Abstract
Aims: Mitochondrial stress and dysfunction within the intestinal epithelium are known to contribute to the pathogenesis of inflammatory bowel disease (IBD). However, the importance of mitophagy during intestinal inflammation remains poorly understood. The primary aim of this study was to investigate how the mitophagy protein BCL2/adenovirus E1B 19 kDa protein-interacting protein 3-like (BNIP3L/NIX) mitigates mitochondrial damage during intestinal inflammation in the hopes that these data will allow us to target mitochondrial health in the intestinal epithelium as an adjunct to immune-based treatment strategies.
Results: In the intestinal epithelium of patients with ulcerative colitis, we found that NIX was upregulated and targeted to the mitochondria. We obtained similar findings in wild-type mice undergoing experimental colitis. An increase in NIX expression was found to depend on stabilization of hypoxia-inducible factor-1 alpha (HIF1α), which binds to the Nix promoter region. Using the reactive oxygen species (ROS) scavenger MitoTEMPO, we were able to attenuate disease and inhibit both HIF1α stabilization and subsequent NIX expression, suggesting that mitochondrially derived ROS are crucial to initiating the mitophagic response during intestinal inflammation. We subjected a global Nix−/− mouse to dextran sodium sulfate colitis and found that these mice developed worse disease. In addition, Nix−/− mice were found to exhibit increased mitochondrial mass, likely due to the inability to clear damaged or dysfunctional mitochondria.
Innovation: These results demonstrate the importance of mitophagy within the intestinal epithelium during IBD pathogenesis.
Conclusion: NIX-mediated mitophagy is required to maintain intestinal homeostasis during inflammation, highlighting the impact of mitochondrial damage on IBD progression.
Keywords: inflammatory bowel disease, reactive oxygen species, HIF1α, hypoxia
Introduction
Inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn's disease, is characterized by chronic and relapsing intestinal inflammation (1). It is estimated that 3.1 million people in the United States are currently diagnosed with IBD, and global incidence rates are increasing (62). Although IBD pathophysiology is believed to be multifactorial in nature, oxidative stress and increased reactive oxygen species (ROS) within the intestinal epithelium are considered two hallmarks of disease (4, 45, 47, 60). Mitochondrially derived ROS (mtROS) have been implicated as key inflammatory intermediates in murine models of colitis and coincide with a dysfunctional mitochondrial network (15, 23, 27, 40, 59). Increasing evidence demonstrates that mitochondrial damage and dysfunction within the intestinal epithelium may play important roles in IBD pathogenesis (14, 25, 40). How the intestinal epithelium mitigates injury and regulates the growing dysfunctional mitochondrial network, however, remains unclear.
During intestinal inflammation, oxidative stress contributes to the upregulation and subsequent stabilization of hypoxia-inducible factor-1 alpha (HIF1α) (22). This transcription factor is critical to maintaining cellular homeostasis in response to a variety of factors, including hypoxia and inflammation. HIF1α has also been shown to drive mitophagy in the setting of mitochondrial stress (11). Mitophagy is a selective method of autophagy by which damaged or dysfunctional mitochondria are targeted for physiologic degradation (26).
Although other mitophagy proteins exist, only BCL2/adenovirus E1B 19 kDa protein-interacting protein 3-like, BNIP3L (NIX) and its homolog, BNIP3, are induced by HIF1α (61). HIF1α has been shown to induce NIX in a variety of cell types, including epithelial and endothelial cells, as well as macrophages and human breast cancer cells (20, 56). In erythroid cells, clearance of old or damaged mitochondria by NIX was shown to play an important role in cellular maturation. Erythroid cells devoid of NIX are unable to incorporate mitochondria into the autophagosome to complete the mitophagic process, resulting in decreased cellular maturation and survival (49, 51). However, the function of NIX in the intestinal epithelium has not been studied.
Innovation
Mitochondrial stress and dysfunction within the intestinal epithelium are now known to play important roles in inflammatory bowel disease pathogenesis. NIX is a key regulator of mitophagy and cellular homeostasis; however, its functions in the intestinal epithelium and intestinal inflammation are yet to be investigated. We outline for the first time a pathway for NIX-mediated mitophagy during human ulcerative colitis and murine experimental colitis as a host response to mitochondrial insult. Preservation of the delicate balance between mitochondrial biogenesis and mitophagy during intestinal inflammation may represent a critical therapeutic goal for future studies.
We now hypothesize that NIX-mediated mitophagy is a key mechanism by which intestinal epithelial cells mitigate mitochondrial stress and dysfunction. Here, we show that under conditions of oxidative stress HIF1α is activated, allowing it to bind directly to the promoter region of Nix, driving transcription. NIX associates with LC3 at the mitochondria, thereby initiating mitophagy. Nix−/− mice exhibit worse clinical disease when subjected to experimental colitis as compared with wild-type (WT) mice similarly treated, exhibiting an enhanced inflammatory profile and loss of mucosal integrity. This implies that NIX-mediated mitophagy is a key homeostatic function during cellular stress in the intestinal epithelium. Modulation of NIX-mediated mitophagy may offer therapeutic benefits for the treatment of patients with IBD.
Results
Mitophagy is induced in human IBD
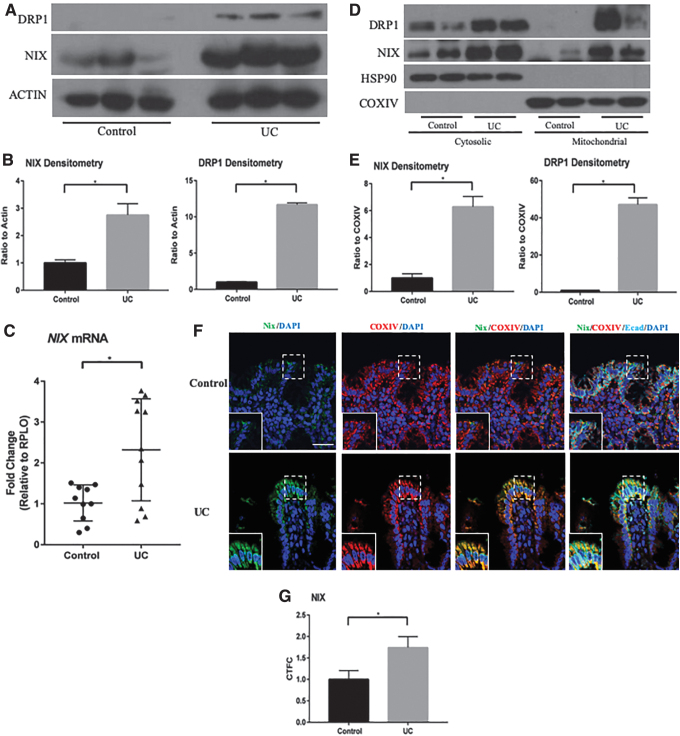
To investigate the mitophagic response to injury within the intestinal epithelium during human IBD, we isolated protein from human tissue and found that NIX was increased in patients with UC when compared with patients of noninflammatory pathology (Fig. 1A, B). Dynamin-related protein-1 (DRP1) protein was also increased in UC patients, suggesting an increase in mitochondrial fission, a common precursor to mitophagy that involves cleavage of dysfunctional mitochondrial sections from the healthy network (58). Although more variable, the mRNA expression of Nix was also increased in inflamed tissues as compared with those from non-IBD controls (Fig. 1C). When activated, NIX forms a stable homodimer that is targeted to the mitochondria to initiate mitophagy (41).
FIG. 1.
NIX expression is upregulated in human ulcerative colitis. (A) Whole-tissue protein lysates from non-IBD control patients (n = 3) and UC patients (n = 3) were examined by Western blot for NIX expression. (B) Densitometry of NIX and DRP1 protein shows a significant increase in UC tissues (n = 3 control, n = 3 UC). (C) qPCR expression is increased in UC tissues (n = 10 control, 11 UC). (D) Mitochondrial protein isolation revealed an increase in dimerized NIX in UC fractions (n = 2 control, n = 2 UC). (E) Densitometry of NIX and DRP1 shows an increase in expression in UC mitochondrial fractions when compared with COXIV (n = 2 control, n = 2 UC). (F) Immunofluorescence of NIX (green) COXIV (red) are shown on noninflammatory controls and UC tissue. E-cadherin (cyan) and DAPI (blue) were stained to identify epithelial cells and nuclei, respectively. NIX expression is increased in the epithelium of UC tissues. Images are representative images (n = 6/group). (G) Expression was analyzed by CTFC (n = 6/group; scale bar = 100 μm). *p ≤ 0.05. CTFC, corrected total cell fluorescence; DAPI, 4′,6-diamino-2-phenylindole; DRP1, dynamin related protein-1; IBD, inflammatory bowel disease; qPCR, quantitative real-time polymerase chain reaction; UC, ulcerative colitis.
To demonstrate that the active form of NIX is, indeed, upregulated during intestinal inflammation, we isolated mitochondria from the colonic tissue of control and dextran sodium sulfate (DSS)-subjected mice. On enriching for mitochondria from UC samples, we observed increased levels of NIX protein in the mitochondrial fractions when compared with similar fractions from control patient samples (Fig. 1D, E). Likewise, we found that DRP1 was predominately found in mitochondrial protein fractions, a hallmark of mitochondrial fission. Using immunofluorescence, we found that NIX co-localized with mitochondrial cytochrome c oxidase, subunit IV (COXIV) protein in the intestinal epithelium, with increased signal in tissue from UC patients (Fig. 1F, G). Orthogonal imaging highlighted co-expression of NIX and COXIV in the epithelium of UC tissue (Supplementary Fig. S1A). Taken together, these data show that the components of NIX-mediated mitophagy are increased in the intestinal epithelium of patients with active UC and that NIX is targeted to the mitochondrion during disease.
Mitophagy is induced in experimental murine colitis
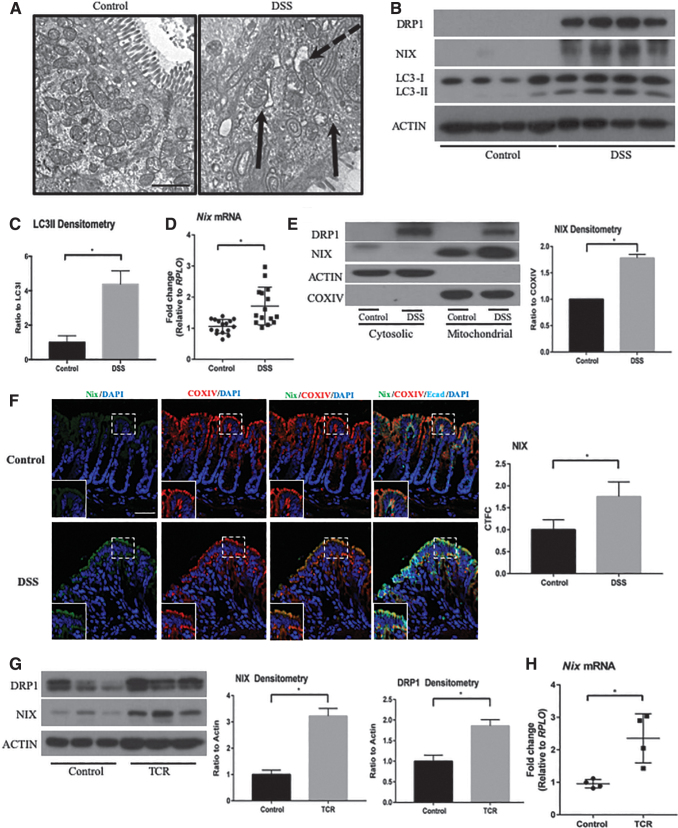
To evaluate the mechanism of NIX-mediated mitophagy during intestinal inflammation, we subjected WT mice to an acute DSS model of colitis for 7 days. We then analyzed the colonic epithelium via transmission electron microscopy (TEM) and found evidence of dysmorphic mitochondria, characterized by a destruction of cristae and overall abnormal architecture. Many of these dysmorphic mitochondria were found in close proximity to autophagic structures (Fig. 2A).
FIG. 2.
Mitophagy-associated proteins are upregulated during murine experimental colitis. (A) Tissue sections were imaged by TEM for mitochondrial morphology during DSS colitis (n = 4/group). Solid arrows indicate dysmorphic mitochondria, and dashed arrow identifies vesicles closely associated with dysmorphic mitochondria (scale bar = 500 nm). (B) Whole-cell protein lysates from control and DSS-treated mice were probed for DRP1, NIX, LC3, and actin. Increased expression was found in DSS tissue (n = 4 control, n = 4 DSS). (C) Densitometry of the LC3II/LC3I ratio indicates an upregulation of autophagy in DSS-treated mice (n = 4 control, n = 4 DSS). (D) Tissue samples were analyzed via qPCR for Nix expression (n = 16 control, 15 DSS). (E) Colon tissue was separated into cytosolic and mitochondrial protein fractions and probed for NIX, actin, and COXIV. Increases in NIX were found in the mitochondrial fraction of DSS mice. Actin and COXIV were used for cytosolic and mitochondrial loading controls, respectively (n = 2 control, n = 2 DSS). The blot shown is representative of two separate experiments. (F) Mouse colons were stained by immunofluorescence for NIX (green) and COXIV (red) expression. E-cadherin (cyan) and DAPI (blue) were stained to identify epithelial cells and nuclei, respectively. DSS-treated mice exhibited an increase in NIX expression in the intestinal epithelium, which co-localized with COXIV, a mitochondrial marker. Expression was analyzed by CTFC (n = 4; scale bar = 100 μm). (G) Rag1−/− mice were adoptively transferred with CD4+ CD45RBhigh cells to establish chronic inflammation. Whole-cell protein lysates were probed for NIX, DRP1, and actin. Densitometry was performed on DRP1 and NIX, showing increased protein expression (n = 3 control, n = 3 TCR). (H) T cell recipient mice were shown to have elevated Nix expression via qPCR analysis of colonic tissue (n = 4/group). *p ≤ 0.05. DSS, dextran sodium sulfate; TCR, T-cell recipients; TEM, transmission electron microscopy.
DRP1, a standard marker for mitochondrial fission, was increased in protein lysates from colonic tissue of DSS-subjected mice as compared with control littermates (Fig. 2B). LC3, a marker for autophagy, was also increased in the same tissues (Fig. 2B, C). We found that Nix gene expression and protein levels were increased in whole cell lysates of DSS-treated mice as compared with those from control mice (Fig. 2B, D). Further, NIX and DRP1 proteins were found to be increased within mitochondrial fractions from DSS-treated mice, suggesting that both DRP1 and NIX are targeted to the mitochondria during inflammation (Fig. 2E). These findings suggest an upregulation of both mitochondrial fission and NIX-mediated mitophagy within the intestinal epithelium during murine colitis.
To further evaluate the association of NIX with the mitochondria, we performed immunofluorescence on tissue sections for NIX and COXIV (Fig. 2F). We found increased co-localization of NIX and COXIV during inflammation, suggesting an increase in mitophagy. This is further demonstrated in orthogonal imaging of DSS tissue (Supplementary Fig. S1B). To validate our findings in a nonchemically mediated model of experimental colitis, we performed the adoptive T cell transfer model of disease. On observation of disease, such as weight loss and loose stools, T cell recipient mice were sacrificed and whole cell colonic lysates were prepared. Similar to our findings from DSS experiments, we found an increase in DRP1 and NIX protein in the intestines of T cell recipient mice as well as a significant increase in Nix mRNA expression (Fig. 2G, H). Altogether, these data suggest that damaged or dysfunctional mitochondria are targeted for NIX-mediated mitophagy during experimental colitis.
NIX binds to LC3 to initiate clearance of damaged mitochondria
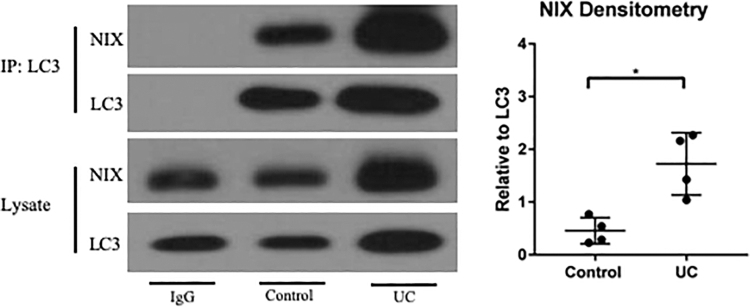
LC3 is a critical component of both autophagy and mitophagy, recruiting the autophagosome to degrade an identified target. NIX has been shown to contain an LC3-interacting region in its cytoplasmic domain, which allows for direct binding of the two proteins (38, 41). To determine whether NIX is bound to LC3 to a greater degree during experimental colitis and UC, we assessed protein interactions via co-immunoprecipitation. We found an increased association of NIX and LC3 in UC samples as compared with control samples (Fig. 3). Based on these data, we deduce that NIX is responsible for the recruitment of LC3 and the autophagosome to dysfunctional mitochondria during human IBD.
FIG. 3.
NIX binds to LC3 in murine and human colitis. Immunoprecipitation was performed on protein lysates from human tissues. NIX and LC3 were shown to pull down together. IgG was used as a negative control. Blots are representative of multiple samples analyzed where densitometry was performed (n = 3/group). *p ≤ 0.05.
HIF1α binds to hypoxia response elements in the Nix promoter region during human and murine experimental colitis
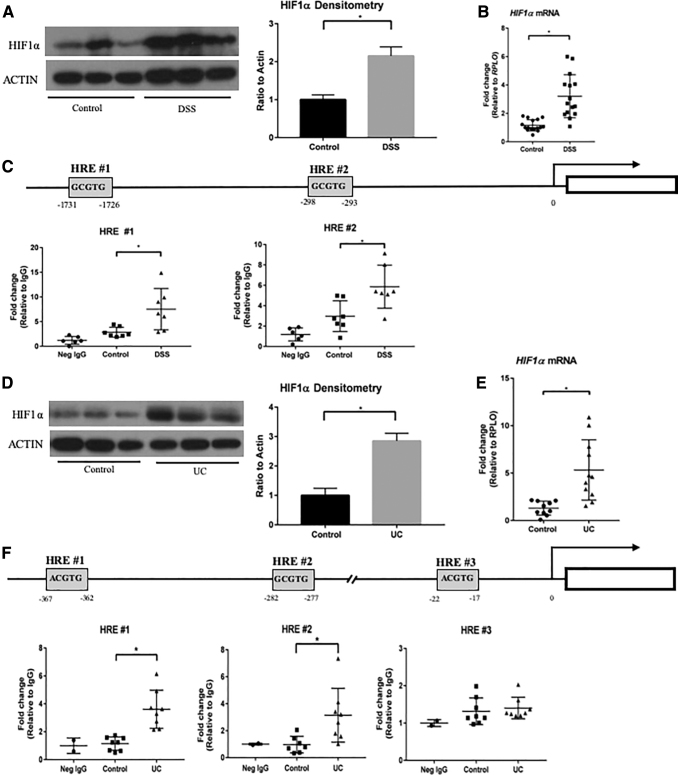
To understand the mechanism of Nix expression, we investigated transcriptional activators associated with Nix. Recent studies have shown that HIF1α is induced during experimental colitis, providing a physiologic response to and protection from inflammation (22, 31, 42, 46, 53). Our results are consistent with these findings. We found increased protein and RNA levels of HIF1α in the colons of mice subjected to DSS colitis (Fig. 4A, B). The Nix promoter region has been analyzed for transcriptional binding elements, including those specific for HIF1α. Gálvez et al. located two hypoxia response elements (HREs) in the Nix promoter region of mice (21). These HRE regions consist of a consensus DNA sequence, 5′-[A/G]CGTG-3′, which HIF1α binds to induce gene expression (37, 52) (Fig. 4C). Therefore, we hypothesized that HIF1α may be responsible for the transcriptional upregulation of Nix during IBD.
FIG. 4.
HIF1α is responsible for NIX expression. (A) Nuclear extracts were probed for HIF1α expression (n = 3 control, n = 3 DSS). (B) Hif1α mRNA expression was increased in DSS colon samples (n = 16 control, 15 DSS). (C) Diagram of Nix promoter region in mice shows two HRE regions. Increased binding of HIF1α to both regions in DSS tissue was demonstrated by ChIP (n = 6 neg IgG, n = 7 control, n = 7 DSS). (D) Nuclear extracts from human control and UC tissues (n = 3 control, n = 3 UC). (E) HIF1α mRNA expression by qPCR was also upregulated in UC tissue (n = 10 control, n = 11 UC). (F) Diagram of Nix promoter region in humans, which contains three HRE regions. HIF1α had increased binding to two HRE regions, as shown by ChIP analysis (n = 2 neg IgG, n = 8 control, n = 8 UC). *p ≤ 0.05. ChIP, chromatin immunoprecipitation; HIF1α, hypoxia-inducible factor-1α; HRE, hypoxia response element.
Using a chromatin immunoprecipitation (ChIP) assay, we were able to detect an increase in HIF1α protein binding to both Nix HRE regions in mice subjected to DSS colitis as compared with controls (Fig. 4C). We were also able to recapitulate these findings in human UC samples, observing an increase in HIF1α protein and transcription levels (Fig. 4D, E). We then evaluated HREs in the promoter region of human NIX, as described in human erythroid cells (2). We saw an increase in HIF1α binding at two of the three human HRE sites of the NIX promoter in humans with UC (Fig. 4F). These data suggest that during intestinal inflammation, HIF1α binds to the promoter region of NIX, upregulating expression.
ROS induced by hydrogen peroxide initiates Nix mitophagy
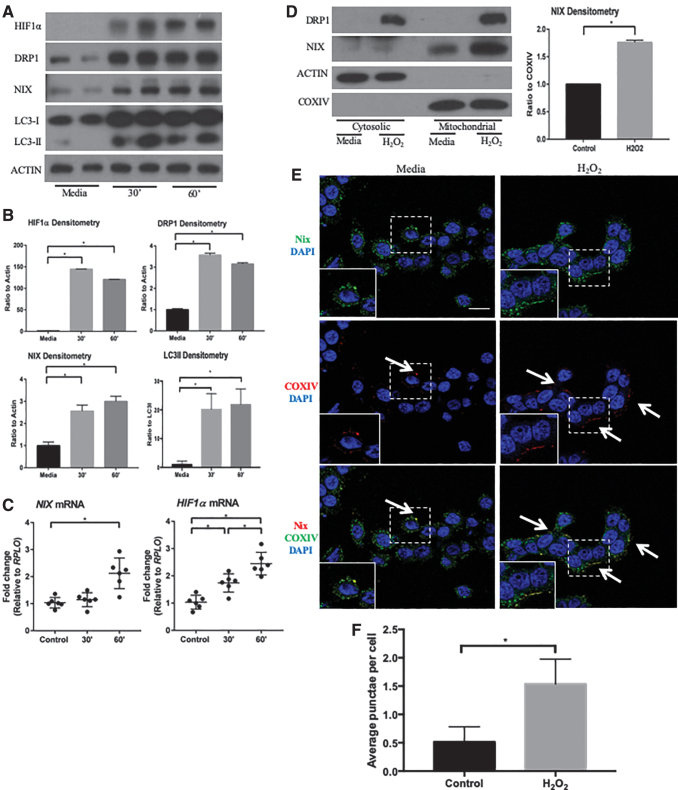
To elucidate the mechanism by which HIF1α is activated during inflammation, we treated colonic epithelial HT-29 cells with 10 μM hydrogen peroxide (H2O2), which has been previously shown to induce mitochondrial stress and mitochondrial ROS release, as well as HIF1α expression and stabilization (30, 44, 67). HT-29 cells treated with H2O2 demonstrated an early induction of HIF1α and a subsequent increase in NIX expression (Fig. 5A). Interestingly, the increase in dimerized NIX protein occurred before the increase in Nix expression. This may represent an early increase the dimerization and thus activation of existing NIX protein before an actual increase in transcription.
FIG. 5.
ROS stimulates HIF1α/NIX pathway in vitro. (A) Protein lysates of control and 10 μM hydrogen peroxide-treated cells were analyzed by Western blot, showing an increase in HIF1α, DRP1, NIX, and LC3 after 30 and 60 min. Blots shown are representative of several experiments (n = 2 for all groups). (B) Densitometry analysis highlights upregulation of HIF1α, DRP1, NIX, and LC3II/LC3I (n = 4 for all groups). (C) qPCR analysis showed an upregulation of NIX and HIF1α after 60 min of hydrogen peroxide treatment (n = 6 for all groups). (D) HT-29 protein lysates were separated into cytosolic and mitochondrial fractions, showing an accumulation of NIX in the mitochondria of hydrogen peroxide-treated cells for 1 h. Densitometry of NIX expression in mitochondrial fractions compared with COXIV depicts increased expression of NIX in hydrogen peroxide-treated cells (n = 2 for all groups). (E) HT-29 cells were treated with hydrogen peroxide for 60 min and stained by immunofluorescence for COXIV (green), NIX (red), and DAPI (blue). Immunofluorescence shows an increase in the protein levels of NIX and increased co-localization of NIX with COXIV in treated cells (n = 3/group; scale bar = 1 μm; arrows highlight areas of co-localization). Images are representative of three experiments. (F) The number of co-localized punctae per cell were counted, showing an increase in the hydrogen peroxide-treated cells (at least 50 cells/group per experiment were counted; n = 3/group). *p ≤ 0.05. ROS, reactive oxygen species.
An increase in DRP1 protein suggests an upregulation of mitochondrial fission, whereas an increase in LC3 suggests an upregulation in autophagic pathways (Fig. 5B). Transcriptional increases in HIF1α and NIX were also found, supporting our previous in vivo findings (Fig. 5C). Similar to findings from our human and murine in vivo studies, we found an increase in both NIX and DRP1 in the mitochondrial fractions of H2O2-treated cells, indicating an association of mitophagy proteins with mitochondria (Fig. 5D). To visualize the location of NIX in vitro, we performed immunofluorescence on H2O2-treated cells and found NIX in close association with COXIV (Fig. 5E and Supplementary Fig. S1C). H2O2-treated cells also demonstrated in an increase in Nix- and COXIV-positive punctae per cell (Fig. 5F). These findings suggest that HIF1α is induced by an increase in ROS, resulting in the upregulation of NIX.
Antioxidant treatment with MitoTEMPO reduces NIX-mediated mitophagy
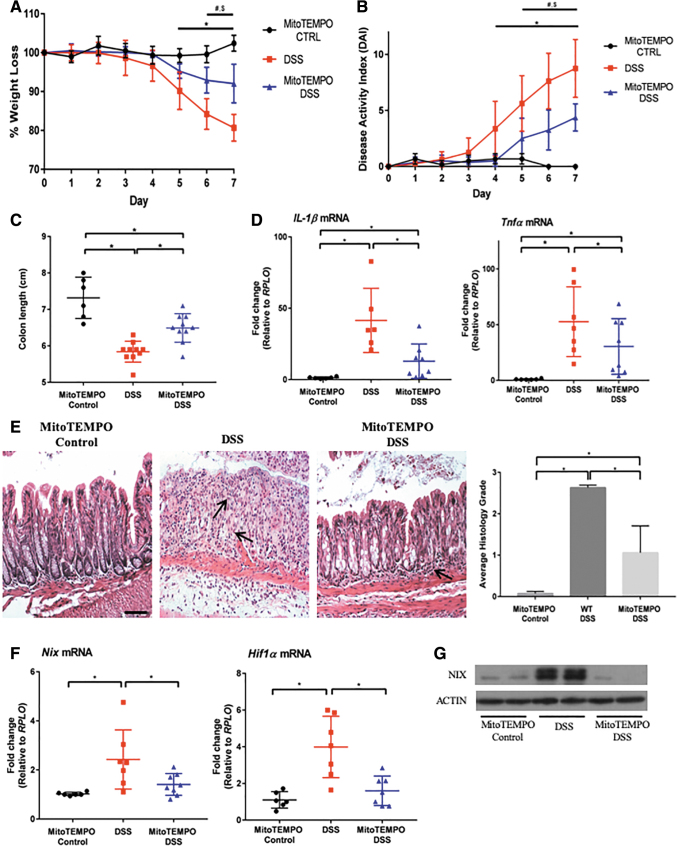
A reduction in ROS production has proven therapeutic in multiple murine studies of intestinal inflammation. Recently, the mitochondrial-specific ROS modulator MitoTEMPO was shown to protect mice from DSS colitis (59). However, its effects on HIF1α and NIX expression have not been analyzed. Therefore, we treated mice subjected to DSS with MitoTEMPO and found that these mice were protected from DSS colitis, in accordance with previous reports. Weight loss and disease activity index (DAI) scores were reduced, whereas colonic shortening was lessened (Fig. 6A–C). Expression levels of the inflammatory cytokines Tnfα and Il-1β were also reduced in mice subjected to DSS and treated with MitoTEMPO (Fig. 6D).
FIG. 6.
MitoTEMPO protects from DSS-induced colitis by scavenging mitochondrial ROS in vivo. (A) Mice were subjected to 3% DSS for 7 days, with MitoTEMPO administered daily via intraperitoneal injection. MitoTEMPO-treated mice were protected from weight loss and exhibited a lower DAI score (B) than DSS only. Significant changes were observed between MitoTEMPO control versus 3% DSS mice (*p ≤ 0.05), MitoTEMPO control versus MitoTEMPO 3% DSS (#p ≤ 0.05), and 3% DSS versus MitoTEMPO 3% DSS ($p ≤ 0.05) (n = 6 control, n = 10 DSS, n = 10 MitoTEMPO DSS). (C) MitoTEMPO 3% DSS mice also displayed longer colons than DSS only mice (n = 6 control, n = 10 DSS, n = 10 MitoTEMPO DSS). (D) Inflammatory cytokines were analyzed via qPCR, showing a reduction in Il-1β and Tnfα in MitoTEMPO 3% DSS mice (n = 6 control, n = 7 DSS, n = 8 MitoTEMPO DSS). (E) H&E staining shows a preservation of structure and a decrease in inflammatory infiltrate in MitoTEMPO 3% DSS mice with a significant decrease in the histological scoring, as performed by a blinded pathologist (20 × magnification, scale bar = 100 μm) (n = 6 control, n = 10 DSS, n = 10 MitoTEMPO DSS). Arrows point out areas of immune cell infiltration. (F) Nix and Hif1α were significantly decreased in MitoTEMPO 3% DSS-treated mice when compared with DSS only mice (n = 6 control, n = 7 DSS, n = 8 MitoTEMPO DSS). (G) Protein lysates were analyzed via Western blot, showing a decrease in NIX in MitoTEMPO 3% DSS-treated mice compared with DSS alone (n = 2 for all groups). Blots are representative of several experiments. Please note that the y-axis in (A) and (C) are not set to 0 to aid in the visual representation of the data. *p ≤ 0.05. H&E, hematoxylin and eosin.
Histologic analysis demonstrated a preservation of intestinal architecture, as well as a reduction in inflammatory infiltrate (Fig. 6E). Nix and Hif1α expression was significantly reduced, suggesting that mtROS contribute to activation of the HIF1α-NIX axis during inflammation (Fig. 6F). NIX dimer (active NIX) protein was also not increased in mice subjected to DSS colitis and treated with MitoTEMPO relative to those given DSS alone (Fig. 6G). These data suggest that MitoTEMPO treatment reduces mitochondrial ROS release, leading to decreased HIF1α stabilization and, thus, NIX expression.
Nix−/− mice are more susceptible to experimental colitis
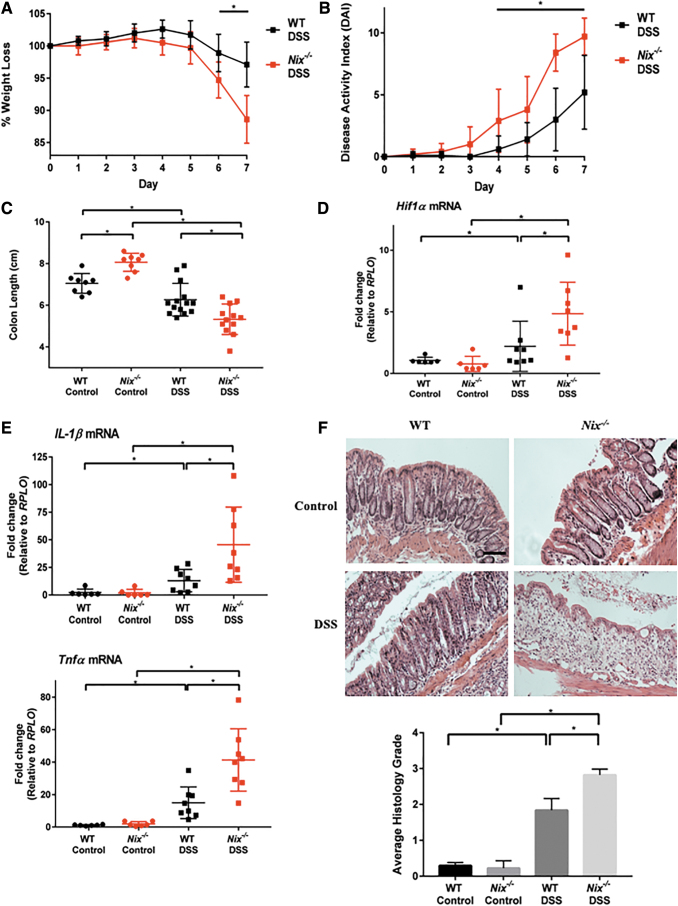
To determine the role of NIX in intestinal homeostasis and the pathogenesis of IBD, we employed the Nix global knockout mouse (Nix−/−) in our DSS colitis model (17). We first utilized a 3% DSS model and found increased susceptibility to disease in Nix−/− DSS mice compared with WT controls as early as day 5. However, this effect was diminished by day 7 as the disease continued to progress in the WT DSS mice (data not shown). To better differentiate disease progression between Nix−/− and WT mice, we subjected mice to a milder 2% DSS model. Nix−/− mice exhibited greater weight loss and an increased DAI relative to WT littermates at day 6 and 7 of the model (Fig. 7A, B). Nix−/− control mice interestingly had significantly longer colons than WT littermates at baseline; however, the colon lengths of Nix−/− mice were significantly shorter after DSS exposure compared with WT mice similarly treated (Fig. 7C).
FIG. 7.
Nix−/− mice are more susceptible to DSS colitis. (A) Mice were subjected to a 7-day 2% DSS model with Nix−/− mice exhibiting a significant decrease when compared with WT mice (n = 14 WT DSS, n = 12 Nix−/− DSS). (B) DAI scores of Nix−/− DSS mice were significantly increased when compared with WT DSS mice (n = 14 WT DSS, n = 12 Nix−/− DSS). (C) Colon length was measured. Nix−/− DSS mice displayed a significant shortening of the colon when compared with WT DSS mice. Nix−/− control mice also had longer colons than WT control mice (n = 8 control, n = 8 Nix−/− control, n = 14 WT DSS, n = 12 Nix−/− DSS). (D, E) Nix−/− mice were further analyzed via qPCR. Nix−/− DSS mice had higher expression levels of Hif1α, Il-1β, and Tnfα than WT DSS mice (n = 6 control/group, 8 DSS/group). (F) Histology showed a worsening of immune cell infiltrate and disruption of normal architecture in the Nix−/− DSS mice (20 × magnification scale bar = 100 μm) (n = 8 control, n = 8 Nix−/− control, n = 14 WT DSS, n = 12 Nix−/− DSS). Please note that the y-axis in (A) and (C) are not set to 0 to aid in the visual representation of the data. *p ≤ 0.05. DAI, disease activity index; WT, wild-type.
We found via quantitative real-time polymerase chain reaction (qPCR) that Hif1α expression was elevated in Nix−/− mice when compared with WT mice subjected to DSS treatment (Fig. 7D). Likewise, qPCR revealed increased Il-1β and Tnfα expression in DSS-treated Nix−/− mice (Fig. 7E). Histologically, there was an increase in immune infiltrate and disruption of architecture in experimental Nix−/− mice as compared with WT littermates (Fig. 7F). Based on these observations, we conclude that NIX is critical for intestinal homeostasis during inflammation.
Nix−/− mice experience a higher damaged mitochondrial burden
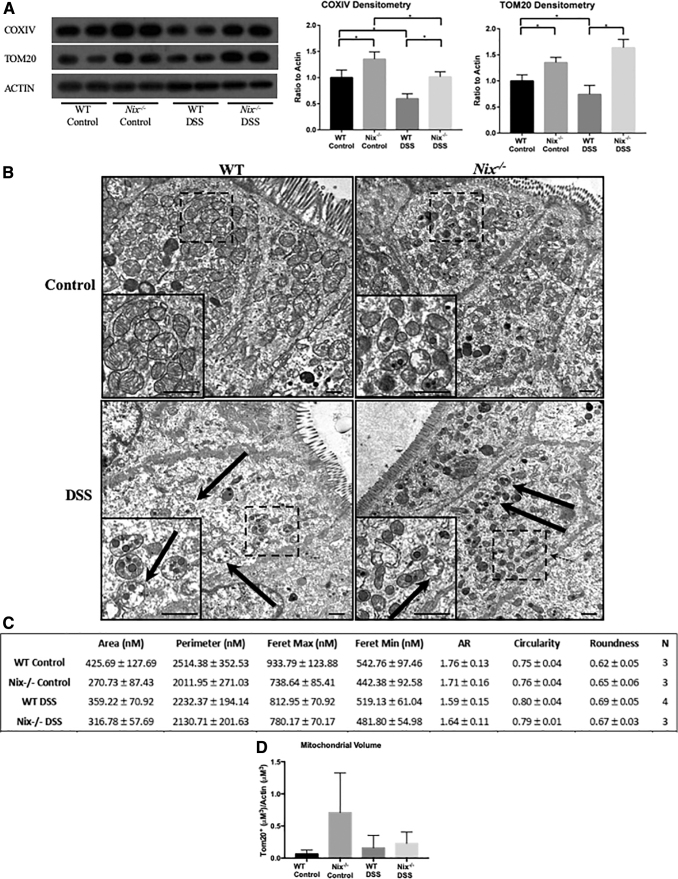
To evaluate the effects of Nix deletion during intestinal inflammation on mitochondrial content, we first evaluated mitochondrial protein levels via Western blot. Interestingly, COXIV and TOM20, two mitochondrial-associated proteins, were increased in Nix−/− control mice as compared with WT control mice. This finding is in agreement with studies identifying an increase in mitochondrial content in Nix−/− mouse tissues, including the retina, reticulocytes, and cardiomyocytes (7, 16–19, 49). In addition, there was a significant decrease in these proteins in WT DSS mice, but not in Nix−/− DSS mice (Fig. 8A). Likewise, we observed decreased protein levels of COXIV and TOM20 via immunofluorescence in the colonic epithelium of WT DSS mice (Supplementary Fig. S2A, B).
FIG. 8.
Nix−/− mice have a deficiency in mitophagy, leading to a higher dysfunctional mitochondrial burden. (A) Western blot analysis of protein lysates from WT and Nix−/− mice reveals an increase in COXIV and TOM20 protein content in Nix−/− mice when compared with WT littermates (n = 2/group); images shown are representative of several experiments. COXIV and TOM20 proteins were decreased in WT DSS samples, whereas this decrease was not found in Nix−/− protein lysates (Densitometry analysis is n = 6/group). *p ≤ 0.05. (B) TEM imaging of colon tissue epithelium show an increased number of mitochondria in Nix−/− mice, with a higher number in Nix−/− DSS tissue when compared with WT DSS tissue. Solid arrows identify dysmorphic mitochondria (n = 4 control, n = 5 DSS; scale bar = 500 nm). (C) Analysis of mitochondrial morphology was completed by manually tracing individual mitochondria and analyzed with ImageJ software (n = 3 WT control, Nix−/− control, and Nix−/− DSS; n = 4 WT DSS). Feret's diameters are defined as the longest/shortest distance between two points in an individual mitochondrion. Aspect ratio, circularity, and roundness determine the roundness of an object, with 1 representing a perfect circle. Area, perimeter, and Feret's diameter are shown in nm. (D) Analysis of mitochondrial volume was completed by immunofluorescence staining using TOM20 as a mitochondrial marker, actin as a cytoskeletal marker, and Hoechst as a nuclear marker (n = 3 WT control, Nix−/− control, and WT DSS; n = 4 Nix−/− DSS). The TOM20+ content and area of actin was measured through Z-stacks (at least 6 μm of tissue) over a large area equivalent to four unique fields. The TOM20+ content (μM3) was then normalized to the area of actin (μM3).
To obtain a clearer picture of the cellular landscape, we examined these tissues by TEM (Fig. 8B). In comparison to mitochondria from WT control mice, there was a trend toward smaller mitochondria in DSS-treated WT mice as shown by their area, perimeter, and Feret's maximum diameter, a measurement of the longest distance between two points in an individual mitochondrion (Fig. 8C). There was also a trend toward smaller area, perimeter, and Feret's diameter in mitochondria from Nix−/− DSS mice as compared with WT DSS mice. We also noted that mitochondria from WT DSS and Nix−/− DSS mice, as compared with their respective controls, exhibited mitochondria that trended toward a more rounded appearance by measurements of aspect ratio, circularity, and roundness based on a designation of 1, which represents a perfectly spherical shape (34, 35). Mitochondria from Nix−/− control mice trended toward decreased area, perimeter, and Feret's diameter when compared with mitochondria from WT control mice. However, none of these results was statistically significant.
We also performed immunofluorescence to analyze mitochondrial volume in the experimental groups (Supplementary Fig. S3D). Although not statistically significant, our results demonstrated a clear trend of increased mitochondrial volume in the Nix−/− control versus Nix−/− DSS and Nix−/− DSS versus WT DSS (Fig. 8D). In agreement with our hypothesis, these data suggest a buildup of damaged or otherwise abnormal mitochondria in the Nix−/− mice, which are not properly cleared from the epithelium. This accumulation appears to add to the oxidative stress in the intestinal epithelium of Nix−/− mice, as noted by nitrotyrosine immunofluorescence, a marker of nitric oxide radicals (Supplementary Fig. S3A). In addition, inducible nitric oxide synthase (inos) was significantly increased in Nix−/− DSS mice, suggesting induction of nitric oxide, a common marker of intestinal inflammation (Supplementary Fig. S3B).Then, 8-Hydroxy-2′-deoxyguanosine (8-OHdg), a marker of oxidative DNA damage, was also increased in Nix−/− DSS tissue, lending weight to the hypothesis that impairment of the physiologic mitophagic response leads to elevated cellular damage during inflammation (Supplementary Fig. S3C). Overall, these data suggest that the deletion of Nix results in a failure to clear damaged or dysfunctional mitochondria from the intestinal epithelium, further exacerbating disease through dysregulated mtROS production.
Discussion
Our data suggest that NIX may play a key role in maintaining intestinal epithelial homeostasis during murine colitis and human IBD by initiating the mitophagic response to mitochondrial injury. Specifically, we demonstrated that NIX is upregulated within the intestinal epithelium during inflammation and translocates to the mitochondria where it interacts with the autophagy protein LC3. NIX expression is activated, in part by HIF1α, which binds to the hypoxia response elements within the Nix promoter. The mtROS scavenger, MitoTEMPO, ameliorated disease and decreased both HIF1α and NIX expression during DSS colitis in WT mice, suggesting that mtROS may be critical to HIF1α stabilization and subsequent transcription of Nix during intestinal inflammation (Fig. 9). Nix−/− mice were subsequently found to be highly susceptible to experimental colitis. Thus, we show that NIX-mediated mitophagy may be an intriguing therapeutic target in the setting of human IBD.
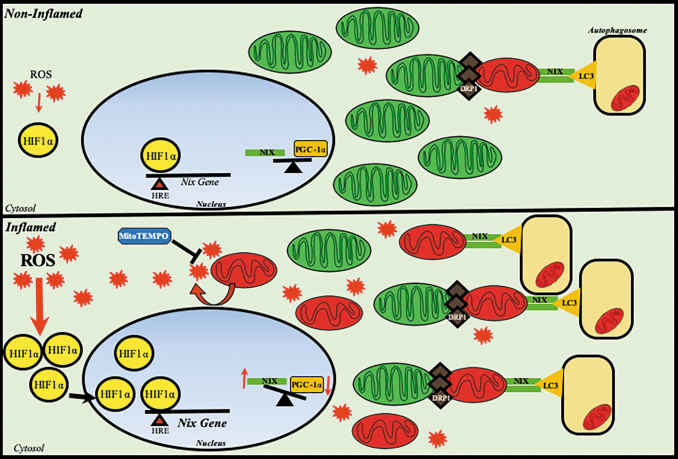
FIG. 9.
Proposed mechanism of NIX-induced mitophagy in the intestinal epithelium. Mechanistically, HIF1α becomes stabilized via an ROS insult, with mitochondrial injury largely contributing to this phenomenon. As the mtROS (red symbol) burden increases, this allows for further stabilization of HIF1α (yellow symbol), allowing it to translocate to the nucleus to induce upregulation of target genes, including NIX (green symbol). NIX expression is upregulated (bold text) and is targeted to an increasingly dysfunctional mitochondrial network. Meanwhile, mitochondria are primed for mitophagy through fission, which is mediated by DRP1 (brown symbol). Mitochondria are targeted for degradation; however, as mitochondrial biogenesis is also impaired via downregulation of PGC1α (orange symbol), fewer new mitochondria are generated to replace damaged mitochondria that are cleared. This tips the balance in favor of more dysfunctional mitochondria (red) than healthy mitochondria (green), which further exacerbates injury. Targeting mtROS with MitoTEMPO (blue symbol) can ameliorate this effect, whereas modulation of biogenesis and mitophagy prove to be potential therapeutic targets of interest. mtROS, mitochondrially derived ROS.
NIX has been previously shown to play an important role in mitochondrial clearance in erythrocytes and cardiomyocytes (10, 17, 51, 65); however, its role in intestinal inflammation is unknown. Here, we show for the first time the presence of NIX within the intestinal epithelium of both mice and humans. We also demonstrate an upregulation of NIX within the intestinal epithelium during UC, as well as localization of NIX to the mitochondria during disease. NIX expression appears to closely mirror disease severity, with little to no increase in expression in less severe disease.
Previous studies have described an LC3-interacting region found in the amino acid sequence of NIX, which facilitated the interaction of mitochondria to the autophagosome (26, 48). We were able to show an increase in LC3 in 3% DSS tissue, suggesting an upregulation in the autophagic machinery. LC3II is the activated form of LC3, responsible for binding to its target protein. We show that the LC3II/LC3I ratio was increased in DSS tissue. In conjunction with this, we confirmed that NIX binds to LC3 in human disease, suggesting that the mitochondrion is targeted to the autophagosome for removal from the cell when damaged during intestinal inflammation.
A growing body of literature describes mitochondrial dysfunction as an important phenotypic feature of human IBD and murine colitis (5, 14, 50, 55). Under this paradigm, mitochondrial damage and dysfunction contribute to cellular stress within the intestinal epithelium and perpetuate inflammation. Mitophagy is a mechanism for the selective removal of mitochondria that are old, damaged, dysfunctional, or otherwise unnecessary. Therefore, we hypothesized that mitophagy may be activated in an attempt to remove damaged mitochondria from the cellular network in response to oxidative stress during intestinal inflammation. ROS are known to play critical roles in the development of human IBD, and their reduction has been shown to provide protection in multiple murine models of disease (3, 32, 42, 45, 59, 63). However, translation to human disease has proven difficult. It is possible that the early removal of damaged mitochondria may more effectively mitigate ongoing oxidative stress during inflammation, since mitochondria are responsible for the majority of cellular ROS production (68).
The observed increase in Nix expression appears to result largely from ROS-mediated HIF1α stabilization. mtROS and reactive nitrogen species (RNS) have been implicated in the stabilization of HIF1α in previous reports (9, 24, 28). Both mtROS and nitric oxide can stabilize HIF1α by inhibition of prolyl hydroxylases (PHD) that are responsible for degradation of HIF1α, mostly under normoxic conditions (8, 36, 39). Therefore, an ROS/RNS insult may induce HIF1α stabilization during inflammation to mitigate the effects of oxidative and nitrosyl stress. In support of this paradigm, HIF1α has been shown to be protective in a murine model of 2,4,6-trinitrobenzenesulfonic acid (TNBS) colitis, where constitutively active HIF1α via von Hippel Lindau deletion conveyed protection from disease (31). Likewise, PHD inhibition via the hydroxylase inhibitor dimethyloxalylglycine, or genetic deletion of PHD1 protected mice from DSS colitis (12, 57).
Here, we show that use of the mtROS scavenger, MitoTEMPO resulted in protection from DSS-induced colitis, as others have shown. However, we also show a decrease in HIF1α and NIX expression in mice subjected to DSS and treated with MitoTEMPO, lending weight to the importance of the ROS-HIF1α-NIX axis in epithelial health. Further, we observed an increase in HIF1α expression in Nix−/− DSS mice when compared with WT DSS mice. This may be a result of a buildup of damaged mitochondria, leading to an increased mtROS insult and further feeding into HIF1α stabilization and activation. Taken together, these data suggest that mtROS production initiates HIF1α stabilization and subsequent NIX-mediated mitophagy during UC.
These investigations have several potential limitations. First, it is important to note that the Nix−/− mice used in this study are global knockout mice. Although we have found these mice to be healthy at baseline, Nix deletion in other cell types, such as erythrocytes or immune cells, could theoretically affect the development of colitis (17). The use of an epithelial-specific knockout mouse would be ideal to further confirm the findings of this study (43). Second, IBD is a heterogenous disease with multiple phenotypes. A more exhaustive evaluation of Nix expression during disease may reveal a more nuanced mitophagic response in disease subtypes. Finally, we recognize that mitochondria in immortalized cell lines may be phenotypically different than those in primary cells. Our goals in pursuing an in vitro approach, however, were limited and our findings mirrored those seen in vivo. Despite these limitations, our data extend our knowledge of the roles that mitochondrial dynamics play in the maintenance of intestinal epithelial health and how mitochondrial damage contributes to the pathogenesis of IBD.
In summary, we show that mitophagy is upregulated within the intestinal epithelium during IBD. Mitophagy appears to represent a physiologic response to mitochondrial stress and damage during disease pathogenesis, allowing for the removal of dysfunctional mitochondria from the cell and preventing ongoing ROS accumulation. NIX is a critical protein in this pathway and is transcriptionally upregulated upon stabilization of its transcription factor, HIF1α. Deletion of Nix exacerbates disease, leading to a buildup of damaged mitochondria and an increase in mtROS within the cell. Our data suggest that NIX-mediated mitophagy represents a physiologic response to mitochondrial injury, offering an attempt to mitigate the inflammatory insult. Clearly, however, an upregulation of NIX-mediated mitophagy is not enough to fully mitigate the inflammatory response. Thus, activators of mitophagy may offer attractive therapeutic targets in human IBD; however, the delicate balance between mitophagy and mitochondrial biogenesis must be considered. Assisting the cell in the removal of dysfunctional mitochondria while simultaneously enhancing mitochondrial biogenesis may prove to be a desirable strategy in future studies.
Materials and Methods
Materials and reagents
Antibodies were obtained from: 8-OHdg (ab62623) and nitrotyrosine (ab61392) (Abcam, Cambridge, MA); NIX (sc-28240 and sc-166332), TOM20 (sc-11415), and IgG (sc-2025) (Santa Cruz Biotechnology, Dallas, TX); β-ACTIN (4970), DRP1 (8570), HIF1α (14179), HSP90 (4877), and COXIV (11967) (Cell Signaling Technologies, Danvers, MA); and LC-3B (NB600-1384) (Novus, Littleton, CO).
Human tissue
Human tissue samples were obtained from the UPMC Children's Hospital of Pittsburgh after Institutional Review Board (IRB) approval at the University of Pittsburgh. Human surgical samples were obtained from patients (<21 years of age) undergoing bowel resection surgery at the UPMC Children's Hospital for a diagnosis of IBD. Age-matched, non-diseased control bowel was obtained from patients undergoing bowel resection surgery for another diagnosis. Patients were excluded from this study only if they met any of the following criteria: (1) lack of parental consent, (2) congenital anomalies, and (3) pregnant females. Each sample was accompanied by minimal clinical data, including a pathology report that was de-identified by an honest broker. Samples were grouped according to control or UC based on the pathology report.
Murine models of colitis
C57BL/6J mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and housed in accordance with the Institutional Animal Care and Use Committee (IACUC) at the University of Pittsburgh and in accordance with both federal guidelines for the care and use of laboratory animals and guidelines set forth by the Animal Research and Care Committee at the University of Pittsburgh. Bnip3ltm1Gwd (Nix−/−) mice were a gift from Dr. Ales Cvekl (Albert Einstein College of Medicine, Bronx, NY) with permission from Dr. Gerald Dorn II (Washington University, St. Louis, MO) (17). Male mice aged 8–10 weeks old and weighing 20–25 g were provided water ad libitum containing 2% or 3% dextran sodium sulfate (DSS; 36–50,000 kDa; Thermo Fisher Scientific, Waltham, MA) for 7 days. Signs of colitis were recorded daily, and mice were euthanized after 7 days or earlier if weight loss exceeded 20% of their starting weight. DAI was also recorded daily. Briefly, this score is based on weight loss, stool consistency, and presence of blood in the stool. Scores were calculated as follows, weight loss (0: <1%, 1: 1%–5%. 2: 5%–10%, 3: 11%–15%, 4: >15%), stool consistency (0: normal, 2: loose stool, 4: diarrhea), and stool blood (0: no blood, 2: positive in stool, 4: gross bleeding). MitoTEMPO (Sigma-Aldrich, St. Louis, MO) was administered 100 μg/day via intraperitoneal injection. T cell transfer model of chronic colitis was performed as previously described (14). Briefly, immunocompromised Rag−/− male mice at 8–10 weeks of age were injected retro-orbitally with one million CD4+CD45RBhigh cells purified from donor spleens of male C57BL/6J mice. Cells were purified by using Dynal Mouse CD4 Negative Isolation Kit (Invitrogen, Carlsbad, CA) utilizing Dyna-Mag 50 (Thermo Fisher Scientific) magnetic separation system. Flow-through cells were further purified and sorted by using CD4+ (L3T4; BD Pharmingen, San Diego, CA) and CD45RBhigh (16A; BD Pharmingen) antibodies using an FACSAria cell sorter (BD Biosciences, San Jose, CA). The CD45RBhigh population was identified as the 40% of cells exhibiting the brightest CD45RBhigh staining.
Quantitative real-time polymerase chain reaction
qPCR was performed as previously described by using the Bio-Rad CFX96 real-time system (Bio-Rad, Hercules, CA) (12). The gene expression was measured relative to the housekeeping gene Ribosomal Protein L15 (RPLO). Total RNA was isolated from whole intestinal tissue from mouse and human intestinal samples or cell culture by using the Zymo RNA kit (Zymo, Irvine, CA). cDNA (500 μg) was synthesized by using the QuantiTect Reverse Transcription Kit (Qiagen, Valencia, CA), according to the manufacturer's instructions. Primer sequences used for qPCR and ChIP assays are as follows: Mouse Hif1α, 5′-GCT TGG TGC TGA TTT GTG AAC CCA-3′ and 5′-TCC GGC TCA TAA CCC ATC AAC TCA-3′; Human HIF1α, 5′-GTC TGA GGG GAC AGG AGG AT-3′ and 5′-AAA GGC AAG TCC AGA GGT GG-3′; Mouse Il-1β, 5′-AGT GTG GAT CCC AAG CAA TAC CCA-3′ and 5′-TGT CCT GAC CAC TGT TGT TTC CCA-3′; Mouse inos, 5′-CTG CTG GTG GTG ACA AGC ACA TTT-3′ and 5′-ATG TCA TGA GCA AAG GCG CAG AAC-3′; Mouse Nix, 5′-CTC GAG AGC CGG ATA CTG TC-3′ and 5′-GCC ATT GCT GCT GTT CAT GG-3′; Human NIX, 5′-TGT TGT GTT GCT GCC TGA GT-3′ and 5′-GCT GTT CAT GGG TAG CTC CA-3′; Mouse Nix HRE 1, 5′-CTG CTC CCA GTG TTT CTA GTC TG-3′ and 5′-CAT CTT ATT CTC TGA CCT CCA CAG-3′; Mouse Nix HRE 2, 5′-CTT TAA AGC CAC GGG TGA GTT C-3′ and 5′-CTT TAA AGC CAC GGG TGA GTT C-3′; Human NIX HRE 1, 5′-CGT ATG GAG TGT TGA TGT TTC AGC-3′ and 5′-GTC TTG GGG ACA CAA AGG AAT C-3′; Human NIX HRE 2, 5′-CCT ATT GCA GAA GTG AGT CCT AAG-3′ and 5′-GAA GTG CGC TCC TGT GTT C-3′; Human NIX HRE 3, 5′-GTC CGG TCG CTT GTT GTG T-3′ and 5′-GCA ACA CAA CAA GCC GAG T-3′; Mouse and Human RPLO, 5′-GGC GAC CTG GAA GTC CAA CT-3′ and 5′-CCA TCA GCA CCA CAG CCT TC-3′; Mouse Tnfα, 5′-CAT CTT CTC AAA ATT CGA GTG ACA A-3′ and 5′-TGG GAG TAG ACA AGG TAC AAC CC-3′.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis
Tissue or cell culture lysates were homogenized by using 1 × RIPA buffer supplemented with protease and lysosomal inhibitors (Boston Bio, Ashland, MA). Nuclear extracts for HIF1α were collected by using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific), per the manufacturer's instructions. Protein concentration was determined by using a bicinchoninic acid (BCA) assay (Sigma-Aldrich). Protein lysates were separated on 8%–12% sodium dodecyl sulfate (SDS) gel by SDS–polyacrylamide gel electrophoresis (PAGE) and transferred to a 0.45 μM polyvinylidene difluoride membrane (Millipore Sigma, Burlington, MA). Membranes were blocked with 5% nonfat dry milk in Tris buffered saline with Tween 20 and incubated with primary antibody overnight at 4°C. A secondary horseradish peroxidase (HRP)-conjugated antibody (Cell Signaling) was used, followed by incubation with a chemiluminescent HRP substrate (Thermo Fisher Scientific). Densitometry was measured by using ImageJ as previously described (14).
In vitro upregulation of HIF1α and NIX
Human colonic HT-29 cells (ATCC, Manassas, VA) were cultured with DMEM (Lonza, Basel, Switzerland) and 10% FBS (Atlanta, Flowery Branch, GA) with 1% penicillin/streptomycin. Cells were seeded in a 6- or 12-well plates overnight. Each well was treated with 10 μM H2O2 for 1 h. Protein or RNA was then isolated as previously described (see Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis section). Experiments were repeated in biological triplicate.
Immunoprecipitation
Whole cell lysates of colonic tissue from control and DSS-subjected mice or human surgical specimens were prepared by homogenizing ∼50 mg of intestinal tissue in 250 μL of T-PER Tissue Protein Extraction Reagent (Thermo Fisher Scientific). The protein solution was centrifuged for 15 min at 13,000 rpm at 4°C. The supernatant was transferred to a new tube, and the concentration was determined via BCA assay. Protein G Dynabeads (Thermo Fisher Scientific) were conjugated to an antibody by using bis(sulfosuccinimidyl)suberate (BS3) (Thermo Fisher Scientific) before the incubation of 500 μg protein lysate overnight on a rotator at 4°C. The antibody-protein complex was then eluted according to the manufacturer's instructions. Immunoblot of the eluted proteins was performed as described earlier (see Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis section).
Mitochondrial isolation from cells and tissues
Mitochondria were isolated by using a Mitochondria Isolation Kit (Abcam), per the manufacturer's instructions. Briefly, tissues were placed in a Dounce homogenizer to lyse cells. Lysates were then centrifuged to separate the cytosolic fraction. The remaining mitochondrial pellet was then centrifuged and resuspended. Cytosolic and mitochondrial protein fractions were then analyzed by BCA assay to determine protein concentration.
ChIP assay
ChIP assay was performed by using SimpleChIP Plus Enzymatic Chromatin IP Kit (Cell Signaling), per the manufacturer's instructions. Briefly, 100 mg human or mouse colonic tissue was minced and then fixed with 1.5% formaldehyde for 20 min. Fixation was quenched with 200 μL glycine. Tissue was washed with 1 × phosphate-buffered saline (PBS) for 5 min in triplicate, then lysed by using a Dounce homogenizer, and finally treated with a micrococcal nuclease for 20 min at 37°C. Samples were sonicated for 20 s for four cycles, resulting in DNA base pair fragments between 100 and 500 bp. Samples were then incubated with HIF1α or a negative control IgG antibody overnight on a rotator at 4°C. Before this, 10 μL was collected as the 2% input sample. The next day, the samples were incubated with 30 μL protein G agarose beads for 2 h at 4°C. The beads were washed three times with low- and high-gradient salt washes for 5 min by rotation; then, they were centrifuged to pellet the beads. Chromatin complexes were eluted from the beads at 65°C for 30 min with gentle agitation. The beads were then pelleted, and the supernatant was moved to a clean tube. To digest protein from the chromatin complex, 20 mg/mL proteinase K and 5 M NaCl was added to each sample and incubated at 65°C for 2 h. The DNA was then purified by using the DNA cleanup kit provided. In triplicate, 2 ng of DNA was loaded to a 96-well plate and analyzed via qPCR to determine promoter binding by comparing the signal to the 2% input. Results were quantified by using the Percent Input Method, using the equation: Percent Input = 2% × 2(C[T]2%Input Sample – C[T] IP Sample), where C[T] = CT = threshold cycle of qPCR reaction.
Histological analysis and immunofluorescence
Tissues were fixed with 4% paraformaldehyde (PFA), dehydrated, and embedded in paraffin blocks. Tissue sections were then cut at 5 μm on a Microm HM325 (Thermo Fisher Scientific) microtome. Sections were deparaffinized and rehydrated through a gradient of xylene and ethanol baths. For hematoxylin and eosin (H&E) staining, sections were stained with H&E (Sigma), dehydrated, and mounted by using tolulene (Thermo Fisher Scientific). A blinded pathologist evaluated tissues for signs of disease, as previously described (6, 13, 14). Briefly, tissue sections were graded on a 0 to 4 scale, with 0 representing normal tissue with no inflammation and normal tissue architecture, to 4 representing severe inflammation and damage of tissue morphology. For immunofluorescence, 10 mM citric acid was used as an antigen retrieval step. Samples were microwaved in a 900 W microwave at full power for 2 min, and they were then boiled for an additional 6 min at 30% power. Sections were blocked in 1% BSA and 5% donkey serum for 1 h. Sections were incubated with primary antibody overnight at 4°C and probed with an Alexa Fluor secondary antibody (Life Technologies) and 4′, 6-diamino-2-phenylindole the next day. The sections were mounted by using a mounting reagent, allowed to dry, and finally imaged by using a Leica SP8 confocal microscope (Leica Microsystems, Inc., Buffalo Grove, IL). For each animal, three to four paraffin-embedded tissue sections were analyzed, and six to eight images total were taken for analysis. For in vitro experiments, experiments were completed three times, with ∼150 cells analyzed per experiment. The fluorescence signal in a defined region of interest was determined by using the following equation: corrected total cell fluorescence (CTFC) = integrated density − (area of selected cell × mean fluorescence of background readings). The CTFC values were graphed, and standard deviations (SDs) were calculated as previously described (14).
Mitochondrial measurement in vivo
Colonic tissue was perfused with PBS and 2% PFA. Tissues were then placed in 2% PFA for an additional 2 h and then switched to 30% sucrose in distilled water solution for 24 h. Cryopreservation of the tissue was accomplished with liquid nitrogen cooled 2-methylbutane. Tissue sections (6 mm; 4–6 sections per mouse) were incubated with 5% normal donkey serum in PBS for 1 h, followed by five washes with PBS containing 0.5% BSA. The samples were then incubated overnight at 4°C with the primary antibody TOM20 (5 mg/mL, rabbit; PA5-52843; Invitrogen). The secondary antibody Alexa Cy3-conjugated Donkey anti-rabbit IgG (1:500, red for anti-TOM20 antibody; 711-165-152, Jackson Immunoresearch) was incubated for 1 h at room temperature in combination with Alexa Fluor-conjugated 647 phalloidin (1:500, A22287; Invitrogen). Nuclear stain was accomplished by using 1 mg/mL Hoechst (B-2883; Sigma-Aldrich). Images were collected as large area stacks with a 60 × objective (NA of 1.4) with the Nyquist digital zoom of 1.55 for the equivalent of four fields (Nikon A1 confocal microscope purchased with 1S10OD019973-01 awarded to Dr. Simon C. Watkins). Analysis was performed by using NIS Elements (Nikon).
Electron microscopy and analysis
Samples obtained from both control and experimental groups were fixed in 2.5% glutaraldehyde for analysis by electron microscopy. Each sample was cut into 1–2 mm rings of intestinal tissue. They were then placed on 1 mm3 blocks and postfixed with 1% osmium tetroxide containing 1% potassium ferricyanide. Tissues were dehydrated with propylene oxide and then infiltrated with a 1:1 mix of propylene oxide and epon overnight. Samples were then infiltrated with pure epon overnight at 4°C, embedded in pure epon for 24 h at 37°C, and cured for 48 h at 60°C. Samples were cut into 70 nm sections on an Ultra Microtome, placed on copper grids, and stained with urinal acetate and lead citrate. TEM was performed by using a JEOL electron microscope (model number JSM6335F; JEOL, Peabody, MA). For each sample, four tissue rings were embedded and sectioned, with six to eight images taken per tissue ring to evaluate multiple areas of the intestine. Individual mitochondria were manually traced in ImageJ and quantified by using the following morphological categories: area (nm2), perimeter (nm), Feret's maximum/minimum diameter (the longest/shortest distance between any two points within a mitochondrion, nm), aspect ratio (Feret's maximum/Feret's minimum, nm), circularity [4π × (area/perimeter × 2)], and roundness [perimeter/(4π × area)]. Aspect ratio, circularity, and roundness with a measurement of one represent a perfect circle (34, 35).
Statistical analysis
Results are expressed as the mean ± SD. Statistical analysis was performed by using Graphpad Prism Software (La Jolla, CA). Two-tailed Student's t-test was used for comparison in experiments consisting of two experimental groups. Analysis of variance was used for comparison of experiments consisting of more than two experimental groups. A Mann–Whitney nonparametric test was used to analyze the data across several Western blots. Statistical significance was accepted at p ≤ 0.05 between groups in all cases.
Supplementary Material
Acknowledgments
The authors would also like to thank members of the Center of Biological Imaging (CBI) at the University of Pittsburgh, Jonathan Franks, Mara Sullivan, and Ming Sun for their assistance with TEM tissue processing and imaging.
Abbreviations Used
- 8-OHdg
8-hydroxy-2′-deoxyguanosine
- BCA
bicinchoninic acid
- ChIP
chromatin immunoprecipitation
- COXIV
cytochrome c oxidase, subunit IV
- CTFC
corrected total cell fluorescence
- DAI
disease activity index
- DRP1
dynamin related protein-1
- DSS
dextran sodium sulfate
- H&E
hematoxylin and eosin
- H2O2
hydrogen peroxide
- HIF1α
hypoxia-inducible factor-1α
- HRE
hypoxia response element
- HRP
horseradish peroxidase
- IBD
inflammatory bowel disease
- inos
inducible nitric oxide synthase
- mtROS
mitochondrially derived ROS
- NIX
BCL2/adenovirus E1B 19 kDa protein-interacting protein 3-like, BNIP3L
- PBS
phosphate-buffered saline
- PFA
paraformaldehyde
- PGC1α
peroxisome proliferator-activated receptor-γ coactivator 1-α
- PHD
prolyl hydroxylases
- qPCR
quantitative real-time polymerase chain reaction
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- RPLO
Ribosomal Protein L15
- SD
standard deviation
- TEM
transmission electron microscopy
- TNBS
2,4,6-trinitrobenzenesulfonic acid
- UC
ulcerative colitis
- WT
wild-type
Author Disclosure Statement
No competing financial interests exist.
Funding Information
Support for this study was provided by NIDDK DK120986 (K.P.M.), DK101753 (K.P.M.), and DK114464 (K.P.M.).
Supplementary Material
References
- 1. Abraham C and Cho JH. Inflammatory bowel disease. N Engl J Med 361: 2066–2078, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aerbajinai W, Giattina M, Lee YT, Raffeld M, and Miller JL. The proapoptotic factor Nix is coexpressed with Bcl-xL during terminal erythroid differentiation. Blood 102: 712–717, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Amrouche-Mekkioui I and Djerdjouri B. N-acetylcysteine improves redox status, mitochondrial dysfunction, mucin-depleted crypts and epithelial hyperplasia in dextran sulfate sodium-induced oxidative colitis in mice. Eur J Pharmacol 691: 209–217, 2012 [DOI] [PubMed] [Google Scholar]
- 4. Balmus IM, Ciobica A, Trifan A, and Stanciu C. The implications of oxidative stress and antioxidant therapies in Inflammatory Bowel Disease: clinical aspects and animal models. Saudi J Gastroenterol 22: 3–17, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bär F, Bochmann W, Widok A, von Medem K, Pagel R, Hirose M, Yu X, Kalies K, König P, Böhm R, Herdegen T, Reinicke AT, Büning J, Lehnert H, Fellermann K, Ibrahim S, and Sina C. Mitochondrial gene polymorphisms that protect mice from colitis. Gastroenterology 145: 1055–1063.e3, 2013 [DOI] [PubMed] [Google Scholar]
- 6. Berg DJ, Davidson N, Kühn R, Müller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, and Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest 98: 1010–1020, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brennan LA, McGreal-Estrada R, Logan CM, Cvekl A, Menko AS, and Kantorow M. BNIP3L/NIX is required for elimination of mitochondria, endoplasmic reticulum and Golgi apparatus during eye lens organelle-free zone formation. Exp Eye Res 174: 173–184, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cao Y, Eble JM, Moon E, Yuan H, Weitzel DH, Landon CD, Nien CY-C, Hanna G, Rich JN, Provenzale JM, and Dewhirst MW. Tumor cells upregulate normoxic HIF-1α in response to doxorubicin. Cancer Res 73: 6230–6242, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, and Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A 95: 11715–11720, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen Y, Lewis W, Diwan A, Cheng EH-Y, Matkovich SJ, and Dorn GW. Dual autonomous mitochondrial cell death pathways are activated by Nix/BNip3L and induce cardiomyopathy. Proc Natl Acad Sci U S A 107: 9035–9042, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chourasia AH, Tracy K, Frankenberger C, Boland ML, Sharifi MN, Drake LE, Sachleben JR, Asara JM, Locasale JW, Karczmar GS, and Macleod KF. Mitophagy defects arising from BNip3 loss promote mammary tumor progression to metastasis. EMBO Rep 16: 1145–1163, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cummins EP, Seeballuck F, Keely SJ, Mangan NE, Callanan JJ, Fallon PG, and Taylor CT. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology 134: 156–165, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Cunningham KE, Novak EA, Vincent G, Siow VS, Griffith BD, Ranganathan S, Rosengart MR, Piganelli JD, and Mollen KP.. Calcium/calmodulin-dependent protein kinase IV (CaMKIV) activation contributes to the pathogenesis of experimental colitis via inhibition of intestinal epithelial cell proliferation. FASEB J fj201800535R, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cunningham KE, Vincent G, Sodhi CP, Novak EA, Ranganathan S, Egan CE, Stolz DB, Rogers MB, Firek B, Morowitz MJ, Gittes GK, Zuckerbraun BS, Hackam DJ, and Mollen KP. Peroxisome proliferator-activated receptor-γ coactivator 1-α (PGC1α) protects against experimental murine colitis. J Biol Chem 291: 10184–10200, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dashdorj A, Jyothi KR, Lim S, Jo A, Nguyen MN, Ha J, Yoon K-S, Kim HJ, Park J-H, Murphy MP, and Kim SS. Mitochondria-targeted antioxidant MitoQ ameliorates experimental mouse colitis by suppressing NLRP3 inflammasome-mediated inflammatory cytokines. BMC Med 11: 178, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ding W-X, Ni H-M, Li M, Liao Y, Chen X, Stolz DB, Dorn GW, and Yin X-M. Nix is critical to two distinct phases of mitophagy, reactive oxygen species-mediated autophagy induction and Parkin-ubiquitin-p62-mediated mitochondrial priming. J Biol Chem 285: 27879–27890, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Diwan A, Koesters AG, Odley AM, Pushkaran S, Baines CP, Spike BT, Daria D, Jegga AG, Geiger H, Aronow BJ, Molkentin JD, Macleod KF, Kalfa TA, and Dorn GW. Unrestrained erythroblast development in Nix−/− mice reveals a mechanism for apoptotic modulation of erythropoiesis. Proc Natl Acad Sci U S A 104: 6794–6799, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Diwan A, Wansapura J, Syed FM, Matkovich SJ, Lorenz JN, and Dorn GW. Nix-mediated apoptosis links myocardial fibrosis, cardiac remodeling, and hypertrophy decompensation. Circulation 117: 396–404, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Esteban-Martínez L, Sierra-Filardi E, McGreal RS, Salazar-Roa M, Mariño G, Seco E, Durand S, Enot D, Graña O, Malumbres M, Cvekl A, Cuervo AM, Kroemer G, and Boya P. Programmed mitophagy is essential for the glycolytic switch during cell differentiation. EMBO J 36: 1688–1706, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Formentini L, Santacatterina F, Núñez de Arenas C, Stamatakis K, López-Martínez D, Logan A, Fresno M, Smits R, Murphy MP, and Cuezva JM. Mitochondrial ROS production protects the intestine from inflammation through functional M2 macrophage polarization. Cell Rep 19: 1202–1213, 2017 [DOI] [PubMed] [Google Scholar]
- 21. Gálvez AS, Brunskill EW, Marreez Y, Benner BJ, Regula KM, Kirschenbaum LA, and Dorn GW. Distinct pathways regulate proapoptotic Nix and BNip3 in cardiac stress. J Biol Chem 281: 1442–1448, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Giatromanolaki A, Sivridis E, Maltezos E, Papazoglou D, Simopoulos C, Gatter K, Harris AL, and Koukourakis MI. Hypoxia inducible factor 1 and 2 overexpression in inflammatory bowel disease. J Clin Pathol 56: 209–213, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gioscia-Ryan RA, LaRocca TJ, Sindler AL, Zigler MC, Murphy MP, and Seals DR. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J Physiol (Lond) 592: 2549–2561, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, and Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab 1: 401–408, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Haberman Y, Karns R, Dexheimer PJ, Schirmer M, Somekh J, Jurickova I, Braun T, Novak E, Bauman L, Collins MH, Mo A, Rosen MJ, Bonkowski E, Gotman N, Marquis A, Nistel M, Rufo PA, Baker SS, Sauer CG, Markowitz J, Pfefferkorn MD, Rosh JR, Boyle BM, Mack DR, Baldassano RN, Shah S, Leleiko NS, Heyman MB, Grifiths AM, Patel AS, Noe JD, Aronow BJ, Kugathasan S, Walters TD, Gibson G, Thomas SD, Mollen K, Shen-Orr S, Huttenhower C, Xavier RJ, Hyams JS, and Denson LA. Ulcerative colitis mucosal transcriptomes reveal mitochondriopathy and personalized mechanisms underlying disease severity and treatment response. Nat Commun 10: 38, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hamacher-Brady A and Brady NR. Mitophagy programs: mechanisms and physiological implications of mitochondrial targeting by autophagy. Cell Mol Life Sci 73: 775–795, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ho G-T, Aird RE, Liu B, Boyapati RK, Kennedy NA, Dorward DA, Noble CL, Shimizu T, Carter RN, Chew ETS, Morton NM, Rossi AG, Sartor RB, Iredale JP, and Satsangi J. MDR1 deficiency impairs mitochondrial homeostasis and promotes intestinal inflammation. Mucosal Immunol 11: 120–130, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hwang AB, Ryu E-A, Artan M, Chang H-W, Kabir MH, Nam H-J, Lee D, Yang J-S, Kim S, Mair WB, Lee C, Lee SS, and Lee S-J. Feedback regulation via AMPK and HIF-1 mediates ROS-dependent longevity in Caenorhabditis elegans. Proc Natl Acad Sci U S A 111:E4458–E4467, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. This reference has been deleted
- 30. Jung S-N, Yang WK, Kim J, Kim HS, Kim EJ, Yun H, Park H, Kim SS, Choe W, Kang I, and Ha J. Reactive oxygen species stabilize hypoxia-inducible factor-1 alpha protein and stimulate transcriptional activity via AMP-activated protein kinase in DU145 human prostate cancer cells. Carcinogenesis 29: 713–721, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, and Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest 114: 1098–1106, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim H-R, Lee A, Choi E-J, Kie J-H, Lim W, Lee HK, Moon B-I, and Seoh J-Y. Attenuation of experimental colitis in glutathione peroxidase 1 and catalase double knockout mice through enhancing regulatory T cell function. PLoS ONE 9: e95332, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. This reference has been deleted
- 34. Koopman WJH, Visch H-J, Smeitink JAM, and Willems PHGM. Simultaneous quantitative measurement and automated analysis of mitochondrial morphology, mass, potential, and motility in living human skin fibroblasts. Cytometry A 69: 1–12, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Leduc-Gaudet J-P, Picard M, St-Jean Pelletier F, Sgarioto N, Auger M-J, Vallée J, Robitaille R, St-Pierre DH, and Gouspillou G. Mitochondrial morphology is altered in atrophied skeletal muscle of aged mice. Oncotarget 6: 17923–17937, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Metzen E, Zhou J, Jelkmann W, Fandrey J, and Brüne B. Nitric oxide impairs normoxic degradation of HIF-1alpha by inhibition of prolyl hydroxylases. Mol Biol Cell 14: 3470–3481, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mojsilovic-Petrovic J, Callaghan D, Cui H, Dean C, Stanimirovic DB, and Zhang W. Hypoxia-inducible factor-1 (HIF-1) is involved in the regulation of hypoxia-stimulated expression of monocyte chemoattractant protein-1 (MCP-1/CCL2) and MCP-5 (Ccl12) in astrocytes. J Neuroinflammation 4: 12, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ney PA. Mitochondrial autophagy: origins, significance, and role of BNIP3 and NIX. Biochim Biophys Acta 1853: 2775–2783, 2015 [DOI] [PubMed] [Google Scholar]
- 39. Niecknig H, Tug S, Reyes BD, Kirsch M, Fandrey J, and Berchner-Pfannschmidt U. Role of reactive oxygen species in the regulation of HIF-1 by prolyl hydroxylase 2 under mild hypoxia. Free Radic Res 46: 705–717, 2012 [DOI] [PubMed] [Google Scholar]
- 40. Novak EA and Mollen KP. Mitochondrial dysfunction in inflammatory bowel disease. Front Cell Dev Biol 3: 62, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Novak I, Kirkin V, McEwan DG, Zhang J, Wild P, Rozenknop A, Rogov V, Löhr F, Popovic D, Occhipinti A, Reichert AS, Terzic J, Dötsch V, Ney PA, and Dikic I. Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep 11: 45–51, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Novak S, Drenjancevic I, Vukovic R, Kellermayer Z, Cosic A, Tolusic Levak M, Balogh P, Culo F, and Mihalj M. Anti-inflammatory effects of hyperbaric oxygenation during DSS-induced colitis in BALB/c mice include changes in gene expression of HIF-1α, proinflammatory cytokines, and antioxidative enzymes. Mediators Inflamm 2016: 7141430, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. O'Sullivan TE, Johnson LR, Kang HH, and Sun JC. BNIP3- and BNIP3L-Mediated mitophagy promotes the generation of natural killer cell memory. Immunity 43: 331–342, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ogawa Y, Kobayashi T, Nishioka A, Kariya S, Ohnishi T, Hamasato S, Seguchi H, and Yoshida S. Reactive oxygen species-producing site in hydrogen peroxide-induced apoptosis of human peripheral T cells: involvement of lysosomal membrane destabilization. Int J Mol Med 13:383–388, 2004 [PubMed] [Google Scholar]
- 45. Rezaie A, Parker RD, and Abdollahi M. Oxidative stress and pathogenesis of inflammatory bowel disease: an epiphenomenon or the cause? Dig Dis Sci 52: 2015–2021, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Robinson A, Keely S, Karhausen J, Gerich ME, Furuta GT, and Colgan SP. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology 134: 145–155, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Roessner A, Kuester D, Malfertheiner P, and Schneider-Stock R. Oxidative stress in ulcerative colitis-associated carcinogenesis. Pathol Res Pract 204: 511–524, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Rogov VV, Suzuki H, Marinković M, Lang V, Kato R, Kawasaki M, Buljubašić M, Šprung M, Rogova N, Wakatsuki S, Hamacher-Brady A, Dötsch V, Dikic I, Brady NR, and Novak I. Phosphorylation of the mitochondrial autophagy receptor Nix enhances its interaction with LC3 proteins. Sci Rep 7: 1131, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sandoval H, Thiagarajan P, Dasgupta SK, Schumacher A, Prchal JT, Chen M, and Wang J. Essential role for Nix in autophagic maturation of erythroid cells. Nature 454: 232–235, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Santhanam S, Rajamanickam S, Motamarry A, Ramakrishna BS, Amirtharaj JG, Ramachandran A, Pulimood A, and Venkatraman A. Mitochondrial electron transport chain complex dysfunction in the colonic mucosa in ulcerative colitis. Inflamm Bowel Dis 18: 2158–2168, 2012 [DOI] [PubMed] [Google Scholar]
- 51. Schweers RL, Zhang J, Randall MS, Loyd MR, Li W, Dorsey FC, Kundu M, Opferman JT, Cleveland JL, Miller JL, and Ney PA. NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proc Natl Acad Sci U S A 104: 19500–19505, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Semenza GL. Hypoxia-inducible factor 1 and the molecular physiology of oxygen homeostasis. J Lab Clin Med 131: 207–214, 1998 [DOI] [PubMed] [Google Scholar]
- 53. Shah YM, Ito S, Morimura K, Chen C, Yim S-H, Haase VH, and Gonzalez FJ. Hypoxia-inducible factor augments experimental colitis through an MIF-dependent inflammatory signaling cascade. Gastroenterology 134: 2036–2048, 2048.e1, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. This reference has been deleted
- 55. Sifroni KG, Damiani CR, Stoffel C, Cardoso MR, Ferreira GK, Jeremias IC, Rezin GT, Scaini G, Schuck PF, Dal-Pizzol F, and Streck EL. Mitochondrial respiratory chain in the colonic mucosal of patients with ulcerative colitis. Mol Cell Biochem 342: 111–115, 2010 [DOI] [PubMed] [Google Scholar]
- 56. Sowter HM, Ratcliffe PJ, Watson P, Greenberg AH, and Harris AL. HIF-1-dependent regulation of hypoxic induction of the cell death factors BNIP3 and NIX in human tumors. Cancer Res 61: 6669–6673, 2001 [PubMed] [Google Scholar]
- 57. Tambuwala MM, Cummins EP, Lenihan CR, Kiss J, Stauch M, Scholz CC, Fraisl P, Lasitschka F, Mollenhauer M, Saunders SP, Maxwell PH, Carmeliet P, Fallon PG, Schneider M, and Taylor CT. Loss of prolyl hydroxylase-1 protects against colitis through reduced epithelial cell apoptosis and increased barrier function. Gastroenterology 139: 2093–2101, 2010 [DOI] [PubMed] [Google Scholar]
- 58. Tilokani L, Nagashima S, Paupe V, and Prudent J. Mitochondrial dynamics: overview of molecular mechanisms. Essays Biochem 62: 341–360, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang A, Keita ÅV, Phan V, McKay CM, Schoultz I, Lee J, Murphy MP, Fernando M, Ronaghan N, Balce D, Yates R, Dicay M, Beck PL, MacNaughton WK, Söderholm JD, and McKay DM. Targeting mitochondria-derived reactive oxygen species to reduce epithelial barrier dysfunction and colitis. Am J Pathol 184: 2516–2527, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang Z, Li S, Cao Y, Tian X, Zeng R, Liao D-F, and Cao D. Oxidative stress and carbonyl lesions in ulcerative colitis and associated colorectal cancer. Oxid Med Cell Longev 2016: 9875298, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wu H and Chen Q. Hypoxia activation of mitophagy and its role in disease pathogenesis. Antioxid Redox Signal 22: 1032–1046, 2015 [DOI] [PubMed] [Google Scholar]
- 62. Xu F, Dahlhamer JM, Zammitti EP, Wheaton AG, and Croft JB. Health-risk behaviors and chronic conditions among adults with inflammatory bowel disease—United States, 2015 and 2016. MMWR Morb Mortal Wkly Rep 67: 190–195, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. You Y, Fu J-J, Meng J, Huang G-D, and Liu Y-H. Effect of N-acetylcysteine on the murine model of colitis induced by dextran sodium sulfate through up-regulating PON1 activity. Dig Dis Sci 54: 1643–1650, 2009 [DOI] [PubMed] [Google Scholar]
- 64. This reference has been deleted
- 65. Yussman MG, Toyokawa T, Odley A, Lynch RA, Wu G, Colbert MC, Aronow BJ, Lorenz JN, and Dorn GW. Mitochondrial death protein Nix is induced in cardiac hypertrophy and triggers apoptotic cardiomyopathy. Nat Med 8: 725–730, 2002 [DOI] [PubMed] [Google Scholar]
- 66. This reference has been deleted
- 67. Zmijewski JW, Banerjee S, Bae H, Friggeri A, Lazarowski ER, and Abraham E. Exposure to hydrogen peroxide induces oxidation and activation of AMP-activated protein kinase. J Biol Chem 285: 33154–33164, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zorov DB, Juhaszova M, and Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 94: 909–950, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.