Abstract
Importance:
The association of hypertension with late-life hearing impairment (HI) is unclear, and the relative importance of mid-life versus late-life hypertension is unknown.
Objective:
To determine whether late-life HI is associated with mid-life hypertension
Design:
To investigate the association between hypertension and late-life HI, we used data from the Atherosclerosis Risk in Communities Study, an ongoing prospective, longitudinal study (baseline 1987–89). Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured at each study visit and hypertension defined by SBP, DBP and antihypertensive medication use. A 4-frequency (0.5–4 kHz) better-hearing ear pure-tone average (PTA) in decibels hearing loss (dB HL) calculated from pure tone audiometry measured in late life (2013). A cutoff of 40 dB HL was used to indicate clinically significant moderate-to-severe hearing loss. Hearing thresholds at 5 frequencies (0.5–8 kHz) were also considered separately.
Setting:
Washington County, MD research field site of the Atherosclerosis Risk in Communities Study
Participants:
248 subset of community-dwelling men and women in Washington County, MD from the Atherosclerosis Risk in Communities Study cohort.
Exposure:
Hypertension measured at visit 1 (1987–1989).
Main Outcomes:
HI measured at visit 5 (2011–2013).
Results:
In 248 community-dwelling men and women in Washington County, MD, 47 participants (19%) had hypertension at baseline (1987–89) compared to 183 (74%) at when hearing was measured (2013). The SBP association with late-life PTA differed by the time when SBP was measured, with greater hearing loss associated with SBP measured at earlier visits (difference in PTA per 10mm Hg SBP measured at baseline (1987–89): 1.43 dB HL (95% CI: 0.32, 2.53) vs. a visit in 2013: −0.43 dB HL (95% CI: −1.41, 0.55). Hypertension at baseline was significantly associated with higher thresholds (i.e., with poorer hearing) at 4 tone frequencies (1, 2, 4, 8 kHz).
Conclusions and Relevance:
Mid-life SBP was associated with poorer hearing measured 25 years later which warrants further analysis into the longitudinal relationship between hypertension and HI.
INTRODUCTION
Hearing impairment (HI) is highly prevalent in older adults, affecting two-thirds of the population ages 70 and older.1 While HI can lead to communication difficulties and decreased health-related quality of life,2 an increasing body of evidence links HI with a variety of poor health outcomes including cognitive decline,3,4 physical disability,5–7 falls,8,9 hospitalization,10,11 and mortality.12 HI is responsible for an estimated $3.1 billion in additional medical expenditures in the United States alone.13 Current initiatives at the National Academies of Sciences, Engineering, and Medicine (NAS) and the President’s Council of Advisors on Science and Technology (PCAST) have begun to study hearing loss as a key public health issue for older adults. 14–16
A potential target for mitigation of the adverse public health effects of hearing loss is prevention. Aside from noise exposure, few modifiable risk factors for hearing loss have been identified.17–20 Models of the mechanisms underlying age-related hearing loss have suggested that cochlear circulatory insufficiency such as that caused by vascular disease or hypertension, may affect the inner ear in a way that results in disruption of action potential signal transduction and accelerated cell loss.21,22 The stria vascularis at the basal end of the cochlea, which is responsible for high-frequency sound encoding, may be particularly vulnerable to capillary degeneration and basement membrane thickening due to its distance from the main cochlear blood supply.23 These data suggest that vascular factors may increase risk of hearing loss, but the role of hypertension is unclear. Some studies have identified cross-sectional associations between hypertension and HI, but other studies, both cross-sectional and longitudinal, have not,20,24–26 and the relative importance of mid-life versus late-life hypertension is unknown.
We used data from a hearing pilot study within the Atherosclerosis Risk in Communities (ARIC) study, an ongoing longitudinal prospective study of 15,792 participants in four communities across the United States, to quantify the association between hypertension and HI measured via pure-tone audiometry, and to examine possible differences in that association by timing of hypertension (mid-life vs. late-life). Secondarily, we tested the hypothesis that hypertension is more strongly associated with high-frequency, rather than low-frequency HI.
METHODS
Study Population
ARIC began in the 1980s to examine the risk factors and complications of atherosclerosis. To date, there have been 5 clinic visits for ARIC participants: Visit 1 (1987–89), Visit 2 (1990–92), Visit 3 (1993–95), Visit 4 (1996–98), and Visit 5 (2011–2013). A pilot study collected audiometric data from 255 participants at the Washington County, MD site in 2013. Audiometric testing was offered to 307 ARIC participants presenting for their regularly-scheduled ARIC visit. The last participants scheduled for Visit 5 were invited. Though scheduling corresponded to the order of Visit 1 scheduling (which was randomized), hard-to-schedule participants were likely over-represented. Six did not participate and 46 did not complete the exam (primarily due to impacted cerumen). Compared to other ARIC Visit 5 participants from Washington County, participants in the hearing pilot study were more likely to be female (61% vs. 53%, P=0.01) and to have >high school education (41% vs. 34%, P=0.02).
For the 255 participants with hearing data, 4 were excluded from analysis due to missing hypertension (N=3) and diabetes (N=1) data. Because of the small number of non-white participants in the hearing pilot study (1 Asian and 1 Black), the analysis was restricted to participants self-reporting white race, yielding a final analytic sample of 248 after the exclusion of 1 outlier with very low systolic blood pressure (61 mmHg).
Assessment of blood pressure and hypertension
Blood pressure was measured using a random zero sphygmomanometer (Visits 1–4) or automated sphygmomanometer (Visit5) with 5 minutes of rest before each measurement, and recorded as the average of the second and third of 3 blood pressure measurements within a visit (excluding Visit 4, which was the average of the first and second of 2 measurements). Hypertension was defined according to standardized algorithms and in our primary analyses, defined as systolic blood pressure (SBP) ≥140 mmHg, diastolic blood pressure (DBP) ≥90 mmHg, or antihypertensive use. In secondary analyses, blood pressure was modeled continuously per 10 mmHg.27
Audiometric Assessment
Pure tone air conduction audiometry was conducted by a trained technician during the final year of Visit 5 (2013) in a sound-treated booth in a quiet room using an Interacoustics AD-629 audiometer with insert ER3A earphones. Pure tone audiometry is the gold-standard test to determine the faintest tones a person can detect for a range of frequencies. A speech frequency Pure Tone Average (PTA) in decibels hearing loss (dB HL) was calculated by averaging audiometric thresholds at 0.5, 1, 2, and 4 kilohertz (kHz) in the better-hearing ear,28 with higher PTA’s indicating worse hearing. The primary analysis categorized the PTA using a clinically relevant threshold: normal hearing or mild HI: <40 dB HL vs. moderate/severe HI: ≥40 dB HL. Additionally, PTA was modeled as a continuous variable with higher PTA values indicating greater hearing loss.
Other covariates
Demographic information was collected at Visit 1, including age (years) and sex. Self-reported information on cigarette smoking status was collected at each study visit and classified as never or ever. Diabetes was considered present if fasting (≥ 8 hours) blood glucose level was ≥ 126 mg/dL, nonfasting blood glucose level ≥ 200 mg/dL, or if the participant self-reported a physician diagnosis of or medication use for diabetes.
At each visit, participants were asked to bring all medications they had taken in the past 2 weeks, which were transcribed and coded by trained personnel according to a pre-specified coding system. In analyses of continuous blood pressure, we adjusted for medications coded as having anti-hypertensive effects, although some may have been prescribed off-label rather than for hypertension.
Information on midlife occupation was collected at Visit 1 (1987–89), coded according to the 1980 US Census Alphabetical Index of Industries and Occupations,29 and categorized as for the 1980 US Census:30 managerial and professional specialty occupations; technical, sales, and administrative support occupations; service occupations; farming, forestry, and fishing occupations; precision production, craft, and repair occupations; operators, fabricators, and laborers; homemakers; and retired.31 For analysis, a binary occupation variable was created according to possible noise exposure: farming, forestry, and fishing occupations; precision production, craft, and repair occupations; and operators, fabricators, and laborers vs. all other occupations.
Statistical Analysis
Multivariable logistic and linear regression were used to estimate the association of hypertension measured at Visits 1 and at Visit 5 with HI measured at Visit 5, and of SBP and DBP (per 10 mmHg) at each visit with PTA in the better-hearing ear at Visit 5, respectively. Model assumptions of linearity and constant residual variance were assessed graphically using lowess and residual diagnostic plots and through statistics including the Akaike Information Criterio (AIC), Bayesian Information Criterion (BIC) and likelihood ratio tests. All models were adjusted for age (using both linear and quadratic components), sex, occupation, smoking history and diabetes history. Linear regression models were run with and without adjustment for antihypertensive medication use. In order to test whether the estimated association between SBP or DBP and HI differed based on antihypertensive medication use, an interaction term between hypertension and medication use was included in the models. Because no statistical evidence of interaction was observed (data not shown), the combined estimates are presented. No adjustment was performed for multiple comparisons.
Linear mixed models were used to estimate the association of hypertensive status at a given visit with hearing thresholds at each audiometric test frequency (0.5–8 kHz). Fixed effects of frequency and hypertensive status and their interaction were used to test if audiometric thresholds varied by hypertensive status and how any observed effects vary by frequency. Models were adjusted for age, midlife occupation, sex, smoking and diabetes, and accounted for repeated measures within subjects using random intercept and slopes with an unstructured correlation matrix for residuals. Time-varying covariates included in the models were measured at the same time as hypertensive status.
Data were analyzed using Stata (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP) and R version 3.2.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Of the 248 individuals in the analytic cohort, 47 (19%) had hypertension at Visit 1, and 183 (74%) had hypertension at Visit 5 (Table 1). Compared to participants without hypertension at Visit 5, participants with hypertension at Visit 5 were more likely to have diabetes (38% vs. 22%) (Table 1). 83 (33%) of participants had hearing impairment at Visit 5. Compared to participants without HI, participants with HI were older (56 vs. 52 years at Visit 1), more likely to be male (54% vs. 31%) and more likely to have diabetes at Visit 1 (10% vs. 4%) (Table 1).
Table 1.
Demographic Characteristics by Hypertension,a Hearing Impairment,b and Visit, Atherosclerosis Risk in Communities Study, N=248
| Hypertension at Visit 1 | Hypertension at Visit 5 | Hearing Impairment at Visit 5 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No (N=201) | Yes (n=47) | P-valuec | No (N=65) | Yes (N=183) | P-valuec | No (n=165) | Yes (N=83) | P-valuec | |
| Age (years) | Mean (SD) | Mean (SD) | Mean (SD) | ||||||
| Visit 1 | 53.4 (5.3) | 54.1 (5.1) | 0.40 | 52.6 (5.3) | 53.9 (5.2) | 0.10 | 52.3 (4.7) | 56.0 (5.4) | <0.001 |
| Visit 5 | - | - | - | 76.2 (5.5) | 77.7 (5.3) | 0.07 | 76.0 (4.8) | 79.7 (5.7) | <0.001 |
| N (%) | N (%) | N (%) | |||||||
| Sex (male) | 73 (36) | 23 (49) | 0.11 | 27 (42) | 69 (38) | 0.59 | 51 (31) | 45 (54) | <0.001 |
| Education (< high school) | 29 (14) | 6 (13) | 0.77 | 9 (14) | 26 (14) | 0.94 | 21 (13) | 14 (17) | 0.38 |
| Smoking status (ever) | |||||||||
| Visit 1 | 84 (42) | 21 (45) | 0.72 | 23 (36) | 82 (45) | 0.19 | 67 (41) | 38 (46) | 0.44 |
| Visit 5 | - | - | - | 27 (42) | 91 (50) | 0.26 | 75 (46) | 43 (52) | 0.35 |
| Diabetes | |||||||||
| Visit 1 | 12 (6) | 2 (4) | 0.65 | 4 (6) | 10 (6) | 0.84 | 6 (4) | 8 (10) | 0.05 |
| Visit 5 | - | - | - | 14 (22) | 70 (38) | 0.01 | 51 (31) | 33 (40) | 0.17 |
| Occupation (manufacturing/laborers) | 41 (20) | 10 (22) | 0.89 | 11 (17) | 40 (22) | 0.40 | 32 (19) | 19 (23) | 0.52 |
| Household Income (< $25,000) | |||||||||
| Visit 1 | 44 (23) | 7 (15) | 0.24 | 17 (28) | 34 (19) | 0.14 | 35 (22) | 16 (21) | 0.81 |
| Visit 5 | 52 (28) | 9 (22) | 0.43 | 14 (24) | 47 (28) | 0.59 | 40 (27) | 21 (27) | 0.92 |
Hypertension defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or antihypertensive medicatio n use.
Hearing impairment was measured at Visit 5 and defined as a pure tone average of 40 dB or greater in the better hearing ear
P-values calculated with t-test for age and fisher exact test for all other characteristics
After full adjustment, the estimated association of hypertension with HI was positive but not statistically significant: Odds ratio (OR) = 1.67 (95% CI: 0.82, 3.40) at Visit 1 and 1.81 (95% CI: 0.89, 3.66) at Visit 5 (Table 2).
Table 2.
Multivariable-adjusted a Association of Hypertension with Hearing Impairment,b Atherosclerosis Risk in Communities Study, N=248
| Hypertension at Visit 1 | Hypertension at Visit 5 | |||||
|---|---|---|---|---|---|---|
| Hypertension (binary)c | N | OR (95% CI) | P-value | N | OR (95% CI) | P-value |
| Normotensive | 201 | ref | -- | 65 | ref | -- |
| Hypertensive | 47 | 1.67 (0.82, 3.40) | 0.16 | 183 | 1.81 (0.89, 3.66) | 0.10 |
| Blood pressure (continuous)e | ||||||
| SBP per 10 mmHg | 248 | 1.18 (0.96, 1.46) | 0.11 | 245 | 0.95 (0.79, 1.13) | 0.55 |
| DBP per 10 mmHg | 248 | 1.09 (0.77, 1.52) | 0.63 | 245 | 0.98 (0.73, 1.32) | 0.88 |
Adjusted for age, age2, sex, occupation, smoking history, and diabetes history
Hearing impairment was measured at Visit 5 and defined as a four-frequency pure tone average of 40 dB or greater in the better hearing ear
Hypertension defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥90 mmHg, or antihypertensive use
When hearing was modeled continuously as the better-hearing ear PTA, we observed a significant positive relationship between SBP and PTA when SBP was measured in mid-life (Visits 1–3) (Figure 1). In other words, higher midlife SBP was associated with poorer late-life hearing. The strength of this relationship decreased in later visits, and was not significant for SBP measured in Visits 4 and 5. After adjustment, differences in PTA per 10mm Hg SBP were: Visit 1 (1987–89): 1.43 dB HL (95% CI: 0.32, 2.53), Visit 2 (1990–92): 0.99 dB HL (95% CI: −0.04, 2.03), Visit 3 (1993–95): 1.10 dB HL (95% CI: 0.07, 2.14), Visit 4 (1996–98): 0.44 dB HL (95% CI: −0.63, 1.52), and Visit 5 (2011–13): −0.43 dB HL (95% CI: −1.41, 0.55). Additional adjustment for antihypertensive medication use did not alter estimates (Figure 1), and an interaction term between hypertension and medication use did not reach statistical significance. Associations between DBP and better-hearing ear PTA were similar in magnitude to SBP, but none reached statistical significance (Figure 1).
Figure 1.
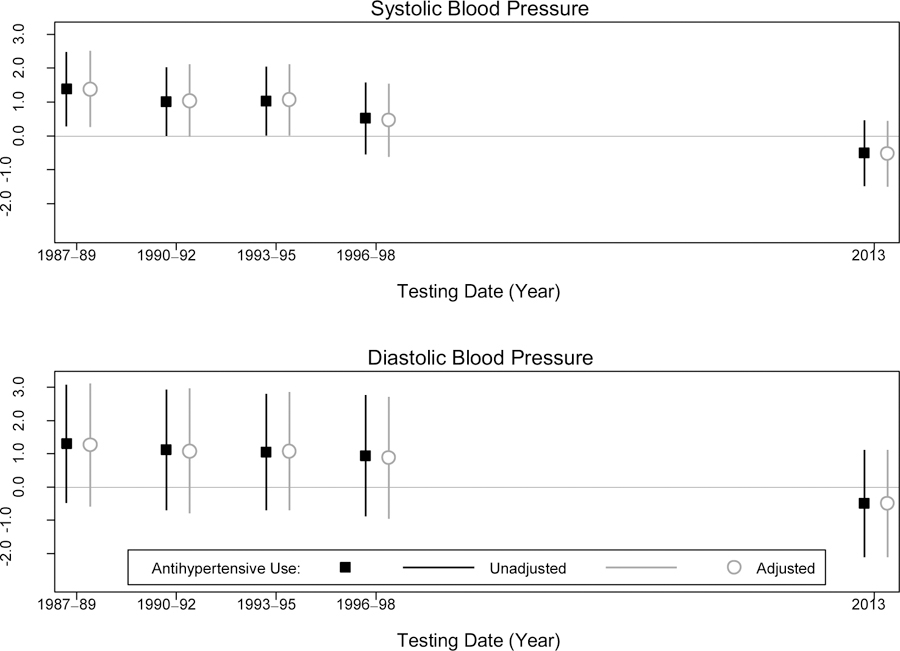
Multivariable-adjusted Association Between Blood Pressure (per 10 mmHg) at each Study Visit and Better-Hearing Ear Pure Tone Average (db HL) in 2013, Atherosclerosis Risk in Communities Study, N=248
Adjusted for age, age2, sex, occupation, smoking status and diabetes status. Results unadjusted for antihypertensive use are indicated with squares. Results adjusted for antihypertensive use are indicated with circles.
Hypertension at Visit 1 was significantly associated with higher thresholds (i.e., with poorer hearing) at 4 tone frequencies: 1 kHz (3.4 dB HL [95% CI: 0.7, 6.1]), 2 kHz (4.2 dB HL [95% CI: −0.4, 8.8]), 4 kHz (7.7 dB HL [95% CI: 2.4, 12.9]), and 8 kHz (9.0 dB HL [95% CI: 3.1, 14.9]) (Table 3). Estimated hypertension-HI associations were greater at higher frequencies, and the p-value for the interaction to test whether the association between HI and hearing differed by frequency was significant at Visit 1 (P=0.006) (Table 3). There were no significant associations of Visit 5 hypertension with hearing thresholds.
Table 3.
Multivariable-adjusted Estimated Differencesa in Hearing Thresholds Comparing Participants with and without Hypertension,b Atherosclerosis Risk in Communities Study, N=248
| Frequency (kHz) | Hypertension at Visit 1 | Hypertension at Visit 5 | ||
|---|---|---|---|---|
| Estimate [dB HL] (95% CI) | P-value | Estimate [dB HL] (95% CI) | P-value | |
| 0.5 | −2.57 (−6.65, 1.52) | 0.219 | 2.48 (−1.13, 6.10) | 0.179 |
| 1 | 3.39 (0.71, 6.06) | 0.013 | 1.40 (−1.00, 3.81) | 0.252 |
| 2 | 4.18 (−0.42, 8.78) | 0.075 | 1.18 (−2.92, 5.28) | 0.572 |
| 4 | 7.68 (2.41, 12.95) | 0.004 | −1.91 (−6.63, 2.81) | 0.428 |
| 8 | 9.01 (3.12, 14.89) | 0.003 | 0.55 (−4.74, 5.85) | 0.838 |
| P-interactionc | 0.006 | 0.309 | ||
Estimated from linear mixed effects models
Hearing impairment was measured at Visit 5. Hypertension defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥90 mmHg, or antihypertensive use. Model adjusts for age, sex, education, smoking status and diabetes status
P-interaction to test whether the association between hypertension and hearing loss differs by frequency
DISCUSSION
In 248 men and women from Washington County, MD, we found that higher midlife SBP (Visits 1–3) was associated with poorer hearing in late-life (estimates of the difference in PTA associated with increasing systolic blood pressure ranging from 1.01–1.42 dB HL per 10 mmHg). These associations persisted with additional adjustment for antihypertensive therapy. Associations between DBP and PTA were similar in magnitude to those observed for SBP and PTA, but unlike SBP, were not statistically significant. This null finding could be due to limited sample size, or could perhaps be indicative of the adverse vascular effects of elevated pulse pressure, although we did not directly test this hypothesis.
By contrast, we did not find statistical evidence for an association between continuous blood pressure and hearing impairment (defined as a PTA categorized at ≥40 dB HL) (Table 2). Given the relatively small number of participants in this pilot study (83 with moderate or severe hearing impairment), we may have been underpowered to detect this difference with the categorical outcome.
As with our study, previous studies have failed to find a cross-sectional relationship between hypertension and hearing loss in older adults.20,24 By contrast, a study of 1,662 participants from the Framingham Heart Study documented a cross-sectional, age-adjusted association between prevalent hypertension and high-frequency (average of 4, 6, and 8 kHz) hearing impairment in men (N=676) but not in women (better-hearing ear PTA in men with and without hypertension: 24.7 vs. 22.6 dB HL, p=0.01).25 For that analysis, however, hypertension was defined according to higher blood pressure cutpoints than in our study: systolic blood pressure > 160 mmHg and diastolic blood pressure > 95 mmHg.25
Results from studies that have evaluated the relationship between continuous blood pressure and hearing loss are not consistent.26,33 In the Framingham Heart Study, SBP and DBP, modeled as an average of blood pressure values measured over 6 biennial visits, were both associated with greater PTA (hearing loss) in women, but not in men; in women, higher SBP was associated with low-frequency (but not high-frequency) hearing loss, as compared to DBP, which was only significantly associated with high-frequency hearing loss.25 In 1,984 men and women enrolled in the Beaver Dam Offspring Study aged 21–79 years at baseline, SBP was not associated with incident hearing impairment (age- and sex-adjusted RR: 1.03, 95% CI: 0.95, 1.12). However, participants were followed for only 5 years and the study’s definition of hearing impairment included mild loss (>25 dB hearing level) as compared to our study, which defined hearing impairment as a moderate or severe loss (PTA >40 dB hearing level).
In contrast to prior studies, we evaluated the association between midlife blood pressure and late-life hearing impairment up to 25 years later, and in our threshold analysis, utilized novel statistical methods (linear mixed models) that both account for within-person correlation of hearing thresholds at different frequencies, and allow for hypothesis testing of the differential associations of hypertension with hearing thresholds by frequency.
The pathophysiology of hearing impairment is multifactorial, with both intrinsic (e.g. hypertension) and extrinsic (e.g. noise exposure) factors.22,34,35 Impaired circulation (perhaps associated with hypertension) to the highly-vascularized stria vascularis has previously been proposed as an indirect mechanism of HI.21,36 Normal strial function is required to maintain inner ear ion homeostasis for endocochlear potential transmission. The loss of strial tissue disrupts K+ production and recycling, resulting in an impairment of signal transduction, and possibly increasing free-radical production in the inner ear.22,35,37 Furthermore, the stria vascularis at the high-frequency encoding portion of the cochlea may be particularly vulnerable to significant capillary degeneration and basement membrane thickening due to distance from the main cochlear blood supply.23 This combination of changes in homeostasis and oxidative stress in a hypoxic environment secondary to hypertension may enhance the negative effect of extrinsic factors such as excessive noise exposure and result in accelerated high-frequency HI, an area of the tonotopically organized cochlea already susceptible to age-related HI. Consistent with our a priori hypotheses based on the biology, we observed a strong monotonic relationship between mid-life hypertension and hearing impairment at higher frequencies.
With these potential mechanisms, it is not surprising that damage to the cochlea may take many years to develop. Mid-life SBP was associated with higher PTA (i.e., poorer hearing) in late-life in our study, and the association between late-life SBP and hearing impairment was weaker at Visit 4 and even inverse (not significant) when measured concurrently, perhaps because low blood pressure in late life represents a mix of persons with lifelong favorable blood pressure levels, and those whose blood pressure has recently fallen because of underlying comorbidity or functional decline which may also cause hearing loss.
There are several limitations to this study. The generalizability of the results may be limited due to the single-center, all-white study cohort. Hearing data was not collected until 2013. Because hypertension was first measured 25 years earlier, presumably hypertension preceded the development of HI for most participants, but the prevalence of mild to moderate HI (bilateral and unilateral) in adults in the US aged 50–59 years is 29%.1 As the mean baseline age for this analysis was 53 years, we cannot establish temporality of exposure and outcome with this study design, although reverse causation (i.e., hearing loss causing hypertension) is unlikely to be a concern in this study. Additionally, because ARIC participants from Washington County with hypertension at Visit 1 were more likely than participants without hypertension to die or be lost to follow-up (67% vs. 51%, respectively, data not shown), the associations we estimated may be attenuated compared to associations if hearing had been measured more frequently (i.e., participants with higher blood pressure in Visits 1–4 may have been more likely than participants without hypertension to die earlier or to be lost to follow-up, but if they had attended Visit 5, may have been more likely to have hearing impairment).
If causal, the association of HI with mid-life hypertension could possibly suggest that better lifelong control of hypertension may be protective for late-life HI. In our analysis, antihypertensive medication use did not modify the association of blood pressure and PTA. However, given the influence of selection factors related to medication use in observational studies, randomized studies designed specifically to study risk modification would be necessary to conclude that blood pressure control in mid-life is protective for late-life HI. Additionally, the potential mechanisms through which elevated blood pressure may damage hearing mimic those of other diseases, supporting that lifelong control of hypertension contributes to health maintenance.
In conclusion, increased mid-life SBP was associated with poorer hearing in late-life. Increased blood pressure, particularly in mid-life, may be a risk factor for hearing loss in older adults.
KEY POINTS.
Question:
What is association between hypertension at mid- and late life with late life peripheral hearing loss?
Findings:
Among a community dwelling cohort of 248 adults, systolic blood pressure at mid-life was significantly associated with hearing loss measured 25 years later in life.
Meaning:
Hypertension may contribute to age-related hearing impairment and further study into the contribution to the onset and degree of presbycusis is warranted.
ACKNOWLEDGEMENTS
FUNDING
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Collection of pilot audiometric data was supported by National Institute on Deafness and Other Communicative Disorders K23DC011279 and the Eleanor Schwartz Charitable Foundation. The authors thank the staff and participants of the ARIC study for their important contributions.
Footnotes
No relevant conflicts of interest.
REFERENCES
- 1.Lin FR. Hearing Loss Prevalence in the United States. Arch Intern Med. 2011;171(20):1851 10.1001/archinternmed.2011.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joo Y-H, Han K, Park KH. Association of Hearing Loss and Tinnitus with Health-Related Quality of Life: The Korea National Health and Nutrition Examination Survey. Malmierca MS, ed. PLOS ONE. 2015;10(6):e0131247 10.1371/journal.pone.0131247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin FR, Yaffe K, Xia J, et al. Hearing Loss and Cognitive Decline in Older Adults. JAMA Intern Med. 2013;173(4):293 10.1001/jamainternmed.2013.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deal JA, Sharrett AR, Albert MS, et al. Hearing Impairment and Cognitive Decline: A Pilot Study Conducted Within the Atherosclerosis Risk in Communities Neurocognitive Study. Am J Epidemiol. 2015;181(9):680–690. 10.1093/aje/kwu333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen DS, Betz J, Yaffe K, et al. Association of Hearing Impairment with Declines in Physical Functioning and the Risk of Disability in Older Adults. J Gerontol A Biol Sci Med Sci. 2015;70(5):654–661. 10.1093/gerona/glu207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strawbridge WJ, Wallhagen MI, Shema SJ, Kaplan GA. Negative Consequences of Hearing Impairment in Old Age A Longitudinal Analysis. The Gerontologist. 2000;40(3):320–326. 10.1093/geront/40.3.320. [DOI] [PubMed] [Google Scholar]
- 7.Deal JA, Richey Sharrett A, Bandeen-Roche K, et al. Hearing Impairment and Physical Function and Falls in the Atherosclerosis Risk in Communities Hearing Pilot Study. J Am Geriatr Soc. 2016;64(4):906–908. 10.1111/jgs.14075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin FR. Hearing Loss and Falls Among Older Adults in the United States. Arch Intern Med. 2012;172(4):369 10.1001/archinternmed.2011.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viljanen A, Kaprio J, Pyykko I, et al. Hearing as a Predictor of Falls and Postural Balance in Older Female Twins. J Gerontol A Biol Sci Med Sci. 2009;64A(2):312–317. 10.1093/gerona/gln015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Genther DJ, Betz J, Pratt S, et al. Association Between Hearing Impairment and Risk of Hospitalization in Older Adults. J Am Geriatr Soc. 2015;63(6):1146–1152. 10.1111/jgs.13456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Genther DJ, Frick KD, Chen D, Betz J, Lin FR. Association of Hearing Loss With Hospitalization and Burden of Disease in Older Adults. JAMA. 2013;309(22):2322 10.1001/jama.2013.5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Genther DJ, Betz J, Pratt S, et al. Association of Hearing Impairment and Mortality in Older Adults. J Gerontol A Biol Sci Med Sci. 2015;70(1):85–90. 10.1093/gerona/glu094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foley DM, Frick KD, Lin FR. Association Between Hearing Loss and Healthcare Expenditures in Older Adults. J Am Geriatr Soc. 2014;62(6):1188–1189. 10.1111/jgs.12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hearing Loss and Healthy Aging: Workshop Summary. http://www.nap.edu/openbook.php?record_id=18735. Accessed August 21, 2015. [PubMed]
- 15.President’s Council of Advisors on Science and Technology. PCAST Report on Hearing Technologies. The White House. https://www.whitehouse.gov/node/8725. Published October 26, 2015. Accessed December 9, 2015.
- 16.Committee on Accessible and Affordable Hearing Health Care for Adults, Board on Health Sciences Policy, Health and Medicine Division, National Academies of Sciences, Engineering, and Medicine Hearing Health Care for Adults: Priorities for Improving Access and Affordability. (Blazer DG, Domnitz S, Liverman CT, eds.). Washington, D.C.: National Academies Press; 2016. http://www.nap.edu/catalog/23446. Accessed June 8, 2016. [PubMed] [Google Scholar]
- 17.Helzner EP, Cauley JA, Pratt SR, et al. Race and Sex Differences in Age-Related Hearing Loss: The Health, Aging and Body Composition Study: HEALTH ABC: HEARING LOSS RISK FACTORS. J Am Geriatr Soc. 2005;53(12):2119–2127. 10.1111/j.1532-5415.2005.00525.x. [DOI] [PubMed] [Google Scholar]
- 18.Brant LJ, Gordon-Salant S, Pearson JD, et al. Risk factors related to age-associated hearing loss in the speech frequencies. J Am Acad Audiol. 1996;(7):152–60. [PubMed] [Google Scholar]
- 19.Cruickshanks KJ, Klein R, Klein BEK, Wiley TL, Nondahl DM, Tweed TS. Cigarette Smoking and Hearing Loss: The Epidemiology of Hearing Loss Study. JAMA. 1998;279(21):1715 10.1001/jama.279.21.1715. [DOI] [PubMed] [Google Scholar]
- 20.Cruickshanks KJ, Nondahl DM, Dalton DS, et al. Smoking, Central Adiposity, and Poor Glycemic Control Increase Risk of Hearing Impairment. J Am Geriatr Soc. 2015;63(5):918–924. 10.1111/jgs.13401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuknecht HF, Watanuki K, Takahashi T, et al. Atrophy of the stria vascularis, a common cause for hearing loss. The Laryngoscope. 1974;84(10):1777–1821. 10.1288/00005537-197410000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Ohlemiller KK. Mechanisms and genes in human strial presbycusis from animal models. Brain Res. 2009;1277:70–83. 10.1016/j.brainres.2009.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gates GA, Mills JH. Presbycusis. Lancet Lond Engl. 2005;366(9491):1111–1120. 10.1016/S0140-6736(05)67423-5. [DOI] [PubMed] [Google Scholar]
- 24.Lin FR, Thorpe R, Gordon-Salant S, Ferrucci L. Hearing Loss Prevalence and Risk Factors Among Older Adults in the United States. J Gerontol A Biol Sci Med Sci. 2011;66A(5):582–590. 10.1093/gerona/glr002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gates GA, Cobb JL, D’Agostino RB, Wolf PA. The relation of hearing in the elderly to the presence of cardiovascular disease and cardiovascular risk factors. Arch Otolaryngol Neck Surg. 1993;119(2):156–161. [DOI] [PubMed] [Google Scholar]
- 26.Fischer ME, Schubert CR, Nondahl DM, et al. Subclinical atherosclerosis and increased risk of hearing impairment. Atherosclerosis. 2015;238(2):344–349. 10.1016/j.atherosclerosis.2014.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gottesman RF, Schneider ALC, Albert M, et al. Midlife Hypertension and 20-Year Cognitive Change: The Atherosclerosis Risk in Communities Neurocognitive Study. JAMA Neurol. 2014;71(10):1218 10.1001/jamaneurol.2014.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO | Grades of hearing impairment. http://www.who.int/pbd/deafness/hearing_impairment_grades/en/. Accessed July 9, 2015.
- 29.US Bureau of the Census. 1980 Census of Population: Alphabetical Index of Industries and Occupations. Final Edition. Washington, DC: US GPO, 1982. (Publication no. PHC80-R3). [Google Scholar]
- 30.US Bureau of the Census. 1980 Census of Population: Classified Index of Industries and Occupations. Final Edition. Washington, DC: US GPO, 1982. (Publication no. PHC80-R4). [Google Scholar]
- 31.Diez-Roux AV, Nieto FJ, Tyroler HA, Crum LD, Szklo M. Social inequalities and atherosclerosis. The atherosclerosis risk in communities study. Am J Epidemiol. 1995;141(10):960–972. [DOI] [PubMed] [Google Scholar]
- 32.Lin BM, Curhan SG, Wang M, Eavey R, Stankovic KM, Curhan GC. Hypertension, Diuretic Use, and Risk of Hearing Loss. Am J Med. 2016;129(4):416–422. 10.1016/j.amjmed.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Helzner EP, Patel AS, Pratt S, et al. Hearing Sensitivity in Older Adults: Associations with Cardiovascular Risk Factors in the Health, Aging and Body Composition Study: HEARING SENSITIVITY AND CVD. J Am Geriatr Soc. 2011;59(6):972–979. 10.1111/j.1532-5415.2011.03444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schuknecht HF, Gacek MR. Cochlear pathology in presbycusis. Ann Otol Rhinol Laryngol. 1993;102(1 Pt 2):1–16. [DOI] [PubMed] [Google Scholar]
- 35.Yamasoba T, Lin FR, Someya S, Kashio A, Sakamoto T, Kondo K. Current concepts in age-related hearing loss: Epidemiology and mechanistic pathways. Hear Res. 2013;303:30–38. 10.1016/j.heares.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chisolm TH, Willott JF, Lister JJ. The aging auditory system: anatomic and physiologic changes and implications for rehabilitation. Int J Audiol. 2003;42 Suppl 2:2S3–10. [PubMed] [Google Scholar]
- 37.Mills DM, Schmiedt RA. Metabolic presbycusis: differential changes in auditory brainstem and otoacoustic emission responses with chronic furosemide application in the gerbil. J Assoc Res Otolaryngol JARO. 2004;5(1):1–10. 10.1007/s10162-003-4004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


