Abstract
Oleanolic acid (OA) and its derivatives are widely found in diverse plants and are naturally effective pentacyclic triterpenoid compounds with broad prophylactic and therapeutic roles in various diseases such as ulcerative colitis, multiple sclerosis, metabolic disorders, diabetes, hepatitis and different cancers. This review assembles and presents the latest in vivo reports on the impacts of OA and OA derivatives from various plant sources and the biological mechanisms of OA activities. Thus, this review presents sufficient data proposing that OA and its derivatives are potential alternative and complementary therapies for the treatment and management of several diseases.
Keywords: Oleanolic acid, Prophylactic, Anti-inflammatory, Anti-diabetics, Neuroprotective, Hepatoprotective
Core tip: Oleanolic acid (OA) is plentiful in many fruits and vegetables. Studies have shown that OA and its derivatives exert promising pharmacological actions including anti-inflammatory, neuroprotective, hepatoprotective, anti-osteoporotic and anti-diabetics at low doses. However, it is not a “cure-all” drug or drug candidate and could exert adverse effects at high doses, particularly its derivatives. In addition, information elucidating the drug-drug/drug-herb interactions associated with OA and its derivatives is inadequate. Nevertheless, there is a reasonable amount of literature, as fully explored in this review that OA and its derivatives have crucial prophylactic and therapeutic potential for diseases including ulcerative colitis, diabetes and cardiovascular diseases.
INTRODUCTION
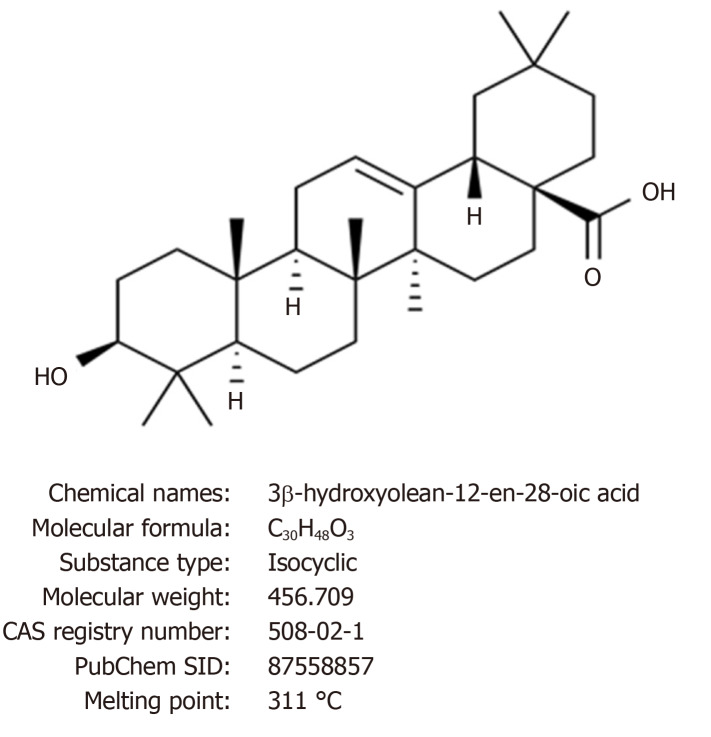
Oleanolic acid (OA: 3β-hydroxyolean-12-en-28- oic acid, Figure 1) is a biologically active natural pentacyclic triterpenoid compound that is present in over 2000 plant species, as well as numerous food and medicinal plants[1]. The compound is particularly common in the Oleaceae family, among which olive (Olea europaea), the plant species after which the compound was entitled, is still the primary supply of mercantile OA.
Figure 1.
Chemical structure and properties of oleanolic acid.
OA is plentiful in apple skin, papaya fruit, persimmon fruit and leaf, plum, loquat, soybeans, filamentous fungi (Table 1)[2-4]. Several medicinal herbs such as ginseng contain OA as one of the active ingredients. The concentrations of OA are often as high as 1% in olive fruit, apple skin, ginseng, papaya fruit and dark plums[5]. It is not solely present as a free compound but also occurs as an aglycone precursor for triterpenoid saponins, in which it is bonded to one or more sugar chains[1,5]. As a triterpenoid, OA belongs to an oversized cluster of structurally diverse natural products, including sterols, steroids, and triterpenoid saponins[6].
Table 1.
| Fruit | Content |
| Apple skin | 0.96 mg/dry skin |
| Apples | 16-28 µg/dm |
| Bilberries whole fruit | 1679.2-2029.6 µg/dm |
| Grapes peel | 176.2 µg/g dw |
| Jujube pulp | 360 ± 10.7 µg/g dw |
| Lemon | 0.62 ± 0.01 µg/dm |
| Loquat skin | 1.46 mg/dry skin |
| Mandarin | 1.05 ± 0.04 µg/dm |
| Olives pulp | 27-29 µg/g fw |
| Olives skin | 3094-4356 µg/g fw |
| Peach skin | 1.49 mg/dry skin |
| Pear skin | 1,25 mg/dry skin |
| Pears | 164.3-3066.6 µg/g fw |
| Pears pulp | 34.0-156.0 µg/g fw |
| Persimmon flesh | 17.2 µg/g dw |
| Persimmon peel | 367.7 µg/g dw |
| Pomegranate | 1.12 - 26.96 µg/dm |
| Quince skin | 0,25 mg/dry skin |
| S. adenocaulon | 12.7 ± 0.2 µg/dm |
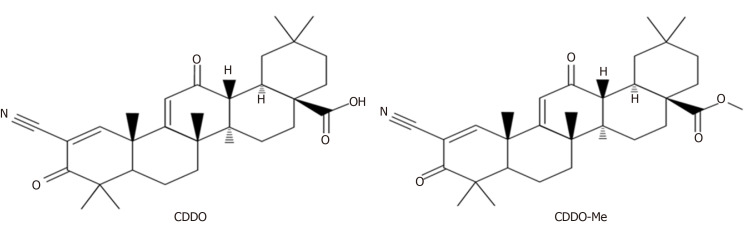
The artificial modification of OA on its three ‘‘reactive’’ regions; the C3-OH, the C12=C13 double bond, and the C28-COOH, has led to a series of new synthetic oleanane triterpenoids[7-9]. Compared to OA, some of these compounds showed increased biological activity such as anti-inflammatory and hepatoprotective activities. One such compound with increased biological activity is 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO) or its C-28 methyl ester (CDDO-Me; Figure 2)[1,7,10,11].
Figure 2.
Structures of 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid and its C-28 methyl ester. CDDO: 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid; CDDO-Me: C-28 methyl ester of CDDO.
PHARMACOLOGY
OA and its derivatives have plenty of useful effects; including remarkable antioxidant, anti-inflammatory, antiviral, and anti-diabetic effects. They are efficacious against proliferation in tumour-bearing mice, such as breast cancer.
Anti-inflammatory effects
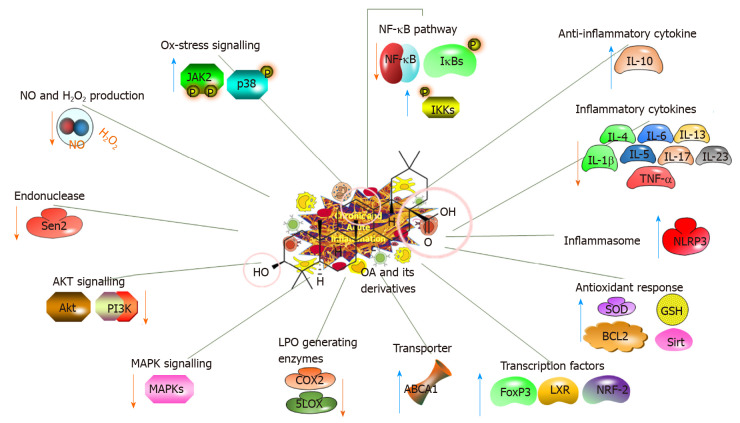
Inflammatory processes are characterised by extreme reactive oxygen species (ROS) levels and are related to many pathological conditions, including ulcerative colitis, AD, PD and cancer[12-14]. Table 2 summarises the recent studies investigating the in vivo anti-inflammatory effects and related mechanisms of action of OA and its natural or synthetic derivatives[14-26]. A proposed potential strategy is to examine the roles of OA and its derivatives in preventing inflammatory responses involving the nuclear factor erythroid-2-related factor 2 (NRF-2) and nuclear factor-κB (NF-κB) pathways[15,27] (Figure 3).
Table 2.
In vivo anti-inflammatory effects and related mechanisms of action of oleanolic acid and its natural and synthetic derivatives (2014-2020)
| Disease model/physiology | Effect |
Mechanism |
Compound | Dose | Ref. | |
| ↑↑↑ | ↓↓↓ | |||||
| Ulcerative colitis (mice, DDS) | Anti-ulcerative colitis restoring the balance of Th17/Treg cells and inhibiting NF-κB signaling | FOXP-3, IL-10, ZO-1, Occludin, Claudin-1, pJNK, pP38 | MPO, Th17, RORγt, IL-17, TNF-α, IL-1β, MAPK, pIKB, pTAK, pP65, iNOS, COX-2 | OA | 5-10 mg/kg·d, 3 d after DSS | [14] |
| Experimental mammary carcinogenesis | Anti-inflammatory | cP65, cIKB-α | COX-2, HSP90, NF-ĸB, npP65 | OA-Xs | 0.8-1.6 mg/kg·2 d, 2 wk before 16 wk after DSS | [15] |
| Colonic inflammation (mice, HFD) | Prevent colon inflammation | CD206, IL-10, #goblet cells | NF-қB, pNF-қB, IL-6, TNF-α, COX-2, KI67 | OA-Xs (CDDO-Me) | 10 mg/kg in drinking water, 21 wk | [16] |
| Ulcerative colitis (mice, DDS) | Anti-ulcerative colitis, anti-inflammatory via inhibiting STAT3 | - | IL-17, STAT3 | OA-Xs (CDDO-Im) | 0.5-2 µmol/L | [17] |
| Anti-inflammation and antinociception (rats) | Anti-inflammatory, anti-nociceptive | Pain latency | Paw volume | OA-Xn | 40 mg/kg once | [18] |
| Anti-inflammation (rats) | Membrane stabilization | - | Paw volume, hemolysis | OA-Xs | 20-40 µg | [19] |
| Anti-inflammation (rats, hPMBCs) | Anti-inflammatory | - | COX-2, 5-LOX, NOS, MPO, edema, IL-6, NF-ĸB, PGE-2 | OA-Xn | 50 mg/kg, 100 µg | [20] |
| Anti-inflammation (mouse skin) | anti-inflammatory properties | - | IL-1α, IL-1β, IL-6, IL-23 | OA-X | 2 µmol | [21] |
| Allergic airway inflammation (rats) | Anti-inflammatory and immunomodulatory | IL-6, IL-8 | DTH, NO, IL-4, 5, 13, 17, TLR2, NF-ĸB and TNF-α; sIgE, COX-2, and 5-LOX | Fe-OA and Zn-OA | 2 mg/kg | [22] |
| Anti-inflammation and antinociception (mice) | Analgesic action and expressed strong anti-inflammatory activity | - | IL-6 | OA-Xs, OA-ASA | 0.3-300.0 mg/kg, p.o. | [23] |
| Lung injury (MLE-12, NDMA) | Anti-inflammatory, anti-oxidative stress and anti-apoptotic effects | SOD, GSH, SIRT-1, NRF-2, BCL-2, | TNF-α, IL-6, IL-1β, MDA, BAX, NF-ĸB, NRLP-3, LDH, Ac-P65, BAX/BCL-2 | OA | 10-20 mg/kg | [24] |
| Pulmonary inflammation and fibrosis (mice) | Anti-inflammatory response and anti- pulmonary fibrosis in the lungs | NLRP3 | IL-1β, IL-6, TNF-α, TGF-β1, and fibronectin, NRLP-3, ASC, CASP-1 | OA | 0.001-1 mg/kg·d, 5 d (nc) | [25] |
| Subarachnoid haemorrhage (rats) | Alleviated SAH-induced vasogenic edema | VE-Cadherins, P120, ZO-1, Occludin- | HO-1 | OA | 5-20 mg/kg | [26] |
DDS: Diaminodiphenyl sulfone; NF-κB: Nuclear factor-κB; JNK: cJUN NH2-terminal kinase; IL: Interleukin; TNF-α: Tumor necrosis factor-α; OA: Oleanolic acid; OA-Xn: Natural derivatives of oleanolic acid; OA-Xs: Synthetic derivatives of oleanolic acid; HFD: High-fat diet; CDDO: 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid; CDDO-Me: C-28 methyl ester of CDDO; CDDO-Im: COOD-imidazole; STAT3: Signal transducer and activator of transcription 3; GSH: Glutathione; LDH: Lactic dehydrogenase; NRF-2: Nuclear factor erythroid-2-related factor 2.
Figure 3.
Anti-inflammatory impacts of oleanolic acid and its derivatives, illustrating the molecular mechanisms. OA: Oleanolic acid; NF-κB: Nuclear factor-κB; IL: Interleukin; TNF-α: Tumour necrosis factor-α; Akt: Serine/threonine kinase; GSH: Glutathione; LXR: Liver X receptor; NRF-2: Nuclear factor erythroid-2-related factor 2.
OA significantly inhibited DSS-induced colitis, as verified by the inhibition of Th17 cells and the downregulation of the expression of interleukin (IL)-1, NF-ĸB, MAPK and RORγt in the colon, whilst the FOXP3 and IL-10 expression, macroscopic score, colon shortening, and myeloperoxidase activity increased. Thus, OA prevents and relieves inflammatory diseases such as colitis[14]. Similarly, a multifunctional semisynthetic OA-derivative, i.e., CDDO-Me prevented the high-fat diet (i.e., modelling obesity)-induced chronic low-grade inflammation in the rodent colon. It reduced the expression of F4/80, CD11c, COX-2, IL-6, KI67, NF-қB, and tumour necrosis factor (TNF)-α but increased CD206 and IL-10, showing an anti-inflammatory mechanism[16]. Likewise, another synthetic OA derivative 1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28- oyl] imidazole (CDDO-Im) inhibited IL-6 and IL-17 and relieved DSS-induced colitis in mice. CDDO-Im also notably inhibited the signal transducer and activator of transcription-3 activation. Thus, OA and its derivatives have a unique anti-inflammatory potential as pharmacological therapies for inflammatory bowel disease[14,17].
Acetylated and methylated derivatives of OA isolated from Syzygium aromaticum L. generated a better anti-inflammatory response in models of inflammation in male Wistar rats than did OA[18,19]. Another natural OA derivative isolated from the leaves of Costus igneus showed anti-inflammatory action in a carrageenan-provoked rat model. This derivative inhibited inflammation-associated enzyme activities such as COX, LOX, MPO and NOS[20]. Maslinic acid and 3-epi-maslinic acid were assessed for their capacity to repress inflammatory gene expression in a mouse model of 12-O-tetradecanoylphorbol-13 acetic acid (TDPAA)-induced skin inflammation. All examined compounds had the capacity to repress the expression of at least one or more inflammatory genes provoked by TDPAA in mouse skin, which were more effective than the OA[21]. These results suggest that OA could be a potential prophylactic and therapeutic agent for the treatment of induced inflammatory responses[22-29].
Neuroprotective effects
Considering the pervasiveness of ageing-related diseases, studies investigating the neuroprotective impacts of natural compounds and their derivatives have become popular during recent years. The signalling pathways engaged with neuroprotection are the focus of studies their mechanism of the activity and intervene in their pleiotropic prophylactic action against neuronal harm. In the present review, the molecular mechanisms of the neuroprotection provided by OA and its derivatives are revised. By acting upon various systems simultaneously, OA is the highlight as a promising multi-targeting operator.
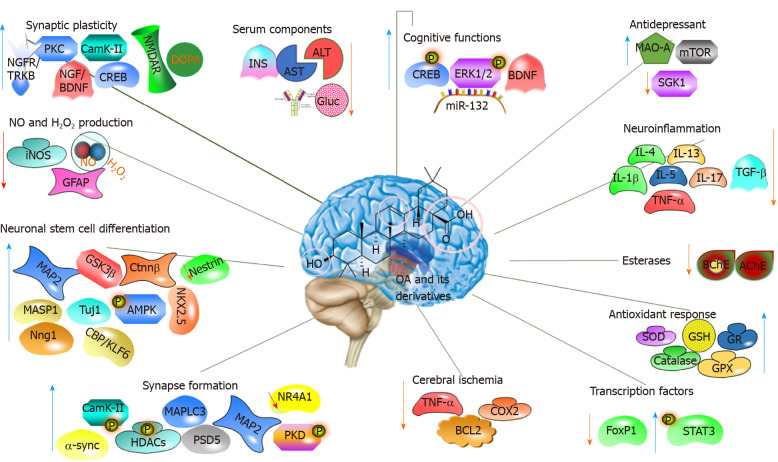
Several studies have shown that OA possesses neuroprotective effects (Table 3)[30-41]. The prophylactic role of OA and its derivatives has been examined using different in vivo models of hydroxydopamine-induced neurodegeneration, Aβ25-35 injection-induced memory deficit in Alzheimer’s disease models, Parkinsonian rat models, stem cell differentiation, and brain slice model of neurodegeneration and ischemic stroke (Table 3 and Figure 4).
Table 3.
In vivo neuroprotective effects and related mechanisms of action of oleanolic acid and its natural and synthetic derivatives (2014-2020)
| Disease model/ physiology | Effect |
Mechanism |
Compound | Dose | Ref. | |
| ↑↑↑ | ↓↓↓ | |||||
| Focal brain hypoxia (rats) | Neuroprotective, IBI, decreased neural damage suppressing glial activities | S-100b, MAP-2 | GFAP, NADP-Diaphorase, iNOS | OA | 6 mg/kg·d, 6 d | [30] |
| Parkinsonian model (rats) | Prevents AIM, anti-PD, ameliorated dyskinesis | CAT | Affected limbs, AIMs, ROS | OA | 100 mg/kg·2 d, 8 d | [31] |
| Neuro-degeneration (rats, hydroxydopamin) | Protects against neurodegeneration | Cerabral doapamine, contralateral limb use | OA | 100 mg/kg·2 d, 7 d pre or post | [32] | |
| Brain damage (rats, fluoride) | Brain damage | GSH, SOD, CAT, GPX, GST, GR | sALT, sAST, LPO, NO | OA | 5 mg/kg·d, last 14 d, | [33] |
| Alzheimer’s disease model (rats, Aβ25-35) | Anti-alzheimer, increased synaptic plasticity, decreased Aβ25-35 toxicity | NMDAR-2B, CREB | CaMKII, PKC, BDNF, TRK-B, Ca2+, Latency time | OA | 21.6 mg/kg | [34] |
| Rat coronal brain slice | Neuroprotective, anti-alzheimer, | BDNF | APP (TAU) toxicity, | OA-Xn | [35] | |
| Cognitive dysfunction (mice) | Ameliorates cognitive dysfunction | pERK-1,2; pCREB, BNDF, TRK-B | - | OA | 0.625-5 mg/kg | [36] |
| Chronic unpredictable mild stress (mice) | Anti-deprassant | pERK-1,2; pCREB, BNDF, miR-132, PSD-95, SYN-1 | - | OA | 2.5-40 mg/kg·d | [37] |
| Cerabral IRI (mice, PC12 cells) | Cerabral protection and prevent IRI | Body weights, sTG, pAMPK, pGSK-3β, APN, Adipo-R1, Adipo-R2, pLKB-1, MAO | sGLU, sINS, Neurological scores, BAX/BCL2, MDA, TNF-α, IL-6, CASP-3, | OA-X (CHS) | pretreatment 30,60, 120 mg/kg·d | [38] |
| Exprerimenal stress (mice, corticoid) | Anti-depressant | AKT/mTOR, BNDF | SGK1, GR | OA | 10 mg/kg | [39] |
| Mice | Anti-depressant | - | MAO-A | OA | 0.1 mL/10g | [40] |
| Mice | Anti-depressant | BNDF, sleep duration | Behavioral tests, MAO | OA | 5-40 mg/kg | [41] |
OA: Oleanolic acid; GFAP: Glial fibrillary acidic protein; APP: Amyloid precursor protein; AIM: Abnormal involuntary movements; CREB: cAMP response element-binding; GSH: Glutathione; ERK: Extracellular-signal-regulated kinase; IRI: Ischemia-reperfusion injury; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase.
Figure 4.
Molecular mechanism of the action of oleanolic acid and its derivatives on the nervous system. OA: Oleanolic acid; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; IL: Interleukin; TNF-α: Tumour necrosis factor-α; GSH: Glutathione; STAT3: Signal transducer and activator of transcription 3.
OA amazingly advanced the migration and proliferation of neural stem cells (NSCs). Differentiation included the increased expression of MAP-2, neuron-explicit marker tubulin-bIII and Mash1, while the astrocyte-explicit marker glial fibrillary acidic protein and Nestin diminished significantly. Moreover, both the phosphorylation of GSK-3β at Ser9 and β-catenin expression were promoted by OA[42-44]. In a DNA microarray investigation, OA was found to differentially controlled 183 genes, and 87 of which were anticipated to share typical NKX-2.5 binding sequences[42]. These outcomes demonstrated that OA is a viable inducer of NSCs differentiation into neurons via NKX-2.5 related components to some extent. Additionally, OA and its derivatives induce neural differentiation and synapse plasticity through a pathway involving histone deacetylase (HDAC) 5 phosphorylation[45]. These results strongly suggest that OA might be a significant therapeutic for the treatment of neurodegenerative diseases under normal conditions or in response to tissue damage.
Animals treated with 6-hydroxydopamine (HDA) showed functional deficiency in a forelimb use asymmetry test and had less dopamine in the striatum, these effects were improved with OA treatment 7-d pre-injury and 1-d post-injury. In addition, pre- or post-injury OA treated rats recovered from HDA-caused membrane depolarisation, indicating that that pre-administration of OA protects dopamine neurons from the toxic effects of HDA[31,32]. Similarly, OA exerted neuroprotective effects on HDA-induced PD in rats by alleviating microglial activation[46,47]. In addition, OA derivatives displayed neuroprotective actions by repressing the expression of α-synuclein and the generation of ROS provoked by rotenone treatment. Additionally, an autophagy biomarker i.e., microtubule-associated protein 1A/1B-light chain 3 (LC3II), was increased significantly. These results suggest that OA and its derivatives could be a new class of prophylactic or therapeutic compounds for PD therapy[48].
OA injection during the last 14 d of fluoride treatment considerably recuperated the fluoride-induced brain injury by modulating brain metabolism. The beneficial neuroprotective impacts of OA in ischemic brain injury suppressed glial activities that promote neurotoxicity while raising glial activities that promote neuronal survival[30,33,47].
The pretreatment of rats with OA before the induction of cortical hypoxia by cobalt chloride injection produced a decreased neuronal degeneration and glial activation and improved brain injury[30]. Moreover, OA mitigated the neuronal degeneration and synaptic changes produced by Aβ25-35 in an AD model. OA treatment significantly increased the expression levels of brain-derived neurotrophic factor (BDNF), CaMKII, cAMP response element-binding (CREB) NMDAR2B, PKC and TRKB in an AD model. Thus, the ameliorative effect of OA was displayed as to maintain synaptic plasticity of the hippocampus in the Aβ-induced memory loss of AD rats[34].
Furthermore, it was reported that OA significantly hinders the Aβ23-35 induced differentiation of NSCs into astrocyte by down-regulating the JAK/STAT signalling pathway through increasing NGN1 expression. These outcomes suggest that OA might impede the progress of AD[44]. Finally, OA confers specific neuroprotection against amyloid precursor protein and TAU-induced neurodegeneration and ischemic injury modelled by oxygen-glucose deprivation in organotypic brain slice models[35].
OA mitigated the memory deficits in a cholinergic blockade-induced cognitive deficit mouse model. A single injection of OA significantly improved the latency in a passive avoidance learning assay, spontaneous alternation behaviour in the Y-maze and the exploration time on the novel object recognition assay. These behavioural results implied that OA reverses the cognitive impairment caused by scopolamine. At the molecular level, it was revealed that OA intensified CREB protein and extracellular-signal-regulated kinase 1/2 (ERK1/2) phosphorylation and BDNF expression in the hippocampus[36]. Similarly, augmented ERK/2, CREB and BNDF phosphorylation which was associated with the upregulation of miR-132 was reported for the antidepressant-like effect of OA. Yi et al[37] showed that a 3 wk of OA treatment in a chronic unpredictable mild stress model attenuated anhedonic and anxiogenic behaviours. All these studies confirm that OA might be a potential therapeutic means for the treatment of cognitive deficits and depression.
OA treatment inhibited the development of experimental autoimmune encephalomyelitis (EAE) in mice by reducing the activation of microglial cells, protecting blood-brain barrier (BBB) integrity, and preventing the infiltration of inflammatory cells into the CNS[26,49-51]. EAE mice treated with OA exhibited decreased levels of TNF-α and cytokines in CNS tissue without toxicity[52-56]. Similar results were also observed with a natural derivative isolated from caper[57]. OA and its derivatives improved neuroinflammation by suppressing the secretion of pro-inflammatory cytokines CCL-5, CXCL-9, CXCL-10, IL-6, IL-1β, NF-κB and TNF-α[57-59]. Additionally, the expression of genes involved in myelination/remyelination was increased significantly. Therefore, these studies have shown that OA possesses neuroprotective effects)[30-59].
Hepatoprotective effects
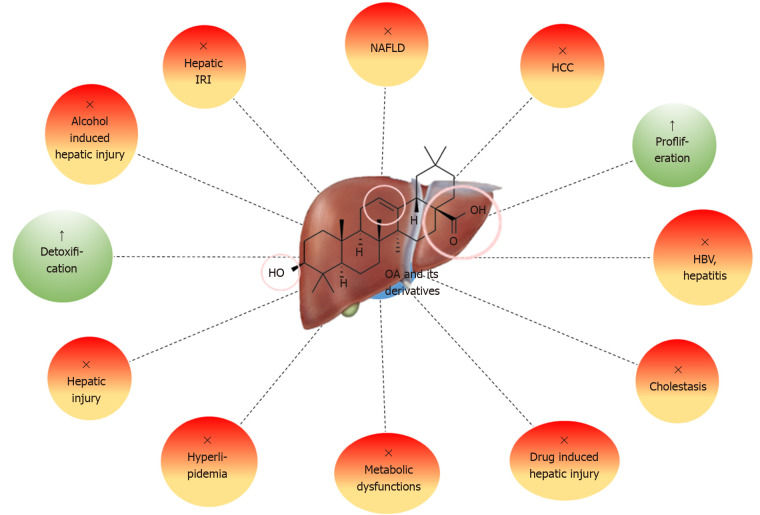
One of the most remarkable pharmacological impacts of OA and its derivatives is hepatoprotection (Figure 5). OA protects against diverse range of hepatotoxic agents, including metals, alcohol, bile acids, natural and synthetic toxins, drugs, viral or microbial agents and ischaemic perturbations. OA and its derivatives perform important protective roles in the instigation of acute liver injury induced by alcohol, carbon tetrachloride (CCl4), acetaminophen (APAP) and phalloidin (Table 4)[59-70].
Figure 5.
Hepatoprotective effects of oleanolic acid and its natural and synthetic derivatives. OA: Oleanolic acid; IRI: Ischemia-reperfusion injury; NAFLD: Non-alcoholic fatty liver disease; HCC: Hepatocellular carcinoma; HBV: Hepatitis B virus.
Table 4.
In vivo hepatoprotective effects and related mechanisms of action of oleanolic acid and its natural and synthetic derivatives (2014-2020)
| Disease model/ physiology | Effect |
Mechanism |
Compound | Dosei | Ref. | |
| ↑↑↑ | ↓↓↓ | |||||
| Hepatic injury (mice, EtOH) | Prevents ethanol induced liver injury, hepatoxicity | nNRF-2, HO-1, SOD-1, CAT, GR, hepatic GSH, ATP | sALT, sAST, CYP2E, ADH, TNF-α, IL-6, sTG, sLDH | OA | 10 mg/kg·d, 30 d | [60] |
| Hepatic injury (rats, CCl4) | Hepatoprotective | SOD, GPX | ALT, AST, LDH | OA, OA-Xs | 15 mg/kg | [61] |
| Hepatic fibrosis (HSCs, HEPG2, BEL-7402, LO-2; mice, CCl4) | Hepatoprotection | Apoptosis, Ca2+ | MitMP, sALT, sAST | OA-amino acids | 20 mg/kg, IC50 > 50 µmol/L | [62] |
| Hepatic fibrosis (rast, CCl4) | Anti-hepatic fibrosis | - | sALT, sAST, Liver indices | OA-Xs | 14-28 mg/kg·3 d, 9 wk | [63] |
| Hepatic injury (mice) | Hepatoprotective | NQO1 | mKC, MIP-2, OATP-1B2, GADD-45, CHOP-10, sALT, sMDA, pJNK, HO-1. | OA | 22.5 mg/kg·d, 3 d | [64] |
| Cholestasis (HEPG2) | Obstructive cholestasis | urinary BA, MRP-3, MRP-4, MRP-2, NRF-2 | sBA, sBil, sAST, sALT, sALP, nNRF-2, BSEP, | OA | 20 mg/kg, i.p, 1-50 µmol/L | [65] |
| Cholestasis (mice, LCA) | Cholestasis | MRP-2, MRP-3, MRP-4, NRF-2 | sALT, sALP, sAST, tBA, tBIL, SULT-2A1 | OA | 5-20 µg/kg | [66] |
| Hepatic NAFLD (rats, HFD) | Anti-NAFLD via AMPK-related pathways | HGF, ICAM, IGF-1, IGFBP-3, IGFBP-5, IGFBP-6, lipocalin-2, MCP-1, M-CSF, PREF-1, RAGE, GLUT-2, LDLR, pAMPK, pAKT, pGSK-3β, | TC, TG, LDL-C | OA-Xs | 60 mg/kg·d, 4 wk | [67] |
| Hepatic IRI (mice) | HO-1/Sesn2 signaling pathway | PI3K, HO-1, pAKT | sAST, sALT | OA | 30 mg/kg·d, 7 d | [68] |
| Hepatic IRI (rat) | Protects agaist hepatic IRI | pPI3K, pAKT, pGSK-3β | SALT, IL-1β | OA | 100 mg/kg·d, 7 d before IRI | [69] |
| Hepatic IRI, (mice) | Alleviate hepatic IRI | BCL-2 | apoptosis and autophagy, ALT, AST, CASP-3, CAPS-9, BAX, Beclin 1, LC3, TNF-α, HMG-B1, TLR-4, pJNK | OA | 30-60 mg/kg, 7 d | [70] |
OA: Oleanolic acid; OA-Xs: Natural derivatives of oleanolic acid; IRI: Ischemia-reperfusion injury; NRF-2: Nuclear factor erythroid-2-related factor 2; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; IL: Interleukin; TNF-α: Tumor necrosis factor-α; NAFLD: Non-alcoholic fatty liver disease; HFD: High-fat diet; LDH: Lactic dehydrogenase; MRPs: Multidrug resistance-associated proteins; JNK: cJUN NH2-terminal kinase.
The hepatoprotective effects of OA and its derivatives against CCl4-caused liver injury involved decreasing the increased serum levels of alanine aminotransferase (ALT), lactic dehydrogenase, aspartate aminotransferase (AST) and hepatic malondialdehyde (MDA) levels and increasing SOD and GPX activities. These biochemical attenuations were further supported by histochemical analyses[61-63].
Esculentoside A (EsA) is an OA derivative that treatment attenuated CCl4- and GalN/LPS-induced acute liver damage in mice. The prophylactic impact of EsA involved the inhibition of the inflammatory response such as IL-1β, IL-6 and TNF-α and oxidative stress, and the underlying mechanism included the peroxisome proliferator-activated receptor (PPAR)-γ, NF-κB and ERK signalling pathways[63]. EsA also exhibited protective effects against APAP, which is known to account for overdose toxicity for the majority of acute liver failure cases. EsA treatment attenuated APAP-induced serum AST and ALT levels and stimulated NRF-2 activation and glutathione (GSH) production. Additionally, it significantly increased the phosphorylation of AMP-activated protein kinase (AMPK) and serine/threonine kinase (Akt), as well as glycogen synthase kinase-3 beta (GSK-3β) suggesting that EsA potentiates the NRF-2-controlled survival process through the AMPK/AKT/GSK-3β pathway[71]. Similarly, the induction of antioxidant defence and suppression of ER stress and inflammatory responses by the NRF-2 battery as an OA-induced protection against phalloidin-induced hepatotoxicity were reported[64]. OA reduced the liberation of inflammatory agents and liver enzymes and prevented ConA-induced liver injury. OA treatment decreased the phosphorylation of cJUN NH2-terminal kinase (JNK) and increased the expression levels of PPAR-α[72]. Another NRF-2 mediated protective role of OA was reported against LCA-induced hepatotoxicity and obstructive cholestasis, whereby NRF-2-mediated upregulation of multidrug resistance-associated proteins was possibly involved[65,66].
Alcoholic liver disease (ALD) is one of the main causes of death worldwide, and oxidative stress was found to be an important factor in the pathogenesis of ALD damage. OA plays an important role in preventing alcohol-induced oxidative injury by decreasing the upregulation of serum AST, ALT and ATP levels while increasing the reduced hepatic GSH level and SOD and CAT activity. The protective effect of OA involved the uprising of anti-oxidative pathways such as NRF-2, HO-1, SOD-1 and GR expression and the suppression of pro-inflammatory cytokines, for instance, TNF-α and IL-6[60]. One of the important enzymes in alcohol-instigated toxicity is CYP2E1, which produces both toxic aldehydes and free radicals from ethanol and is suppressed by OA[73].
Non-alcoholic fatty liver disease (NAFLD) is another highly prevalent liver disease involving disrupted metabolism. It was found that the neonatal administration of OA exhibited hepatoprotective effects on the subsequent development of dietary fructose-induced NAFLD in adulthood, as evidenced by lower NAFLD scores for inflammation and steatosis and liver lipid content[74]. In addition, OA significantly inhibited the transactivation of liver X receptor α and its target genes, resulting in the selective decrease in hepatocellular lipid content, which is beneficial in the treatment of NAFLD[75]. In addition, OA enhanced the phosphorylation of AMPK in hepatocytes. Similarly, 3-Acetyl-OA (AOA) exerted a protective effect on hyperlipidemia in NAFLD rats via AMPK-regulated pathways[67]. Thus, OA shows prophylactic and therapeutic effects against NAFLD complications and shows great promise as a possible natural therapeutic agent for the treatment of liver diseases[60-70,76].
Anti-diabetic effects
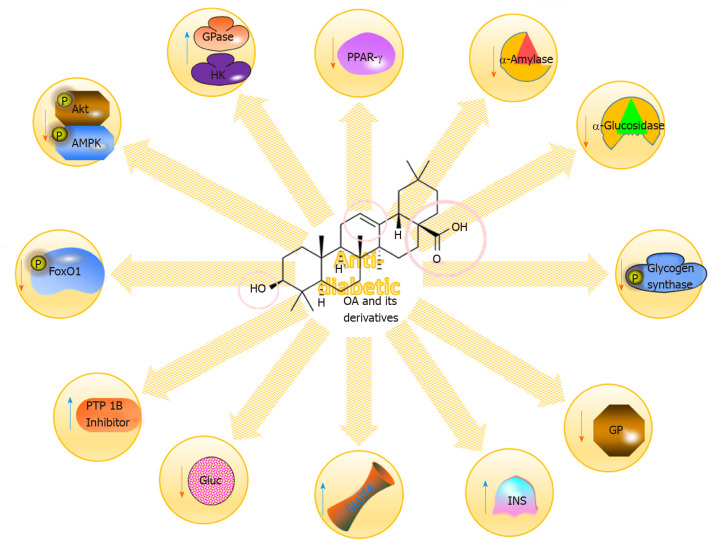
Diabetes is a complicated, progressive and chronic disorder that results from impaired insulin secretion or sensitivity. Type 2 diabetes (T2DM) is a common form of diabetes that is described as hyperglycaemia resulting from either insulin resistance or insufficient insulin secretion by pancreatic β-cells. Increasing evidence illustrates that T2DM is correlated with obesity, as well as with the development of several comorbidities, including cardiac, hepatic, and renal disorders. It is also consolidated with different metabolic complications affecting organs such as the arteries, eyes, kidney and nerves (Figure 6)[77-79].
Figure 6.
Some of the molecular mechanisms for the anti-diabetic impacts of oleanolic acid and its derivatives. OA: Oleanolic acid; PPAR: Peroxisome proliferator-activated receptor; Akt: Serine/threonine kinase; AMPK: AMP-activated protein kinase.
Plant-derived OA alleviated hyperglycaemia by decreasing HBA-1c and EPO concentrations in streptozotocin (STZ)-induced diabetic rats. Furthermore, it notably increased RBC count and other RBC indices, increased the antioxidant status of the RBCs and decreased oxidative stress[80]. In addition, the anti-diabetic effect on the insulin signalling pathway in the skeletal muscle of STZ-induced rats was fully elucidated. It was found that phosphorylated (p)-AKT and p-glycogen synthase (pGS) expression was increased and that the activation of the insulin signalling pathway was enhanced by OA[81-83]. The protective effect of OA is also associated with therapeutic memory, as evidenced by the maintenance of reduced glycaemic levels in mice 4 wk after the termination of OA treatment. This therapeutic memory was associated with FOXO-1 acetylation[84]. Additionally, HDACs 4 and 5 and G6Pase expressions were suppressed while histone acetyltransferase 1 expression was increased, suggesting that enzymes involved in epigenetics may have a role in sustained glycaemic control in T2DM, particularly with OA treatment[84-86]. The anti-diabetic action of OA is mediated in part through the reduction of ghrelin expression, reduced food intake[87]. Furthermore, OA prevents and ameliorates the insulin resistance induced by Aroclor 1254 treatment in mice. It notably suppressed the Aroclor 1254-induced increase in ROS, oxidative agents, and NADPH oxidase 4 (NOX-4) expression while upregulating the decreased expressions of glutamate-cysteine ligase catalytic subunit (GC-LC), glutamate-cysteine ligase modifier (GC-LM) GPX-1, SOD-1 and SOD-2[88]. These effects were suggested to be mediated by an increase in PPAR-γ signalling through the upregulation of hepatocyte nuclear factor 1b[88]. These results strongly indicate the prophylactic effect of OA on insulin resistance and related metabolic dysfunctions (Table 5)[80,81,84,87-101].
Table 5.
In vivo antidiabetic effects and related mechanisms of action of oleanolic acid and its natural and synthetic derivatives (2014-2020)
| Disease model/ physiology | Effect |
Mechanism |
Compound | Dose | Ref. | |
| ↑↑↑ | ↓↓↓ | |||||
| STZ-induced diabetic rat | STZ ind diabetes | RBC, SOT, GPX | sGLU, HBA-1c, EPO, MDA | OA | 80 mg/kg, twice, 5 wk | [80] |
| STZ-induced T2DM rats | Antidiabetic | p-AKT | pGS, GP | OA | 80 mg/kg, 14 d | [81] |
| T2DM mice | Glycemic control | pFOXO-1, AcFOXO-1, HAT-1, pHDAC-1, pAKT, pGSK-3β | sGLU, G6Pase, HDAC5/4, pAMPK, pSIRT-1, PEPCK, SCD-1,SREBP-1c | OA | 100 mg/kg·d, 4 wk | [84] |
| STZ-induced T2DM rats | Antidiabetic | - | sGLU, sGhrelin, | OA-Xn | 80 mg/kg·2 d, 5 wk | [87] |
| Aroclor 1254-treated mice | OA-stimulated HNF-1b-endogenous antioxidant activity, protects against adioposity | SOD1, SOD2, GC-LC, GC-LM, GPX-1 CAT, HNF-1b, GLUT-4 | ROS, oxidant products, NOX-4, PPAR-γ, Adionopectin, AGP-AT2, αP2, CD36 | OA | 50 mg/kg, 1 h before Aroclor 1254 treatment every 3 d for 10 wk | [88] |
| STZ-induced and db/db diabetic mouse models; NCI-H716 | Antidiabetic and hepatoprotective effects | GLP-1, pPKA, sINS | sGSP, sALT, sAST, sGLU, sFBG, sTG, sHDL-C | OA, OA-Xs | 100 mg/kg·d | [89] |
| STZ-nicotinamide-induced type 2 diabetes in mice; C2C12 cells | Anti-diabetic | pAMPK, GLUT4, CPT1 | sGLU, sLDL-C, sFFA, ACC, pPKB | OA-Xn (CHS) | 25-200 mg/kg·d, 14 d; 0.1-10 µg/mL | [90] |
| STZ-nicotinamide-induced type 2 diabetes in mice | Against diabetes induced hiperlipidemia and hypergylcemis | HK, G6Pase, GK, GSH, sHDL-C, SOD, CAT, GPX | SALP, sAST, sALT, sTC, sTG, LDL, IL-6, TNF-α | OA-Xn | 20 mg/kg | [91] |
| HF diet-induced metabolic dysfunctions (rats) | Strategic intervention for the long-term prevention of metabolic diseases such as T2D and obesity via AMP-Activated Protein Kinase patway | AMPK, GLUT-4, CPT-1, AdipoR1, AdipoR2, | TNF-α, IL-6, MCP-1, VEGF | OA | 60 mg/kg, 14 d | [92] |
| HF diet-induced metabolic dysfunctions (rats) | Potentially protects against the development of fructose-induced metabolic dysfunction | GLUT-4, GLUT-5 NRF-1, CPT-1, ALDO-B, FFAs | ACC-1, FAS | OA | 60 mg/kg, 7 d | [93] |
| HFF diet-induced metabolic dysfunctions (rats) | Protected against the development of health outcomes associated with fructose | terminal body mass, visceral fat mass, epididymal fat | sINS | OA | 60 mg/kg, 7 d | [94] |
| HFF diet-induced metabolic dysfunctions | Nano-OA was able to attenuate HFF diet-induced lipid accumulation in the liver | CAT, SOD | MDA, NO | Nano-OA | 25 mg/kg·2 d, wk | [95] |
| T2DM in prediabetic patients (Human) | Prevention of type 2 diabetes in prediabetic patients | - | sGLU, T2DM incidence | OA | 30 mg/kg | [96] |
| α-glucosidase inhibition | α-glucosidase inhibition, decreased blood glucose | - | α-glucosidase | OA-Xs | 0.330.98 µmol/L | [97] |
| db/dc T2DM mice | Anti-diabetic | GS, pPI3K, pAKT, pAMPK, pACC | sLDL, sTG, sTC, GP, PGC1a, PEPCK1, GLUT-2, G6Pase, pmTOR, PCREB, sGLU, sINS | OA + Metmorfin | 250 mg/kg·d, 28 d | [98] |
| Diet-induced pre-diabetic rat model | Prevent the onset of CVDs during pre-diabetes stage | - | TGs, LDL-C, IL-6, TNF-α, CRP, MAP, hearts weights | OA | 80 mg/kg·3 d, 12 wk | [99] |
| Diet-induced pre-diabetic rat model | Anti-diabetic | - | Body weights, sGhrelin, HBA-1c, sGLU, sINS, muscle Glycogen | OA | 80 mg/kg·3 d, 12 wk | [100] |
| MetS | Protects against fructose-induced oxidative damage; against MetS | GPX, SOD, CAT, GSH | OA | 60 mg/kg | [101] | |
OA: Oleanolic acid; OA-Xn: Natural derivatives of oleanolic acid; OA-Xs: Synthetic derivatives of oleanolic acid; STZ: Streptozotocin; HAT-1: Histone acetyltransferase 1; FFA: Free fatty acid; CVDs: Cardiovascular diseases; PGC-1b: Peroxisome proliferator-activated receptor-g coactivator-1b; NRF-1: Nuclear factor erythroid-2-related factor 1; HNF: Hepatocyte nuclear factor; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; IL: Interleukin; TNF-α: Tumor necrosis factor-α; PPAR: Peroxisome proliferator-activated receptor; T2DM: Type 2 diabetes; MetS: metabolic syndrome; GSH: Glutathione.
OA derivatives also exhibit significant anti-diabetic effects. 12,13 DihydroOA methyl ester (DKS26) reduced the plasma levels of glucose, glycosylated serum protein, ALT and AST. DKS26 also alleviated the glucose tolerance and plasma lipid profiles while raising plasma insulin levels and glucagon like peptide 1 (GLP-1) release, which was accompanied by increased levels of cAMP and phosphorylated PKA. Thus, DKS26 is a hypoglycaemic therapeutic that augments the release and expression of GLP-1 mediated by the activation of the cAMP/PKA signalling cascade[89,102]. Similarly, the natural OA derivative CHS isolated from the root bark of Aralia taibaiensis exerted an anti-diabetic effect by decreasing blood glucose, triglyceride, free fatty acid and LDL-cholesterol levels in STZ/nicotinamide-induced T2DM rats by activating AMPK[90]. One new OA derivative, 2a,3b,23a,29a tetrahydroxyolean-12(13)-en-28-oic acid, purified from Malva parviflora demonstrated a similar anti-diabetic effect on a T2DM mice model[91]. Furthermore, a series of synthetic OA derivatives showed inhibitory activity on protein tyrosine phosphatase 1B, which is known to be involved in insulin resistance[103,104].
The long-term neonatal intake of OA significantly increased AMPK, adiponectin and GLUT-4 expression while decreasing TNF-α and IL-6 in rats that were fed a high fructose diet, suggesting a potential treatment for the long-term prevention of metabolic diseases such as T2DM and obesity[92-94]. Additionally, a nanoformulation of OA efficaciously mitigated the increased levels of NO and MDA and serum CAT and SOD activities in rats fed a high fat and fructose diet[95]. Thus, OA is a remarkable prophylactic agent for the long term prevention of diabetes.
In addition to animal models, pre-diabetic human patients were randomised to receive OA-enriched olive oil (equivalent dose, 30 mg OA/d) [intervention group (IG)] or the same oil not enriched with OA [control group (CG)] and followed for the incidence of new-onset of T2DM. The results showed that in total, 38 new T2DM onset events occurred, 31 in the CG and 17 in the IG. Therefore, the intake of OA-enriched olive oil reduced the risk of developing T2DM in pre-diabetic patients, suggesting that OA can be used as a functional food and therapeutic for the prevention of T2DM[92-101].
Anti-osteoporotic effects
Osteoporosis is a persistent skeletal disorder characterised by bone microarchitectural deterioration[105]. It has become a significant health issue within the elderly population and has led to a considerable socioeconomic burden in society. Scientists are working to develop new therapeutics to treat the development of the disease, and natural products become widespread worldwide[106].
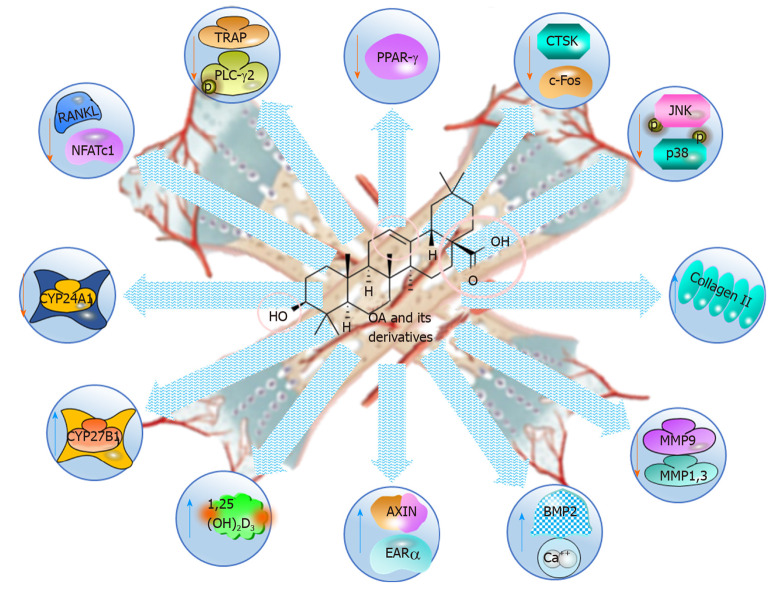
OA is shown to be an anti-osteoporotic natural product, as it increases bone density and remodelling by regulating calcium and vitamin D metabolisms (Table 6 and Figure 7)[107-116]. Rats fed OA-enriched diets had improved bone characteristics, higher serum concentrations of 1,25(OH)2D3 and less endogenous calcium excretion than did the control group resulting in higher calcium mass[108]. Furthermore, the density and microarchitectural characteristics of the bones were significantly improved, 1,25(OH)2D3 was increased, the renal expression of CYP27B1 and increased, and urinary of Ca2+ excretion was increased in mature C57BL/6 ovariectomised (OVX) mice[107]. In addition, OA significantly induced the mRNA and protein expression of renal CYP27B1 while suppressing CYP24A1 in human proximal tubule HKC-8 cells, suggesting that its effects were associated with calcium and vitamin D metabolism. Additionally, OA acetate promoted the development and reshaping of bones by properly modulating osteoblast, osteoclast and inflammatory activities with TGF-β regulatory measures in an experimental periodontitis model in mice[109].
Table 6.
In vivo anti-osteoporotic and related mechanisms of action of oleanolic acid and its natural and synthetic derivatives (2014-2020)
| Disease model/ physiology | Effect |
Mechanism |
Compound | Dose | Ref. | |
| ↑↑↑ | ↓↓↓ | |||||
| OVX-mice | Increased bone mineral density | 1,25(OH)2D3, renal CYP27B1 | Urinary Ca excretion, CYP24A1 | OA | 50 or 100 mg/kg·d, 6 wk | [107] |
| OVX-mice | Better bone density | 1,25(OH)2D3 | Decreased urinary excreation of Ca | OA | 0.67 g/kg in diet, 6 wk | [108] |
| Glucocorticoid-induced osteoporosis (rats) | Bone protection | Bone density of lumbar and femur were reversed, osteocalcin, sCa2+ | - | OA | 9 mg/kg, 14 d | [110] |
| Bone marrow macrophage (mice) | Inhibit osteoclastogen-esis | - | c-FOS, NFAT-c1, TRAP, CTSK,MMP-9 | OA | 10 mg/kg·2 d, 12wk | [111] |
| OVX- mice | Inhibit osteoclastogen-esis | - | NFAT-c1, c-FOS, MMP-9, CTSK, TRAP, CAR-2 | OA | 10 mg/kg·2 d,3 mo | [113] |
| Cartilage degeneration in osteoarthritis (rats) | Anti-cartilage damage | Collagen II | MMP-3, MMP-1, MMP-13, ADAMTS-4, -5, | OA | 1-100 µmol/L, 50-100 µmol/L/rat single | [115] |
| Experimental periodontitis (mice) | Bone formation and remodeling through proper modulation of osteoblast and osteoclast | BMP-2,6,7; AXIN-2, β-CAT, LEFT, TWIST | IL-6, | OA-Xs | 2µL (50 ng/µL)/d, 1-3 wk | [116] |
OVX: Ovariectomised; OA: Oleanolic acid; TRAP: Tartrate-resistant acid phosphatase; CTSK: Cathepsin K; MMP: Matrix metalloproteinase; CAR: Constitutive androstane receptor; IL: Interleukin.
Figure 7.
Anti-osteoporotic and bone protective effects of oleanolic acid and its derivatives, illustrating the molecular mechanisms. OA: Oleanolic acid; PPAR: Peroxisome proliferator-activated receptor; CTSK: Cathepsin K; JNK: cJUN NH2-terminal kinase; MMP: Matrix metalloproteinase; NFAT-c1: Nuclear factor of activated T-cells c1; TRAP: Tartrate-resistant acid phosphatase.
As demonstrated by the reversal of biochemical markers and bone density of the lumbar and femur, the OA defends against the osteoporosis caused by prednisone[110]. In a glucocorticoid-induced model of rat osteoporosis, a total of 25 possible biomarkers were identified, and OA had a regulatory effect on 17 of these biomarkers associated with some important metabolic pathways, for instance, linoleic acid, valine and isoleucine metabolism, phenylalanine, tyrosine, tryptophan, cysteine and methionine biosynthesis[110].
OA also suppressed the osteoclastogenesis at the early stage and possibly at the late stages in bone marrow macrophages (BMMs), suggesting as a prophylactic and therapeutic agent for bone loss in postmenopausal women[111,112]. Mechanical studies revealed that the key parameters inhibited by OA were the c-FOS and nuclear factor of activated T-cells c1 (NFAT-c1), both in vitro RANKL-pretreated BMMs and in vivo in OPG-knockout mice[111]. In fact, reproducible results demonstrated that OA inhibited the functions of the osteoclastic genes, including tartrate-resistant acid phosphatase, cathepsin K, and matrix metalloproteinase 9, in the late stage of osteoclastogenesis[111,113]. Interestingly, the inhibition of RANKL- induced osteoclastic differentiation in BMMs with the OA acetate (OAA) derived from Vigna angularis without cytotoxicity was also reported[114]. RANKL-induced osteoclastogenesis was blocked by OAA through PLCγ2-Ca2+-NFAT-c1 signalling[113,114]. The findings suggest that OA is a potential drug candidate for the management of postmenopausal osteoporosis and bone loss[107-116].
Anti-cancer effects
Cancer is surpassing cardiovascular diseases as the leading cause of death worldwide[117]. Thus, the search for the compounds that selectively kill cancer cells with a mild or no influence on healthy cells is still in progress. In this sense, OA and its derivatives have been observed to exert many anti-cancer actions on various types of tumours. Their molecular mechanisms of these substances are diverse, such as inhibiting the proliferation of cancer cells, preventing cancer cell migration and invasion, restraining angiogenesis, and inducing autophagy and apoptosis. Although a very large number of in vitro studies have been carried out showing the inhibition of carcinogenesis, only a few in vivo studies have confirmed that OA and its derivatives are promising anti-cancer agents (Table 7)[118-125]. Researchers introduced various R groups, particularly at the C3 and C28 positions, to increase the anti-cancer potential of OA[11,126,127]. Angiogenesis is one of the hallmarks of cancer and is targeted by OA[128-130]. Angiogenesis is an essential means of cancer progression, and OA treatment significantly reduced the intratumoural microvessel density (MVD) in CRC mice and inhibited tumour growth[131-133]. The anti-metastatic impact of novel synthetic OA derivatives might have resulted from the downregulation of the VEGF/ pFAK/pJNK/pERK/NF-κB cascade[132]. Therefore, OA inhibited the proliferation of highly invasive cells and acted as a chemopreventive agent in cancer[8,11,118-126,134-137].
Table 7.
In vivo anticancer effects and related mechanisms of action of oleanolic acid and its natural and synthetic derivatives (2014-2020)
| Disease model/ physiology | Effect |
Mechanism |
Compound |
Dose/IC50 /Ki. |
Ref. |
|
| ↑↑↑ | ↓↓↓ | |||||
| Liver, lung and prostate cancer | Inhibits proliferation and induces apoptosis | cPARP-1, | pAKT, NF-κB, pmTOR | OA-Xs | 7.5 mg/kg·d; d | [118] |
| PC3 prostate | Inhibits proliferation and induces apoptosis | HIF-1a, NAC-1 | SENP-1 | OA-Xn | 10 mg/kg·d; 20d | [119] |
| Colorectal cancer mouse xenograft model | Induce apoptosis | BAX, P21, P53 | BCL-2, CYC-D1, CDK-4, AKT p70S6K and MAPK | OA | 16 mg/kg·d, 16d | [120] |
| Gastric cancer | Induce autophagy | pAMPK | pmTOR, pPI3K, AKT, pERK1/2, P38, pmTOR | OA | 100 mg/kg·d; 7d | [121] |
| Kras G12D/+ ;Pdx-1-Cre (KC) pancreactic cancer | Inhibits infiltration | IL-6, CCL-2, VEGF, G-CSF | CDDO-imidazolide | 25 or 100 mg/kg diet, 4 or 8 wk | [122] | |
| Lung carcinoma | Inhibits proliferation | miR122, HNF-1a, HNF-3b, HNF-4a, HNF-6 | CCNG-1, MEF-2D | OA | 40, 120 mg/kg·d; 4 wk | [123] |
| Ovarian and endometrial cancer | Inhibition of profiferation | PARP, BCL-2, CASP-8,-3, -7. | OA-Xs | 10-40 mg/kg·d; 21 d | [124] | |
| Prostate cancer | Cell cycle arrest | AKT/mTOR, pAKT, pmTOR | OA-Xs | 8.5-17 mg/kg·d; 21 d | [125] | |
NF-κB: Nuclear factor-κB; OA: Oleanolic acid; OA-Xn: Natural derivatives of oleanolic acid; OA-Xs: Synthetic derivatives of oleanolic acid; CDDO: 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid; HNF: Hepatocyte nuclear factor; ERK: Extracellular-signal-regulated kinase.
Other effects
Although studies have mainly focused on anti-inflammatory, neuroprotective, anti-osteoporosis, anti-diabetes effects, OA and its derivatives are reported to possess broad biological activities such as antibacterial, antioxidant, anti-hyperlipidaemic, nephroprotective, cardiovascular protection, anti-infertility, and anti-obesity (Table 8)[29,116,138-178].
Table 8.
In vivo miscellaneous effects and related mechanisms of action of oleanolic acid and its natural and synthetic derivatives (2014-2020)
| Disease model/ physiology | Effect |
Mechanism |
Compound | Dose/IC50 /Ki. | Ref. | |
| ↑↑↑ | ↓↓↓ | |||||
| Atherosclerosis | Anti-atherosclerotic | Ang1-7, ANG, NO, eNOS | IL-1β, TNF-α, and IL-6 | OA | 0-160 µmol/L | [29] |
| Immune suppression | ZFP-459, FMO-2 | OA-Xs | [116] | |||
| T. cruzi, L. braziliensis, L. infantum | Anti-protozoal | - | OA, OA-X | 3.3-89 µmol/L | [138] | |
| Leishmania species | Anti-parasitic | CYP51, ergosterol synthesis | OA | 30.4-68.7 µmol/L | [139] | |
| P. berghei malaria | Anti-malaria | TNF-α, IL-6, IL-10, hepcidin | OA | 34 mg/kg, 5 d | [140] | |
| HBV | Anti-viral | HBS-Ag, HBE-Ag, HBV DNA replication | OA-Xs | 8.6-38.1 | [141] | |
| Allergic conjunctivitis | Anti-allergic and anti-inflammatory | IL-10 | Allergen-specific IgGs, sPLA2 -IIA, Th2, RWP-T-Cell dif, EOL-1 , IL-33, MCP-1 | OA | 50 mg/kg·d, 5 d after sens | [142] |
| Asthma | Anti-asthmatic | tBET, FOX-P3 | IL-5, IL-13, IL-17, OVA-IgE, GATA-3, RORγt, | OA | 2 or 20 mg/kg·2 d, 5 wk | [143] |
| Atherosclerosis | Anti-artherosclerotic | NRF-2, HO-1, SOX, NO, CAT, GPX, GSH, HDL | LOX, NADPH Ox, LDL, TC, TG, pGP91, pP67, pP7 | OA | 15-50 mg/kg·d, 3 wk; 5-20 µmol/L | [144] |
| Vascular injury | Prevent endothelial oxLDL effect | CASP, NO, pAKT, peNOS, | OA-Xn | 5 and 100 µmol/L | [145] | |
| Low-density lipoprotein receptor knockout (LDLR −/− ) mice | Review Atherosclerotic | AdipoR1, PPAR-γ | AdipoR2, TC, LDL-C | OA | 25 mg/kg·d, 5 wk | [146] |
| Myocardial injury | Cardioprotection, hyperglycemia-induced myocardial injury | CASP-3/9, BAX, pERK1/2, HOMER-1α, ERK1/2, SIRT1 | BCL-2, ROS | OA-Xn | 12.5-50 µmol/L | [147] |
| Carotid artery injury | Proteccts diabetes induced artery injury | body weights, serum NO | endothelin 1, IL-1β, IL-6 , IL-18, NLRP-3, CASP-1 | OA | 100 mg/kg·d, 6 wk | [148] |
| Vascular injury | Hypotensive | physiological data | physiological data | OA, OA-Xn | 0.1-100 µmol/L | [149] |
| Hiperlipidemia | Anti-hiperlipidemic | 17 genes (microarray), CACNA-1B | TC, TG, HDLC, 4 genes | OA | 3 tablets/d , 4 wk | [150] |
| Hiperlipidemia | Anti-hiperlipidemic likely via regulation of the miR-98-5p/PGC-1b axi | TC, TG, LDL, PGC-1b | OA | 20 mg/kg, 4 wk | [151] | |
| Fertility | Recovered fertility | increasing the permeability of the germinal epithelium | OA | 30 mg/kg | [152] | |
| Fertility | Infertility treatment | OCT-4, GDF-9, STRA-8, MVH, ZP-2, ZP-3, ITG-α6, TP-2, | SCP-3, ZP-1, ITG-β1 | OA | 3 µg/mL | [153] |
| Fertility/Reproductive function | Rejuvenates testicular function | BCL-2 | pNF-κB, IL-1β , COX-2 TNF-α, H2AX, pP53, BAX, P38 | OA | 5-25 mg/kg·d, 24 wk | [154] |
| Renal fibrosis | Attenuates renal fibrosis | NRF-2, HO, NQO-1, BAX, HSP-70 | BCL-2, | OA | N.R. | [155] |
| Nephropathy | Prevent diabetic nephropathy | sINS, SOD, adiponectin | TG, BUN, Cr, TGF-β, SMAD1/2 | OA | 100 mg/kg·d, 20 wk | [156] |
| Renal IRI | anti-Renal IRI | SOD, GPX, TT, eNOS, NRF-2, PPAR-γ, DDAHs | Cre, NGAL, TOS, NO, ADMA, NF-κB, ET-1 | OA-Xs | 20 mg/kg, 5 h before IR | [157] |
| Nephritis Lupus/SLE | Inhibition of Th17 differentiation | Th17, IL-17A, serum dsDNA, ROR-γt | OA-Xs | 0-10 µmol/L, 50 mg/kg | [158] | |
| MRSA | Anti-microbial | Microbe concentration | OA-Xs | 10-30 µg/mL | [159] | |
| Circadian clock | Mediates circadian clock | CLOCK, ELO-VL3, TUBB-2A CLDN-1, BMA-1 | AMY-2A5, USP-2, PER-3,THRSP | OA | 0.01% diet | [160] |
| Cisplatin induced nephrotoxicity | Prevent neprotoxicity | MAP-1A/AB, LC1 | CASP-3/9, PARP cleavage, ATG-5, ERK1/2, STAT3, NF-κB | OA | 10-40 mg/kg | [161] |
| Dermatitis/TPA-treated mouse ears | Inhibit dermatitis | MPO, COX-2, iNOS, TNF-a, IL-1β, pP65 | OA-Xn | 2, 5 or 10 µmol/L | [162] | |
| Diabetes induced cardiomyopathy | Prevent diabetic induced cardiomyopathy via Nrf2 | HO-1, SOD, NRF-2, | Glycogen, MDA, p-GS | OA | 80 mg/kg·2 d, 14 d | [163] |
| Diabetic mesangial cell injury | Diabetic renal fibrosis | PI3K/AKT/mTOR | Autophagy, PTEN, | OA | 10 µmol/L | [164] |
| Gut atrophy /piglet model | Prevent gut atrophy | TGR-5, FXR | OA | 50 mg/kg·d, 14 d | [165] | |
| Immune suppression | Immune suppressive, anti-RA | IL-10 | collagen specific sIgG, CD4+ INF-γ, IL-17α, IL-2-/4/6/1β, TNF-α, GM-CSF, MCP-1 , MMP-1/3 | OA-Xs | 1-10 mg/kg 18 times between 28 and 53 d after the initial immunisation | [166] |
| Immune suppression/glucocorticoid resistance | Protecting DEX induced GC impairment | Apoptosis, GR binding | GR-α | OA+I | 100 mg/kd·d, 21 d | [167] |
| Longevity | DAF-16, SOD-3, HSP-16.2 CTL-1 | OA | 0-600 µmol/L·2 d | [168] | ||
| Metal (MeHg) toxicity | Mitigate low-dose MeHg toxicity. | accumulation of metals in organs | OA-Xs | 40 µg/kg | [169] | |
| Muscle Atrophy | Reduces denervation induced muscle atrophy | CNTF, JNK-2, STAT3 | OA-Xs | 0.2-1 µmol/L | [170] | |
| Muscle atrophy | Anti-muscle atrophy | mTORC-1/P70, S6K, PAX-7, MYO-D, Myogenin | FOXO-1, MURF-1, Atrogi-n1 | OA-Xs | 1 µmol/L, 1-10 mg/kg | [171] |
| Myocarditis - myocardial İnjury | EA myocarditis | IL-10, IL-33 | HW/BW, BPN, IK-17, IL-6, TNF-α , Galectin | OA | 50 mg/kg·d, 21 d or 65 d | [172] |
| Obesity | Anti-obesity | octanoylated ghrelin production, PC-1/3, PC-2 | OA | 20-40 mg/kg, 7 d | [173] | |
| Obesity | Improves gustatory perception of lipids and exerts protective effects in obesity | CD36 | blood insulin and glucose, hepat,c TG, IL-6 | OA | 0.005% (w/v) for 16 wk | [174] |
| Renal injury | Prevent nephropathy | nNRF-2/tNRF-2, HO-1, KEAP-1, BAX | urinary 8-OHdG and 8-iso-PGF-2 α, BCL-2 | OA | N.R. | [175] |
| Renal IRI | Anti-Renal IRI; antioxidant, anti-inflammatory, and anti-apoptotic activities | SOD, GPX, GSH, CAT, IL-10, NRF-2, GGLc | BUN, Cr, KIM-1, LDH, MDA, IL-6, INF-γ, MPO, | OA | 12.5-50 mg/kg·d, 15 d | [176] |
| Sepsis | Lung damage, experimental sepsis | SOD, GPX, IL-6, IL-10, KC | iNOS, NRF-2, | OA | 10 mg/kg | [177] |
| Vascular injury | Prevent oxidative stress induced cell injury by with AKT/eNOS signaling pathway | NO, SOD, CAT, CASP-3, FAS, FASL, BCL-2 | MDA, BAX | OA | [178] | |
IL: Interleukin; TNF-α: Tumor necrosis factor-α; OA: Oleanolic acid; OA-Xn: Natural derivatives of oleanolic acid; OA-Xs: Synthetic derivatives of oleanolic acid; LDH: Lactic dehydrogenase; ERK: Extracellular-signal-regulated kinase; IRI: Ischemia-reperfusion injury; NRF-2: Nuclear factor erythroid-2-related factor 2; JNK: cJUN NH2-terminal kinase; FXR: Farnesoid X receptor; MMP: Matrix metalloproteinase; PGC-1b: Peroxisome proliferator-activated receptor-g coactivator-1b; PPAR: Peroxisome proliferator-activated receptor; NF-κB: Nuclear factor-κB; STAT3: Signal transducer and activator of transcription 3; GSH: Glutathione.
Since OA plays an important role in defending against pathogens in plants, it is expected to possess antimicrobial, antiviral, antifungal and antiparasitic activity against a wide range of pathogens. The antibacterial behaviour of OA and its derivatives was tested in specific bacterial strains[179]. Further mechanistic investigations suggested that the antiparasitic effect of OA might have resulted from its intearction with the sterol 14-α-demethylase (CYP51), a therapeutic target for leishmaniasis, which impairs the oxidant capacity of the parasite[138,139]. Importantly, OA also has the ability to improve parasitemia and anaemia through infection as an effective antimalarian agent[140]. The use of an OA-pectin patch removed malaria parasites and improved abnormal HCT values. In comparison, the analysis proved that the levels of IL-6, IL-10 and TNF-a were decreased by day 12. The results indicate that the OA-pectin patch released therapeutic OA doses to alleviate the cytokine release and to ameliorate anaemia caused by malaria. Transdermally administered OA can thus be a potent therapeutic agent for malaria and anaemia treatment[140]. OA and its derivatives are reported to exhibit pathogenic antiviral activities against HIV, hepatitis, porcine epidemic diarrhoea virus and influenza virus[141,180-183]. OA was shown to be a strong regulator of influenza haemagglutinin (HA). The conjugation of glucose with OA revealed that the HA inhibitory activity of OA was significantly increased with no obvious cytotoxic impact on the MDCK cells[180]. Similarly, another OA derivative exhibited anti- HBsAg, anti-HBeAg, and anti-hepatitis B virus antigens secretion activity in HepG2.2.15 cells with inhibitory effect on the viral replication rate superior to that of lamivudine[141,182].
As oxidative stress under different chronic conditions is considered to be involved in the pathogenic processes, the antioxidant impacts of OA have been investigated. For instance, a decreased intracellular oxidative stress in acute myocardial infarction (MI) was partly due to the protective function of OA[184]. OA has been reported to be a potential therapeutic for oxidative stress by inhibiting NO and activating NRF2-ARE signalling pathway[185]. It has also been found that OA exerts an anti-allergic effect in allergic diseases such as allergic conjunctivitis and asthma, that is modulated through the GATA-3 and RORγt pathways and through T-cell proliferation[142,143]. OA can, therefore, provide a modern prophylactic approach for allergic diseases and potential treatments.
Since cardiovascular diseases are among the leading causes of mortality and morbidity worldwide, the prophylactic and therapeutic effects of OA on cardiovascular disease have been observed. OA and OA derivative therapy also mitigated the high-fat diet mediated atherosclerosis in quail and ox-LDL provoked cytotoxicity in HUVECs by modulating LOX-1, through a decrease in NADPH oxidase and an increase in HO-1 and NRF2 expression[144,145,186]. A detailed study used three different animal models, including rabbits that mimicked atherosclerosis, C57BL/6J mice and low-density lipoprotein receptor knockout (LDLR−/−) mice, were applied to study the effect of OA on atherosclerosis[146]. All the models revealed that OA retarded the development of atherosclerosis by influencing serum lipid levels, lipid accumulation in the liver and intimal thickening of the artery, which involve genes in lipid metabolism: PPAR-γ, AdipoR1, and AdipoR2. Similarly, the protective effects of OA and its derivatives on diabetes-induced cardiomyopathy and cardiomyocytes injuries were reported to involve anti-oxidative and anti-inflammatory mechanisms, PPARγ, and NLRP3 inflammasome signalling pathways[147,148,187,188]. Furthermore, the antihypertensive effects of OA synthetic derivatives are attributed to a decrease in vascular resistance with no negative inotropic effect on the heart[149]. OA could ameliorate hyperlipidaemia in animal models by modulating CACNA1B, FCN, STEAP3, AMPH, and NR6A expression levels[150]. In addition, OA significantly decreased the hepatic expression levels of peroxisome proliferator-activated receptor-g coactivator-1b and the serum levels of triglycerides, total cholesterol, and LDL cholesterol[151]. Additionally, a semisynthetic OA derivative at C3 position was designed and synthesised to demonstrate farnesoid X receptor modulatory activity in regulating HDL and LDL levels and was found to be more effective[189].
OA was demonstrated to increase the fertility of mice involving reversible contraception in male mice by increasing the permeability of the germinal epithelium via reconstitution of the paracellular junctions between adjacent Sertoli cells[152]. In addition, OA efficiently restored testicular function by alleviating germ cell DNA damage and apoptosis through the inactivation of the NF-κB, P53 and P38 cascades and differentiating mouse ESCs into germ cells[153,154].
The nephroprotective activity of OA against oxidative stress-induced renal inflammation, renal fibrosis, drug-induced nephropathy and renal injuries was revealed with in vivo studies[155,156,190,191]. The beneficial effects of OA on renal fibrosis include reducing renal oxidative stress, increasing the nuclear translocation of NRF2, and mediating EMT in renal tubular epithelium[155,190]. Similarly, the activation of NRF2/HO-1 signalling with CDDO-Me treatment in chronic cyclosporine-induced kidney injury and renal ischemia-reperfusion injury revealed beneficial effects[191,157]. Furthermore, an acetylate OA derivative reduced RORγT development and prevented SLE pathogenesis in lupus nephritis caused by pristane, suggesting the possible use of OA as an SLE therapy[158]. These results support the nephroprotective, antibacterial, antioxidant, anti-hyperlipidaemic, cardiovascular protection, anti-infertility, and anti-obesity effects of OA and its derivatives[138-191].
Adverse effects
Increasingly, the adverse effects of the application of herbs used as an ACT are of global concern. In this sense, the paradoxical toxic effects of OA at higher doses and during long-term use have been suggested, as evidenced by liver injury characterised by cholestasis[5]. Not only OA but also other OA derivatives, in particular CDDO-Im and CDDO-Me, exhibit this paradoxical hepatotoxicity. Because of these adverse effects, phase-3 clinical trials with CDDO-Me were terminated[192]. Although the toxic potential of OA and OA-type triterpenoids was first observed in primary rat hepatocyte cultures, the major concern comes from in vivo studies[192-194]. Although OA is relatively non-toxic, it was shown that repeated oral OA administration produced cholestatic liver injuries in mice, illustrating the hepatotoxic potential of a presumed hepatoprotective compound[1,195,196].
In addition, interactions with phase I and phase II drug-metabolising enzymes such as cytochrome P450 (CYP450) and UDP-glucuronosyl- transferases (UGTs) or with the transcriptional inducers of these enzymes might cause adverse reactions. It has been demonstrated that OA alters pregnane X receptor and constitutive androstane receptor promoter activities, which regulate the catalytic activities of CYP3A4 and CYP2B6[197]. Additionally, the week inhibition of CYP3A4, UGT1A3 and UGT1A4 and solute carrier transporters activities were reported[198-200]. Therefore, information elucidating the drug-drug/drug-herb interactions associated with OA and its derivatives is essential to prevent these adverse reactions.
CONCLUSION
This review has presented multiple confirmations of the attenuation and amelioration of various diseases by applying either OA derived from plants or its synthetic and natural derivatives from in vivo investigations. OA and its derivatives have demonstrated diverse molecular mechanisms of action. However, it should be emphasised that there are no confirmations of that OA itself is a candidate for clinical trials since significant efforts have been made to synthesise OA derivatives with less toxic, more potent and bioavailable forms. Nevertheless, there is a reasonable amount of literature, as this literature fully explored in this review. OA and its derivatives have crucial prophylactic and therapeutic potential as an alternative and complementary therapies for diseases including ulcerative colitis, diabetes, cardiovascular diseases.
Footnotes
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
Manuscript source: Invited manuscript
Peer-review started: January 12, 2020
First decision: February 24, 2020
Article in press: May 1, 2020
Specialty type: Medicine, research and experimental
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu J, Sun XT S-Editor: Wang YQ L-Editor: A E-Editor: Ma YJ
References
- 1.Pollier J, Goossens A. Oleanolic acid. Phytochemistry. 2012;77:10–15. doi: 10.1016/j.phytochem.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 2.Žiberna L, Šamec D, Mocan A, Nabavi SF, Bishayee A, Farooqi AA, Sureda A, Nabavi SM. Oleanolic Acid Alters Multiple Cell Signaling Pathways: Implication in Cancer Prevention and Therapy. Int J Mol Sci. 2017:18. doi: 10.3390/ijms18030643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalaycıoğlu Z, Uzaşçı S, Dirmenci T, Erim FB. α-Glucosidase enzyme inhibitory effects and ursolic and oleanolic acid contents of fourteen Anatolian Salvia species. J Pharm Biomed Anal. 2018;155:284–287. doi: 10.1016/j.jpba.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Ludeña-Huaman MA, Ramos-Inquiltupa DA. Determination of the content of ursolic and oleanolic acid in the cuticular wax of fruits of different species of Rosaceae. Rev Colomb Química. 2019;48:15–20. [Google Scholar]
- 5.Liu J, Lu YF, Wu Q, Xu SF, Shi FG, Klaassen CD. Oleanolic acid reprograms the liver to protect against hepatotoxicants, but is hepatotoxic at high doses. Liver Int. 2019;39:427–439. doi: 10.1111/liv.13940. [DOI] [PubMed] [Google Scholar]
- 6.Phillips DR, Rasbery JM, Bartel B, Matsuda SP. Biosynthetic diversity in plant triterpene cyclization. Curr Opin Plant Biol. 2006;9:305–314. doi: 10.1016/j.pbi.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Li X, Wang Y, Gao Y, Li L, Guo X, Liu D, Jing Y, Zhao L. Synthesis of methyl 2-cyano-3,12-dioxo-18β-olean-1,9(11)-dien-30-oate analogues to determine the active groups for inhibiting cell growth and inducing apoptosis in leukemia cells. Org Biomol Chem. 2014;12:6706–6716. doi: 10.1039/c4ob00703d. [DOI] [PubMed] [Google Scholar]
- 8.Oprean C, Mioc M, Csányi E, Ambrus R, Bojin F, Tatu C, Cristea M, Ivan A, Danciu C, Dehelean C, Paunescu V, Soica C. Improvement of ursolic and oleanolic acids' antitumor activity by complexation with hydrophilic cyclodextrins. Biomed Pharmacother. 2016;83:1095–1104. doi: 10.1016/j.biopha.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 9.Reyes-Zurita FJ, Medina-O’Donnell M, Ferrer-Martin RM, Rufino-Palomares EE, Martin-Fonseca S, Rivas F, Martínez A, García-Granados A, Pérez-Jiménez A, García-Salguero L, Peragón J, Mokhtari K, Medina PP, Parra A, Lupiáñez JA. The oleanolic acid derivative, 3-O-succinyl-28-O-benzyl oleanolate, induces apoptosis in B16–F10 melanoma cells via the mitochondrial apoptotic pathway. RSC Adv. 2016;6:93590–93601. [Google Scholar]
- 10.Cheng KG, Su CH, Yang LD, Liu J, Chen ZF. Synthesis of oleanolic acid dimers linked at C-28 and evaluation of anti-tumor activity. Eur J Med Chem. 2015;89:480–489. doi: 10.1016/j.ejmech.2014.10.066. [DOI] [PubMed] [Google Scholar]
- 11.Tian T, Liu X, Lee ES, Sun J, Feng Z, Zhao L, Zhao C. Synthesis of novel oleanolic acid and ursolic acid in C-28 position derivatives as potential anticancer agents. Arch Pharm Res. 2017;40:458–468. doi: 10.1007/s12272-016-0868-8. [DOI] [PubMed] [Google Scholar]
- 12.Verdile G, Keane KN, Cruzat VF, Medic S, Sabale M, Rowles J, Wijesekara N, Martins RN, Fraser PE, Newsholme P. Inflammation and Oxidative Stress: The Molecular Connectivity between Insulin Resistance, Obesity, and Alzheimer's Disease. Mediators Inflamm. 2015;2015:105828. doi: 10.1155/2015/105828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khansari N, Shakiba Y, Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat Inflamm Allergy Drug Discov. 2009;3:73–80. doi: 10.2174/187221309787158371. [DOI] [PubMed] [Google Scholar]
- 14.Kang GD, Lim S, Kim DH. Oleanolic acid ameliorates dextran sodium sulfate-induced colitis in mice by restoring the balance of Th17/Treg cells and inhibiting NF-κB signaling pathway. Int Immunopharmacol. 2015;29:393–400. doi: 10.1016/j.intimp.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 15.Mandal A, Bhatia D, Bishayee A. Suppression of inflammatory cascade is implicated in methyl amooranin-mediated inhibition of experimental mammary carcinogenesis. Mol Carcinog. 2014;53:999–1010. doi: 10.1002/mc.22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinh CH, Yu Y, Szabo A, Zhang Q, Zhang P, Huang XF. Bardoxolone Methyl Prevents High-Fat Diet-Induced Colon Inflammation in Mice. J Histochem Cytochem. 2016;64:237–255. doi: 10.1369/0022155416631803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fitzpatrick LR, Stonesifer E, Small JS, Liby KT. The synthetic triterpenoid (CDDO-Im) inhibits STAT3, as well as IL-17, and improves DSS-induced colitis in mice. Inflammopharmacology. 2014;22:341–349. doi: 10.1007/s10787-014-0203-2. [DOI] [PubMed] [Google Scholar]
- 18.Rali S, Oyedeji OO, Aremu OO, Oyedeji AO, Nkeh-Chungag BN. Semisynthesis of Derivatives of Oleanolic Acid from Syzygium aromaticum and Their Antinociceptive and Anti-Inflammatory Properties. Mediators Inflamm. 2016;2016:8401843. doi: 10.1155/2016/8401843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nkeh-Chungag BN, Oyedeji OO, Oyedeji AO, Ndebia EJ. Anti-inflammatory and membrane-stabilizing properties of two semisynthetic derivatives of oleanolic acid. Inflammation. 2015;38:61–69. doi: 10.1007/s10753-014-0007-y. [DOI] [PubMed] [Google Scholar]
- 20.Krishnan K, Mathew LE, Vijayalakshmi NR, Helen A. Anti-inflammatory potential of β-amyrin, a triterpenoid isolated from Costus igneus. Inflammopharmacology. 2014;22:373–385. doi: 10.1007/s10787-014-0218-8. [DOI] [PubMed] [Google Scholar]
- 21.Nelson AT, Camelio AM, Claussen KR, Cho J, Tremmel L, DiGiovanni J, Siegel D. Synthesis of oxygenated oleanolic and ursolic acid derivatives with anti-inflammatory properties. Bioorg Med Chem Lett. 2015;25:4342–4346. doi: 10.1016/j.bmcl.2015.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jehangir A, Shahzad M, Shahid K, Waheed A, Ayub F. Zinc and iron complexes of oleanolic acid, (OA) attenuate allergic airway inflammation in rats. Inflammopharmacology. 2019;27:1179–1192. doi: 10.1007/s10787-019-00597-2. [DOI] [PubMed] [Google Scholar]
- 23.Bednarczyk-Cwynar B, Wachowiak N, Szulc M, Kamińska E, Bogacz A, Bartkowiak-Wieczorek J, Zaprutko L, Mikolajczak PL. Strong and Long-Lasting Antinociceptive and Anti-inflammatory Conjugate of Naturally Occurring Oleanolic Acid and Aspirin. Front Pharmacol. 2016;7:202. doi: 10.3389/fphar.2016.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng XP, Li XH, Li Y, Huang XT, Luo ZQ. The protective effect of oleanolic acid on NMDA-induced MLE-12 cells apoptosis and lung injury in mice by activating SIRT1 and reducing NF-κB acetylation. Int Immunopharmacol. 2019;70:520–529. doi: 10.1016/j.intimp.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 25.Kim MS, Han JY, Kim SH, Jeon D, Kim HY, Lee SW, Rho MC, Lee K. Oleanolic acid acetate attenuates polyhexamethylene guanidine phosphate-induced pulmonary inflammation and fibrosis in mice. Respir Physiol Neurobiol. 2018;252-253:1–9. doi: 10.1016/j.resp.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Han YW, Liu XJ, Zhao Y, Li XM. Role of Oleanolic acid in maintaining BBB integrity by targeting p38MAPK/VEGF/Src signaling pathway in rat model of subarachnoid hemorrhage. Eur J Pharmacol. 2018;839:12–20. doi: 10.1016/j.ejphar.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Li W, Guo Y, Zhang C, Wu R, Yang AY, Gaspar J, Kong AN. Dietary Phytochemicals and Cancer Chemoprevention: A Perspective on Oxidative Stress, Inflammation, and Epigenetics. Chem Res Toxicol. 2016;29:2071–2095. doi: 10.1021/acs.chemrestox.6b00413. [DOI] [PubMed] [Google Scholar]
- 28.Xiang P, Chen T, Mou Y, Wu H, Xie P, Lu G, Gong X, Hu Q, Zhang Y, Ji H. NZ suppresses TLR4/NF-κB signalings and NLRP3 inflammasome activation in LPS-induced RAW264.7 macrophages. Inflamm Res. 2015;64:799–808. doi: 10.1007/s00011-015-0863-4. [DOI] [PubMed] [Google Scholar]
- 29.Pan Y, Zhou F, Song Z, Huang H, Chen Y, Shen Y, Jia Y, Chen J. Oleanolic acid protects against pathogenesis of atherosclerosis, possibly via FXR-mediated angiotensin (Ang)-(1-7) upregulation. Biomed Pharmacother. 2018;97:1694–1700. doi: 10.1016/j.biopha.2017.11.151. [DOI] [PubMed] [Google Scholar]
- 30.Caltana L, Rutolo D, Nieto ML, Brusco A. Further evidence for the neuroprotective role of oleanolic acid in a model of focal brain hypoxia in rats. Neurochem Int. 2014;79:79–87. doi: 10.1016/j.neuint.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 31.Ndlovu BC, Daniels WM, Mabandla MV. Amelioration of L-Dopa-Associated Dyskinesias with Triterpenoic Acid in a Parkinsonian Rat Model. Neurotox Res. 2016;29:126–134. doi: 10.1007/s12640-015-9567-3. [DOI] [PubMed] [Google Scholar]
- 32.Mabandla MV, Nyoka M, Daniels WM. Early use of oleanolic acid provides protection against 6-hydroxydopamine induced dopamine neurodegeneration. Brain Res. 2015;1622:64–71. doi: 10.1016/j.brainres.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 33.Sarkar C, Pal S, Das N, Dinda B. Ameliorative effects of oleanolic acid on fluoride induced metabolic and oxidative dysfunctions in rat brain: Experimental and biochemical studies. Food Chem Toxicol. 2014;66:224–236. doi: 10.1016/j.fct.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 34.Wang K, Sun W, Zhang L, Guo W, Xu J, Liu S, Zhou Z, Zhang Y. Oleanolic Acid Ameliorates Aβ25-35 Injection-induced Memory Deficit in Alzheimer's Disease Model Rats by Maintaining Synaptic Plasticity. CNS Neurol Disord Drug Targets. 2018;17:389–399. doi: 10.2174/1871527317666180525113109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Kanegan MJ, Dunn DE, Kaltenbach LS, Shah B, He DN, McCoy DD, Yang P, Peng J, Shen L, Du L, Cichewicz RH, Newman RA, Lo DC. Dual activities of the anti-cancer drug candidate PBI-05204 provide neuroprotection in brain slice models for neurodegenerative diseases and stroke. Sci Rep. 2016;6:25626. doi: 10.1038/srep25626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeon SJ, Lee HJ, Lee HE, Park SJ, Gwon Y, Kim H, Zhang J, Shin CY, Kim DH, Ryu JH. Oleanolic acid ameliorates cognitive dysfunction caused by cholinergic blockade via TrkB-dependent BDNF signaling. Neuropharmacology. 2017;113:100–109. doi: 10.1016/j.neuropharm.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 37.Yi LT, Li J, Liu BB, Luo L, Liu Q, Geng D. BDNF-ERK-CREB signalling mediates the role of miR-132 in the regulation of the effects of oleanolic acid in male mice. J Psychiatry Neurosci. 2014;39:348–359. doi: 10.1503/jpn.130169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gutiérrez-Rebolledo GA, Siordia-Reyes AG, Meckes-Fischer M, Jiménez-Arellanes A. Hepatoprotective properties of oleanolic and ursolic acids in antitubercular drug-induced liver damage. Asian Pac J Trop Med. 2016;9:644–651. doi: 10.1016/j.apjtm.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 39.Dong SQ, Wang SS, Zhu JX, Mu RH, Li CF, Geng D, Liu Q, Yi LT. Oleanolic acid decreases SGK1 in the hippocampus in corticosterone-induced mice. Steroids. 2019;149:108419. doi: 10.1016/j.steroids.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 40.Fajemiroye JO, Polepally PR, Chaurasiya ND, Tekwani BL, Zjawiony JK, Costa EA. Oleanolic acid acrylate elicits antidepressant-like effect mediated by 5-HT1A receptor. Sci Rep. 2015;5:11582. doi: 10.1038/srep11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fajemiroye JO, Galdino PM, Florentino IF, Da Rocha FF, Ghedini PC, Polepally PR, Zjawiony JK, Costa EA. Plurality of anxiety and depression alteration mechanism by oleanolic acid. J Psychopharmacol. 2014;28:923–934. doi: 10.1177/0269881114536789. [DOI] [PubMed] [Google Scholar]
- 42.Zhang SQ, Lin KL, Law CY, Liu B, Fu XQ, Tse WS, Wong SSM, Sze SCW, Yung KKL. Oleanolic acid enhances neural stem cell migration, proliferation, and differentiation in vitro by inhibiting GSK3β activity. Cell Death Discov. 2018;4:48. doi: 10.1038/s41420-018-0111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ning Y, Huang J, Kalionis B, Bian Q, Dong J, Wu J, Tai X, Xia S, Shen Z. Oleanolic Acid Induces Differentiation of Neural Stem Cells to Neurons: An Involvement of Transcription Factor Nkx-2.5. Stem Cells Int. 2015;2015:672312. doi: 10.1155/2015/672312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang YL, Zhou Z, Han WW, Zhang LL, Song WS, Huang JH, Liu S. Oleanolic Acid Inhibiting the Differentiation of Neural Stem Cells into Astrocyte by Down-Regulating JAK/STAT Signaling Pathway. Am J Chin Med. 2016;44:103–117. doi: 10.1142/S0192415X16500075. [DOI] [PubMed] [Google Scholar]
- 45.Jo HR, Wang SE, Kim YS, Lee CH, Son H. Oleanolic Acid Promotes Neuronal Differentiation and Histone Deacetylase 5 Phosphorylation in Rat Hippocampal Neurons. Mol Cells. 2017;40:485–494. doi: 10.14348/molcells.2017.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castellano JM, Garcia-Rodriguez S, Espinosa JM, Millan-Linares MC, Rada M, Perona JS. Oleanolic Acid Exerts a Neuroprotective Effect Against Microglial Cell Activation by Modulating Cytokine Release and Antioxidant Defense Systems. Biomolecules. 2019:9. doi: 10.3390/biom9110683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moeini R, Memariani Z, Asadi F, Bozorgi M, Gorji N. Pistacia Genus as a Potential Source of Neuroprotective Natural Products. Planta Med. 2019;85:1326–1350. doi: 10.1055/a-1014-1075. [DOI] [PubMed] [Google Scholar]
- 48.Yang JL, Quy Ha TK, Dhodary B, Seo JY, Kim H, Park J, Oh WK. 3,4-seco-28-Nor-oleanane triterpenes from Camellia japonica protect from neurotoxicity in a rotenone model of Parkinson’s disease. Tetrahedron. 2016;72:3240–3249. [Google Scholar]
- 49.Ayeleso TB, Matumba MG, Mukwevho E. Oleanolic Acid and Its Derivatives: Biological Activities and Therapeutic Potential in Chronic Diseases. Molecules. 2017:22. doi: 10.3390/molecules22111915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martín R, Hernández M, Córdova C, Nieto ML. Natural triterpenes modulate immune-inflammatory markers of experimental autoimmune encephalomyelitis: therapeutic implications for multiple sclerosis. Br J Pharmacol. 2012;166:1708–1723. doi: 10.1111/j.1476-5381.2012.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ozgun-Acar O, Celik-Turgut G, Gazioglu I, Kolak U, Ozbal S, Ergur BU, Arslan S, Sen A, Topcu G. Capparis ovata treatment suppresses inflammatory cytokine expression and ameliorates experimental allergic encephalomyelitis model of multiple sclerosis in C57BL/6 mice. J Neuroimmunol. 2016;298:106–116. doi: 10.1016/j.jneuroim.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 52.Acar OO, Sen A, Topcu G, Gazioglu I, Kolak U, Arslan S. Beneficial actions of dichloromethane sub-fraction of the COWE extract for multiple sclerosis: a potential therapeutic role. FEBS J. 2016;283:358. [Google Scholar]
- 53.Ozgun O, Celik G, Arslan S, Sen A. Capparis ovata water extract (MSCov) for the potential treatment of multiple sclerosis without drug interaction: molecular evidences. FEBS J. 2014;281:104–105. [Google Scholar]
- 54.Celik G, Ozgun O, Arslan S, Sen A. Toxicogenomic analysis of Capparis ovata water extract (MSCov) FEBS J. 2014;281:184. [Google Scholar]
- 55.Ozgun O, Turgut GC, Gazioglu I, Kolat U, Ozbal S, Ergur BU, Arslan S, Topcu G, Sen A. Capparis ovate ameliorates experimental allergic encephalomyelitis model of multiple sclerosis in C57BL/6 mice. Mult Scler J. 2015;21:671–672. [Google Scholar]
- 56.Sen A, Topcu G, Ozgun O, Kolak U, Hacibekiroglu I, Celik G, Arslan S. Anti-neuroinflammatory effect of butanolic subextract of Capparis ovata water extract used as an alternative and complementary treatment for multiple sclerosis. J Neuroimmunol. 2014;275:172–173. [Google Scholar]
- 57.Sen A, Senol H, Acar OO, Kale E, Dag A, Topcu G. Synthesis and initial evaluation of efficacy of olean-12-en-28-ol, 3 beta-pentacosanoate for the symptomatic treatment of multiple sclerosis. FEBS Open Bio. 2019;9:74. [Google Scholar]
- 58.Wang R, Yang W, Fan Y, Dehaen W, Li Y, Li H, Wang W, Zheng Q, Huai Q. Design and synthesis of the novel oleanolic acid-cinnamic acid ester derivatives and glycyrrhetinic acid-cinnamic acid ester derivatives with cytotoxic properties. Bioorg Chem. 2019;88:102951. doi: 10.1016/j.bioorg.2019.102951. [DOI] [PubMed] [Google Scholar]
- 59.González-Cofrade L, de Las Heras B, Apaza Ticona L, Palomino OM. Molecular Targets Involved in the Neuroprotection Mediated by Terpenoids. Planta Med. 2019;85:1304–1315. doi: 10.1055/a-0953-6738. [DOI] [PubMed] [Google Scholar]
- 60.Liu J, Wang X, Liu R, Liu Y, Zhang T, Fu H, Hai C. Oleanolic acid co-administration alleviates ethanol-induced hepatic injury via Nrf-2 and ethanol-metabolizing metabolizing modulating in rats. Chem Biol Interact. 2014;221:88–98. doi: 10.1016/j.cbi.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 61.Yu Z, Sun W, Peng W, Yu R, Li G, Jiang T. Pharmacokinetics in Vitro and in Vivo of Two Novel Prodrugs of Oleanolic Acid in Rats and Its Hepatoprotective Effects against Liver Injury Induced by CCl4. Mol Pharm. 2016;13:1699–1710. doi: 10.1021/acs.molpharmaceut.6b00129. [DOI] [PubMed] [Google Scholar]
- 62.Chu F, Zhang W, Guo W, Wang Z, Yang Y, Zhang X, Fang K, Yan M, Wang P, Lei H. Oleanolic Acid-amino Acids Derivatives: Design, Synthesis, and Hepatoprotective Evaluation In Vitro and In Vivo. Molecules. 2018:23. doi: 10.3390/molecules23020322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xiang H, Han Y, Zhang Y, Yan W, Xu B, Chu F, Xie T, Jia M, Yan M, Zhao R, Wang P, Lei H. A New Oleanolic Acid Derivative against CCl₄-Induced Hepatic Fibrosis in Rats. Int J Mol Sci. 2017:18. doi: 10.3390/ijms18030553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lu YF, Liu J, Wu KC, Klaassen CD. Protection against phalloidin-induced liver injury by oleanolic acid involves Nrf2 activation and suppression of Oatp1b2. Toxicol Lett. 2015;232:326–332. doi: 10.1016/j.toxlet.2014.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen P, Li J, Fan X, Zeng H, Deng R, Li D, Huang M, Bi H. Oleanolic acid attenuates obstructive cholestasis in bile duct-ligated mice, possibly via activation of NRF2-MRPs and FXR antagonism. Eur J Pharmacol. 2015;765:131–139. doi: 10.1016/j.ejphar.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 66.Chen P, Zeng H, Wang Y, Fan X, Xu C, Deng R, Zhou X, Bi H, Huang M. Low dose of oleanolic acid protects against lithocholic acid-induced cholestasis in mice: potential involvement of nuclear factor-E2-related factor 2-mediated upregulation of multidrug resistance-associated proteins. Drug Metab Dispos. 2014;42:844–852. doi: 10.1124/dmd.113.056549. [DOI] [PubMed] [Google Scholar]
- 67.Ou-Yang Q, Xuan CX, Wang X, Luo HQ, Liu JE, Wang LL, Li TT, Chen YP, Liu J. 3-Acetyl-oleanolic acid ameliorates non-alcoholic fatty liver disease in high fat diet-treated rats by activating AMPK-related pathways. Acta Pharmacol Sin. 2018;39:1284–1293. doi: 10.1038/aps.2017.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hao BB, Pan XX, Fan Y, Lu L, Qian XF, Wang XH, Zhang F, Rao JH. Oleanolic acid attenuates liver ischemia reperfusion injury by HO-1/Sesn2 signaling pathway. Hepatobiliary Pancreat Dis Int. 2016;15:519–524. doi: 10.1016/s1499-3872(16)60115-7. [DOI] [PubMed] [Google Scholar]
- 69.Gui B, Hua F, Chen J, Xu Z, Sun H, Qian Y. Protective effects of pretreatment with oleanolic acid in rats in the acute phase of hepatic ischemia-reperfusion injury: role of the PI3K/Akt pathway. Mediators Inflamm. 2014;2014:451826. doi: 10.1155/2014/451826. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 70.Wang W, Wu L, Li J, Ji J, Chen K, Yu Q, Li S, Feng J, Liu T, Zhang J, Chen J, Zhou Y, Mao Y, Wang F, Dai W, Fan X, Guo C, Wu J. Alleviation of Hepatic Ischemia Reperfusion Injury by Oleanolic Acid Pretreating via Reducing HMGB1 Release and Inhibiting Apoptosis and Autophagy. Mediators Inflamm. 2019;2019:3240713. doi: 10.1155/2019/3240713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang L, Zhang S, Cheng H, Lv H, Cheng G, Ci X. Nrf2-mediated liver protection by esculentoside A against acetaminophen toxicity through the AMPK/Akt/GSK3β pathway. Free Radic Biol Med. 2016;101:401–412. doi: 10.1016/j.freeradbiomed.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 72.Wang W, Chen K, Xia Y, Mo W, Wang F, Dai W, Niu P. The Hepatoprotection by Oleanolic Acid Preconditioning: Focusing on PPARα Activation. PPAR Res. 2018;2018:3180396. doi: 10.1155/2018/3180396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim KA, Lee JS, Park HJ, Kim JW, Kim CJ, Shim IS, Kim NJ, Han SM, Lim S. Inhibition of cytochrome P450 activities by oleanolic acid and ursolic acid in human liver microsomes. Life Sci. 2004;74:2769–2779. doi: 10.1016/j.lfs.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 74.Nyakudya TT, Mukwevho E, Nkomozepi P, Erlwanger KH. Neonatal intake of oleanolic acid attenuates the subsequent development of high fructose diet-induced non-alcoholic fatty liver disease in rats. J Dev Orig Health Dis. 2018;9:500–510. doi: 10.1017/S2040174418000259. [DOI] [PubMed] [Google Scholar]
- 75.Lin YN, Chang HY, Wang CCN, Chu FY, Shen HY, Chen CJ, Lim YP. Oleanolic Acid Inhibits Liver X Receptor Alpha and Pregnane X Receptor to Attenuate Ligand-Induced Lipogenesis. J Agric Food Chem. 2018;66:10964–10976. doi: 10.1021/acs.jafc.8b03372. [DOI] [PubMed] [Google Scholar]
- 76.Zhang F, Wang X, Qiu X, Wang J, Fang H, Wang Z, Sun Y, Xia Z. The protective effect of Esculentoside A on experimental acute liver injury in mice. PLoS One. 2014;9:e113107. doi: 10.1371/journal.pone.0113107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Taylor R. Insulin resistance and type 2 diabetes. Diabetes. 2012;61:778–779. doi: 10.2337/db12-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, Nathan D, Palmer J, Rizza R, Saudek C, Shaw J, Steffes M, Stern M, Tuomilehto J, Zimmet P Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 79.Malchoff CD. Diagnosis and classification of diabetes mellitus. Conn Med. 1991;55:625–629. [PubMed] [Google Scholar]
- 80.Baloyi CM, Khathi A, Sibiya NH, Ngubane PS. The Haematological Effects of Oleanolic Acid in Streptozotocin-Induced Diabetic Rats: Effects on Selected Markers. J Diabetes Res. 2019;2019:6753541. doi: 10.1155/2019/6753541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mukundwa A, Mukaratirwa S, Masola B. Effects of oleanolic acid on the insulin signaling pathway in skeletal muscle of streptozotocin-induced diabetic male Sprague-Dawley rats. J Diabetes. 2016;8:98–108. doi: 10.1111/1753-0407.12260. [DOI] [PubMed] [Google Scholar]
- 82.Silva FS, Oliveira PJ, Duarte MF. Oleanolic, Ursolic, and Betulinic Acids as Food Supplements or Pharmaceutical Agents for Type 2 Diabetes: Promise or Illusion? J Agric Food Chem. 2016;64:2991–3008. doi: 10.1021/acs.jafc.5b06021. [DOI] [PubMed] [Google Scholar]
- 83.Nazaruk J, Borzym-Kluczyk M. The role of triterpenes in the management of diabetes mellitus and its complications. Phytochem Rev. 2015;14:675–690. doi: 10.1007/s11101-014-9369-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou X, Zeng XY, Wang H, Li S, Jo E, Xue CC, Tan M, Molero JC, Ye JM. Hepatic FoxO1 acetylation is involved in oleanolic acid-induced memory of glycemic control: novel findings from Study 2. PLoS One. 2014;9:e107231. doi: 10.1371/journal.pone.0107231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Takigawa-Imamura H, Sekine T, Murata M, Takayama K, Nakazawa K, Nakagawa J. Stimulation of glucose uptake in muscle cells by prolonged treatment with scriptide, a histone deacetylase inhibitor. Biosci Biotechnol Biochem. 2003;67:1499–1506. doi: 10.1271/bbb.67.1499. [DOI] [PubMed] [Google Scholar]
- 86.McGee SL, Hargreaves M. Histone modifications and skeletal muscle metabolic gene expression. Clin Exp Pharmacol Physiol. 2010;37:392–396. doi: 10.1111/j.1440-1681.2009.05311.x. [DOI] [PubMed] [Google Scholar]
- 87.Luvuno M, Mbongwa HP, Khathi A. The effects of Syzygium aromaticum-derived triterpenes on gastrointestinal ghrelin expression in streptozotocin-induced diabetic rats. Afr J Tradit Complement Altern Med. 2016;13:8–14. doi: 10.21010/ajtcam.v13i4.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Su S, Wu G, Cheng X, Fan J, Peng J, Su H, Xu Z, Cao M, Long Z, Hao Y, Li G, Li S, Hai C, Wang X. Oleanolic acid attenuates PCBs-induced adiposity and insulin resistance via HNF1b-mediated regulation of redox and PPARγ signaling. Free Radic Biol Med. 2018;124:122–134. doi: 10.1016/j.freeradbiomed.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 89.Chen FF, Wang JT, Zhang LX, Xing SF, Wang YX, Wang K, Deng SL, Zhang JQ, Tang L, Wu HS. Oleanolic acid derivative DKS26 exerts anti-diabetic and hepatoprotective effects in diabetic mice and promotes glucagon-like peptide-1 secretion and expression in intestinal cells. Br J Pharmacol. 2017;174:2912–2928. doi: 10.1111/bph.13921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li Y, Zhang T, Cui J, Jia N, Wu Y, Xi M, Wen A. Chikusetsu saponin IVa regulates glucose uptake and fatty acid oxidation: implications in antihyperglycemic and hypolipidemic effects. J Pharm Pharmacol. 2015;67:997–1007. doi: 10.1111/jphp.12392. [DOI] [PubMed] [Google Scholar]
- 91.Gutiérrez RMP. Hypolipidemic and hypoglycemic activities of a oleanolic acid derivative from Malva parviflora on streptozotocin-induced diabetic mice. Arch Pharm Res. 2017;40:550–562. doi: 10.1007/s12272-016-0873-y. [DOI] [PubMed] [Google Scholar]
- 92.Matumba MG, Ayeleso AO, Nyakudya T, Erlwanger K, Chegou NN, Mukwevho E. Long-Term Impact of Neonatal Intake of Oleanolic Acid on the Expression of AMP-Activated Protein Kinase, Adiponectin and Inflammatory Cytokines in Rats Fed with a High Fructose Diet. Nutrients. 2019:11. doi: 10.3390/nu11020226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Molepo M, Ayeleso A, Nyakudya T, Erlwanger K, Mukwevho E. A Study on Neonatal Intake of Oleanolic Acid and Metformin in Rats (Rattus norvegicus) with Metabolic Dysfunction: Implications on Lipid Metabolism and Glucose Transport. Molecules. 2018:23. doi: 10.3390/molecules23102528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nyakudya TT, Mukwevho E, Erlwanger KH. The protective effect of neonatal oral administration of oleanolic acid against the subsequent development of fructose-induced metabolic dysfunction in male and female rats. Nutr Metab (Lond) 2018;15:82. doi: 10.1186/s12986-018-0314-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang S, Du LB, Jin L, Wang Z, Peng J, Liao N, Zhao YY, Zhang JL, Pauluhn J, Hai CX, Wang X, Li WL. Nano-oleanolic acid alleviates metabolic dysfunctions in rats with high fat and fructose diet. Biomed Pharmacother. 2018;108:1181–1187. doi: 10.1016/j.biopha.2018.09.150. [DOI] [PubMed] [Google Scholar]
- 96.Santos-Lozano JM, Rada M, Lapetra J, Guinda Á, Jiménez-Rodríguez MC, Cayuela JA, Ángel-Lugo A, Vilches-Arenas Á, Gómez-Martín AM, Ortega-Calvo M, Castellano JM. Prevention of type 2 diabetes in prediabetic patients by using functional olive oil enriched in oleanolic acid: The PREDIABOLE study, a randomized controlled trial. Diabetes Obes Metab. 2019;21:2526–2534. doi: 10.1111/dom.13838. [DOI] [PubMed] [Google Scholar]
- 97.Zhong YY, Chen HS, Wu PP, Zhang BJ, Yang Y, Zhu QY, Zhang CG, Zhao SQ. Synthesis and biological evaluation of novel oleanolic acid analogues as potential α-glucosidase inhibitors. Eur J Med Chem. 2019;164:706–716. doi: 10.1016/j.ejmech.2018.12.046. [DOI] [PubMed] [Google Scholar]
- 98.Wang X, Chen Y, Abdelkader D, Hassan W, Sun H, Liu J. Combination therapy with oleanolic acid and metformin as a synergistic treatment for diabetes. J Diabetes Res. 2015;2015:973287. doi: 10.1155/2015/973287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gamede M, Mabuza L, Ngubane P, Khathi A. Plant-Derived Oleanolic Acid (OA) Ameliorates Risk Factors of Cardiovascular Diseases in a Diet-Induced Pre-Diabetic Rat Model: Effects on Selected Cardiovascular Risk Factors. Molecules. 2019:24. doi: 10.3390/molecules24020340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gamede M, Mabuza L, Ngubane P, Khathi A. The Effects of Plant-Derived Oleanolic Acid on Selected Parameters of Glucose Homeostasis in a Diet-Induced Pre-Diabetic Rat Model. Molecules. 2018:23. doi: 10.3390/molecules23040794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nyakudya TT, Isaiah S, Ayeleso A, Ndhlala AR, Mukwevho E, Erlwanger KH. Short-Term Neonatal Oral Administration of Oleanolic Acid Protects against Fructose-Induced Oxidative Stress in the Skeletal Muscles of Suckling Rats. Molecules. 2019:24. doi: 10.3390/molecules24040661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yamamoto T, Nakade Y, Yamauchi T, Kobayashi Y, Ishii N, Ohashi T, Ito K, Sato K, Fukuzawa Y, Yoneda M. Glucagon-like peptide-1 analogue prevents nonalcoholic steatohepatitis in non-obese mice. World J Gastroenterol. 2016;22:2512–2523. doi: 10.3748/wjg.v22.i8.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Qian S, Zhang M, He Y, Wang W, Liu S. Recent advances in the development of protein tyrosine phosphatase 1B inhibitors for Type 2 diabetes. Future Med Chem. 2016;8:1239–1258. doi: 10.4155/fmc-2016-0064. [DOI] [PubMed] [Google Scholar]
- 104.Yang L, Chen F, Gao C, Chen J, Li J, Liu S, Zhang Y, Wang Z, Qian S. Design and synthesis of tricyclic terpenoid derivatives as novel PTP1B inhibitors with improved pharmacological property and in vivo antihyperglycaemic efficacy. J Enzyme Inhib Med Chem. 2020;35:152–164. doi: 10.1080/14756366.2019.1690481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shah FA, Stoica A, Cardemil C, Palmquist A. Multiscale characterization of cortical bone composition, microstructure, and nanomechanical properties in experimentally induced osteoporosis. J Biomed Mater Res A. 2018;106:997–1007. doi: 10.1002/jbm.a.36294. [DOI] [PubMed] [Google Scholar]
- 106.Zhang A, Sun H, Wang X. Potentiating therapeutic effects by enhancing synergism based on active constituents from traditional medicine. Phytother Res. 2014;28:526–533. doi: 10.1002/ptr.5032. [DOI] [PubMed] [Google Scholar]
- 107.Cao S, Dong XL, Ho MX, Yu WX, Wong KC, Yao XS, Wong MS. Oleanolic Acid Exerts Osteoprotective Effects and Modulates Vitamin D Metabolism. Nutrients. 2018:10. doi: 10.3390/nu10020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cao S, Wastney ME, Lachcik PJ, Xiao HH, Weaver CM, Wong MS. Both Oleanolic Acid and a Mixture of Oleanolic and Ursolic Acids Mimic the Effects of Fructus ligustri lucidi on Bone Properties and Circulating 1,25-Dihydroxycholecalciferol in Ovariectomized Rats. J Nutr. 2018;148:1895–1902. doi: 10.1093/jn/nxy242. [DOI] [PubMed] [Google Scholar]
- 109.Adhikari N, Neupane S, Aryal YP, Choi M, Sohn WJ, Lee Y, Jung JK, Ha JH, Choi SY, Suh JY, Kim JY, Rho MC, Lee TH, Yamamoto H, An CH, Kim SH, An SY, Kim JY. Effects of oleanolic acid acetate on bone formation in an experimental periodontitis model in mice. J Periodontal Res. 2019;54:533–545. doi: 10.1111/jre.12657. [DOI] [PubMed] [Google Scholar]
- 110.Xu Y, Chen S, Yu T, Qiao J, Sun G. High-throughput metabolomics investigates anti-osteoporosis activity of oleanolic acid via regulating metabolic networks using ultra-performance liquid chromatography coupled with mass spectrometry. Phytomedicine. 2018;51:68–76. doi: 10.1016/j.phymed.2018.09.235. [DOI] [PubMed] [Google Scholar]
- 111.Zhao D, Shu B, Wang C, Zhao Y, Cheng W, Sha N, Li C, Wang Q, Lu S, Wang Y. Oleanolic acid exerts inhibitory effects on the late stage of osteoclastogenesis and prevents bone loss in osteoprotegerin knockout mice. J Cell Biochem. 2020;121:152–164. doi: 10.1002/jcb.28994. [DOI] [PubMed] [Google Scholar]
- 112.Xie BP, Shi LY, Li JP, Zeng Y, Liu W, Tang SY, Jia LJ, Zhang J, Gan GX. Oleanolic acid inhibits RANKL-induced osteoclastogenesis via ER alpha/miR-503/RANK signaling pathway in RAW264.7 cells. Biomed Pharmacother. 2019;117:109045. doi: 10.1016/j.biopha.2019.109045. [DOI] [PubMed] [Google Scholar]
- 113.Zhao D, Li X, Zhao Y, Qiao P, Tang D, Chen Y, Xue C, Li C, Liu S, Wang J, Lu S, Shi Q, Zhang Y, Dong Y, Wang Y, Shu B, Feng X. Oleanolic acid exerts bone protective effects in ovariectomized mice by inhibiting osteoclastogenesis. J Pharmacol Sci. 2018;137:76–85. doi: 10.1016/j.jphs.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 114.Kim JY, Cheon YH, Oh HM, Rho MC, Erkhembaatar M, Kim MS, Lee CH, Kim JJ, Choi MK, Yoon KH, Lee MS, Oh J. Oleanolic acid acetate inhibits osteoclast differentiation by downregulating PLCγ2-Ca(2+)-NFATc1 signaling, and suppresses bone loss in mice. Bone. 2014;60:104–111. doi: 10.1016/j.bone.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 115.Kang DG, Lee HJ, Kim KT, Hwang SC, Lee CJ, Park JS. Effect of oleanolic acid on the activity, secretion and gene expression of matrix metalloproteinase-3 in articular chondrocytes in vitro and the production of matrix metalloproteinase-3 in vivo. Korean J Physiol Pharmacol. 2017;21:197–204. doi: 10.4196/kjpp.2017.21.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kitsukawa M, Tsuchiyama H, Maeda A, Oshida K, Miyamoto Y. Immunosuppressive potential of bardoxolone methyl using a modified murine local lymph node assay (LLNA) J Toxicol Sci. 2014;39:545–550. doi: 10.2131/jts.39.545. [DOI] [PubMed] [Google Scholar]
- 117.Howard J. Cancer now tops heart disease as the No. 1 cause of death in these countries. 2019. Available from: https://edition.cnn.com/2019/09/03/health/leading-cause-of-death-cancer-heart-disease-study/index.html. [Google Scholar]
- 118.Gao X, Deeb D, Liu Y, Liu P, Zhang Y, Shaw J, Gautam SC. CDDO-Me inhibits tumor growth and prevents recurrence of pancreatic ductal adenocarcinoma. Int J Oncol. 2015;47:2100–2106. doi: 10.3892/ijo.2015.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wu J, Lei H, Zhang J, Chen X, Tang C, Wang W, Xu H, Xiao W, Gu W, Wu Y. Momordin Ic, a new natural SENP1 inhibitor, inhibits prostate cancer cell proliferation. Oncotarget. 2016;7:58995–59005. doi: 10.18632/oncotarget.10636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li L, Wei L, Shen A, Chu J, Lin J, Peng J. Oleanolic acid modulates multiple intracellular targets to inhibit colorectal cancer growth. Int J Oncol. 2015;47:2247–2254. doi: 10.3892/ijo.2015.3198. [DOI] [PubMed] [Google Scholar]
- 121.Nie H, Wang Y, Qin Y, Gong XG. Oleanolic acid induces autophagic death in human gastric cancer cells in vitro and in vivo. Cell Biol Int. 2016;40:770–778. doi: 10.1002/cbin.10612. [DOI] [PubMed] [Google Scholar]
- 122.Leal AS, Sporn MB, Pioli PA, Liby KT. The triterpenoid CDDO-imidazolide reduces immune cell infiltration and cytokine secretion in the KrasG12D;Pdx1-Cre (KC) mouse model of pancreatic cancer. Carcinogenesis. 2016;37:1170–1179. doi: 10.1093/carcin/bgw099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhao X, Liu M, Li D. Oleanolic acid suppresses the proliferation of lung carcinoma cells by miR-122/Cyclin G1/MEF2D axis. Mol Cell Biochem. 2015;400:1–7. doi: 10.1007/s11010-014-2228-7. [DOI] [PubMed] [Google Scholar]
- 124.Jo H, Oh JH, Park DW, Lee C, Min CK. Oleanolic acid 3-acetate, a minor element of ginsenosides, induces apoptotic cell death in ovarian carcinoma and endometrial carcinoma cells via the involvement of a reactive oxygen species-independent mitochondrial pathway. J Ginseng Res. 2020;44:96–104. doi: 10.1016/j.jgr.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ai Y, Hu Y, Kang F, Lai Y, Jia Y, Huang Z, Peng S, Ji H, Tian J, Zhang Y. Synthesis and Biological Evaluation of Novel Olean-28,13β-lactams as Potential Antiprostate Cancer Agents. J Med Chem. 2015;58:4506–4520. doi: 10.1021/jm5020023. [DOI] [PubMed] [Google Scholar]
- 126.Pięt M, Paduch R. Ursolic and Oleanolic Acids as Potential Anticancer Agents Acting in the Gastrointestinal Tract. Mini Rev Org Chem. 2018;16:78–91. [Google Scholar]
- 127.Fontana G, Bruno M, Notarbartolo M, Labbozzetta M, Poma P, Spinella A, Rosselli S. Cytotoxicity of oleanolic and ursolic acid derivatives toward hepatocellular carcinoma and evaluation of NF-κB involvement. Bioorg Chem. 2019;90:103054. doi: 10.1016/j.bioorg.2019.103054. [DOI] [PubMed] [Google Scholar]
- 128.Li Y, Xu Q, Yang W, Wu T, Lu X. Oleanolic acid reduces aerobic glycolysis-associated proliferation by inhibiting yes-associated protein in gastric cancer cells. Gene. 2019;712:143956. doi: 10.1016/j.gene.2019.143956. [DOI] [PubMed] [Google Scholar]
- 129.Gao F, Zuo Q, Jiang T, Song H, Zhou J. A newly synthesized oleanolic acid derivative inhibits the growth of osteosarcoma cells in vitro and in vivo by decreasing c-MYC-dependent glycolysis. J Cell Biochem. 2019;120:9264–9276. doi: 10.1002/jcb.28202. [DOI] [PubMed] [Google Scholar]
- 130.Liu J, Zheng L, Wu N, Ma L, Zhong J, Liu G, Lin X. Oleanolic acid induces metabolic adaptation in cancer cells by activating the AMP-activated protein kinase pathway. J Agric Food Chem. 2014;62:5528–5537. doi: 10.1021/jf500622p. [DOI] [PubMed] [Google Scholar]
- 131.Li L, Lin J, Sun G, Wei L, Shen A, Zhang M, Peng J. Oleanolic acid inhibits colorectal cancer angiogenesis in vivo and in vitro via suppression of STAT3 and Hedgehog pathways. Mol Med Rep. 2016;13:5276–5282. doi: 10.3892/mmr.2016.5171. [DOI] [PubMed] [Google Scholar]
- 132.Niu G, Sun L, Pei Y, Wang D. Oleanolic Acid Inhibits Colorectal Cancer Angiogenesis by Blocking the VEGFR2 Signaling Pathway. Anticancer Agents Med Chem. 2018;18:583–590. doi: 10.2174/1871520617666171020124916. [DOI] [PubMed] [Google Scholar]
- 133.Duan L, Yang Z, Jiang X, Zhang J, Guo X. Oleanolic acid inhibits cell proliferation migration and invasion and induces SW579 thyroid cancer cell line apoptosis by targeting forkhead transcription factor A. Anticancer Drugs. 2019;30:812–820. doi: 10.1097/CAD.0000000000000777. [DOI] [PubMed] [Google Scholar]
- 134.Sánchez-Quesada C, López-Biedma A, Gaforio JJ. Oleanolic Acid, a Compound Present in Grapes and Olives, Protects against Genotoxicity in Human Mammary Epithelial Cells. Molecules. 2015;20:13670–13688. doi: 10.3390/molecules200813670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Guo Y, Han B, Luo K, Ren Z, Cai L, Sun L. NOX2-ROS-HIF-1α signaling is critical for the inhibitory effect of oleanolic acid on rectal cancer cell proliferation. Biomed Pharmacother. 2017;85:733–739. doi: 10.1016/j.biopha.2016.11.091. [DOI] [PubMed] [Google Scholar]
- 136.Xu N, Shi YN, Zhong X, Cao Y, Wang L, Jia TZ. A new saikogenin from the roots of Bupleurum bicaule. Chin J Nat Med. 2014;12:305–308. doi: 10.1016/S1875-5364(14)60060-1. [DOI] [PubMed] [Google Scholar]
- 137.Ball MS, Shipman EP, Kim H, Liby KT, Pioli PA. CDDO-Me Redirects Activation of Breast Tumor Associated Macrophages. PLoS One. 2016;11:e0149600. doi: 10.1371/journal.pone.0149600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pertino MW, Vega C, Rolón M, Coronel C, Rojas de Arias A, Schmeda-Hirschmann G. Antiprotozoal Activity of Triazole Derivatives of Dehydroabietic Acid and Oleanolic Acid. Molecules. 2017:22. doi: 10.3390/molecules22030369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Melo TS, Gattass CR, Soares DC, Cunha MR, Ferreira C, Tavares MT, Saraiva E, Parise-Filho R, Braden H, Delorenzi JC. Oleanolic acid (OA) as an antileishmanial agent: Biological evaluation and in silico mechanistic insights. Parasitol Int. 2016;65:227–237. doi: 10.1016/j.parint.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 140.Sibiya H, Musabayane CT, Mabandla MV. Transdermal delivery of oleanolic acid attenuates pro-inflammatory cytokine release and ameliorates anaemia in P. berghei malaria. Acta Trop. 2017;171:24–29. doi: 10.1016/j.actatropica.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 141.Yan W, Zhang C, Li B, Xu X, Liang M, Gu S, Chu F, Xu B, Ren J, Wang P, Lei H. A Series of Oleanolic Acid Derivatives as Anti-Hepatitis B Virus Agents: Design, Synthesis, and in Vitro and in Vivo Biological Evaluation. Molecules. 2016;21:402. doi: 10.3390/molecules21040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Córdova C, Gutiérrez B, Martínez-García C, Martín R, Gallego-Muñoz P, Hernández M, Nieto ML. Oleanolic acid controls allergic and inflammatory responses in experimental allergic conjunctivitis. PLoS One. 2014;9:e91282. doi: 10.1371/journal.pone.0091282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kim SH, Hong JH, Lee YC. Oleanolic acid suppresses ovalbumin-induced airway inflammation and Th2-mediated allergic asthma by modulating the transcription factors T-bet, GATA-3, RORγt and Foxp3 in asthmatic mice. Int Immunopharmacol. 2014;18:311–324. doi: 10.1016/j.intimp.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 144.Jiang Q, Wang D, Han Y, Han Z, Zhong W, Wang C. Modulation of oxidized-LDL receptor-1 (LOX1) contributes to the antiatherosclerosis effect of oleanolic acid. Int J Biochem Cell Biol. 2015;69:142–152. doi: 10.1016/j.biocel.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 145.Lai P, Liu Y. Echinocystic acid, isolated from Gleditsia sinensis fruit, protects endothelial progenitor cells from damage caused by oxLDL via the Akt/eNOS pathway. Life Sci. 2014;114:62–69. doi: 10.1016/j.lfs.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 146.Luo H, Liu J, Ouyang Q, Xuan C, Wang L, Li T, Liu J. The effects of oleanolic acid on atherosclerosis in different animal models. Acta Biochim Biophys Sin (Shanghai) 2017;49:349–354. doi: 10.1093/abbs/gmx013. [DOI] [PubMed] [Google Scholar]
- 147.Duan J, Yin Y, Wei G, Cui J, Zhang E, Guan Y, Yan J, Guo C, Zhu Y, Mu F, Weng Y, Wang Y, Wu X, Xi M, Wen A. Chikusetsu saponin IVa confers cardioprotection via SIRT1/ERK1/2 and Homer1a pathway. Sci Rep. 2015;5:18123. doi: 10.1038/srep18123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.An Q, Hu Q, Wang B, Cui W, Wu F, Ding Y. Oleanolic acid alleviates diabetic rat carotid artery injury through the inhibition of NLRP3 inflammasome signaling pathways. Mol Med Rep. 2017;16:8413–8419. doi: 10.3892/mmr.2017.7594. [DOI] [PubMed] [Google Scholar]
- 149.Madlala HP, Metzinger T, van Heerden FR, Musabayane CT, Mubagwa K, Dessy C. Vascular Endothelium-Dependent and Independent Actions of Oleanolic Acid and Its Synthetic Oleanane Derivatives as Possible Mechanisms for Hypotensive Effects. PLoS One. 2016;11:e0147395. doi: 10.1371/journal.pone.0147395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Luo HQ, Shen J, Chen CP, Ma X, Lin C, Ouyang Q, Xuan CX, Liu J, Sun HB, Liu J. Lipid-lowering effects of oleanolic acid in hyperlipidemic patients. Chin J Nat Med. 2018;16:339–346. doi: 10.1016/S1875-5364(18)30065-7. [DOI] [PubMed] [Google Scholar]
- 151.Chen S, Wen X, Zhang W, Wang C, Liu J, Liu C. Hypolipidemic effect of oleanolic acid is mediated by the miR-98-5p/PGC-1β axis in high-fat diet-induced hyperlipidemic mice. FASEB J. 2017;31:1085–1096. doi: 10.1096/fj.201601022R. [DOI] [PubMed] [Google Scholar]
- 152.Fisher D, Mosaval F, Tharp DL, Bowles DK, Henkel R. Oleanolic acid causes reversible contraception in male mice by increasing the permeability of the germinal epithelium. Reprod Fertil Dev. 2019;31:1589–1596. doi: 10.1071/RD18484. [DOI] [PubMed] [Google Scholar]
- 153.Wan Q, Lu H, Deng Y, Xiang J, Liang L. Oleanolic acid has similar effects as retinoic acid in inducing mouse embryonic stem cell 1B10 to differentiate towards germ cells. Hum Cell. 2014;27:5–11. doi: 10.1007/s13577-013-0084-5. [DOI] [PubMed] [Google Scholar]
- 154.Zhao H, Liu J, Song L, Liu Z, Han G, Yuan D, Wang T, Dun Y, Zhou Z, Liu Z, Wang Y, Zhang C. Oleanolic acid rejuvenates testicular function through attenuating germ cell DNA damage and apoptosis via deactivation of NF-κB, p53 and p38 signalling pathways. J Pharm Pharmacol. 2017;69:295–304. doi: 10.1111/jphp.12668. [DOI] [PubMed] [Google Scholar]
- 155.Chung S, Yoon HE, Kim SJ, Kim SJ, Koh ES, Hong YA, Park CW, Chang YS, Shin SJ. Oleanolic acid attenuates renal fibrosis in mice with unilateral ureteral obstruction via facilitating nuclear translocation of Nrf2. Nutr Metab (Lond) 2014;11:2. doi: 10.1186/1743-7075-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lee ES, Kim HM, Kang JS, Lee EY, Yadav D, Kwon MH, Kim YM, Kim HS, Chung CH. Oleanolic acid and N-acetylcysteine ameliorate diabetic nephropathy through reduction of oxidative stress and endoplasmic reticulum stress in a type 2 diabetic rat model. Nephrol Dial Transplant. 2016;31:391–400. doi: 10.1093/ndt/gfv377. [DOI] [PubMed] [Google Scholar]
- 157.Kocak C, Kocak FE, Akcilar R, Bayat Z, Aras B, Metineren MH, Yucel M, Simsek H. Effects of captopril, telmisartan and bardoxolone methyl (CDDO-Me) in ischemia-reperfusion-induced acute kidney injury in rats: an experimental comparative study. Clin Exp Pharmacol Physiol. 2016;43:230–241. doi: 10.1111/1440-1681.12511. [DOI] [PubMed] [Google Scholar]
- 158.Zhou X, Chen H, Wei F, Zhao Q, Su Q, Liang J, Yin M, Tian X, Liu Z, Yu B, Bai C, He X, Huang Z. 3β-Acetyloxy-oleanolic Acid Attenuates Pristane-Induced Lupus Nephritis by Regulating Th17 Differentiation. J Immunol Res. 2019;2019:2431617. doi: 10.1155/2019/2431617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Blanco-Cabra N, Vega-Granados K, Moya-Andérico L, Vukomanovic M, Parra A, Álvarez de Cienfuegos L, Torrents E. Novel Oleanolic and Maslinic Acid Derivatives as a Promising Treatment against Bacterial Biofilm in Nosocomial Infections: An in Vitro and in Vivo Study. ACS Infect Dis. 2019;5:1581–1589. doi: 10.1021/acsinfecdis.9b00125. [DOI] [PubMed] [Google Scholar]
- 160.Gabás-Rivera C, Martínez-Beamonte R, Ríos JL, Navarro MA, Surra JC, Arnal C, Rodríguez-Yoldi MJ, Osada J. Dietary oleanolic acid mediates circadian clock gene expression in liver independently of diet and animal model but requires apolipoprotein A1. J Nutr Biochem. 2013;24:2100–2109. doi: 10.1016/j.jnutbio.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 161.Potočnjak I, Šimić L, Vukelić I, Domitrović R. Oleanolic acid attenuates cisplatin-induced nephrotoxicity in mice and chemosensitizes human cervical cancer cells to cisplatin cytotoxicity. Food Chem Toxicol. 2019;132:110676. doi: 10.1016/j.fct.2019.110676. [DOI] [PubMed] [Google Scholar]
- 162.Joh EH, Jeong JJ, Kim DH. Inhibitory effect of echinocystic acid on 12-O-tetradecanoylphorbol-13-acetate-induced dermatitis in mice. Arch Pharm Res. 2014;37:225–231. doi: 10.1007/s12272-013-0092-8. [DOI] [PubMed] [Google Scholar]
- 163.Li WF, Wang P, Li H, Li TY, Feng M, Chen SF. Oleanolic acid protects against diabetic cardiomyopathy via modulation of the nuclear factor erythroid 2 and insulin signaling pathways. Exp Ther Med. 2017;14:848–854. doi: 10.3892/etm.2017.4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chen J, Cui Y, Zhang N, Yao X, Wang Z, Yang L. Oleanolic acid attenuated diabetic mesangial cell injury by activation of autophagy via miRNA-142-5p/PTEN signaling. Cytotechnology. 2019;71:925–933. doi: 10.1007/s10616-019-00335-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jain AK, Wen JX, Blomenkamp KS, Arora S, Blaufuss TA, Rodrigues J, Long JP, Neuschwander-Tetri BA, Teckman JH. Oleanolic Acid Improves Gut Atrophy Induced by Parenteral Nutrition. JPEN J Parenter Enteral Nutr. 2016;40:67–72. doi: 10.1177/0148607115583536. [DOI] [PubMed] [Google Scholar]
- 166.Choi JK, Kim SW, Kim DS, Lee JY, Lee S, Oh HM, Ha YS, Yoo J, Park PH, Shin TY, Kwon TK, Rho MC, Kim SH. Oleanolic acid acetate inhibits rheumatoid arthritis by modulating T cell immune responses and matrix-degrading enzymes. Toxicol Appl Pharmacol. 2016;290:1–9. doi: 10.1016/j.taap.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 167.Tang XF, Li XX, Chen YH, Gao YY, Yu P, Xu LP, Liu RH. Combination of icariin and oleanolic acid attenuates: In vivo and in vitro glucocorticoid resistance through protecting dexamethasone-induced glucocorticoid receptor impairment. RSC Adv. 2018;8:230–242. [Google Scholar]
- 168.Zhang J, Lu L, Zhou L. Oleanolic acid activates daf-16 to increase lifespan in Caenorhabditis elegans. Biochem Biophys Res Commun. 2015;468:843–849. doi: 10.1016/j.bbrc.2015.11.042. [DOI] [PubMed] [Google Scholar]
- 169.Nakamura R, Shirahata T, Konishi N, Takanezawa Y, Sone Y, Uraguchi S, Kobayashi Y, Kiyono M. Oleanolic acid 3-glucoside, a synthetic oleanane-type saponin, alleviates methylmercury toxicity in vitro and in vivo. Toxicology. 2019;417:15–22. doi: 10.1016/j.tox.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 170.Cui W, Liu CX, Wang J, Zhang YC, Shen Q, Feng ZH, Wu J, Li JX. An oleanolic acid derivative reduces denervation-induced muscle atrophy via activation of CNTF-mediated JAK2/STAT3 signaling pathway. Eur J Pharmacol. 2019;861:172612. doi: 10.1016/j.ejphar.2019.172612. [DOI] [PubMed] [Google Scholar]
- 171.Cui W, Liu CX, Zhang YC, Shen Q, Feng ZH, Wang J, Lu SF, Wu J, Li JX. A novel oleanolic acid derivative HA-19 ameliorates muscle atrophy via promoting protein synthesis and preventing protein degradation. Toxicol Appl Pharmacol. 2019;378:114625. doi: 10.1016/j.taap.2019.114625. [DOI] [PubMed] [Google Scholar]
- 172.Martín R, Cordova C, San Román JA, Gutierrez B, Cachofeiro V, Nieto ML. Oleanolic acid modulates the immune-inflammatory response in mice with experimental autoimmune myocarditis and protects from cardiac injury. Therapeutic implications for the human disease. J Mol Cell Cardiol. 2014;72:250–262. doi: 10.1016/j.yjmcc.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 173.Nakajima K, Maeda N, Oiso S, Kariyazono H. Decreased Plasma Octanoylated Ghrelin Levels in Mice by Oleanolic Acid. J Oleo Sci. 2019;68:103–109. doi: 10.5650/jos.ess18148. [DOI] [PubMed] [Google Scholar]
- 174.Djeziri FZ, Belarbi M, Murtaza B, Hichami A, Benammar C, Khan NA. Oleanolic acid improves diet-induced obesity by modulating fat preference and inflammation in mice. Biochimie. 2018;152:110–120. doi: 10.1016/j.biochi.2018.06.025. [DOI] [PubMed] [Google Scholar]
- 175.Hong YA, Lim JH, Kim MY, Kim EN, Koh ES, Shin SJ, Choi BS, Park CW, Chang YS, Chung S. Delayed treatment with oleanolic acid attenuates tubulointerstitial fibrosis in chronic cyclosporine nephropathy through Nrf2/HO-1 signaling. J Transl Med. 2014;12:50. doi: 10.1186/1479-5876-12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Long C, Yang J, Yang H, Li X, Wang G. Attenuation of renal ischemia/reperfusion injury by oleanolic acid preconditioning via its antioxidant, anti‑inflammatory, and anti‑apoptotic activities. Mol Med Rep. 2016;13:4697–4704. doi: 10.3892/mmr.2016.5128. [DOI] [PubMed] [Google Scholar]
- 177.Santos RS, Silva PL, de Oliveira GP, Santos CL, Cruz FF, de Assis EF, de Castro-Faria-Neto HC, Capelozzi VL, Morales MM, Pelosi P, Gattass CR, Rocco PR. Oleanolic acid improves pulmonary morphofunctional parameters in experimental sepsis by modulating oxidative and apoptotic processes. Respir Physiol Neurobiol. 2013;189:484–490. doi: 10.1016/j.resp.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 178.Zhang W, Feng J, Cheng B, Lu Q, Chen X. Oleanolic acid protects against oxidative stress‑induced human umbilical vein endothelial cell injury by activating AKT/eNOS signaling. Mol Med Rep. 2018;18:3641–3648. doi: 10.3892/mmr.2018.9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dorota W, Tichaczek-Goska D, Kicia MK. Effect of asiatic and ursolic acids on growth and virulence factors of uropathogenic Escherichia coli strains. Turkish J Biol. 2013;37:556–564. [Google Scholar]
- 180.Su Y, Meng L, Sun J, Li W, Shao L, Chen K, Zhou D, Yang F, Yu F. Design, synthesis of oleanolic acid-saccharide conjugates using click chemistry methodology and study of their anti-influenza activity. Eur J Med Chem. 2019;182:111622. doi: 10.1016/j.ejmech.2019.111622. [DOI] [PubMed] [Google Scholar]
- 181.Yang JL, Ha TK, Dhodary B, Pyo E, Nguyen NH, Cho H, Kim E, Oh WK. Oleanane triterpenes from the flowers of Camellia japonica inhibit porcine epidemic diarrhea virus (PEDV) replication. J Med Chem. 2015;58:1268–1280. doi: 10.1021/jm501567f. [DOI] [PubMed] [Google Scholar]
- 182.Khwaza V, Oyedeji OO, Aderibigbe BA. Antiviral Activities of Oleanolic Acid and Its Analogues. Molecules. 2018:23. doi: 10.3390/molecules23092300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Cheng SY, Wang CM, Cheng HL, Chen HJ, Hsu YM, Lin YC, Chou CH. Biological activity of oleanane triterpene derivatives obtained by chemical derivatization. Molecules. 2013;18:13003–13019. doi: 10.3390/molecules181013003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Miriyala S, Chandra M, Maxey B, Day A, St Clair DK, Panchatcharam M. Arjunolic acid ameliorates reactive oxygen species via inhibition of p47(phox)-serine phosphorylation and mitochondrial dysfunction. Int J Biochem Cell Biol. 2015;68:70–77. doi: 10.1016/j.biocel.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hwang YJ, Song J, Kim HR, Hwang KA. Oleanolic acid regulates NF-κB signaling by suppressing MafK expression in RAW 264.7 cells. BMB Rep. 2014;47:524–529. doi: 10.5483/BMBRep.2014.47.9.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cao J, Li G, Wang M, Li H, Han Z. Protective effect of oleanolic acid on oxidized-low density lipoprotein induced endothelial cell apoptosis. Biosci Trends. 2015;9:315–324. doi: 10.5582/bst.2015.01094. [DOI] [PubMed] [Google Scholar]
- 187.Tian Y, Sun Z, Wang W, Shang H, Wang B, Deng D, Ma G, Wu H, Zhu N, Xu X, Sun G, Sun X. Semisynthesis and Biological Evaluation of Oleanolic Acid 3-O-β-d-Glucuronopyranoside Derivatives for Protecting H9c2 Cardiomyoblasts against H₂O₂-Induced Injury. Molecules. 2018:23. doi: 10.3390/molecules23010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang Z, Jiang M, Xie X, Yang H, Wang X, Xiao L, Wang N. Oleanolic acid ameliorates high glucose-induced endothelial dysfunction via PPARδ activation. Sci Rep. 2017;7:40237. doi: 10.1038/srep40237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wang SR, Xu T, Deng K, Wong CW, Liu J, Fang WS. Discovery of Farnesoid X Receptor Antagonists Based on a Library of Oleanolic Acid 3-O-Esters through Diverse Substituent Design and Molecular Docking Methods. Molecules. 2017:22. doi: 10.3390/molecules22050690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.He WM, Yin JQ, Cheng XD, Lu X, Ni L, Xi Y, Yin GD, Lu GY, Sun W, Wei MG. Oleanolic acid attenuates TGF-β1-induced epithelial-mesenchymal transition in NRK-52E cells. BMC Complement Altern Med. 2018;18:205. doi: 10.1186/s12906-018-2265-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Atilano-Roque A, Aleksunes LM, Joy MS. Bardoxolone methyl modulates efflux transporter and detoxifying enzyme expression in cisplatin-induced kidney cell injury. Toxicol Lett. 2016;259:52–59. doi: 10.1016/j.toxlet.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 192.de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, Goldsberry A, Houser M, Krauth M, Lambers Heerspink HJ, McMurray JJ, Meyer CJ, Parving HH, Remuzzi G, Toto RD, Vaziri ND, Wanner C, Wittes J, Wrolstad D, Chertow GM BEACON Trial Investigators. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med. 2013;369:2492–2503. doi: 10.1056/NEJMoa1306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yuan B, Yang R, Ma Y, Zhou S, Zhang X, Liu Y. A systematic review of the active saikosaponins and extracts isolated from Radix Bupleuri and their applications. Pharm Biol. 2017;55:620–635. doi: 10.1080/13880209.2016.1262433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yang F, Dong X, Yin X, Wang W, You L, Ni J. Radix Bupleuri: A Review of Traditional Uses, Botany, Phytochemistry, Pharmacology, and Toxicology. Biomed Res Int. 2017;2017:7597596. doi: 10.1155/2017/7597596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lu YF, Wan XL, Xu Y, Liu J. Repeated oral administration of oleanolic acid produces cholestatic liver injury in mice. Molecules. 2013;18:3060–3071. doi: 10.3390/molecules18033060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Liu J. Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol. 1995;49:57–68. doi: 10.1016/0378-8741(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 197.Lin YN, Chen CJ, Chang HY, Cheng WK, Lee YR, Chen JJ, Lim YP. Oleanolic Acid-Mediated Inhibition of Pregnane X Receptor and Constitutive Androstane Receptor Attenuates Rifampin-Isoniazid Cytotoxicity. J Agric Food Chem. 2017;65:8606–8616. doi: 10.1021/acs.jafc.7b02696. [DOI] [PubMed] [Google Scholar]
- 198.Sun M, Tang Y, Ding T, Liu M, Wang X. Investigation of cytochrome P450 inhibitory properties of maslinic acid, a bioactive compound from Olea europaea L., and its structure-activity relationship. Phytomedicine. 2015;22:56–65. doi: 10.1016/j.phymed.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 199.Xie H, Wu J, Liu D, Liu M, Zhang H, Huang S, Xiong Y, Xia C. In vitro inhibition of UGT1A3, UGT1A4 by ursolic acid and oleanolic acid and drug-drug interaction risk prediction. Xenobiotica. 2017;47:785–792. doi: 10.1080/00498254.2016.1234087. [DOI] [PubMed] [Google Scholar]
- 200.Li Z, Wang K, Zheng J, Cheung FS, Chan T, Zhu L, Zhou F. Interactions of the active components of Punica granatum (pomegranate) with the essential renal and hepatic human Solute Carrier transporters. Pharm Biol. 2014;52:1510–1517. doi: 10.3109/13880209.2014.900809. [DOI] [PubMed] [Google Scholar]