Abstract
Controlled substance lock-in programs are garnering increased attention from payers and policy makers seeking to combat the epidemic of opioid misuse. These programs require high-risk patients to visit a single prescriber and pharmacy for coverage of controlled substance medication services. Despite high prevalence of the programs in Medicaid, we know little about their effects on patients’ behavior and outcomes aside from reducing controlled substance–related claims. Our study was the first rigorous investigation of lock-in programs’ effects on out-of-pocket controlled substance prescription fills, which circumvent the programs’ restrictions and mitigate their potential public health benefits. We linked claims data and prescription drug monitoring program data for the period 2009–12 for 1,647 enrollees in North Carolina Medicaid’s lock-in program and found that enrollment was associated with a roughly fourfold increase in the likelihood and frequency of out-of-pocket controlled substance prescription fills. This finding illuminates weaknesses of lock-in programs and highlights the need for further scrutiny of the appropriate role, optimal design, and potential unintended consequences of the programs as tools to prevent opioid abuse.
The United States is grappling with a public health crisis of prescription drug abuse fueled by the use of high-risk controlled substance medications—namely, opioids and benzodiazepines.1 Opioid-related prescribing,2–4 substance use disorders,5,6 emergency department visits,7 and overdose deaths5,8 increased dramatically during the 2000s. In North Carolina, opioid deaths more than tripled from 1999 to 2014.9 Nationally, opioids and benzodiazepines were implicated in 18,893 and 7,945 fatal overdoses in 2014, respectively.10 This epidemic is estimated to cost over $50 billion annually.11
Policies that enhance monitoring of risky use of controlled substances or restrict high-risk patients’ access to prescriptions for these medications constitute a major component of an “all-hands-on-deck approach” to combating prescription drug misuse and abuse.12 One such policy is the controlled substance lock-in program.
Lock-in programs are common in Medicaid: At least forty-six state Medicaid programs are currently operating such a program.13 Lock-in programs attempt to mitigate the unsafe use of controlled substances by requiring enrolled patients to seek controlled substance services and prescriptions from, typically, a single prescriber and dispensing pharmacy—in other words, patients are “locked in” to using certain prescribers and pharmacies if they want Medicaid to help pay for their controlled substance prescriptions.14
Patients typically become eligible for enrollment in a lock-in program if they exceed a pre-defined threshold of controlled substance prescription fills, a threshold of controlled substance providers, or both in a specified time period. Restricting access to prescribers and pharmacies is intended to enhance care coordination for these high-risk patients and curb provider and pharmacy “shopping,” which contributes to controlled substance abuse, overdose, and diversion (the transfer of a drug from the person for whom it was precribed to another person, for the purpose of misuse).
Momentum is building for the broader implementation of lock-in programs. Most notably, the Comprehensive Addiction and Recovery Act of 2016 establishes such programs in all Medicare Part D plans, beginning in January 2019.
Despite the prevalence of lock-in programs and increased attention to them from policy makers, very little is known about the programs’ effectiveness in improving public health outcomes related to prescription drug abuse.13 What little is known about the programs comes almost exclusively from the grey literature, including non-peer-reviewed internal evaluations of Medicaid programs. This evidence focuses primarily on economic outcomes and shows lock-in programs to be highly effective at reducing claims for controlled substance prescriptions and lowering Medicaid expenditures.15–18
However, these analyses used only Medicaid claims data to examine programmatic outcomes and did not account for controlled substance prescription fills that circumvented the lock-in program. Such circumvention is possible because of an inherent design flaw in the programs: Enrolled patients can avoid enforcement of the lock-in restrictions by paying the full price out of pocket for controlled substance prescription fills at a pharmacy to which they are not locked in.
Administrators of Medicaid lock-in programs in most states are unaware that these circumvented fills of controlled substance prescriptions occur because the fills do not generate a Medicaid prescription claim. To the extent that circumvention happens, it undermines the mechanism through which the lock-in program is thought to prevent prescription drug abuse and overdose. That is, circumvention represents a failure to maintain care coordination between an enrollee’s lock-in prescriber and pharmacy and a failure to prevent access to prescriptions for high-risk controlled substances among patients motivated to abuse or divert them.
A 2014 qualitative study found that North Carolina pharmacists reported significant concerns about Medicaid circumvention among lock-in program enrollees.19 A recent descriptive study of fifty-seven enrollees in Michigan’s Medicaid lock-in program provided initial evidence for the existence of lock-in program circumvention.20 However, there is a need for additional evidence regarding both the prevalence of circumvention in lock-in program populations and whether and to what extent the programs’ restrictions induce patients to engage in it. This knowledge is necessary to assess the potential threat that such behavior poses to the programs’ effectiveness in preventing prescription drug abuse and overdose.
The purpose of our study was to examine Medicaid claims data and data from North Carolina’s prescription drug monitoring program, to generate new evidence of circumvention behavior in lock-in programs. We hypothesized that the lock-in program’s prescriber and pharmacy restrictions would be associated with increased likelihood and rates of circumvented out-of-pocket controlled substance prescription purchases among enrollees. Our findings were intended to establish a needed evidence base about lock-in programs, identify potential opportunities for improving the programs’ operations, and clarify an appropriate role for the programs in combating the prescription drug abuse epidemic.
Study Data And Methods
NORTH CAROLINA MEDICAID LOCK-IN PROGRAM
The North Carolina Medicaid lock-in program was implemented in October 2010. Medicaid beneficiaries in the state were eligible to enroll in the program if they had filled at least seven opioid claims or at least seven benzodiazepine claims, or had claims from at least four unique prescribers of these medications, in two calendar months. Beneficiaries who had dual Medicare and Medicaid coverage or had cancer were not enrolled in the lock-in program. Enrolled beneficiaries were locked in for twelve months to one prescriber and one pharmacy of their choosing for Medicaid coverage of opioid and benzodiazepine prescriptions. After twelve months, enrollees were evaluated for reenrollment.
Program implementation was phased in over eighteen months. Patients with the most excessive use of controlled substances and prescribers were prioritized for early enrollment.
DATA
We conducted a retrospective cohort study with repeated monthly measures. We used a unique data set that linked North Carolina Medicaid claims data with records from the state’s prescription drug monitoring program, known as the Controlled Substances Reporting System. The data spanned a three-year period from October 2009 through September 2012.
North Carolina Medicaid claims included records of paid prescription, outpatient, inpatient, and emergency care services; Medicaid enrollment; and lock-in program enrollment, as well as detailed demographic information. Data from the Controlled Substances Reporting System provided detailed prescription-level information for nearly all controlled substance prescriptions dispensed from community and outpatient pharmacies in the state. The system failed to capture 6.6 percent of Medicaid prescription claims from our study cohort. These missing data did not appear to be systematic, and we assumed that they caused only a slight underestimation of our primary outcome (for a further description of missing prescription claims, see the online Appendix).21
Linking North Carolina Medicaid claims data and Controlled Substances Reporting System records allowed us to measure lock-in program circumvention behavior because the Controlled Substances Reporting System captured controlled substance prescription fills purchased using any payment source, including Medicaid coverage and out-of-pocket spending. A research assistant at the North Carolina Division of Medical Assistance linked the data by using a protocol to manually match enrollees in North Carolina’s Medicaid lock-in program to their records in the Controlled Substances Reporting System records via names, birth dates, and addresses. Manual linkage was necessary because the two data sources did not use the same patient identifiers and because North Carolina law required that each patient record in the Controlled Substances Reporting System be queried individually. The linked data were deidentified before they were delivered to us for analysis.
We obtained permission to query the system’s data for research purposes from system administrators. Our study was approved by the Institutional Review Board at the University of North Carolina at Chapel Hill.
ANALYTIC SAMPLE
The study sample consisted of North Carolina Medicaid beneficiaries who were enrolled in the state’s lock-in program during its first eighteen months of operation. We excluded people who were younger than eighteen or older than sixty-four at any point during their observation period. We required subjects to have continuous North Carolina Medicaid coverage from the beginning of the study period, on October 1, 2009, through at least six months following their enrollment in the lock-in program (for a further description of the continuous Medicaid coverage requirement, see the Appendix).21 This allowed us to observe circumvention behavior for a full year before the implementation of the lock-in program and to measure subjects’ characteristics during a twelve-month baseline period. It also ensured that we followed subjects for at least six months after their enrollment in the lock-in program. Postenrollment observation ended when a subject had a gap in North Carolina Medicaid coverage, his or her twelve-month program enrollment period ended, or the study period ended—whichever occurred first.
MEASURES
Our outcome variables were measured monthly and included a binary indicator of any circumvented controlled substance prescription fill and a count measure of the number of such circumvented fills. A circumvented controlled substance prescription fill was defined as a record of an opioid or benzodiazepine prescription fill in the reporting system data that lacked a corresponding claim in the Medicaid data—which indicated that the fill had been paid for out of pocket despite the patient’s having Medicaid coverage. Only those controlled substance medications subject to the Medicaid lock-in program restrictions were assessed in our outcome measures (for further details about drug product selection in our study, please see the Appendix).21 The key independent variable was an indicator of whether or not a subject was enrolled in the lock-in program in a given month.
We measured subject characteristics such as age, sex, race, residence in a metropolitan versus nonmetropolitan county, and the number of community pharmacies in the subject’s county of residence. Because neither data source could capture the use of controlled substance prescriptions outside the state, we also measured residence in a county bordering another state, to control for a subject’s likelihood of having un-observed prescription fills across state lines.
Subjects’ baseline clinical characteristics included the following: score on the Charlson Comorbidity Index, which quantifies patients’ comorbid disease burden;22 prescription drug burden, defined as the number of unique medications received during the baseline period in Medicaid claims data; and indicators of baseline diagnoses of chronic noncancer pain, anxiety disorder, substance use disorder, depression, or any other mental illness.23 These diagnoses represented conditions for which opioids and benzodiazepines are commonly prescribed or are known predictors of problematic use of controlled substances. We also created variables indicating which lock-in program eligibility criteria a subject satisfied.
We used multiple measures to account for time and the phased-in implementation of the lock-in program. The measures included monthly indicators denoting periods before or after lock-in program implementation and whether or not an observation was during a period when a subject’s lock-in enrollment was delayed despite the subject’s having become eligible for the program. We accounted for the effects of time as months across the entire study period, months across the program’s period of operation, months of delayed enrollment, and months since enrollment.
STATISTICAL ANALYSIS
We used descriptive statistics to summarize the characteristics of the study cohort. We used bivariate analyses to compare opioid and benzodiazepine use, both circumvented and covered by Medicaid, before and after enrollment in the lock-in program.
Generalized estimating equations modeled the associations of lock-in program enrollment with both the likelihood of obtaining an opioid or benzodiazepine prescription through circumvention in a given month and the estimated number of monthly circumvented controlled substance prescription fills, controlling for subject characteristics and policy and time covariates (for details on the model specification, see Appendix Exhibit A1).21 In addition, generalized estimating equations leveraged our repeated monthly measure study design to estimate population-average effects of independent variables on our outcomes while accounting for within-subject correlation over time.
LIMITATIONS
Our findings should be interpreted in light of several limitations. First, our findings might not be generalizable to other payer populations or other Medicaid lock-in programs, which often vary in beneficiary characteristics, program design, and size.13 Specifically, the North Carolina Medicaid lock-in program uses high thresholds of controlled substance use to determine eligibility, which may mean that its enrollees are more likely to circumvent its restrictions, compared to enrollees in a program with lower eligibility thresholds.
Second, we required study subjects to have an extended period of continuous North Carolina Medicaid coverage to enable longitudinal analysis of controlled substance use before and after enrollment in the lock-in program and during any periods of delayed enrollment. Therefore, our findings reflect the experiences of subjects with long-term Medicaid coverage and may have limited generalizability to Medicaid beneficiaries with more turbulent coverage status.
Third, the claims data furnished by the North Carolina Medicaid program lacked a reliable unique prescriber identifier, which prevented us from constructing an indicator of eligibility for the lock-in program based on the number of controlled substance prescribers. We were able to observe unique pharmacies that dispensed controlled substances, and we created an indicator of use of four or more unique pharmacies in a two-month period in place of prescriber-based eligibility for the lock-in program. Measuring the number of pharmacies a patient used to obtain controlled substances provided a similar metric of provider shopping and controlled substance overuse behaviors. Pharmacy use thresholds are often used to assess eligibility for other Medicaid lock-in programs.13
Study Results
The study analyzed 1,647 enrollees in the North Carolina Medicaid lock-in program. Their mean age was forty, and they were mostly female (73.5 percent), white (75.8 percent), and residents of metropolitan counties (72.7 percent) (Exhibit 1). Nearly all of the subjects had a baseline diagnosis of chronic noncancer pain, while roughly half of the subjects had baseline diagnoses of anxiety disorder, substance use disorder, depression, or any other mental illness.
EXHIBIT 1.
Characteristics of the 1,647 North Carolina Medicaid lock-in program enrollees included in the study cohort
| Characteristics | Number or mean | Percent |
|---|---|---|
| DEMOGRAPHIC | ||
| Mean age, years (SD)a | 39.7 (10.5) | —b |
| Female | 1,211 | 73.5 |
| Race | ||
| White | 1,248 | 75.8 |
| Black | 291 | 17.7 |
| Other or unknown | 108 | 6.6 |
| GEOGRAPHICa,c | ||
| Metropolitan county residenced | 1,197 | 72.7 |
| Residence in border county | 600 | 36.4 |
| Mean community pharmacies in county (SD) | 49.3 (58.6) | —b |
| Mean prescribers in county (SD) | 711.2 (942.7) | —b |
| CLINICALe | ||
| Charlson Comorbidity Index score | ||
| Mean score (SD) | 1.4 (2.0) | —b |
| 0 | 676 | 41.0 |
| 1 | 465 | 28.2 |
| 2 or more | 506 | 30.7 |
| Mean prescription drug burden (SD)f | 19.3 (9.9) | —b |
| Chronic noncancer pain | 1,583 | 96.1 |
| Anxiety disorder | 959 | 58.2 |
| Substance use disorder | 743 | 45.1 |
| Depression | 905 | 55.0 |
| Other mental illness | 836 | 50.8 |
| LOCK-IN PROGRAM | ||
| Eligibility criteria met | ||
| Opioid use | 1,446 | 87.8 |
| Benzodiazepine use | 58 | 3.5 |
| Pharmacy use | 1,141 | 69.3 |
| Enrollment delayed | 1,547 | 93.9 |
| Mean delay, months (SD) | 5.6 (3.9) | —b |
| Mean months observed before enrollment (SD) | 20.6 (5.0) | —b |
| Mean months observed after enrollment (SD) | 10.9 (1.8) | —b |
SOURCEAuthors’ analysis of data from the North Carolina Medicaid program and the North Carolina Controlled Substances Reporting System. NOTESFor the lock-in program, opioid eligibility was defined as having at least seven opioid Medicaid claims in two calendar months; benzodiazepine eligibility was defined as having at least seven benzodiazepine Medicaid claims in two calendar months; and pharmacy eligibility was defined as having Medicaid claims for an opioid, a benzodiazepine, or both from at least four unique pharmacies in two months (a proxy for the unique prescriber measure used in practice). Subjects could be included in multiple eligibility groups. SD is standard deviation.
Age and county-of-residence measures reflect values of the measures at the time of a subject’s enrollment in the lock-in program.
Not applicable.
Characteristics were assessed at the county level.
We used the 2013 version of the Department of Agriculture’s rural-urban continuum codes. The most metropolitan counties are coded 1, and the most rural counties are coded 9. We classified metropolitan counties as those with continuum codes 1–3, and nonmetropolitan counties as those with continuum codes 4–9.
Characteristics were assessed for all subjects during the twelve-month baseline period (October 1, 2009–September 30, 2010).
Number of unique medications received during the baseline period according to Medicaid claims data.
Almost 90 percent of the subjects were eligible for lock-in program enrollment because of their high opioid use. Over two-thirds of them met the threshold of using four or more unique opioid- and benzodiazepine-dispensing pharmacies, which indicates that most subjects were also eligible for the lock-in program because of their potential provider shopping behavior. Enrollment was delayed for nearly all subjects, with a mean delay of almost six months following initial eligibility. Subjects were observed for a mean of twenty-one months before and eleven months after their lock-in enrollment.
Bivariate analyses showed that the mean number of per person per month fills of opioid and benzodiazepine prescriptions (fills covered by Medicaid plus circumvented fills) decreased 17 percent after subjects were enrolled in the lock-in program, while the mean number of pharmacies that dispensed controlled substances used per person per month decreased 46 percent—both significant changes (Exhibit 2). In addition, per person per month prescription claims for opioids and benzodiazepines covered by Medicaid decreased 43 percent after enrollment, from a mean of 2.43 to a mean of 1.39.
EXHIBIT 2.
Bivariate trends in opioid and benzodiazepine prescription fills, before and after enrollment in the North Carolina Medicaid lock-in program
| Overall | Before enrollment | After enrollment | |||||
|---|---|---|---|---|---|---|---|
| Mean, no., % | (SD), % | Mean, no., % | (SD), % | Mean, no., % | (SD), % | Percent change | |
| Mean per person per month fills | |||||||
| Total | 2.56 | (2.06) | 2.72 | (2.13) | 2.26 | (189) | −17 |
| Covered by Medicaid | 2.07 | (186) | 2.43 | (1.94) | 1.39 | (1.50) | −43 |
| Circumvented | 0.50 | (113) | 0.30 | (0.85) | 0.87 | (1.45) | 195 |
| Mean pharmacies used per person per month | 0.99 | (0.81) | 1.18 | (0.87) | 0.64 | (0.52) | −46 |
| No. of subjects with any circumvented fills | 1,566 | 95% | 1,337 | 81% | 1,436 | 87% | 7 |
| Percent of person-months with any circumvented fills | 26.1 | 17.6 | 42.3 | 140 | |||
SOURCEAuthors’ analysis of data from the North Carolina Medicaid program and the North Carolina Controlled Substances Reporting System. NOTES“Circumvented” fills were defined as opioid analgesic or benzodiazepine prescription fills purchased from a community pharmacy in North Carolina and paid for entirely out of pocket despite the purchaser’s having Medicaid coverage. All bivariate associations comparing measures before and after lock-in enrollment were significant (p < 0.001). There were 33,882 person-months observed in the period before lock-in enrollment and 17,916 person-months observed after lock-in enrollment. SD is standard deviation.
However, circumvented controlled substance prescription fills increased 195 percent after enrollment. Increased circumvention behavior was also evident when we compared the percentage of months in which subjects filled a controlled substance prescription through circumvention in periods before and after enrollment. Enrollees had at least one circumvented fill in 42 percent of observed person-months following enrollment, compared to 18 percent of person-months before enrollment (Exhibit 2).
We observed a steady increase in the average number of monthly circumvented controlled substance prescription fills, with a corresponding decrease in Medicaid-covered fills, following implementation of the North Carolina Medicaid lock-in program (Exhibit 3).
EXHIBIT 3. Opioid and benzodiazepine prescription fills per person per month for the study cohort of enrollees in the North Carolina Medicaid lock-in program, October 2009–September 2012.
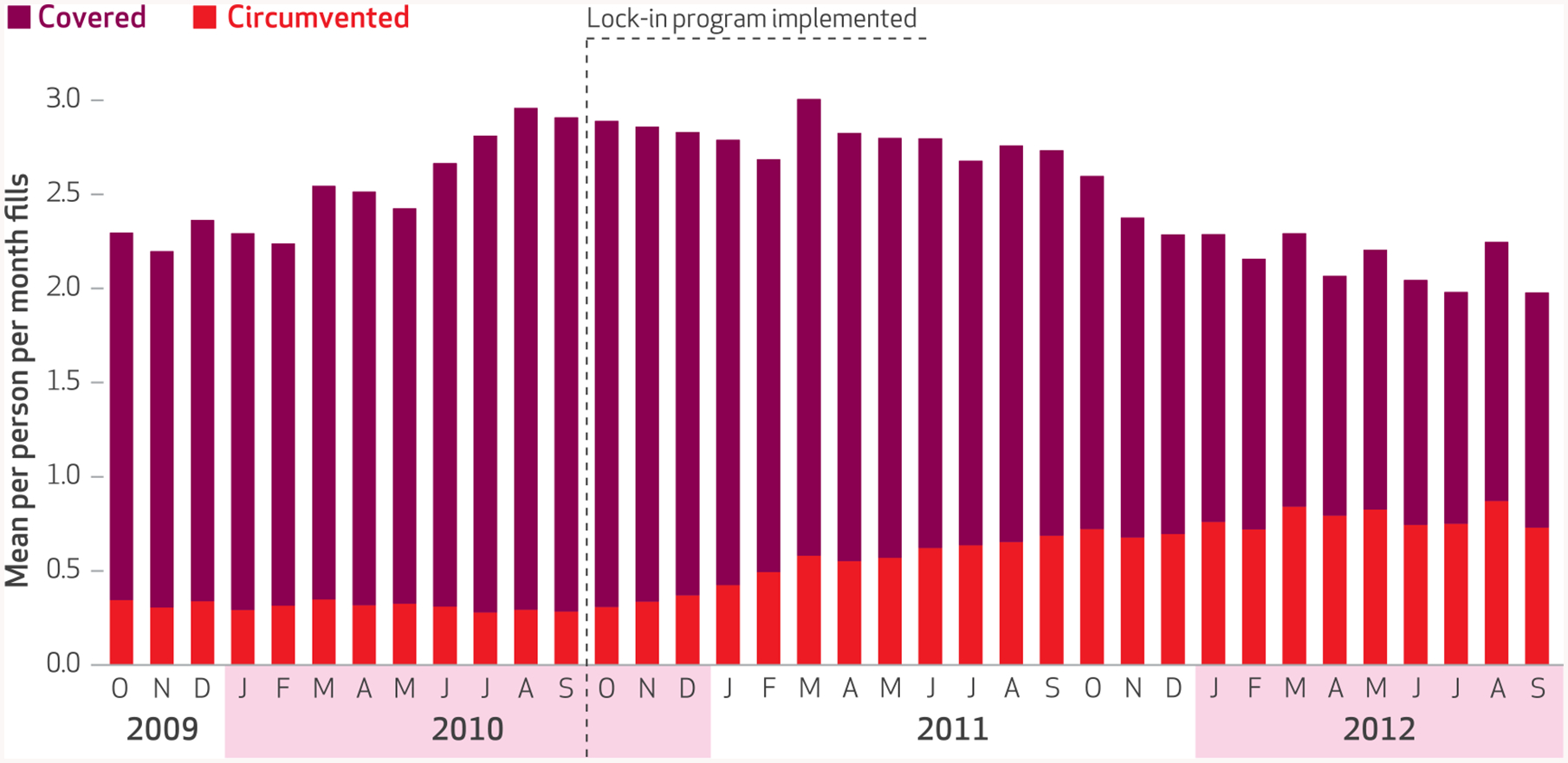
SOURCE Authors’ analysis of data from the North Carolina Medicaid program and the North Carolina Controlled Substances Reporting System. notes October 1, 2010, is the date of implementation of the North Carolina Medicaid lock-in program. “Covered” means that fills were covered by Medicaid. “Circumvented” fills are defined in the Notes to Exhibit 2.
The estimated probability that enrollees would circumvent their Medicaid coverage at least once in a given month to purchase an opioid or benzodiazepine prescription out of pocket was 55 percent (95% confidence interval: 0.51, 0.60) following lock-in enrollment, compared to 16 percent (95% CI: 0.15, 0.17) in the months before lock-in program restrictions took effect (Exhibit 4). Lock-in program enrollment was also associated with a more than fourfold increase in the rate of prescription purchases that circumvented Medicaid, from 0.26 fills per person per month before lock-in program enrollment to 1.16 fills per person per month after enrollment (p < 0.001).
EXHIBIT 4. Estimated likelihood of any Medicaid circumvention and mean number of circumvented controlled substance prescription fills per person per month, before and after enrollment in the North Carolina Medicaid lock-in program.
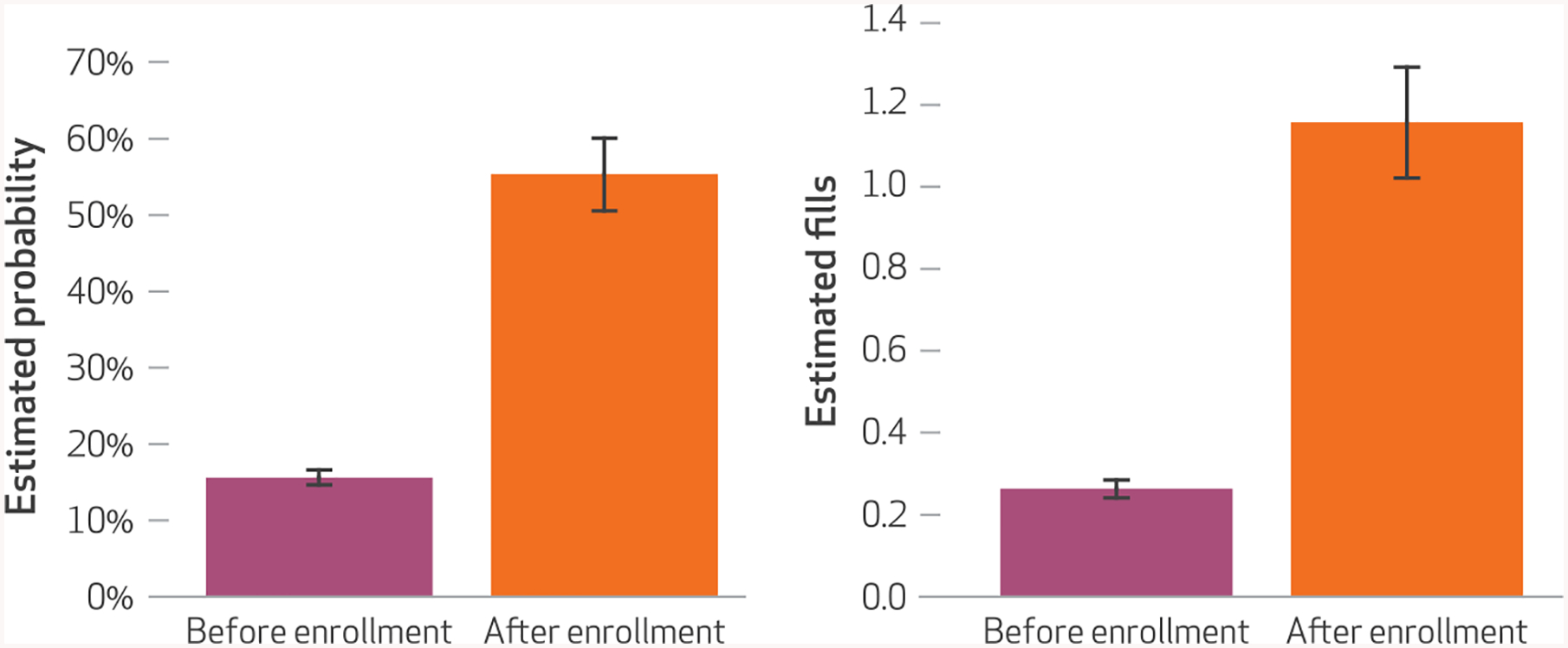
SOURCE Authors’ analysis of data from the North Carolina Medicaid program and the North Carolina Controlled Substances Reporting System. NOTES Probabilities and their 95% confidence intervals (shown by the whiskers) were estimated using a modified Poisson generalized estimating equation model. Numbers of circumvented controlled fills and their 95% confidence intervals (shown by the whiskers) were estimated using a similar model. Both models controlled for time; eligibility for the lock-in program and program eligibility criteria met; age; sex; race; metropolitan residence; county-level supply of pharmacies; residence in a border county; baseline diagnoses of chronic noncancer pain, anxiety disorder, substance use disorder, depression, and other mental illness; Charlson Comorbidity Index score; and prescription drug burden. The models’ full specifications and outputs are provided in Appendix Exhibit A2 (see Note 21 in text). Both the increase in estimated probability of any circumvention and the increase in estimated mean number of monthly circumvented controlled substance prescription fills before and after enrollment were significant (p < 0.001). “Circumvented” fills are defined in the Notes to Exhibit 2.
Of note, both circumvention behavior outcomes were positively associated with longer delays in lock-in program enrollment, program eligibility because of benzodiazepine and pharmacy use, relatively young age, living in a county with more than a hundred pharmacies, and having chronic noncancer pain (Appendix Exhibit A2).21 Having an anxiety disorder and having a high physical comorbidity burden were also associated with increased likelihood of engaging in circumvention.
Discussion
Previous evaluations of Medicaid lock-in programs have generally asserted that prescriber and pharmacy lock-in restrictions mitigate high-risk use of controlled substances because enrollment in a lock-in program reduced Medicaid prescription claims and expenditures.24–27 Our findings provide a new and more comprehensive picture of patients’ controlled substance–seeking behavior following enrollment in a lock-in program by linking Medicaid claims data with prescription drug monitoring program data.
We found that North Carolina Medicaid lock-in program enrollees’ total per person per month opioid and benzodiazepine prescription fills decreased 17 percent following lock-in enrollment. However, our analysis also provides among the first rigorous evidence that enrollment in a lock-in program was associated with significant increases in out-of-pocket controlled substance prescription fills, which circumvent lock-in program restrictions and are not observable in Medicaid claims. Specifically, lock-in program enrollees in our cohort were 3.6 times more likely to purchase a controlled substance prescription out of pocket after lock-in program enrollment than before it. Over half of the observed reduction in Medicaid claims for controlled substance prescriptions in our cohort after lock-in enrollment was offset by new out-of-pocket controlled substance prescription fills. Thus, circumvention behavior poses a serious threat to the effectiveness of lock-in programs.
The root causes of increased out-of-pocket controlled substance prescription purchases following enrollment in a lock-in program are likely multifactorial.19 For some patients, circumvention may stem from new barriers to necessary care that a lock-in program’s prescriber and pharmacy restrictions unintentionally create.
For example, patients may be forced to pay out of pocket for a controlled substance prescription fill because lock-in program restrictions are too inflexible to take certain reasonable and un-avoidable circumstances into account. These may include a patient’s prolonged travel away from his or her approved providers, extended periods of time when providers are unavailable, and a patient with complex health needs requiring controlled substance medication therapy to be managed across multiple specialist providers. In this scenario, circumvention reflects a failure of the lock-in program to maintain desired enhanced care coordination between an enrollee and his or her prescriber and pharmacy. Unintended barriers to care created by program restrictions would also place undue financial burden on patients who must pay the full price of legitimate controlled substance prescription fills dispensed by providers and pharmacies to which they are not locked in by the program.
Lock-in program circumvention may also reflect patients’ efforts to fill controlled substance prescriptions for abuse, misuse, or diversion. If so, increased circumvention following enrollment reveals inherent limitations to the programs’ ability to reduce the supply of controlled substance prescriptions used for illicit purposes. Our data were limited to records of legal prescription fills. However, it is reasonable to be concerned that program restrictions may unintentionally give people incentives to withdraw from legal prescription drug distribution channels and seek controlled substance medications or illegal drugs of abuse through diversion.
Policy Implications
Efforts are well under way to implement lock-in programs more widely to fight the opioid epidemic—despite a dearth of rigorous evidence of the lock-in programs’ intended and unintended effects on clinical outcomes, patients’ behavior, and health care access, such as the circumvention behavior we observed in our study. As noted above, the Comprehensive Addiction and Recovery Act signed into law in July 2016 includes a provision establishing controlled substance lock-in programs in every Medicare Part D prescription drug plan, beginning in January 2019. Additionally, state officials are considering the inclusion of lock-in programs in an aggressive new push to combat the opioid epidemic at the state level.28 In the private sector, the health insurer Anthem recently rolled out lock-in programs in its health plans across fourteen states.29
These efforts were based on limited evidence that serves almost exclusively to show that lock-in programs are cost-saving tools.13 Little to no attention has been paid to the programs’ effects on meaningful public health outcomes—such as rates of opioid overdose or medication-assisted treatment use—or on the extent of prescription and illicit controlled substance use and abuse. Our finding that enrollment in the North Carolina Medicaid lock-in program was associated with a 17 percent reduction in total controlled substance prescription fills and a 46 percent drop in the numbers of pharmacies used per person per month provides a necessary first step toward building a comprehensive understanding of the programs’ potentially promising effects on risky controlled substance use behavior.
However, greater scrutiny is still needed by members of the policy, academic, and managed care communities regarding the effects of controlled substance lock-in programs on patterns of health care use and the programs’ suitability in their present form as public health tools for preventing prescription drug abuse and overdose.
In particular, future research should investigate the predictors and limiting effects of circumvention behavior on the effectiveness of lock-in programs and other potential unintended consequences of the programs’ restrictions. Specifically, to what extent do the restrictions limit access to necessary care, and what is the risk that the programs will unintentionally lead certain patients to continue or newly engage in risky drug use behaviors?
Policy makers and lock-in program administrators should also explore opportunities to improve enrollees’ adherence to their program’s prescriber and pharmacy restrictions. This should include better education of enrollees and providers about the purpose and intended outcomes of the program. Currently, it is common practice for the only communication from lock-in program administrators to enrollees, prescribers, and pharmacies to be a single letter informing them of the patient’s lock-in status. In a 2014 survey, North Carolina pharmacists reported a strong desire for enhanced communication from lock-in program administrators to alleviate a lack of understanding about the program and its purpose.19 Our recommendation echoes a call from a 2012 Centers for Disease Control and Prevention expert panel for clearer communication and educational messages to better demonstrate the value of a lock-in program to patients and providers.14
Additionally, lock-in program administrators should consider reconceptualizing the programs as platforms for delivering more robust and integrated care services to patients with complex physical and mental health needs, as opposed to simply imposing restrictions on provider and pharmacy access for insurance coverage of controlled substance prescriptions. For example, establishing Medicaid health homes for beneficiaries with opioid abuse, misuse, or dependence is an appealing and viable model for states to use in bolstering the positive effects of their Medicaid lock-in programs.
Medicaid health homes are an initiative introduced in the Affordable Care Act. In brief, the federal government provides financial support to create integrated care models that deliver high-quality coordinated medical and behavioral services to high-risk subsets of the Medicaid population with complex conditions. The Centers for Medicare and Medicaid Services recently reported that Maryland, Rhode Island, and Vermont were already operating opioid treatment–specific Medicaid health homes.30
Lastly, our linking of North Carolina Medicaid claims data with records from the North Carolina Controlled Substances Reporting System demonstrates the value of leveraging prescription drug monitoring program data in the operation of managed care interventions, such as lock-in programs, to prevent controlled substance abuse, misuse, and overdose. Administrators and providers tasked with delivering such interventions will benefit from the efficient integration of prescription drug monitoring program records into e-prescribing and pharmacy dispensing software systems.
Our experiences with linked Medicaid claims and North Carolina Controlled Substances Reporting System data reinforced the need to establish interoperable prescription drug monitoring programs across state lines and to mandate that providers use these programs before prescribing or dispensing controlled substance medications—a point on which there is growing consensus.31 Collectively, these efforts will empower providers to deliver higher-quality care for patients at the greatest risk of preventable controlled substance overdose.
Conclusion
Lock-in programs are poised to assume a much larger role in national policy efforts to curb the opioid overdose crisis, despite limited evidence about their effect on controlled substance use and outcomes. Our study of the North Carolina Medicaid lock-in program provides some of the first rigorous evidence that enrollment in such a program increased the likelihood and rate of out-of-pocket controlled substance prescription fills, which circumvent and mitigate the effectiveness of program restrictions. This circumvention threatens the programs’ potential public health benefit and sheds light on the present lack of understanding of whether lock-in programs in their current form are a viable tool for preventing prescription drug abuse and overdose. ■
Supplementary Material
Acknowledgments
Findings from this research were presented at the 21st Annual National Research Services Award Trainee Research Conference, Minneapolis, Minnesota, June 14, 2015; the AcademyHealth Annual Research Meeting, Minneapolis, Minnesota, June 16, 2015; and the American Pharmacists Association Annual Meeting and Exposition, San Diego, California, March 28, 2015. This research was supported by the Centers for Disease Control and Prevention (Cooperative Agreement No. CDC U01 CE002160-01); a Clinical and Translational Sciences Award (Grant No. UL1TR000083); the Injury Prevention Research Center at the University of North Carolina at Chapel Hill; the Agency for Healthcare Research and Quality, via a National Research Service Award T32 Postdoctoral Fellowship for Andrew Roberts, sponsored by the Cecil G. Sheps Center for Health Services Research at UNC-Chapel Hill (Grant No. 5T32 HS000032); and the American Foundation for Pharmaceutical Education, via a Predoctoral Fellowship in the Pharmaceutical Sciences for Roberts. The authors thank Leslie Moss for her invaluable technical support for this research.
Contributor Information
Andrew W. Roberts, is an assistant professor in the Department of Pharmacy Sciences, School of Pharmacy and Health Professions, and a program faculty member in the Center for Health Services Research and Patient Safety, both at Creighton University, in Omaha, Nebraska.
Joel F. Farley, is a professor in the Division of Pharmaceutical Outcomes and Policy, Eshelman School of Pharmacy, at the University of North Carolina at Chapel Hill.
G. Mark Holmes, is an associate professor in the Department of Health Policy and Management, Gillings School of Global Public Health, and director of the the Cecil G. Sheps Center for Health Services Research, both at UNC-Chapel Hill..
Christine U. Oramasionwu, is an assistant professor in the Division of Pharmaceutical Outcomes and Policy, Eshelman School of Pharmacy, at UNC-Chapel Hill.
Chris Ringwalt, is a senior research scientist at the Injury Prevention Research Center, UNC-Chapel Hill..
Betsy Sleath, is the George H. Cocolas Distinguished Professor; chair of the Division of Pharmaceutical Outcomes and Policy, Eshelman School of Pharmacy; and director of the Child and Adolescent Health Services Program at the Sheps Center, all at UNC-Chapel Hill..
Asheley C. Skinner, is an associate professor at the Duke Clinical Research Institute, Duke University, in Durham, North Carolina.
NOTES
- 1.Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013; 309(7):657–9. [DOI] [PubMed] [Google Scholar]
- 2.Dart RC, Surratt HL, Cicero TJ, Parrino MW, Severtson SG, Bucher-Bartelson B, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015; 372(3):241–8. [DOI] [PubMed] [Google Scholar]
- 3.Daubresse M, Chang HY, Yu Y, Viswanathan S, Shah ND, Stafford RS, et al. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000–2010. Med Care. 2013;51(10):870–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kenan K, Mack K, Paulozzi L. Trends in prescriptions for oxycodone and other commonly used opioids in the United States, 2000–2010. Open Med. 2012;6(2):e41–7. [PMC free article] [PubMed] [Google Scholar]
- 5.Han B, Compton WM, Jones CM, Cai R. Nonmedical prescription opioid use and use disorders among adults aged 18 through 64 years in the United States, 2003–2013. JAMA. 2015;314(14):1468–78. [DOI] [PubMed] [Google Scholar]
- 6.Roland CL, Joshi AV, Mardekian J, Walden SC, Harnett J. Prevalence and cost of diagnosed opioid abuse in a privately insured population in the United States. J Opioid Manag. 2013;9(3):161–75. [DOI] [PubMed] [Google Scholar]
- 7.Substance Abuse and Mental Health Services Administration. The DAWN report: drug-related emergency department visits involving pharmaceutical misuse or abuse by older adults: 2009. Rockville (MD): SAMHSA; 2012. July 26. [Google Scholar]
- 8.Centers for Disease Control and Prevention. Vital signs: overdoses of prescription opioid pain relievers—United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2011;60(43): 1487–92. [PubMed] [Google Scholar]
- 9.North Carolina Injury and Violence Prevention Branch. Prescription and drug overdoses [Internet]. Raleigh (NC): The Branch; [cited 2016 Aug 4]. Available from: http://www.injuryfreenc.ncdhhs.gov/About/PrescriptionDrugOverdoseFactSheet-2014-7-JAN-2016.pdf [Google Scholar]
- 10.Centers for Disease Control and Prevention. Vital statistics data available online: Mortality Multiple Cause files [Internet]. Atlanta (GA): CDC; [last updated 2016 Aug 3; cited 2016 Aug 12]. Available for download from: http://www.cdc.gov/nchs/data_access/vitalstatsonline.htm#Mortality_Multiple [Google Scholar]
- 11.Hansen RN, Oster G, Edelsberg J, Woody GE, Sullivan SD. Economic costs of nonmedical use of prescription opioids. Clin J Pain. 2011; 27(3):194–202. [DOI] [PubMed] [Google Scholar]
- 12.National Rx Drug Abuse Summit [Internet]. Orlando (FL): The Summit; Press release, Prescription drug abuse epidemic can be solved through collaborative efforts, officials say during opening of summit; 2013. April 2 [cited 016 Aug 4]. Available from: http://nationalrxdrugabusesummit.org/wp-content/uploads/2013/04/SummitNews-Day1-4-2-13.pdf [Google Scholar]
- 13.Roberts AW, Skinner AC. Assessing the present state and potential of Medicaid controlled substance lock-in programs. J Manag Care Spec Pharm. 2014;20(5):439–46c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Center for Injury Prevention and Control. Patient review and restriction programs: lessons learned from state Medicaid programs: CDC expert panel meeting report [Internet]. Atlanta (GA): The Center; 2012. [cited 2016 Aug 4]. Available from: https://www.cdc.gov/drugoverdose/pdf/pdo_patient_review_meeting-a.pdf [Google Scholar]
- 15.Mercer Health and Benefits LLC Government Human Services Consulting. Recipient Management Lock-In Program analysis: state of North Carolina. Raleigh (NC): State of North Carolina; 2011. November 14. [Google Scholar]
- 16.Centers for Medicare and Medicaid Services. Medicaid Integrity Program: Iowa Comprehensive Program Integrity Review: final report [Internet]. Baltimore (MD): CMS; 2008. November [cited 2016 Aug 4]. Available from: http://www.cms.gov/Medicare-Medicaid-Coordination/Fraud-Prevention/FraudAbuseforProfs/downloads/iacompfy08pireviewfinalreport.pdf [Google Scholar]
- 17.Hatchett EB Jr. Medicaid’s Recipient Lock-In Program: December 1997–performance audit [Internet]. 1997. December 10 [cited 2016 Aug 4]. Available from: http://apps.auditor.ky.gov/Public/Audit_Reports/Archive/medicaid_Lock_In.pdf [Google Scholar]
- 18.Keast S Evaluation of the Oklahoma SoonerCare Lock-In Program. CDC Expert Panel Meeting; August 28, 2012; Atlanta, GA. [Google Scholar]
- 19.Werth SR, Sachdeva N, Roberts AW, Garrettson M, Ringwalt C, Moss LA, et al. North Carolina Medicaid recipient management lock-in program: the pharmacist’s perspective. J Manag Care Pharm. 2014;20(11): 1122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dreyer TR, Michalski T,Williams BC. Patient outcomes in a Medicaid managed care lock-in program. J Manag Care Spec Pharm. 2015; 21(11):1006–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.To access the Appendix, click on the Appendix link in the box to the right of the article online.
- 22.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–9. [DOI] [PubMed] [Google Scholar]
- 23.Agency for Healthcare Research and Quality. CCS software and user’s guide [Internet]. Rockville (MD): AHRQ; [revised 2016 Apr 18]. Appendix C, Clinical Classifications Software—expanded diagnosis categories (January 1980 through September 2015) [cited 2016 Aug 10]. Available from: http://www.hcup-us.ahrq.gov/toolssoftware/ccs/AppendixCMultiDX.txt [Google Scholar]
- 24.Blake SG, Feldhaus JF, Hunter TS, Rappaport H, Holt G, Medon PJ. PMD2 the effect of the Louisiana Medicaid lock-in on prescription drug utilization and expenditures. Value Health. 1998;1(1):72. [Google Scholar]
- 25.Hladilek MK, Howe MJ, Carr RM. An overview of Wisconsin Medicaid quality. WMJ. 2004;103(3):58–62. [PubMed] [Google Scholar]
- 26.Kachur S, Schuster A, Fagan PJ, Lu Y, LeNoach E, Fatodu H, et al. Impact of a single-provider lock-in program for opiates in a managed Medicaid population. Paper presented at: AcademyHealth Annual Research Meeting; 2013. Jun 23–25; Baltimore, MD. [Google Scholar]
- 27.Mitchell L Pharmacy lock-in program promotes appropriate use of resources. J Okla State Med Assoc. 2009;102(8):276. [PubMed] [Google Scholar]
- 28.Pear R Governors devise bipartisan effort to reduce opioid abuse. New York Times. 2016. February 21. [Google Scholar]
- 29.Business Wire. Pharmacy programs that tackle inappropriate opioid and Rx drug use can improve drug safety and health care quality. Business Wire [serial on the Internet]. 2016. May 25 [cited 2016 Aug 4]. Available from: http://www.businesswire.com/news/home/20160525005960/en/Pharmacy-Programs-Tackle-Inappropriate-Opioid-Rx-Drug
- 30.Moses K, Klebonis J. Designing Medicaid health homes for individuals with opioid dependency: considerations for states [Internet]. Baltimore (MD): Centers for Medicare and Medicaid Services; 2015. January [cited 2016 Aug 4]. (Brief). Available from: https://www.medicaid.gov/state-resource-center/medicaid-state-technical-assistance/health-homes-technical-assistance/downloads/health-homes-for-opiod-dependency.pdf [Google Scholar]
- 31.Greenwood-Ericksen MB, Poon SJ, Nelson LS, Weiner SG, Schuur JD. Best practices for prescription drug monitoring programs in the emergency department setting: results of an expert panel. Ann Emerg Med. 2016;67(6):755–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


