Abstract
Background: The state of prediabetes comprises atherosclerotic changes leading to decreased vascular function in humans. This study examined the effects on incretin mimetics on vascular physiology in the prediabetic postprandial state.
Methods: Fifteen obese adults with prediabetes participated in a randomized, crossover, double-blinded trial comparing the postprandial effects of exenatide, saxagliptin, and placebo on peripheral vasodilation. All studies utilized a standardized high-fat meal. Resting and peak forearm blood flow (FBF) were measured via strain gauge venous occlusion plethysmography, and makers of vascular dysfunction were measured in plasma.
Results: Exenatide attenuated resting FBF at 3 hr (P = 0.003) and 6 hr (P = 0.056) postmeal, compared to placebo. Nonsignificant reductions in resting FBF were observed between saxagliptin and placebo at the same time points. No group differences were observed for peak FBF, plasma nitrotyrosine, and plasma 8-iso-prostaglandin F2alpha. A transient increase in plasma triglyceride was abated in the exenatide group, when compared to saxagliptin and placebo groups. Only exenatide group showed no significant upsurge in plasma insulin. Plasma-free fatty acids significantly declined in all three groups, although less markedly for exenatide. Postmeal glucose increased at 2 hr with placebo and saxagliptin, but simultaneously decreased with exenatide.
Conclusions: Acute treatment with exenatide blunted the postprandial vasodilatory effect of a high-fat meal in prediabetes. Exenatide's acute effects derived primarily from multiple endothelium-independent processes. Trial Registration Number: NCT02104739.
Keywords: exenatide, prediabetes, postprandial, vasodilation, endothelium
Introduction
Prediabetes—a condition associated with increased cardiovascular mortality1—is characterized by increased insulin resistance, dynamic insulin secretion changes, and reduced incretin hormone secretion.2 Compared to healthy subjects, prediabetes exacerbates endothelial dysfunction3 via the loss of vasodilatory function, dysregulation of vascular permeability, and impairment of fibrinolysis.4 These metabolic derangements largely occur in the postprandial state.5,6
Glucagon-like peptide-1 (GLP-1) receptor agonists (GLP-1RAs) lead to reductions in major adverse cardiovascular events when added as treatment for type 2 diabetes mellitus (T2DM).7–9 Furthermore, GLP-1RAs and the related dipeptidyl peptidase-IV (DPP-IV) inhibitors exert relevant beneficial effects on levels of plasma triglycerides, free fatty acids (FFAs), and glucose.10 The exact mechanisms by which these incretin mimetic therapies improve cardiovascular and metabolic parameters are not fully established.
The postprandial state—which reflects the majority of the day for humans—accounts for many GLP-1RA effects on coronary and peripheral vascular function. Measures of peripheral vascular function – including venous occlusion plethysmography, flow mediated dilation (FMD), and peripheral arterial tonometry (PAT)—function as surrogate measures for coronary artery vascular function.10 Studies show conflicting results on the effects of fatty meals on peripheral vasculature function.11–15
Most prior studies have examined the effects of incretin therapies on vascular endothelial function in the fasting state, with some studies showing improvement16,17 and other studies showing no improvement.18–22 A few studies suggest that exenatide improves postprandial endothelial function in T2DM.23–25
The aim of this study was to examine the effects of two incretin mimetics – exenatide (GLP-1RA) and saxagliptin (DPP-IV inhibitor) – on postprandial vascular physiology and relevant metabolic responses in humans with prediabetes.
Methods and Research Design
The protocol was approved by the Committee for the Protection of Human Subjects at The University of Texas Health Science Center at Houston. All subjects provided written informed consent before participation.
Subjects
Eligible subjects were obese men and women, aged 30 to 70 years, with prediabetes. Prediabetes was defined as either impaired fasting glucose (fasting glucose of 100–125 mg/dL), impaired glucose tolerance (IGT) (2-hr postprandial blood glucose of 140–199 mg/dL after 75-gram oral glucose challenge), and/or a hemoglobin A1C (HbA1c) ranging from 5.7% to 6.4%. Fasting glucose and HbA1c were tested. Subjects were asked to bring any oral glucose tolerance test (OGTT) data completed during the last 3 months. All subjects had a BMI of 30–35 mg/kg2 (±1 mg/kg2). Women of childbearing age agreed to a nonhormonal pregnancy prevention method. Pregnant and breastfeeding women were excluded. Required laboratory values were hematocrit ≥34%, serum creatinine <1.5 mg/dL in men or <1.4 mg/dL in women, aspartate aminotransferase <2.5 times upper limit of normal (ULN), alanine aminotransferase <2.5 times ULN, and alkaline phosphatase <2.5 times ULN. Subjects on statins, angiotensin-converting enzyme inhibitors, and angiotensin-receptor blockers were on stable doses for at least 3 months before study enrollment, and remained on these doses. No subjects were on metformin, DPP-IV inhibitors, GLP-1RAs, sodium-glucose cotransporter-2 (SGLT-2) inhibitors, thiazolidinediones, insulin, sulfonylureas, corticosteroids, lipid-lowering medications other than statins, hormone replacement therapy, nor immunosuppressive therapy for at least 3 months before study initiation. Nonsteroidal anti-inflammatory drugs (NSAIDS) and antioxidant vitamins were stopped 1 week before study initiation. Subjects with significant cardiac, hepatic, or renal disease, current tobacco use, active malignancy, acute infectious conditions, diabetes mellitus, history of pancreatitis, history of medullary thyroid cancer, and multiple endocrine neoplasia 2 (MEN2) were excluded.
Fifteen subjects completed the study (Fig. 1).
FIG. 1.
Flowchart of participant enrollment, randomization, allocation, follow up, and analysis.
Study design
The study was a single center, randomized, crossover, double-blinded, placebo-controlled trial. Simple randomization (for study drug order in each participant) was achieved via a computer-generated randomization, and allocation concealment was maintained by independent study pharmacists. Blinding of intervention sequence (to investigators and subjects) was achieved through the use of identical-appearing placebos. Normal saline served as the exenatide placebo. Saxagliptin and saxagliptin placebo were placed in identical-appearing opaque capsules. Primary study outcomes for this pilot study were postprandial changes in resting and peak postprandial forearm blood flow (FBF). Secondary outcomes were changes in postprandial plasma markers of endothelial dysfunction and metabolic changes. All analyses were conducted as intention-to-treat.
Potential subjects participated in a screening visit, where a complete history and physical examination was performed, followed by laboratory testing
Qualified subjects completed three separate, daylong outpatient studies at the Clinical Research Unit (CRU). On each study day, subjects were given a single dose of exenatide 10 mcg by subcutaneous (sc) injection, saxagliptin 5 mg orally, or placebo (sc and orally). Each study was performed ≥10 days apart to ensure washout of study medication before next study day.
The study day began at 8:00 AM after an overnight fast and avoidance of alcohol and excessive exercise for 24 hr. An intravenous catheter was placed in a stable vein in an upper extremity. Baseline blood draws were collected at 10:45 AM. Then study medication was given. At 11:00 AM, a timed standardized test meal which consisted of a hamburger, French fries, small apple pies, and diet soda (1550 kcal; 700 kcal from fat; 60% carbohydrate, 30% fat, and 10% protein) was initiated. Venous blood samples were collected every 2 hr for total of 6 hr postmeal. Forearm venous occlusion plethysmography was measured every 3 hr during the same postmeal period. These procedures were repeated for the remaining study arms.
Venous occlusion plethysmography
Resting and peak FBF were measured by strain gauge venous occlusion plethysmography with calibrated mercury strain gauges, per the method described by Skilton et al.15 In brief, the nondominant forearm was positioned at the level slightly above the right atrium. A blood pressure cuff was placed on the upper arm and another cuff around the wrist. The wrist cuff was inflated to a supra-systolic pressure (250 mmHg) and clamped to prevent circulation to the hand. The strain gauge was positioned around the widest portion of the forearm and connected to a Hokanson EC6 plethysmograph (Hokanson, Bellevue, WA). The plethysmograph was connected to a PowerLab 4/35 data acquisition system, which was connected to a computer operating LabChart7 data analysis software (AD Instruments, Colorado Springs, CO).
To obtain resting FBF, an automatic rapid cuff inflator intermittently inflated the upper arm cuff at 60 mmHg (7 sec on, 7 sec off for 3 min) to periodically occlude the venous outflow from the distal portion of the arm without obstructing the arterial inflow. Flow curves were recorded on LabChart. We used an average of six acceptable flow curves for our measurements. The slopes of the flow curves were used to calculate FBF (mL/min/100 mL of tissue).
Then to obtain peak FBF, we initiated automatic rapid inflation of the upper arm cuff to a suprasystolic pressure (250 mmHg) for 5 min continuously, to induce forearm ischemia. This was followed by intermittent inflations of the upper arm cuff at 60 mmHg (7 sec on, 7 sec off for 3 min). Flow curves were again recorded on LabChart, and we used the same methods to calculate FBF.
Plasma measurements
Commercial ELISA kits were used to measure plasma nitrotyrosine (Cell Biolabs, Inc., San Diego, CA) and plasma 8-iso-prostaglandin F2alpha (8-iso-PGF2a) (Cayman Chemical, Ann Arbor, MI). Plasma triglycerides and cholesterol were determined using an enzymatic method. Plasma insulin was measured by chemiluminescent immunoassay. A hexokinase assay was used to measure plasma glucose (Beckman Coulter, Indianapolis, IN). Plasma FFA concentration was determined by an enzymatic colorimetric quantification method (Fujifilm Wako Diagnostics, Mountain View, CA).
Statistical analyses
Considering that this was a crossover design with repeated measures at multiple times, linear mixed-effects model was used to account for the within-subject correlation. The outcomes included resting FBF, peak FBF, and plasma measurements. We analyzed each one independently. The factors used in the model included the drug type, time of measurement, the interaction of drug and time, and order of drug assignment. The order factor had a significant effect on the outcomes of resting FBF, FFA, and total cholesterol; hence it was included in the mixed effect model. The P values for the main effect of drug and interaction of drug and time from the linear mixed-effect model are shown in the study figures. Pairwise group comparison was performed at each measurement time, adjusted for multiple testing by Tukey's method. Outcomes for each drug at each subsequent measurement times were compared with their corresponding values at baseline, adjusted for multiple testing. Area under the curve (AUC) was calculated using the trapezoidal rule and adjusted for multiple testing. We calculated the correlation between resting FBF and insulin and triglycerides for exenatide arm by Spearman method. Insulin and triglycerides were imputed at 3 hr by averaging the values from hours 2 and 4. Results with significant P values (P < 0.05) were marked in study figures. All analyses were performed in SAS 9.4 software (Cary, NC).
Results
Table 1 shows baseline clinical characteristics. Eight male and seven female participants were studied. Ethnicities were Black (8/15), Hispanic (4/15), and Caucasian (3/15). The median age was 53 years (interquartile range 45–55 years), with median body mass index of 33.4 kg/m2 (interquartile range 30.5–34.2 kg/m2) and mean HbA1c of 5.96% ± 0.06%. The median fasting glucose level was 93 mg/dL. No subjects presented with OGTT data. The majority of subjects met prediabetes criteria by HbA1c alone.
Table 1.
Baseline Clinical Characteristics of Study Participants
| Variable | n = 15 | Range |
|---|---|---|
| Ethnicity (Caucasian/Black/Hispanic) | 3/8/4 | — |
| Gender (male/female) | 8/7 | — |
| Age (years) | 51 | 45–55 |
| Body mass index (kg/m2) | 32.7 | 30.5–34.2 |
| Systolic BP (mm Hg) | 138 | 121–148 |
| Diastolic BP (mm Hg) | 79 ± 3 | — |
| Fasting glucose (mg/dL) | 93 | 85–99 |
| Hemoglobin A1C (%) | 5.99 ± 0.6 | — |
| Triglycerides (mg/dL) | 122 | 86–137 |
| Total cholesterol (mg/dL) | 194 | 167–226 |
| HDL cholesterol (mg/dL) | 55 ± 2 | — |
| LDL cholesterol (mg/dL) | 115 | 87–151 |
| AST (U/L) | 21 | 16–27 |
| ALT (U/L) | 22 | 14–25 |
| Creatinine (mg/dL) | 0.99 ± 0.7 | — |
| Hemoglobin (g/dL) | 13.9 | 12.9–14.9 |
| Platelets ( × 109/L) | 228 | 183–254 |
Data for ethnicity and gender are presented as absolute numbers. All other data are presented as mean ± standard error of mean (SEM) if normally distributed, or median and interquartile range if not normally distributed.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BP, blood pressure; HDL, high-density lipoprotein; LDL, low density lipoprotein; SEM, standard error of mean.
Similar baseline (premeal) measurements between groups were observed for all study measures.
FBF measurements
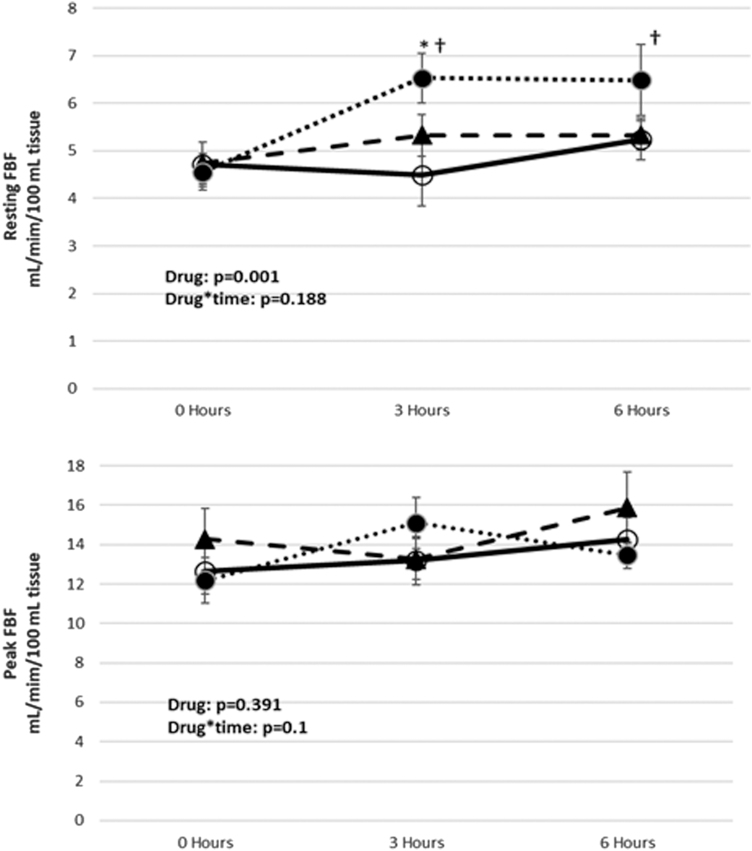
Postmeal resting FBF increased significantly from baseline in the placebo group only, at 3 and 6 hr (P < 0.01 for both). Resting FBF was significantly lower at 3 hr in exenatide compared to placebo (P = 0.003), with trend toward lower values in saxagliptin versus placebo (P = 0.095). There were no significant differences between groups at 6 hr, although there was trend toward lower values in exenatide versus placebo (P = 0.056). In contrast, there were neither significant increases from baseline nor any postmeal FBF differences between the three groups (Fig. 2).
FIG. 2.
Changes of resting and peak FBF in prediabetes subjects after high-fat meal. Data are expressed as mean ± SEM; ○, exenatide; ▲, saxagliptin; ●, placebo; *P < 0.005 exenatide versus placebo; †P < 0.01 versus baseline. n = 15. FBF, forearm blood flow.
Plasma measurements
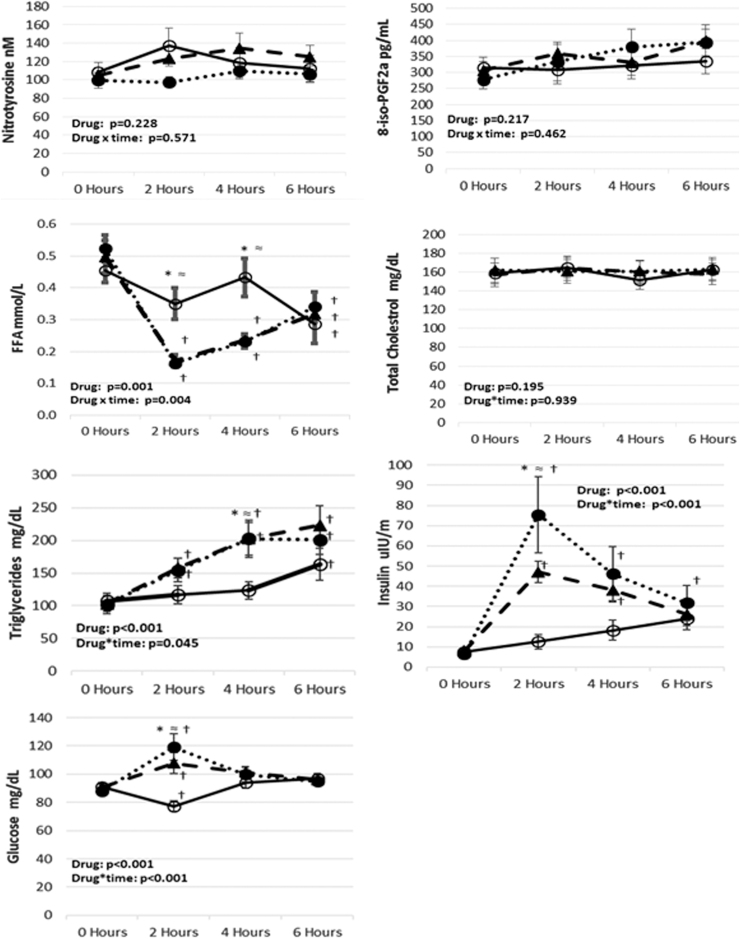
Postmeal nitrotyrosine, 8-iso-PGF2a, and total cholesterol levels did not differ between groups (Fig. 3).
FIG. 3.
Changes of plasma nitrotyrosine, 8-iso-PGF2a, triglycerides, total cholesterol, insulin, FFAs, and glucose in prediabetes subjects after high-fat meal. Data are expressed as mean ± SEM; ○, exenatide; ▲, saxagliptin; ●, placebo. *P < 0.05 exenatide versus placebo, ≈P < 0.05 exenatide versus saxagliptin, †P < 0.05 versus baseline. n = 15. 8-iso-PGF2a = 8-iso-prostaglandin F2alpha. FFA, free fatty acid.
Postmeal triglyceride levels increased significantly from baseline for saxagliptin and placebo at 2 hr (P = 0.031 and P = 0.013) and 4 hr (P < 0.0001 for both). These levels increased at 6 hr for exenatide, saxagliptin, and placebo (P = 0.043, P < 0.0001, and P < 0.0001, respectively). Triglycerides increased significantly at 4 hr in saxagliptin and placebo compared to exenatide (P = 0.005 and P = 0.009, respectively). There were no significant differences between groups at 2 and 6 hr (Fig. 3). AUC measurements showed significant differences between exenatide and placebo (P = 0.032) and between exenatide and saxagliptin (P = 0.024) (Supplementary Table S1).
Postmeal insulin levels significantly increased from baseline for saxagliptin and placebo at 2 hr (P < 0.0001 for both) and 4 hr (P = 0.004 and P < 0.0001, respectively), and at 6 hr for the placebo group only (P = 0.032). Insulin levels increased significantly at 2 hr in saxagliptin and placebo compared to exenatide (P = 0.006 and P < 0.001, respectively). There were no significant differences between groups at 4 and 6 hr (Fig. 3). AUC measurements showed significant differences in percent change between exenatide and placebo (P = 0.0006) (Supplementary Table S1).
Postmeal FFA levels decreased significantly from baseline at 2 hr in the saxagliptin and placebo groups (P < 0.0001 for both), at 4 hr in the saxagliptin and placebo groups (P < 0.0001 for both), and at 6 hr for all groups (P = 0.012 for exenatide, P = 0.007 for saxagliptin, and P = 0.004 for placebo). FFA decreased significantly in saxagliptin and placebo when compared to exenatide at 2 hr (P = 0.023 and P = 0.0004, respectively) and 4 hr (P = 0.008 and P = 0.001, respectively). There were no significant differences between groups at 6 hr (Fig. 3).
Postmeal glucose levels increased significantly from baseline for saxagliptin and placebo at 2 hr (P = 0.007 and P < 0.0001), and simultaneously decreased from baseline in exenatide (P = 0.029). No differences from baseline were seen at 4 and 6 hr. Glucose levels decreased significantly at 2 hr in the exenatide group when compared to saxagliptin and placebo (P < 0.0001 for both comparisons). No significant differences were seen between groups at 4 and 6 hr (Fig. 3).
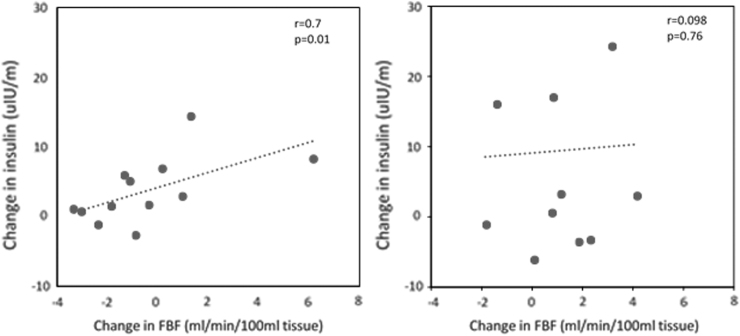
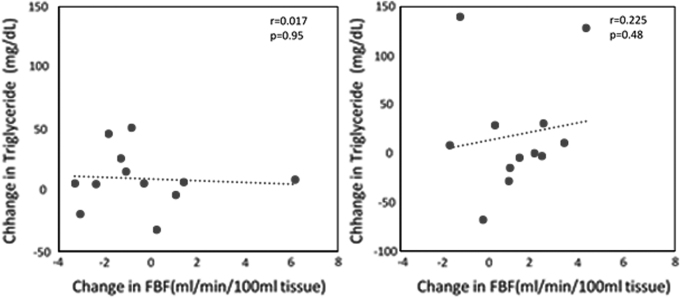
With exenatide therapy, the 0 to 3 hr postprandial increase in resting FBF was closely correlated with increases in plasma insulin (r = 0.7, P = 0.01) (Fig. 4). There were no significant correlations between resting FBF and triglycerides (Fig. 5).
FIG. 4.
(Left) Relationships between postprandial changes (0 to 3 hr) in insulin level and resting FBF in prediabetes subjects after high-fat meal in the exenatide group. (Right) Relationships between postprandial changes (3 to 6 hr) in insulin level and resting FBF in prediabetes subjects after high-fat meal in the exenatide group.
FIG. 5.
(Left) Relationships between postprandial changes (0 to 3 hr) in triglyceride level and resting FBF in prediabetes subjects after high-fat meal in the exenatide group. (Right) Relationships between postprandial changes (3 to 6 hr) in triglyceride level and resting FBF in prediabetes subjects after high-fat meal in the exenatide group.
Discussion
We show that acute exenatide therapy blunts the vasodilatory effect of a high-fat meal in obese humans with prediabetes. A high-fat meal alone (represented by placebo) is known to increase resting FBF, but not peak FBF, in healthy subjects.14 In prediabetes, our placebo findings show a similar increase in postprandial resting FBF. Exenatide lowered resting FBF at 3 hr (P = 0.003) and 6 hr (P = 0.056). A nonsignificant similar trend was seen with saxagliptin at 3 hr (P = 0.095). No effects were seen in peak FBF for any study arm. Interestingly, neither postmeal changes in plasma nitrotyrosine nor 8-iso-PGF2a were detected in any study arm; this contrasts prior findings suggesting positive endothelial effects for GLP-1 therapy.26
Relevant metabolic changes accompanied vasodilatory changes. Exenatide attenuated plasma triglyceride levels as seen previously in early insulin resistance.23,27, Plasma insulin levels increased in all groups, but much less with exenatide. Notably there was a correlation between rises in resting FBF insulin between 0 and 3 hr. FFAs decreased in all groups as anticipated after fatty meals (suggesting skeletal muscle uptake),28 although less with exenatide.
Prior studies frequent used PAT to examine effects of acute exenatide therapy on postprandial endothelial function. Findings range from attenuation of endothelial dysfunction in DM223–25 and IGT24 to no acute effects in prediabetes.20 PAT measures endothelial function via finger arterial pulse volume amplitude after induction of reactive hyperemia, calculating a reactive hyperemic index (RHI). While automated and relatively easy to use, there are concerns about suboptimal within-day variability, and it is unknown if the RHI is exclusively a measure of endothelial function or simply a generalized measure of microvascular function. Furthermore, PAT cannot assess endothelium-independent vasodilation.29
Our results show a predominantly endothelium-independent effect of exenatide on vasodilation, as shown by decreased resting FBF and no change in peak FBF. Resting FBF measures blood flow through the skeletal muscle and skin vasculature. It is influenced by sympathetic nervous system responses and vascular smooth muscle tone.30 Peak FBF is mostly affected by endothelium-derived nitric oxide (NO), prostaglandins, lactic acid, pH, adenosine, carbon dioxide, and potassium and not influenced by sympathetic tone.31,32 Peak FBF measurement captures endothelium-dependent and endothelium-independent effects. The separation of endothelium-dependent and -independent effects is best delineated by the intra-arterial infusions of acetylcholine and sodium nitroprusside; we did not perform these studies due to their invasive nature.
Exenatide attenuated the rise in plasma triglycerides, although no correlation was seen with change in resting FBF. Prior PAT studies have generally shown links among GLP-1 therapy, decreased postprandial endothelial dysfunction, and triglyceride reduction.23,24,27 Studies using strain-gauge venous occlusion plethysmography and FMD suggest no improvement of either measure after GLP-1 therapy, through these were not done in the postprandial state.19,21 Apart from this study, we are not aware of any other study, which utilizes strain gauge venous occlusion plethysmography to examine these relationships in the postmeal state. Further studies are needed to better understand the role of triglycerides in endothelial function.
Exenatide therapy led to higher plasma FFA levels, representing decreased uptake,28 likely due at least, in part, to decreased insulin levels. This leads to less adipose tissue lipolysis, resulting in higher FFA levels.33 Increased FFA reduces vasodilation by increased phosphorylation of endothelial NO synthase, decreased ATP-induced calcium uptake in endothelial cells, and increased reactive oxygen species production.4
The insulin response (along with vasodilation) was reduced with exenatide. Another trial observed identical results in a similar population undergoing OGTT; overall physiological findings suggested that exenatide either inhibited lipolysis by a direct suppressive effect or augmented the antilipolytic effect of insulin.34 Insulin, which vasodilates via endothelial NO,35 can also vasoconstrict via an endothelin-1 pathway.36 Further studies are needed to learn the roles of these mechanisms in the context of GLP-1 therapy.
Exenatide also slows the rate of gastric emptying, which leads to slower intestinal nutritional transport in T2DM.37 Other studies suggest this effect with exenatide at 2 hr after high-fat meal and resolution after 4 hr; the effect is not seen in DPP-IV inhibitors.23,38 Some changes in fat-induced vasodilation may be explained by gastric emptying. However, this unlikely explains all effects, as glucose level decreased at 2 hr with exenatide.
There are some limitations to this study. Although the same meal was used for all study arms, we cannot rule out an interaction between exenatide and high-fat meal. The results only reflect acute exenatide action and cannot be extrapolated to chronic therapy. There was a 32% attrition rate. The small trial size and the use of a predominantly minority population decreases generalizability in populations seen at several clinics. Exenatide can induce nausea. The numbers of uncompleted meals were three for exenatide, three for saxagliptin, and two for placebo.
The study also has some unique strengths. Venous occlusion plethysmography has excellent reproducibility when testing the effects of drugs on vasculature.32 The crossover randomized controlled trial (RCT) design eliminates multiple confounders, including age, which affects vasodilation in both nondiabetic and diabetic adults.15 A near-equal number of females and males were studied. Furthermore, our study population—primarily Black—is very different from similar prior studies.20,23–25 This is the only RCT which utilizes venous occlusion plethysmography to examine postprandial vascular physiology in prediabetes.
In conclusion, acute exenatide therapy reduced vasodilation via resting FBF, but not peak FBF, when compared to placebo in obese adults with prediabetes. Exenatide also reduced postprandial triglycerides and increased FFAs, with no significant rise in insulin, and no changes in nitrotyrosine or 8-isoPGF2a when compared to placebo. Contrasting prior studies, exenatide attenuates vasodilation primarily from endothelium-independent effects, which may include changes in vascular smooth muscle tone, direct effects on intestinal absorption, direct effects on lipolysis and/or insulin action, delay in gastric emptying, and alterations in sympathetic innervation. Future studies can address this testing in chronic incretin mimetic therapy and may further delineate the mechanisms differentiating endothelium-independent and -dependent effects.
Supplementary Material
Acknowledgments
We thank Kathy Franco, Theresa Danczak, Monika Ruscheinsky, Vu Ta, Christine Wong, Truong Lam, Hiba Ali, Nitya Kumar, the UT Houston Clinical Research Unit, Memorial Hermann Investigational Drug Services, Memorial Hermann Food and Nutrition Services, and Quest Laboratories for excellent clinical trial support and laboratory assistance. We also thank Steve Platts and the NASA Johnson Space Center for technical support with vascular physiology studies. We thank Jon Tyson and Philip Orlander for assistance in protocol development and oversight. We also thank all of the study participants, who willingly gave their time for this study.
Authors' Contributions
V.H. and K.R. wrote the article and contributed to data collection and interpretation. L.Z. conducted the statistical analyses and revised the article. K.B.S.M. revised the article and contributed to data collection and interpretation. C.W. analyzed and the interpreted the vascular physiology studies and provided article assistance. S.C., A.D., and H.W. contributed to data collection and interpretation. C.M. assisted with protocol development and statistical analyses. H.T. contributed to protocol development and oversight, and revised the article. A.D.G. designed and oversaw the study, obtained funding, interpreted the data, and wrote the article. A.D.G. is the guarantor of this work, and, as such, had full access to all the study data and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Author Disclosure Statement
A.D.G. previously served on the Speakers' Bureau for AstraZeneca Pharmaceuticals. No competing financial interests exist for any of the other authors.
Funding Information
This work was supported by the Center for Clinical and Translational Sciences, which is funded by National Institutes of Health Clinical and Translational Award UL1 TR000371 and KL2 TR000370 from the National Center for Advancing Translational Research. A.D.G. received support from the above awards. H.T. receives support from R01-HL-61483-15. AstraZeneca provided study medications, but was neither involved in the design or conduct of the study nor in article preparation.
Supplementary Material
References
- 1. Barr EL, Zimmet PZ, Welborn TA, et al. . Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: The Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation 2007;116:151–157 [DOI] [PubMed] [Google Scholar]
- 2. Papaetis GS. Incretin-based therapies in prediabetes: Current evidence and future perspectives. World J Diabetes 2014;5:817–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Su Y, Liu XM, Sun YM, et al. . Endothelial dysfunction in impaired fasting glycemia, impaired glucose tolerance, and type 2 diabetes mellitus. Am J Cardiol 2008;102:497–498 [DOI] [PubMed] [Google Scholar]
- 4. Wasserman DH, Wang TJ, Brown NJ. The vasculature in prediabetes. Circ Res 2018;122:1135–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bae JH, Schwemmer M, Lee IK, et al. . Postprandial hypertriglyceridemia-induced endothelial dysfunction in healthy subjects is independent of lipid oxidation. Int J Cardiol 2003;87:259–267 [DOI] [PubMed] [Google Scholar]
- 6. Temelkova-Kurktschiev TS, Koehler C, Henkel E, et al. . Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1c level. Diabetes Care 2000;23:1830–1834 [DOI] [PubMed] [Google Scholar]
- 7. Marso SP, Bain SC, Consoli A, et al. . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–1844 [DOI] [PubMed] [Google Scholar]
- 8. Marso SP, Daniels GH, Brown-Frandsen K, et al. . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gerstein HC, Colhoun HM, Dagenais GR, et al. . Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 2019;394:121–130 [DOI] [PubMed] [Google Scholar]
- 10. Ansar S, Koska J, Reaven PD. Postprandial hyperlipidemia, endothelial dysfunction and cardiovascular risk: Focus on incretins. Cardiovasc Diabetol 2011;10:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Giannattasio C, Zoppo A, Gentile G, et al. . Acute effect of high-fat meal on endothelial function in moderately dyslipidemic subjects. Arterioscler Thromb Vasc Biol 2005;25:406–410 [DOI] [PubMed] [Google Scholar]
- 12. Lane-Cordova AD, Witmer JR, Dubishar K, et al. . High trans but not saturated fat beverage causes an acute reduction in postprandial vascular endothelial function but not arterial stiffness in humans. Vasc Med 2016;21:429–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vogel RA, Corretti MC, Plotnick GD. Effect of a single high-fat meal on endothelial function in healthy subjects. Am J Cardiol 1997;79:350–354 [DOI] [PubMed] [Google Scholar]
- 14. Raitakari OT, Lai N, Griffiths K, et al. . Enhanced peripheral vasodilation in humans after a fatty meal. J Am Coll Cardiol 2000;36:417–422 [DOI] [PubMed] [Google Scholar]
- 15. Skilton MR, Lai NT, Griffiths KA, et al. . Meal-related increases in vascular reactivity are impaired in older and diabetic adults: Insights into roles of aging and insulin in vascular flow. Am J Physiol Heart Circ Physiol 2005;288:H1404–H1410 [DOI] [PubMed] [Google Scholar]
- 16. van Poppel PC, Netea MG, Smits P, et al. . Vildagliptin improves endothelium-dependent vasodilatation in type 2 diabetes. Diabetes Care 2011;34:2072–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nakamura K, Oe H, Kihara H, et al. . DPP-4 inhibitor and alpha-glucosidase inhibitor equally improve endothelial function in patients with type 2 diabetes: EDGE study. Cardiovasc Diabetol 2014;13:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ayaori M, Iwakami N, Uto-Kondo H, et al. . Dipeptidyl peptidase-4 inhibitors attenuate endothelial function as evaluated by flow-mediated vasodilatation in type 2 diabetic patients. J Am Heart Assoc 2013;2:e003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nandy D, Johnson C, Basu R, et al. . The effect of liraglutide on endothelial function in patients with type 2 diabetes. Diab Vasc Dis Res 2014;11:419–430 [DOI] [PubMed] [Google Scholar]
- 20. Kelly AS, Bergenstal RM, Gonzalez-Campoy JM, et al. . Effects of exenatide vs. metformin on endothelial function in obese patients with pre-diabetes: A randomized trial. Cardiovasc Diabetol 2012;11:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nomoto H, Miyoshi H, Furumoto T, et al. . A comparison of the effects of the GLP-1 analogue liraglutide and insulin glargine on endothelial function and metabolic parameters: A randomized, controlled trial Sapporo Athero-Incretin Study 2 (SAIS2). PLoS One 2015;10:e0135854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Faber R, Zander M, Pena A, et al. . Effect of the glucagon-like peptide-1 analogue liraglutide on coronary microvascular function in patients with type 2 diabetes—A randomized, single-blinded, cross-over pilot study. Cardiovasc Diabetol 2015;14:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koska J, Sands M, Burciu C, et al. . Exenatide protects against glucose and lipid-induced endothelial dysfunction: Evidence for direct vasodilation effect of GLP-1 receptor agonists in humans. Diabetes 2015;64:2624–2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koska J, Schwartz EA, Mullin MP, et al. . Improvement of postprandial endothelial function after a single dose of exenatide in individuals with impaired glucose tolerance and recent-onset type 2 diabetes. Diabetes Care 2010;33:1028–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Torimoto K, Okada Y, Mori H, et al. . Effects of exenatide on postprandial vascular endothelial dysfunction in type 2 diabetes mellitus. Cardiovasc Diabetol 2015;14:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ceriello A, Esposito K, Testa R, et al. . The possible protective role of glucagon-like peptide 1 on endothelium during the meal and evidence for an “endothelial resistance” to glucagon-like peptide 1 in diabetes. Diabetes Care 2011;34:697–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schwartz EA, Koska J, Mullin MP, et al. . Exenatide suppresses postprandial elevations in lipids and lipoproteins in individuals with impaired glucose tolerance and recent onset type 2 diabetes mellitus. Atherosclerosis 2010;212:217–222 [DOI] [PubMed] [Google Scholar]
- 28. Kelley DE, Simoneau JA. Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitus. J Clin Invest 1994;94:2349–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bruno RM, Gori T, Ghiadoni L. Endothelial function testing and cardiovascular disease: Focus on peripheral arterial tonometry. Vasc Health Risk Manag 2014;10:577–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Benjamin N, Calver A, Collier J, et al. . Measuring forearm blood flow and interpreting the responses to drugs and mediators. Hypertension 1995;25:918–923 [DOI] [PubMed] [Google Scholar]
- 31. Joyner MJ, Dietz NM, Shepherd JT. From Belfast to Mayo and beyond: The use and future of plethysmography to study blood flow in human limbs. J Appl Physiol (1985) 2001;91:2431–2441 [DOI] [PubMed] [Google Scholar]
- 32. Wilkinson IB, Webb DJ. Venous occlusion plethysmography in cardiovascular research: Methodology and clinical applications. Br J Clin Pharmacol 2001;52:631–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meier JJ, Gethmann A, Gotze O, et al. . Glucagon-like peptide 1 abolishes the postprandial rise in triglyceride concentrations and lowers levels of non-esterified fatty acids in humans. Diabetologia 2006;49:452–458 [DOI] [PubMed] [Google Scholar]
- 34. Gastaldelli A, Gaggini M, Daniele G, et al. . Exenatide improves both hepatic and adipose tissue insulin resistance: A dynamic positron emission tomography study. Hepatology 2016;64:2028–2037 [DOI] [PubMed] [Google Scholar]
- 35. Steinberg HO, Chaker H, Leaming R, et al. . Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest 1996;97:2601–2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mather KJ, Steinberg HO, Baron AD. Insulin resistance in the vasculature. J Clin Invest 2013;123:1003–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cervera A, Wajcberg E, Sriwijitkamol A, et al. . Mechanism of action of exenatide to reduce postprandial hyperglycemia in type 2 diabetes. Am J Physiol Endocrinol Metab 2008;294:E846–E852 [DOI] [PubMed] [Google Scholar]
- 38. DeFronzo RA, Okerson T, Viswanathan P, et al. . Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: A randomized, cross-over study. Curr Med Res Opin 2008;24:2943–2952 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.