | ND | ND | Yes | Culture |
| ACTA3186 | Male | Yes | 5 | 356 | 320 | 35 | 3 | ND | ND | Yes | Culture |
ACTA, Advancing Cryptococcal meningitis Treatment for Africa; CM, cryptococcal meningitis; CSF, cerebrospinal fluid; HIV, human immunodeficiency virus; ND, not done; SGA, single-genome amplification.
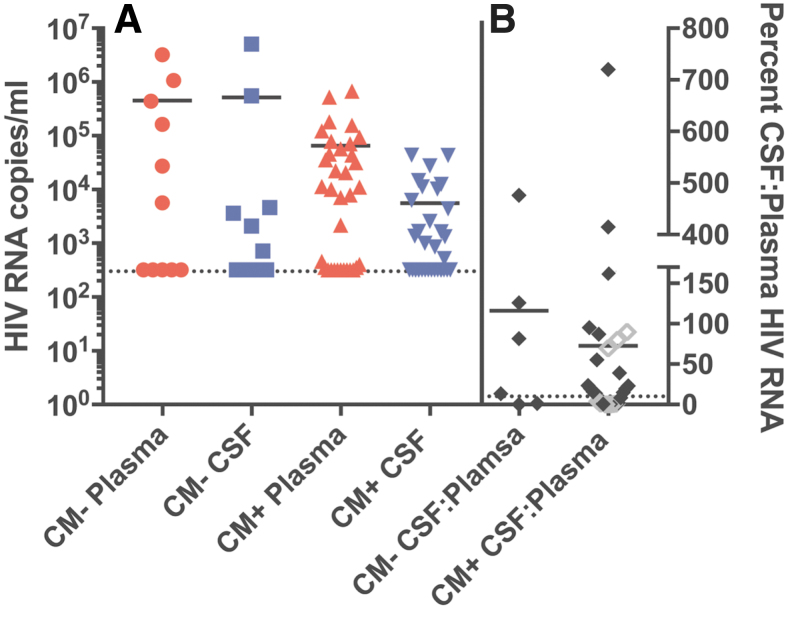
In the CM+ cohort a total of 6 (of 34) participants had HIV-1 viral loads below the level of detection (<320 copies/mL in the assay used) in the blood plasma and CSF indicating this subset was likely on suppressive ART (Table 1 and Fig. 1A). For the remaining 28 participants there was a range of viral load values for plasma and CSF, with the plasma viral load levels being about 10-fold higher than the viral load levels in the CSF. A total of 5 people in the CM− cohort were on suppressive ART, and another 2 of the 11 participants in this group had very high viral loads in the blood and CSF (around 106 copies of viral RNA or higher). Aside from the two CM− participants with very high viral loads, the rest of the viremic CM− participants had a distribution of viral loads in the blood and CSF that was similar to those participants in the CM+ cohort not on suppressive therapy.
FIG. 1.
(A) HIV RNA copies/mL in blood plasma (red circles) and CSF (blue squares) of CM− participants, and blood plasma (red triangles) and CSF (blue triangles) of CM+ participants. The dashed line denotes the limit of detection (320 RNA copies/mL) of the viral load assay used in the study. (B) Percent CSF:plasma HIV-1 RNA ratio (calculated by taking the absolute value of the CSF viral load divided by the blood plasma viral load and multiplying by 100). Gray, open symbols indicate a ratio calculated with one measurement set at the limit of detection if that sample was negative. The dashed line at 10% represents a typical CSF:plasma HIV-1 RNA ratio. CM, cryptococcal meningitis; CSF, cerebrospinal fluid.
We found a relatively lower CD4 count (30 cells/μL of blood; range: 2–294 cells/μL of blood) in CM+ cohort in comparison with the CM− cohort (248 cells/μL of blood; range: 51–406 cells/μL of blood) (Table 1). Among those with detectable viremia in the blood the CD4+ T cell concentrations were significantly higher in the CM− group (mean 46 cells/μL versus 193 cells/μL; p = .0015, Mann–Whitney rank-sum test), while in the subset that was on suppressive therapy there was no statistical difference in the distribution of CD4+ T cell counts (mean 117 cells/μL versus 268 cells/μL; not significant, Mann–Whitney rank-sum test). This observation is consistent with CM being associated with lower CD4+ T cell counts, and with ART being associated with some restoration of CD4+ T cell levels.
Blood plasma versus CSF HIV-1 RNA levels
Typically, the viral load in the CSF is between 1% and 10% the viral load in the blood in the absence of local production of virus within the CNS or an influx of blood cells through pleocytosis.17–20 We compared the number of participants with viral loads in the CSF >10% of the value in the blood (termed elevated CSF viral load) (Fig. 1B). For this analysis, we examined only those participants with a viral load of at least 1,000 copies per mL in either the blood or CSF compartment to allow a comparison of viral loads in people with actively replicating virus. We found that a comparable 67% (4 of 6) of the CM− and 58% (14 of 24) of the CM+ cohorts had elevated CSF viral loads (Table 1 and . 1B). Thus, it appears that in the presence of CM there is not an elevated viral load in the CSF relative to that seen in participants without a CM infection after taking into account the viral load in the blood plasma.
Of note, three out of nine CM+ participants had detectable CSF HIV-1 RNA despite plasma RNA suppression (Participant ACTA3164, 1,328 copies/mL; ACTA3168, 520 copies/mL; ACTA3169, 4,224 copies/mL). All three of the participants with CSF virus escape had elevated levels of white blood cells in the CSF (ranging from 12 to 210 cells/μL; data not shown). However, three of the six CM+ participants suppressed in blood and CSF also had elevated CSF white blood cell counts (ranging from 82 to 120 cells/μL; data not shown) but without elevated virus, suggesting that pleocytosis by itself cannot explain the three cases of CSF virus escape.
Analysis of HIV-1 compartmentalization comparing viral populations in blood and CSF
We have previously described a deep sequencing approach for assessing virus diversity.18 This involves making a cDNA transcript of purified virion RNA where each cDNA primer has a unique sequence tag (Primer ID), thus providing a tag for each RNA template copied. The cDNA is amplified using PCR and the PCR product sequenced using the MiSeq platform. In this way, all sequences with the same Primer ID sequence tag are collapsed to make a TCS; thus, the number of RNA templates that were actually sequenced is validated by the number of unique Primer ID tags, and the error rate is reduced by making a consensus sequence for each original RNA template.
To monitor viral diversity and assess viral compartmentalization between the blood and CSF, we applied this approach to the V1 to V3 region of the viral env gene. We were able to generate HIV-1 sequences for the blood and the CSF for five of the CM− participants and twelve of the CM+ participants (Table 1; NGS [next-generation sequencing] Data). In addition, we used endpoint dilution PCR/SGA21 to generate full-length env genes from blood and CSF for five of the participants from Nigeria (four CM−, one CM+), all of whom had sequences generated using Primer ID/NGS. Based on the full length and V1 to V3 region of the env gene, four of the sequenced viruses from Nigeria were most closely related to subtype G (participants A02, A04, A05, and A13) and two to the CRF02_AG recombinant (participants A10 and A12). As expected, all viruses sequenced from the ACTA Malawi cohort were most closely related to HIV-1 subtype C.
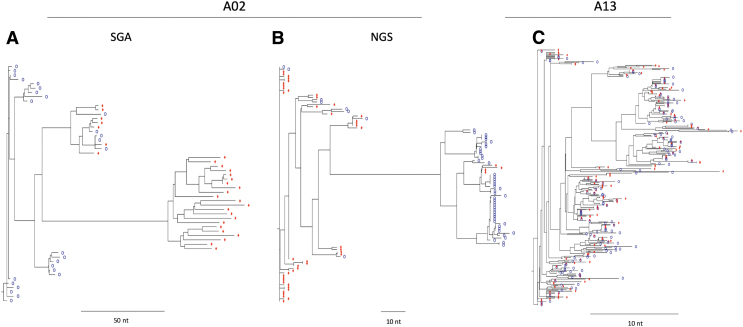
The main goal of analyzing the viral populations in CSF compared with blood was to determine if CM was associated with increased compartmentalization of HIV-1 within the CNS. This could be the case if CM was a marker for immunodeficiency specifically within the CNS, or due to an influx of inflammatory cells into the CNS that could serve as targets for HIV-1 infection. Phylogenetic trees were built for the NGS sequences and separately for the SGA sequences. Examples of each type of tree showing compartmentalized populations are shown in Figure 2A and B for participant A02. Examples of each type of tree showing mixing of virus between the blood and CSF (i.e., equilibrated) along with a strong clonal expansion of one lineage of virus in the CSF is shown for participant A10 in Figure 3A and B. We assessed the trees for all of the paired sequences for compartmentalization in three ways: (1) we used the standard Slatkin-Maddison test (Standard S-M)22; (2) we used a variant of the S-M designed to be more informative for the high-density sequence information obtained with deep sequencing (Structured S-M; Kosakovsky Pond et al., in preparation); and (3) we inspected the trees to visually assess the patterns for compartmentalization (Table 2).
FIG. 2.
Phylogenetic trees generated from SGA (A) and NGS (B) sequence data showing virus compartmentalization in participant A02. An example of a phylogenetic tree showing equilibration between CSF-derived (blue circles) and blood-derived (red diamonds) virus (C). NGS next-generation, sequencing; SGA, single-genome amplification.
FIG. 3.
Phylogenetic trees generated from SGA (A) and NGS (B) sequence data showing mixing of virus between the blood (red diamonds) and the CSF (blue circles) along with a strong clonal expansion of one lineage of virus in the CSF in participant A10.
Table 2.
Results of Next-Generation Sequencing and Single-Genome Amplification Sequences Analyzed to Determine Compartmentalization in HIV-Infected Cohorts from Nigeria and Malawi
| Sample ID | NGS data |
SGA data |
||||
|---|---|---|---|---|---|---|
| Structured S-M, p-value | Standard S-M, p-value | Inspection | Structured S-M, p-value | Standard S-M, p-value | Inspection | |
| CM antigen − | ||||||
| A02 | 0 | 0 | Compartmentalized | .024 | 0 | Compartmentalized |
| A04 | .17 | .0001 | Partial compartmentalization | .293 | .0016 | Partial compartmentalization |
| A05 | .84 | .0016 | Equilibrated | ND | ND | ND |
| A10 | 0 | 0 | Equilibrated, clonal amplification | 0 | .0001 | Equilibrated, clonal amplification |
| A13 | .88 | 0 | Equilibrated | .407 | .0036 | Minor compartmentalization |
| CM antigen + | ||||||
| A12 | .257 | .00059 | Equilibrated, clonal amplification | 1 | 1 | Equilibrated |
| ACTA3171 | 1 | 1 | Equilibrated | ND | ND | ND |
| ACTA3179 | .128 | .0001 | Minor compartmentalization | ND | ND | ND |
| ACTA3181 | .932 | .0201 | Minor compartmentalization | ND | ND | ND |
| ACTA3182 | .001 | 0 | Equilibrated, clonal amplification | ND | ND | ND |
| ACTA3183 | .426 | .0002 | Equilibrated | ND | ND | ND |
| ACTA3185 | .773 | .2028 | Equilibrated | ND | ND | ND |
HIV, human immunodeficiency virus; ND, not determined; NGS, next-generation sequencing; S-M, Slatkin-Maddison test.
There are three points to make about the assessment of compartmentalization between blood and CSF in these participants. First, there was a range of states describing the viral populations between the blood and CSF: a compartmentalized viral population in the CSF relative to the blood; equilibrated viral populations that were well mixed between the blood and CSF; equilibrated populations with minor or partial compartmentalization or with clonal amplification of a single viral sequence. Second, the Standard S-M generated small p-values for virtually all of the samples suggesting more compartmentalization than was apparent based on inspection. Conversely, the Structured S-M p-values were more in line with inspection with the exception that clonal amplification of a single viral sequence generated a strong signal in this test (a type of compartmentalization but distinct from a diverse population). Third, the range of outcomes in comparing the blood and CSF viral populations was similar between the CM− and CM+ participants, (Supplementary Figs. S1, S2) suggesting CM does not by itself contribute to a strong likelihood of having an independently replicating HIV-1 population within the CNS, at least as assessed by sequencing virus in the CSF. Finally, we were able to document compartmentalized viral populations in the context of a subtype G infection.
Discrete lineages containing sequences predicted to use the CXCR4 coreceptor (using the geno2pheno algorithm on the V3 region of env and with an FPR of 2.5%) were observed in the blood and CSF of two CM+ individuals. In a third CM+ individual, CXCR4-using variants were observed in the blood, but not the CSF. Importantly, this individual had compartmentalized CSF-derived virus, whereas the two individuals in whom CXCR4-using variants were found in both the blood and CSF had equilibrated viral populations in the blood and CSF. The observation that 3/12 participants had X4 variants is not surprising, given the low CD4 counts of these 3 people (2, 16, and 72 CD4+ T cells/μL).
Blood plasma and CSF cytokine levels
HIV-1 infection by itself elicits the production of a broad range of cytokines and chemokines as part of the inflammatory response.7 Also, CM is known to induce a cytokine response in the host that in part predicts the clinical outcome of the infection.8,9 We attempted to define the cytokine/chemokine response of the host in both the blood plasma and the CSF by measuring the concentration of a set of cytokines/chemokines, specifically TNF-α, MMP-9, CXCL10, CCL2, IL-4, IL-17A, CD25, IFN-γ, IL-13, CD14, and sCD163. In an effort to have a comparator/control group of samples, we used the blood and plasma samples from the participants that had tested negative for CM in the context of clinical care. While these control samples (blood and CSF) had been collected because of a clinical indication (ultimately of undetermined etiology) and cannot be considered a normal control, they do show significant differences with the CM+ samples.
Because of the unknown etiology of the symptoms that led to their CSF collection, they likely represent a greater spread of normal values than would be observed in an asymptomatic cohort; however, since it was not possible to obtain a true parallel set of samples from asymptomatic participants (i.e., voluntary LPs), we have used this more heterogeneous set of CM− CSF and blood plasma samples as the control group. Also, since the host response to CM is likely to be affected if the person is on suppressive ART, we analyzed the data based on whether the person was viremic (greater than the 320 HIV-1 RNA copies per mL, which was the limit of detection for the viral load test used) or suppressed for their HIV-1 infection (320 copies per mL).
Overall, the cytokine profile indicates a proinflammatory environment, with IFN-γ elevated in the plasma and IL-17A elevated in the plasma and CSF, with the exception that IL-17A was not elevated in the CSF of ART-treated people (Fig. 4, and summarized in Fig. 5). Similarly, TNF-α, MMP9, CXCL10, and CD14 were all elevated in the CSF regardless of ART status. However, there is evidence of a fairly mature immune response with the expression of IL-4 and IL-13 in all compartments (with the exception that IL-4 was not elevated in the plasma of ART-treated people). IL-4 and IL-13 play similar roles in differentiation of naive T cells to the Th2 pathway and also the maturation of macrophages to the M2/repair macrophage lineage. A surprising finding was that sCD163 levels (generally associated with macrophage activation) are suppressed in the CSF (but not in the blood) even while another marker of macrophage activation, sCD14, is elevated.
FIG. 4.
Cytokine levels in the plasma (top two charts) and CSF (bottom two charts) samples of the CM− (white circle) and CM+ (red circle). The analysis was further divided into participants with detectable HIV-1 RNA in the blood (left two panels) or with undetectable viral RNA in the blood (right two panels, with the level of detection for this study being 320 copies/mL). Statistical comparisons were made between the two groups for each cytokine in each compartment using an unpaired t test of log-transformed data. *p < .05, **p < .01, ***p < .001.
FIG. 5.

Venn diagram showing cytokine variation in the plasma and CSF of CM− and CM+ HIV-infected cohorts from Nigeria and Malawi, with the cytokines shown being significantly elevated in the CM+ participants (with the exception of sCD163 in the CSF). Blue represents cytokines in the CSF, red represents cytokines in the blood. Darker shades are from viremic participants, lighter shades are from participants with suppressed HIV-1 viral loads. The arrows indicate the direction of change for sCD163 relative to the CM− group.
Discussion
In this study we examined the effect of CM on HIV-1 populations in the CNS/CSF relative to the blood; the study participants included participants in Nigeria and Malawi who were infected with different subtypes of HIV-1 (G and C) and also the CRF02_AG recombinant (Supplementary Fig. S3). HIV-1 subtype G and the CRF02_AG recombinant are the most prevalent subtypes in West Africa,17,23 while subtype C is the predominant subtype in Southern African countries.16,24 The samples from Nigeria were collected because a LP was clinically indicated, but 11 of the 13 samples collected were subsequently shown to be negative for cryptococcal infection (CM−); we used the CM− samples as a comparator group for the CM+ samples. The remaining CM+ samples were collected in Malawi as part of the ACTA study,14,15 where all enrollees were known to have a cryptococcal infection.
In the two cohorts, between 25% and 50% of the participants were on suppressive ART. These cross-sectional samples allow us to determine if there are changes in the presence of CM that have a large impact on HIV-1 populations; however, smaller effects could not be detected given the heterogeneity in the comparator group and the overall small sample size. Given the heterogeneity in the samples, beyond whether they were CM+ or CM−, we cannot account for other potential variables such as different HIV-1 subtypes, which may or may not have an effect.
Overall, we did not find evidence for any large-effect changes in HIV-1 during CM. There was a similar distribution of the ratio of CSF/plasma viral loads, suggesting CM does not lead to elevated CSF viral load beyond what would be expected after normalizing for the level of virus in the blood. The range of blood plasma and CSF viral loads seen in the CM+ participants not on therapy is similar to that seen previously in a larger cohort.25 While there were examples of elevated CSF viral load relative to the blood, this was also true in the comparator CM− group. We did find three examples of CSF escape (i.e., CSF viral load detected while suppressed in the blood) in the CM+ group (three of nine ART-suppressed participants), but not in the CM− ART-suppressed group (zero of five); the sample size is too small for comparative analysis based on this study alone (not significant, Fisher's exact test). Nevertheless, HIV-1 CSF escape has been reported to occur in only 5%–10% of participants on suppressive ART,26–32 making the 33% occurrence observed in the CM+ ART-suppressed group notable as potentially being at significant risk of CSF/CNS escape due to the inflammatory environment created by CM.33,34
Studies with larger sample size will elucidate whether the level of escape is significantly higher in these CM+ participants. We did not examine drug resistance in these samples which can contribute to CSF escape.29,30,32 Given that many people still start therapy late they might still be susceptible to CM and thus potentially to CSF escape, making this an important question for future studies. The three CSF escape participants also had pleocytosis, although pleocytosis was also present in a majority of the CM+ participants who were on suppressive ART therapy without CSF escape. Thus, while pleocytosis may have contributed to the increase in viral load, by itself pleocytosis does not explain the elevated viral load in the CSF. White blood cell counts were not available for the CM− participants precluding a comparison of this parameter. While therapy regimen information with these groups is incomplete, most were likely on TDF, 3TC, EFV. This is not a regimen that scores high in CNS penetration, although no correlation has been found between CNS penetration scores and neuroAIDS conditions beyond an association between HIV-associated dementia and a regimen with a high CNS penetration score.35
We did not see a strong impact on the nature of viral populations in the CSF in the presence of CM in the participants not on therapy. CSF viral populations can be similar to those in the blood (equilibrated), can have clonal amplification of a specific viral genotype, or can be genetically distinct from the virus in the blood suggestive of long-term local replication of virus within the CNS. We found an overlapping range of outcomes in comparing blood and CSF viral populations in CM− and CM+ groups of participants. Thus, we did not find evidence that the presence of CM is a strong determinant of local viral replication within the CNS that would be reflected as a complex compartmentalized viral population in the CSF; this is similar to a previous study that did not find significant compartmentalization in the CSF in a cohort of CM+ participants.36 Our findings are, however, limited by the clinical characteristics of the CM− comparator arm. Specifically, all the CM− participants had substantial CNS symptoms that indicated diagnostic testing with a LP. Accordingly, it is possible that they had opportunistic or other CNS processes that confounded our observations in the CM+ group. Studies involving an asymptomatic CM− comparator group would provide additional insight.
Immune responses play a significant role in CM pathogenesis. High fungal burden is associated with reduced levels of CD4+ and CD8+ T cells, with mortality associated with reduced INF-γ concentrations.37 Peripheral CD4+ T cells producing IFNγ and TNFα and specific to cryptococcal antigen have been shown to be important to treatment outcome.38 A number of studies have examined cytokine levels in blood and CSF in the context of disease and treatment outcome, with a general consensus that IFNγ, IL-2, and IL-12 have protective effects against more severe disease; a supportive role has been suggested for the following cytokines: TNFα, IL-6, IL-8, IL-18, IL-23, and IP10/CXCL10 (reviewed in Mukaremera and Nielsen39). There is also evidence that cytokines associated with a Th2 differentiation pathway for T cells are not protective (reviewed in Mukaremera and Nielsen39).
While we did not find significant differences in viral populations in the presence of CM, we did observe effects on host cytokines/chemokines in the presence of a cryptococcal infection. Overall, we observed an inflammatory state with the cryptococcal infection that was apparent whether the person was on suppressive ART or not. Since a number of these cytokines are increased with HIV-1 infection, and at least partially resolved with ART, the persistence of the inflammatory state in those on and off ART indicates that the inflammation was being driven by the cryptococcal infection. The inflammatory state was more pronounced in the CSF of these CM participants than in the blood, in comparing the CM+ to the CM− groups, which could be due to an enhanced immune response in this compartment or simply due to less dilution/turnover of the cytokines in the CSF compared with the blood. In a study design that was analogous to ours, Mora et al. found elevated CSF cytokine levels in untreated, HIV-positive people who had CM versus those who did not, with the elevated cytokines being IL-2, IL-8, IL-17A, IFNγ, and TNFα.40 Although we did not measure all these cytokines, we did observe elevated levels of IL-17A, IFNγ, and TNFα as well as elevated soluble CD25 (the IL-2 receptor).
However, the pattern of cytokines was more complex than a simple inflammatory state. IL-4 and IL-13 were elevated suggestive of a more mature immune response, although their role in CM pathogenesis is not clear.39 Soluble markers of macrophage activation have been less well studied. Higher levels of MCP-1/CCL-2 have been associated with immune reconstitution inflammatory syndrome (IRIS) after therapy initiation,41 although in our study, we did not detect elevated MCP-1/CCL-2 in the CM population as a group. In contrast, elevated TNF-α and IL-17a have also been associated with IRIS42 and these were elevated in the six people with viral suppression in both the blood and CSF with CM, raising the possibility that IRIS could explain some of the cytokine variability.
There was the unexpected observation of suppression of sCD163 in the CSF in the CM+ group relative to the CM− group (Fig. 4). CD163 binds hemoglobin with its expression elevated with the activation of macrophages; similarly sCD14, which was elevated in the CSF, is also produced by inflammatory macrophages, making the suppression of one of these markers (sCD163) in the face of elevated expression of the other (sCD14) unexpected. IL-4 has been shown to repress expression of the transmembrane form of CD163,43 providing a potential mechanistic explanation for the reduction in sCD163. This effect must be at least in part independent of HIV-1 since it is seen in both the viremic individuals and in the ART-suppressed individuals. However, IL-4 is also a driver of the M2 differentiation state of macrophages which is associated with increased sCD163 expression.44 We do not know if suppression of sCD163 is due to suppression of expression of CD163 by IL-4 in the absence of an M2 differentiation state, or if sCD163 is upregulated with M2 differentiation driven by IL-4, but that there is an absence of the protease needed to release the sCD163 version of the protein. Micronutrients such as iron are important for infecting organisms,45 thus it is also possible that Cryptococcus is manipulating the levels of sCD163 to some advantage with this effect enhanced within the context of the CNS.
In summary, we have compared HIV-1 populations and cytokine profiles in the blood and CSF of groups of participants with and without CM. We found no differences in the frequency of viral compartmentalization in the presence or absence of CM, although in the subset of participants on ART there were several examples of CSF escape but only in those with CM. The cytokine profile was generally inflammatory but with the unexpected observation that sCD163 was suppressed in the presence of CM, suggesting a complex interaction between the cryptococcal infection and the immune response. We confirmed the potential of subtype C HIV-1 to establish compartmentalized infections in the CSF/CNS, and observed a compartmentalized infection with subtype G HIV-1. The limited size and heterogeneity of the study populations limit the sensitivity in effect size for drawing strong conclusions but suggest several phenomena worthy of follow-up studies.
Supplementary Material
Acknowledgments
The authors appreciate study participants from Jos University Teaching Hospital, Nigeria and ACTA UNC Project-Malawi for their cooperation. They are grateful to Dr. Thomas Harrison and the ACTA study team for helpful discussion and sharing of samples. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Sequence Data
NGS sequence raw reads and TCS have been submitted to NCBI's SRA with accession number PRJNA602884. SGA sequence data have been submitted to GenBank under the accession numbers MN944109-MN944384.
Author Disclosure Statement
UNC is pursuing IP protection for Primer ID with R.S. listed as a coinventor, for which he has received nominal royalties. The remaining authors have no conflicts of interest to declare.
Funding Information
Postdoctoral training for O.M.A. and the study were supported by the Fogarty International Center of the National Institutes of Health under award number 5D43TW009608-03.
Supplementary Material
References
- 1. Maartens G, Celum C, Lewin SR: HIV infection: Epidemiology, pathogenesis, treatment, and prevention. Lancet 2014;384:258–271 [DOI] [PubMed] [Google Scholar]
- 2. Swanson PA 2nd, McGavern DB: Viral diseases of the central nervous system. Curr Opin Virol 2015;11:44–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Joseph SB, Arrildt KT, Sturdevant CB, Swanstrom R: HIV-1 target cells in the CNS. J Neurovirol 2015;21:276–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schnell G, Joseph S, Spudich S, Price RW, Swanstrom R: HIV-1 replication in the central nervous system occurs in two distinct cell types. PLoS Pathog 2011;7:e1002286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gorry PR, Taylor J, Holm GH, et al. : Increased CCR5 affinity and reduced CCR5/CD4 dependence of a neurovirulent primary human immunodeficiency virus type 1 isolate. J Virol 2002;76:6277–6292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peters PJ, Bhattacharya J, Hibbitts S, et al. : Biological analysis of human immunodeficiency virus type 1 R5 envelopes amplified from brain and lymph node tissues of AIDS patients with neuropathology reveals two distinct tropism phenotypes and identifies envelopes in the brain that confer an enhanced tropism and fusigenicity for macrophages. J Virol 2004;78:6915–6926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thomas ER, Dunfee RL, Stanton J, et al. : Macrophage entry mediated by HIV Envs from brain and lymphoid tissues is determined by the capacity to use low CD4 levels and overall efficiency of fusion. Virology 2007;360:105–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Joseph SB, Arrildt KT, Swanstrom AE, et al. : Quantification of entry phenotypes of macrophage-tropic HIV-1 across a wide range of CD4 densities. J Virol 2014;88:1858–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arrildt KT, LaBranche CC, Joseph SB, et al. : Phenotypic correlates of HIV-1 macrophage tropism. J Virol 2015;89:11294–11311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Forrester JV, McMenamin PG, Dando SJ: CNS infection and immune privilege. Nat Rev Neurosci 2018;19:655–671 [DOI] [PubMed] [Google Scholar]
- 11. Rajasingham R, Smith RM, Park BJ, et al. : Global burden of disease of HIV-associated cryptococcal meningitis: An updated analysis. Lancet Infect Dis 2017;17:873–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Colombo AC, Rodrigues ML: Fungal colonization of the brain: Anatomopathological aspects of neurological cryptococcosis. An Acad Bras Cienc 2015;87(2 Suppl):1293–1309 [DOI] [PubMed] [Google Scholar]
- 13. Vu K, Garcia JA, Gelli A: Cryptococcal meningitis and anti-virulence therapeutic strategies. Front Microbiol 2019;10:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Molloy SF, Kanyama C, Heyderman RS, et al. ; ACTA Trial Study Team: Antifungal combinations for treatment of cryptococcal meningitis in Africa. N Engl J Med 2018;378:1004–1017 [DOI] [PubMed] [Google Scholar]
- 15. Kanyama C, Molloy SF, Chan AK, et al. : One year mortality outcomes from the ACTA trial of cryptococcal meningitis treatment in Malawi. Clin Infect Dis. 2020;70:521–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peeters M, Jung M, Ayouba A: The origin and molecular epidemiology of HIV. Expert Rev Anti Infect Ther 2013;11:885–896 [DOI] [PubMed] [Google Scholar]
- 17. Chaplin B, Eisen G, Idoko J, et al. : Impact of HIV type 1 subtype on drug resistance mutations in Nigerian patients failing first-line therapy. AIDS Res Hum Retroviruses 2011;27:71–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhou S, Jones C, Mieczkowski P, Swanstrom R: Primer ID validates template sampling depth and greatly reduces the error rate of next-generation sequencing of HIV-1 genomic RNA populations. J Virol. 2015;89:8540–8555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Spudich SS, Nilsson AC, Lollo ND, et al. : Cerebrospinal fluid HIV infection and pleocytosis: Relation to systemic infection and antiretroviral treatment. BMC Infect Dis 2005;5:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sturdevant CB, Joseph SB, Schnell G, Price RW, Swanstrom R, Spudich S: Compartmentalized replication of R5 T cell-tropic HIV-1 in the central nervous system early in the course of infection. PLoS Pathog 2015;11:e1004720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Salazar-Gonzalez JF, Bailes E, Pham KT, et al. : Deciphering human immunodeficiency virus type 1 transmission and early envelope diversification by single-genome amplification and sequencing. J Virol 2008;82:3952–3970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Slatkin M, Maddison WP: A cladistic measure of gene flow inferred from the phylogenies of alleles. Genetics. 1989;123:603–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heipertz RA Jr., Ayemoba O, Sanders-Buell E, et al. : Significant contribution of subtype G to HIV-1 genetic complexity in Nigeria identified by a newly developed subtyping assay specific for subtype G and CRF02_AG. Medicine (Baltimore) 2016;95:e4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wilkinson E, Engelbrecht S, de Oliveira T: History and origin of the HIV-1 subtype C epidemic in South Africa and the greater southern African region. Sci Rep 2015;5:16897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chang CC, Kangethe R, Omarjee S, et al. : Relationship of human immunodeficiency virus viral load in cerebrospinal fluid and plasma in patients co-infected with cryptococcal meningitis. Open Forum Infect Dis 2017;4:ofx032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Canestri A, Lescure FX, Jaureguiberry S, et al. : Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis 2010;50:773–778 [DOI] [PubMed] [Google Scholar]
- 27. Peluso MJ, Ferretti F, Peterson J, et al. : Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load. AIDS 2012;26:1765–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dahl V, Peterson J, Fuchs D, Gisslen M, Palmer S, Price RW: Low levels of HIV-1 RNA detected in the cerebrospinal fluid after up to 10 years of suppressive therapy are associated with local immune activation. AIDS 2014;28:2251–2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nightingale S, Geretti AM, Beloukas A, et al. : Discordant CSF/plasma HIV-1 RNA in patients with unexplained low-level viraemia. J Neurovirol 2016;22:852–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mukerji SS, Misra V, Lorenz D, et al. : Temporal patterns and drug resistance in CSF viral escape among ART-experienced HIV-1 infected adults. J Acquir Immune Defic Syndr 2017;75:246–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mukerji SS, Misra V, Lorenz DR, et al. : Impact of antiretroviral regimens on cerebrospinal fluid viral escape in a prospective multicohort study of antiretroviral therapy-experienced human immunodeficiency virus-1-infected adults in the United States. Clin Infect Dis 2018;67:1182–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Joseph SB, Kincer LP, Bowman NM, et al. : Human immunodeficiency virus type 1 RNA detected in the central nervous system (CNS) after years of suppressive antiretroviral therapy can originate from a replicating CNS reservoir or clonally expanded cells. Clin Infect Dis 2019;69:1345–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Soulie C, Grude M, Descamps D, et al. ; ANRS AC11 Resistance Study Group: Antiretroviral-treated HIV-1 patients can harbour resistant viruses in CSF despite an undetectable viral load in plasma. J Antimicrob Chemother 2017;72:2351–2354 [DOI] [PubMed] [Google Scholar]
- 34. Kugathasan R, Collier DA, Haddow LJ, et al. : Diffuse white matter signal abnormalities on magnetic resonance imaging are associated with human immunodeficiency virus type 1 viral escape in the central nervous system among patients with neurological symptoms. Clin Infect Dis 2017;64:1059–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Caniglia EC, Cain LE, Justice A, et al. ; HIV-CAUSAL Collaboration: Antiretroviral penetration into the CNS and incidence of AIDS-defining neurologic conditions. Neurology 2014;83:134–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sojane K, Kangethe RT, Chang CC, et al. : Individuals with HIV-1 subtype C infection and cryptococcal meningitis exhibit viral genetic intermixing of HIV-1 between plasma and cerebrospinal fluid and a high prevalence of CXCR4-using variants. AIDS Res Hum Retroviruses 2018;34:607–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Scriven JE, Graham LM, Schutz C, et al. : The CSF immune response in HIV-1-associated cryptococcal meningitis: Macrophage activation, correlates of disease severity, and effect of antiretroviral therapy. J Acquir Immune Defic Syndr 2017;75:299–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jarvis JN, Casazza JP, Stone HH, et al. : The phenotype of the Cryptococcus-specific CD4+ memory T-cell response is associated with disease severity and outcome in HIV-associated cryptococcal meningitis. J Infect Dis 2013;207:1817–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mukaremera L, Nielsen K: Adaptive immunity to Cryptococcus neoformans infections. J Fungi (Basel) 2017;3:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mora DJ, Fortunato LR, Andrade-Silva LE, et al. : Cytokine profiles at admission can be related to outcome in AIDS patients with cryptococcal meningitis. PLoS One 2015;10:e0120297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jarvis JN, Meintjes G, Bicanic T, et al. : Cerebrospinal fluid cytokine profiles predict risk of early mortality and immune reconstitution inflammatory syndrome in HIV-associated cryptococcal meningitis. PLoS Pathog 2015;11:e1004754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Meya DB, Manabe YC, Boulware DR, Janoff EN: The immunopathogenesis of cryptococcal immune reconstitution inflammatory syndrome: Understanding a conundrum. Curr Opin Infect Dis 2016;29:10–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Etzerodt A, Moestrup SK: CD163 and inflammation: Biological, diagnostic, and therapeutic aspects. Antioxid Redox Signal 2013;18:2352–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mosser DM, Edwards JP: Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008;8:958–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Potrykus J, Ballou ER, Childers DS, Brown AJ: Conflicting interests in the pathogen-host tug of war: Fungal micronutrient scavenging versus mammalian nutritional immunity. PLoS Pathog 2014;10:e1003910. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.