Abstract
Cardiac rehabilitation through center-based programs is an effective multicomponent intervention for the secondary prevention of cardiovascular diseases. Despite the benefits it brings, patients’ participation in rehabilitation programs remains low. In this work, the latest relevant literature regarding remotely monitored cardiac telerehabilitation (TR) was reviewed considering its efficiency and utilization. The main objective was to assess whether TR has the potential to be an appropriate alternative form of rehabilitation. A total of 105 publications on this topic were screened out of 747 full-text articles that were read and evaluated, of which 12 were considered suitable for inclusion in the final review. Feasibility, efficiency, and safety were assessed for each TR intervention. The results of our evaluation indicate that TR seems to be a usable, effective, and safe alternative rehabilitation for patients with heart disease. Most of the currently published articles have studied remotely monitored TR intervention offering a comprehensive approach, which indicates the significant development and steps forward in this field of study. Our research evidence supports the implementation of TR, which could positively influence barriers in participating in cardiac rehabilitation programs.
Keywords: Telerehabilitation, Cardiac rehabilitation, Wearable devices, Cardiovascular diseases, Telemonitoring, Telecoaching, Telemedicine
Core tip: Telerehabilitation (TR) and mobile technologies are becoming potentially suitable alternatives through which it is possible to fill the gap over barriers in participation in cardiac rehabilitation programs. Based on this review, it is estimated that TR may be an effective and safe alternative rehabilitation for patients with heart disease. Most of the TR interventions currently published provide a comprehensive approach, indicating the significant development and step-forward in this field of study.
INTRODUCTION
Cardiovascular diseases remain significant causes of death worldwide, which represents a substantial economic burden on health care systems[1]. Guidelines for cardiovascular disease prevention focus on a multifaceted approach in controlling cardiovascular risks, including patient participation in cardiac rehabilitation programs[2,3]. Cardiac rehabilitation is strongly recommended as an effective multicomponent intervention, which includes systematic support and consultancy in achieving key preventative components (Figure 1) and which helps reduce mortality and numbers of rehospitalization in patients after acute coronary syndrome, coronary revascularization, or heart failure[4,5]. Exercise training is one of the essential components of cardiac rehabilitation. Despite the benefits it brings, the attendance of patients in cardiac rehabilitation programs remains low (about 20%-30%). There are numerous barriers that prevent patients from enrolling in cardiac rehabilitation, such as transportation problems and distance to rehabilitation centers, time, and financial costs[6,7]. The home-based model of cardiac rehabilitation copes with a few of these barriers regarding possibilities to take part in outpatient or center-based programs. Comparable effects of home-based and center-based approaches are very encouraging[8].
Figure 1.
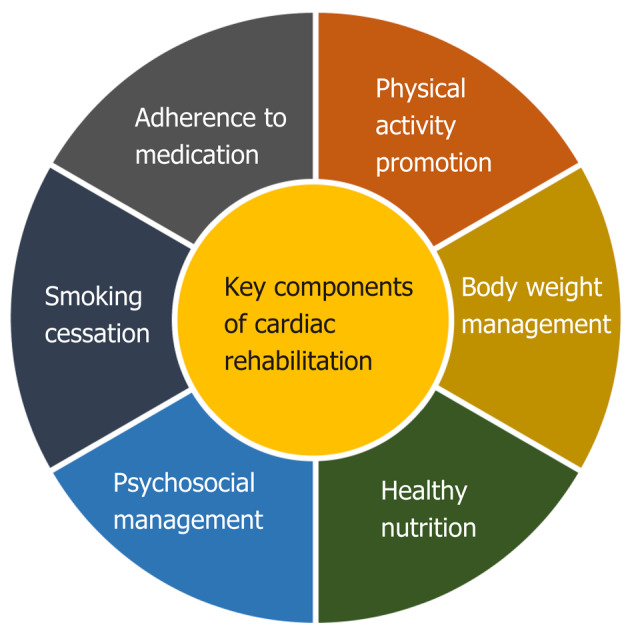
Key preventive components of cardiac rehabilitation. Providing secondary prevention programs of cardiac rehabilitation supports patients in setting goals of increasing levels of physical activity, healthy nutrition, optimal adherence to medication, bodyweight management, smoking cessation, and optimal psychosocial well-being, thereby helping them reduce their risk of a future cardiovascular disease event.
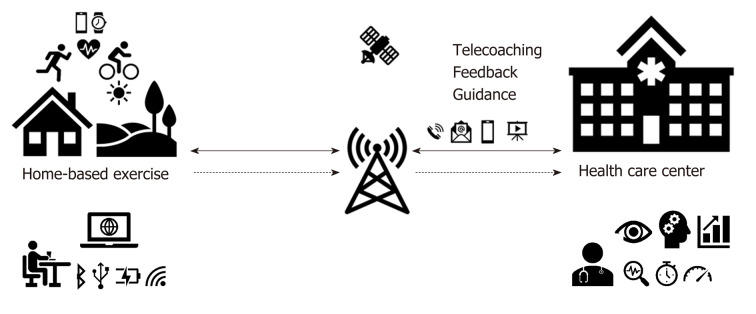
Nevertheless, a lot of home-based programs are unable to include specialist supervision over patients’ performance. Multinational cardiological societies support research studies to increase patients’ attendance in cardiac rehabilitation programs according to their preferences and needs[8-10]. Telerehabilitation (TR) and mobile technologies are becoming potentially suitable alternatives through which it is possible to fill the gap over limited participation. The concept of TR relies on information and communication technologies, such as the internet, telephone, or videoconferencing, to expand home-based cardiac rehabilitation programs and enables enough feedback, coaching, and specialist consultancy to be provided[11] (Figure 2). Thanks to TR, patients do not necessarily have to commute to hospitals and health care centers to take part in cardiac rehabilitation, but they can perform the rehabilitation program in their home environment.
Figure 2.
Scheme of remotely monitored telerehabilitation. The diagram shows remotely monitored home-based exercises from the health care center through telemonitoring and telecoaching. Images and icons are depicted as telerehabilitation form using telemedicine technologies.
The availability of mobile phones and smartphones is still increasing globally. In many developed countries, almost 75% of the population owns mobile devices and nearly 80% can easily connect to the Internet. Furthermore, mobile Internet connection coverage and the Internet subscriptions reach high rates, nearly 80%, and it becomes almost ubiquitous[12,13]. Such a high rate of the Internet and mobile phone accessibility suggests that it is appropriate to start implementing sophisticated telemedicine and mobile health methods that can optimize accessibility, individualization, and utilization of cardiac rehabilitation programs. Using remote technologies and wearable sensors enables monitoring patient performance and exercise information, such as intensity, time, distance, heart rate, and many other relevant factors. Medical staff can provide regular feedback and guidance to patients. The integration of wearable sensors and mobile telecommunication technologies have not been appropriately explored in home-based cardiac rehabilitation programs so far.
Nevertheless, many benefits are expected. Wireless devices are adaptive and enable providing intervention to broader environment. This review examines recent literature on the use of remotely monitored cardiac TR and evaluates its efficiency and utilization, and the safety of these interventions.
LITERATURE SEARCH
We searched for articles on MEDLINE and Web of Science from January 2012 to October 2019, while using a combination of key words as a searching process. The key words were the following: “telerehabilitation”, “telemonitoring”, “home-based”, or “training” or “Internet” or “smartphone” or “mobile” or “remote” and (“cardiac” or “cardiovascular” or “heart”). Initially, the relevant articles were independently selected and evaluated by two reviewers based on their abstracts. In the next step, the full text was assessed for those articles which met the inclusion criteria. The available publications had to be written in English, to have included TR with a wearable sensor in cardiac patients, to be randomized controlled studies with an active or usual care group, to have involved telemedicine surveillance, and to have at least 6 wk for the intervention period.
Studies included in both publication databases as duplicates, describing alternative methods of cardiac rehabilitation without using remote telemonitoring, were excluded from the review. Systematic reviews, meta-analyses, or expert commentaries were excluded as well, but their appropriate references and studies that met the inclusion criteria were evaluated. The selected studies underwent a full evaluation. If there was a disagreement between the two reviewers, the lead author decided on suitability for the analysis.
PATIENT DISTRIBUTION AND INTERVENTION DESCRIPTION
We identified two groups of patients with heart disease divided into ischemic heart disease and heart failure groups. The first group was patients with myocardial infarction, angina pectoris, aortocoronary bypass, and coronary revascularization. The second group consisted of patients with chronic heart failure with preserved or reduced left ventricular ejection fraction. In the next step, we described the phase of cardiac rehabilitation (Phase II, Phase III, or heart failure) and the distribution of intervention participants according to the risk of cardiovascular events.
The two main areas of TR intervention were telemonitoring and telecoaching. Each of the evaluated studies monitored whether TR intervention contains one or both areas of focus. Description of TR intervention with remote monitoring of physical fitness consisted of identifying the component of telemonitoring, through which the data were collected and analyzed from sensors in order to obtain precise and complete patient data. That concretely meant intensity description and duration of physical fitness while using wearable sensors (heart rate monitors, accelerometers, pedometers). In another area of telecoaching focus, we evaluated the variability of techniques that control, educate, and motivate patients to do exercises and the principles of secondary prevention. At the same time, it was necessary to specify all possible ways of how often the information was transmitted from patients to specialists in medical centers. Telecommunication channels allowed providing patients with feedback in real-time or after performance.
STUDY AND REPORTING QUALITY
The quality of the described studies was evaluated by the Tool for the assEssment of Study qualiTy and reporting in EXercise (TESTEX), which is used for the assessment of study quality and reporting in exercise training studies[14]. The TESTEX tool was chosen due to its reliability for exercise scientists, which facilitates a comprehensive review of exercise training trials. The advantage of this scale, compared to traditional study quality criteria, is that it considers criteria such as participant or researcher blinding, which is extremely difficult to be implemented in these study types. The scale includes 12 criteria, some of which are given more than one possible point, allowing a maximum of 15 points, whereby 5 points can be earned for the quality of the study, while 10 points can be earned for reporting. Higher scores reflect better study quality and reporting. Here, we have classified average score studies as "High Quality" for 12 points or more, "Good Quality" for 7 to 11 points, and "Low Quality" for 6 points or less.
High intervention quality was defined as highly relevant, reproducible, and very well methodically-described with precise results reporting. Good intervention quality was defined as medium relevant and with valuable reproduction possibilities in further studies but with limitations in results reporting. Low intervention quality was defined as having substantial limitations regarding relevance, poor reproducing possibilities, and insufficient results reporting. This self-approach was chosen because validated scales of marginal results are not recommended.
OUTCOMES DESCRIPTION AND EVALUATION
All eligible studies were assessed regarding primary and secondary outcomes used in TR intervention publications. Primarily, studies described TR efficiency as an exercise capacity before and after intervention (at the baseline and after intervention). Second, the feasibility of TR has been described by the percentage of completion, and the safety assessment focused on the occurrence of adverse events and mortality. Adverse events were identified as mild, moderate events that require medical help or treatment and could lead to further hospitalization. Mortality was defined as a severe adverse event.
In this review, the focus of TR interventions on key preventive components of cardiac rehabilitation has also been assessed. As recommended by multinational cardiology societies, it is essential to include a combination of these preventive components of cardiac rehabilitation to reduce the burden of the disease[2,3]. We assessed whether TR interventions considered only one, two, or more key preventative components for cardiac rehabilitation. We also focused on methods and forms of delivery for comprehensive cardiac TR.
LITERATURE SEARCH RESULTS
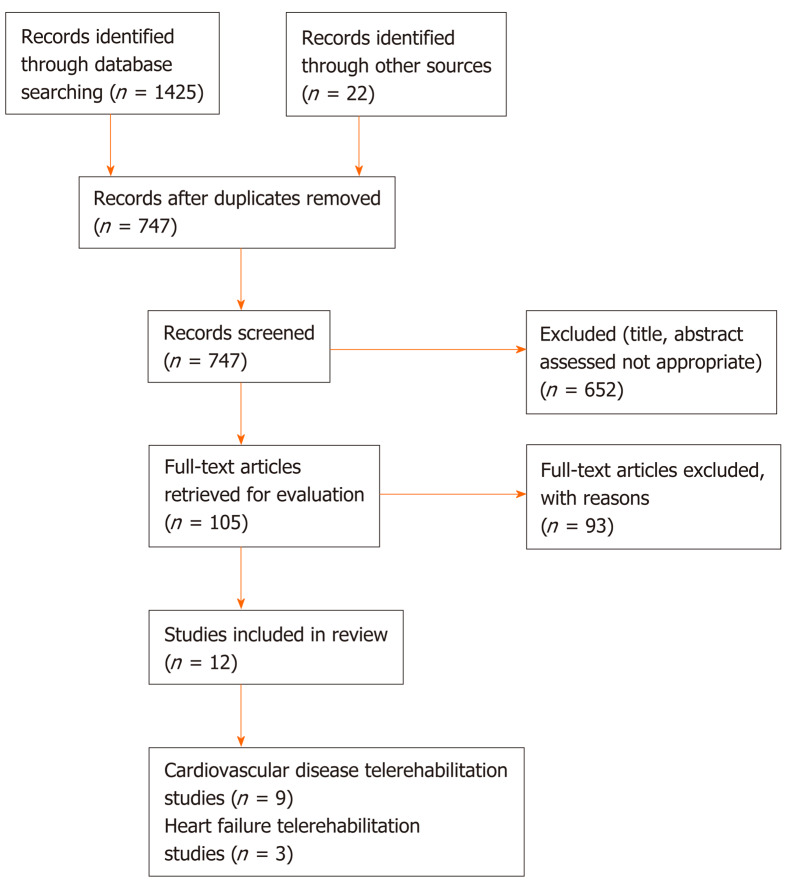
We identified 1425 unique publications through the MEDLINE and Web of Science databases searching and 22 publications from other sources. A total of 700 duplicates were recognized and excluded from the review. We screened the abstracts and titles of the remaining 747 publications and found 652 inappropriate works (other study focus); the 105 publications left were studied in detail as full texts and another 93 works were excluded. The main reasons to exclude these works were lack of remote monitoring (54 publications), insufficient TR description (30 publications), and not including exercise effectivity (9 publications). Finally, 12 eligible randomized controlled studies were accepted, including 9 that were cardiovascular disease TR studies[15-23] and 3 heart failure TR studies[24-26]. An overview of the search and publication selection process is shown in Figure 3.
Figure 3.
Flowchart of the publication selection process.
Two each from among the twelve studies were conducted in Australia[23,24], Belgium[15,18], and China[17,25]; one each was conducted in Korea[20], the Netherlands[19], New Zealand[21], Poland[26], and Spain[16], and one was multi-centrally conducted in Germany, the United Kingdom and Spain[22]. Five of the eligible studies were published between 2012 and 2016, and the other seven works between 2016 and 2020. The sample size of TR interventions ranged from 14 patients to 82 patients (total sample 545) followed by an intervention period ranging from 6 wk to 24 wk (most commonly, a 12-wk follow-up was used). Six studies included the active control group of patients in center-based cardiac rehabilitation, and another six studies included a control group of patients with usual care. Most studies included patients with an uncomplicated myocardial infarction or after coronary revascularization in phase II of cardiac rehabilitation with a low or medium risk of cardiovascular complications. Only three studies included patients with heart failure (Table 1).
Table 1.
Description of telerehabilitation studies
| Ref. | Design | TR group patients |
TR intervention |
Outcomes |
||
| Remote monitoring | Exercise prescription | Feedback | I-primary II-secondary | |||
| Avila et al[15], 2018 | RCT, 12-wk III-phase CR active CBCR | n = 30 age 59 I-II CVR | Post-exercise, chest strap HR monitor, internet connection | Target HR zone 70%-80% HRR, 150 min/wk | Phone call or email 1/wk | I-exercise capacity II-daily PA, strength CVR factors |
| Bravo-Escobar et al[16], 2017 | RCT, 8-wk II-phase CR active CBCR | n = 14 age 56 II - CVR | Post-exercise, ECG, biometric vest, smartphone internet | Target HR zone 70%-80% HRR, 60 min > 2/wk | Supervised CR in center 1/wk | I-exercise capacity II-CVR factors, HRQL, adherence |
| Fang et al[17], 2018 | RCT, 6-wk II-phase CR usual care CG | n = 40 age 60 I - CVR | Real-time belt strap sensor, smartphone internet connection | Physical activity booklet, walking 3/wk | Phone call 1/wk | Exercise capacity, anxiety, depression CVR factors |
| Frederix et al[18], 2015 | RCT, 24-wk II-III phase CR usual care CG | n = 70 age 61 | Post-exercise accelerometer, webservice, internet | Target HR zone 1VT, 45-60 min 2/wk | Email or SMS 1/wk | I-exercise capacity II-daily PA, HRQL, CVR factors |
| Hwang et al[24], 2017 | RCT, 12-wk HF-CR active CBCR | n = 24 age 68 | Real-time video platform via internet, educational lectures | Target RPE zone 9-13 RPE, 60 min 2/wk | 15 min real-time video feedback | I-walking distance II-balance, strength, HRQL |
| Kraal et al[19], 2014 | RCT, 12-wk II-phase CR active CBCR | n = 25 age 59 I-II CVR | Post-exercise, chest strap HR monitor, internet platform | Target HR zone 70%-85% HRmax 45%-60% min > 2/wk | Weekly coaching via telephone | I-exercise capacity II-HRQL, training adherence |
| Lee et al[20], 2013 | RCT, 12-wk II-phase CR usual care CG | n = 26 age 54 I-II CVR | Wearable ECG equipment via mobile phone | Target HR zone 40%-80% HRR, 50 min, 4-5/wk | Phone call 1/wk | I-exercise capacity II-HRQL |
| Maddison et al[21], 2019 | RCT, 12-wk II-phase CR active CBCR | n = 82 age 61 | Chest-worn wearable ECG sensor, internet smartphone app, accelerometry | Target HR and RPE zone 40%-65% HRR, 11-13 RPE 30-60 min, 3/wk | Real-time audio service via earphones | I-exercise capacity II-daily PA, HRQL, CVR factors, costs |
| Peng et al[25], 2018 | RCT, 8-wk HF-CR usual care CG | n = 49 age 66 | Real-time, HR monitor smartphone, internet platform | Target HR zone 40%-70% HRR 20 min, 3/wk | Text, audio service via webcam | I-HRQL II-walking distance, anxiety, depression |
| Piotrowicz et al[26], 2014 | RCT, 8-wk HF-CR usual care CG | n = 77 age 54 | Real-time, ECG, BP and BW monitor, mobile phone | Target HR zone 40%-70% HRR 45-60 min 5/wk | Real-time telephone call | I-exercise capacity II-walking distance HRQL, adherence |
| Skobel et al[22], 2017 | RCT, 24-wk III-phase CR usual care CG | n = 55 age 60 | Real-time, ECG sensor vest, smartphone, internet platform | Target RPE zone 11-13 RPE, 10-60 min 3/wk | Real-time via smartphone | I-exercise capacity II-HRQL, CVR factors, anxiety |
| Varnfield et al[23], 2014 | RCT, 24-wk II-phase CR active CBCR | n = 53 age 56 | Post-exercise, step-counter, BP and BW monitor, web portal, internet | Target RPE zone 11-13 RPE, > 30 min most d/wk | Text message telephone consultation first 6 wk | I-uptake, adherence II-HRQL, CVR factors, functional capacity |
BP: Blood pressure; BW: Body weight; CBCR: Center-based cardiac rehabilitation; CG: Control group; CR: Cardiac rehabilitation; CVR: Cardiovascular risk; ECG: Electrocardiogram; HF: Heart failure; HR: Heart rate; HRQL: Health-related quality of life; HRR: Heart rate reserve; n: Number of participants; PA: Physical activity; RCT: Randomized controlled trial; RPE: Ratings of perceived exertion; SMS: Short message service; TR: Telerehabilitation.
All TR studies we investigated contained physical exercise. The prescription of TR physical activity was determined in the frequency range from 2 sessions to 5 sessions per wk, with a duration from 30 min to 300 min per week. Physical activity prescription was set from 45 min to 60 min per one session, including a warming up phase and cooling down phase with the modality of physical fitness activities (walking, Nordic walking, jogging, cycling).
In most rehabilitation interventions, all participants started the program under the direct supervision of a specialist in a hospital center (the number of sessions varied in each study), followed by a remotely monitored TR exercise. The intensity of physical exercise ranged from 40% to 80% heart rate reserve or from 9 to 13 ratings of perceived exertion. In some cases, intensity or modality was not reported.
Furthermore, in most TR studies, the intensity of physical activity was monitored by electrocardiogram telemetry (n = 5) or through wearable monitor or heart rate sensor with chest strap fixing. In half of the study trials, the telemonitoring was done in real-time. Exercise protocols included telecoaching with feedback provided via telephone calls (n = 7), videoconferences (n = 2), text messages, emails or short message services (commonly known as SMSs) (n = 2). In one case, the feedback was provided by the specialist (n = 1) (Table 1).
In six studies, post-exercise telecoaching was done once a week, with the Internet as a tool for data transmission. In the other six studies, real-time telecoaching was used. In these cases, the physical activity data and communication with patients were provided through videoconferences or mobile phones.
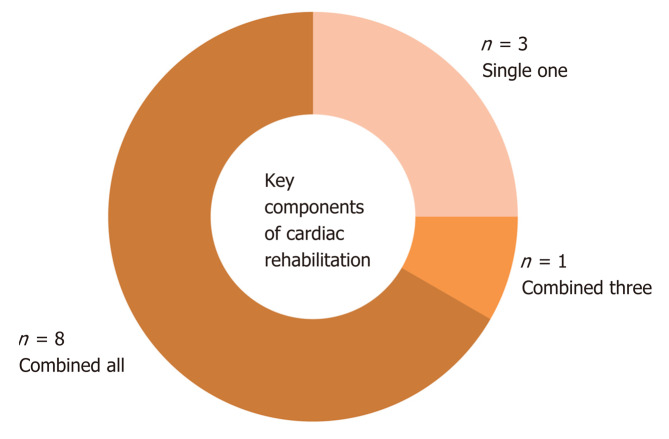
Evaluation of studied publications showed that most of the TR interventions (8 studies, 67%) included a combination of key preventive components (Figure 4). Only three published studies included only one component of physical activity. Also, the physical activity was the most evaluated cardiac rehabilitation component. Comprehensive TR interventions delivered preventive components to participants at the beginning of the intervention by the initial examinations or during the intervention period by telecoaching.
Figure 4.
Use of key components in telerehabilitation interventions. The number of studies that evaluated one, three, or all key preventive components of cardiac rehabilitation are shown. Blue represents the use of a single component (physical activity); Red represents the combination of three components; Green represents the use of all preventive components in the telerehabilitation intervention.
Feedback methods used in the studies were: Group educative session[16]; individual educative session[19-21,25]; educational brochure/booklet[17,25]; telecoaching: Regular consultations via email/SMS[18]; motivational telephone call[19]; audiovisual electronic presentation with possible chat[24]; and health smartphone applications for delivering motivational and educational materials to participants (messages and audio-visual files)[23].
STUDY AND REPORTING QUALITY
Results of the study quality assessment showed that total study and reporting quality were good, with an average score if 11 points (range: 5-15 points). The study quality was mainly evaluated as high, being 4 points on average (range: 2-5 points) and reporting of the study results was rated as good, being 7 points on average (range: 3-10 points). Only two studies were evaluated for low overall intervention quality (Table 2).
Table 2.
Study quality using Tool for the assEssment of Study qualiTy and reporting in EXercise scale
| Ref. | Study quality, Max 5 | Study reporting, Max 10 | Total score, Max 15 |
| Avila et al[15], 2018 | 4 | 9 | 13 |
| Bravo-Escobar et al[16], 2017 | 4 | 7 | 11 |
| Fang et al[17], 2018 | 2 | 3 | 5 |
| Frederix et al[18], 2015 | 5 | 8 | 13 |
| Hwang et al[24], 2017 | 5 | 10 | 15 |
| Kraal et al[19], 2014 | 4 | 8 | 12 |
| Lee et al[20], 2013 | 2 | 3 | 5 |
| Maddison et al[21], 2019 | 4 | 6 | 10 |
| Peng et al[25], 2018 | 4 | 7 | 11 |
| Piotrowicz et al[26], 2014 | 4 | 6 | 10 |
| Skobel et al[22], 2017 | 5 | 5 | 10 |
| Varnfield et al[23], 2014 | 4 | 9 | 13 |
Max: Maximum.
ENDPOINT ASSESSMENT
In most TR studies (n = 10, 83%), the primary outcome was to compare the effect of the intervention in exercise capacity. A large portion of these studies used peak oxygen uptake to describe exercise capacity effect. Besides using peak oxygen uptake, some other studies explored other measurements, such as a 6-min walking test or exercise stress test, with peak metabolic equivalents improvement.
Almost all of the studies evaluating TR intervention in a group of patients reached a statistically significant change in exercise capacity (P < 0.05). In six studies we identified similar improvements in comparison to the active control group undergoing their training in medical center. On the contrary, six studies showed a statistically significant improvement in exercise capacity in the TR group in comparison to the usual care group (Table 3).
Table 3.
Studies outcomes
| Ref. | Follow-up, in week |
Completion rate, % |
Adverse events, n | Exercise capacity between group differencesa | |
| TR | CG | ||||
| Avila et al[15], 2018 | 12 | 94 | 1001 | No adverse events | TR = CBCR |
| Bravo-Escobar et al[16], 2017 | 8 | 93 | 1001 | 3 angina type pain during TR, no ECG changes; no serious CV events | TR = CBCR |
| Fang et al[17], 2018 | 6 | 83 | 85 | Not reported | TR > UC |
| Frederix et al[18], 2015 | 24 | 89 | 91 | Not reported | TR > UC |
| Hwang et al[24], 2017 | 12 | 100 | 901 | Minor adverse events: 3 angina, 2 palpitation, 3 diaphoresis; no serious CV events | TR = CBCR |
| Kraal et al[19], 2014 | 12 | 86 | 961 | No adverse events | TR = CBCR |
| Lee et al[20], 2013 | 12 | 87 | 97 | Not reported | TR > UC |
| Maddison et al[21], 2019 | 12 | 88 | 921 | Mild: 12 non-serious; moderate: 6 medically important event, hospitalization; no serious CV complications | TR = CBCR |
| Peng et al[25], 2018 | 8 | 86 | 84 | No adverse events | TR > UC |
| Piotrowicz et al[26], 2014 | 8 | 97 | 94 | Minor: 4 skin reactions due to the ECG electrodes; no serious CV events | TR > UC |
| Skobel et al[22], 2017 | 24 | 35 | 67 | No CV events associated to CR adverse events: 9; 2 chest infection, 3 angina, 2 chest pain before training, 1 atrial fibrillation, 1 palpitation | TR > UC |
| Varnfield et al[23], 2014 | 6 | 91 | 681 | 10 deterioration in health and/or medical care needs unrelated to CR; no serious CV events | TR = CBCR |
P < 0.05.
Center-based cardiac rehabilitation. CBCR: Center-based cardiac rehabilitation; CG: Control group; CR: Cardiac rehabilitation; CV: Cardiovascular; ECG: Electrocardiogram; TR: Telerehabilitation; UC: Usual care.
These data are supported by the high percentage of completion rate of all TR interventions, having 86% on average (range: 35%-100%) in comparison to control group, having 89% on average (range: 67%-100%).
Adverse events were assessed in most of the studies; only three studies did not include them in their report. No cardiovascular severe complications or deaths were recorded during interventions described in the studies. Only mild to moderate cardiovascular events occurred in six studies but were not reported as a complication during TR exercise (Table 3).
Secondary outcome measures included modifiable lifestyle, biomedical cardiovascular risk factors (daily physical activity levels and energy expenditure, dietary habits, nicotine dependence, psychosocial functioning, blood pressure, heart rate, body weight, body mass index, waist circumference, fasted blood lipids, glucose concentrations, exercise-related motivation, acceptance and satisfaction of the TR intervention, health-related quality of life), cost efficiency through questionnaires, clinical assessments, evaluations, and blood sample testing. Comparing secondary outcomes was difficult due to various measurement methods and monitoring devices. Nevertheless, comparing secondary outcomes was not our research aim.
DISCUSSION
This review brings the newest insight into relevant literary sources regarding TR intervention in cardiac patients. The purpose of this work was to explore whether remotely monitored TR can be an adequate form of rehabilitation compared to traditional center-based cardiac rehabilitation.
The main findings of the review were that TR appears to be at least as effective in improving cardiovascular risk factors and exercise capacity as traditional center-based cardiac rehabilitation. Study results significantly correspond with previous publications comparing intervention between home-based and center-based cardiac rehabilitation[8,27,28]. TR studies included in this review mention completion rates comparable to those monitored in center-based cardiac rehabilitation. The total incompletion intervention rate followed by exclusion from the study, which is mentioned in the final review, is comparable to that in previous studies[29,30]. Study outcomes support our assumption that remotely monitored cardiac TR can be an effective alternative method that is suitable, especially for those participants who face some barriers or limitations and are not able to take part in center-based programs. As already proved in many previous cardiac rehabilitation studies, improving exercise capacity corresponded with the following prognosis, mortality included[5,31,32].
The fact that participants of remotely monitored TR also had similar results to participants of center-based cardiac rehabilitation speaks well of the potential of this effective intervention in improving long-term prognosis after significant heart events. The quality evaluation of the studies showed an above-average level of study design and reporting. Only two of thirteen studies were below average, indicating this collection of studies a good sample with strong research basics and likelihood to be well-reproduced. This finding shows that the studies’ quality levels in this review are better than those assessed in previous research[33].
Besides monitoring physical activity, most TR interventions included some other key components of cardiac rehabilitation. A combination of telemonitoring and telecoaching was the most common TR form. This process is in line with recommendations of the American and European Preventive Societies to a multidisciplinary approach in cardiac rehabilitation, and there is no reason to underestimate TR intervention, which can deliver highly relevant practice[34-36].
In addition to monitoring and coaching, comprehensive programs can support cardiac patients in their rehabilitation process through social networks and e-learning. Social networks support and setting goals through e-learning can help individuals who lack motivation or interest in participating in TR programs. E-learning tools can provide patients with important medical and scientific information to improve knowledge of their condition and eventually lead to better compliance with therapy. Comprehensive TR programs, including all the above areas of key preventive components, can have an even greater impact on patient health[37]. The next part of our review deals with the question of TR intervention safety. As patients rehabilitate without “face-to-face” surveillance, as is common in traditional center-based models, there are some concerns and doubts about the safety of such interventions. Not much about this topic has been published so far, particularly for high-risk patients, such as those with heart failure. Our review includes nine studies that considered the question of safety. Of the total number of participating patients (n = 545), there was no cause of death or serious complications associated with TR intervention. Three of these studies included patients (n = 150) with heart failure[24-26].
Although these results support the implementation of TR intervention in all risk groups of patients with heart disease, more extensive research in this area is needed to decide on the level of control. The ongoing “TELEREH-HF” study provides new data on TR effects on hospitalization and mortality in patients with heart failure. A total of 850 patients with TR intervention period of 8 wk, followed by a 24-mo follow-up, may provide more substantial evidence in this field[38].
The cost-effectiveness of remotely monitored TR was examined in a study by Maddison et al[21] and indicated that the intervention reduced the total cost of rehabilitation by 70% compared to a traditional center-based program. The FIT@home study confirmed that a TR program of the same duration as a center-based program could be a cost effective alternative[39]. Finally, this promising assumption is supported by a study from Belgium, which showed that if the patients’ participation in TR cardiac program increases, it can reduce the burden of disease and the results will overtop the costs[40]. This research describes TR as a cost-effective complement, particularly for those patients who cannot undergo center-based cardiac rehabilitation.
Long-term maintenance after the adaptation phase of cardiac rehabilitation is a critically important issue, and there were only two studies in our review, which monitored long-term effects 1 year after the intervention[39,41]. Although it is reasonable to presume that TR interventions (in case they were effective) lead to improving clinical outcomes, further studies are needed to see the long-time impact of this intervention. However, promising results from individual studies suggest that TR is at least as effective in maintaining multiple intervention outcomes as center-based programs.
Besides, it is not clear how much the use of TR can fill the gap in participation in cardiac rehabilitation programs. An important potential benefit of TR is its flexibility, which can help improve the low level of cardiac rehabilitation participation and compliance reported in studies at center-based programs[42,43]. At the same time, strategies based on improving participation in cardiac rehabilitation, such as a systematic or automatic enrollment may also be successful in cardiac TR models[44,45]. For example, it is interesting that when the patients could choose from participating in a home-based or center-based program, almost one-half of eligible patients preferred a home-based approach[46]. It can also indicate TR preference, which extends current home intervention and enables remotely monitored supervision in order to fill many patients’ needs. At the same time, we estimate that TR can increase the number of participating patients who have difficulties commuting to rehabilitation, have personal reasons for non-participation, or have inflexible working hours[7,47].
Usage and optimization of the TR tool offered through telemedical platforms can lead to enhancing their attractiveness and usefulness. The fact that our review includes patients who are 60-years-old on average (in half of the studies, being even more than 60-years-old in age) reflects the attractiveness and willingness of older people to engage in using new technologies.
However, acceptance and usefulness of the application can be limited by some other factors. Smartphone-based heart rate monitors may produce mixed results in accuracy between applications[48]. This unreliability of data may limit the use of mobile health applications for telemonitoring purposes. On the other hand, the validity of heart rate monitors (wrist or chest strap sensors) has produced satisfactory results in accuracy[49]. This fast-developing technological field of wearable sensors will probably provide more opportunities to be more precise and to optimize remotely monitored TR possibilities[50].
LIMITATIONS
Some limitations of this review result from heterogeneity and lack of details in the publications we analyzed. Most studies included heterogenous study population, used various TR forms, modalities of physical fitness, monitoring times, and evaluations of intervention effect. Various methodologies used in the studies limited us from comparing them in detail.
Another limitation is the disunity among the studies regarding the cardiac rehabilitation phase. The studies mention the adaptation phase (II), stabilization, and maintenance phase (III), and a combination of both, and some studies did not provide information about phases, which limits us from providing a better comparison. It is difficult to be transparent in results’ interpretation. Further problems might occur in developed countries, where the elderly are not capable of using high-tech technology.
Regardless of these limitations, the results show improvement and offer the possibility to find a place for TR in preventing heart disease.
OPPORTUNITIES FOR FUTURE DEVELOPMENT
Although TR has been explored from many points of views, there are some more key questions, which are necessary to be answered in further investigations in this field of study. Concretely, TR studies published do not include enough women in order to decide about specific sex-related differences as an intervention factor. What is more, we have not found any study exploring implementation of TR in patients with low socioeconomic status. In these cases, the intervention might decrease barriers in attendance and bring more essential advantages. At the same time, we need to study TR impact on various age groups, especially for the elderly, who often have individual needs that can limit them from reaching optimal intervention effect[51].
The future requires more studies that will implement TR tools for a various spectrum of eligible patients, including those who have more difficult life conditions (rural living, comorbidity, low socioeconomic status, older age) and who may not achieve similar safety outcomes or effectiveness of TR intervention. The challenge for further development and implementation of TR can be keeping and securing the data regarding the personal information of participants and providers. It is necessary to think about the area of cybernetic security as well.
Previous data have indicated that inclusion of other training modalities, such as interval training with high intensity, can bring further health benefits and shorter time of physical performance[52]. On the other hand, implementing this new method in the home environment can be more difficult and less precise in comparison to possibilities in medical centers under supervision. The use of such a form in the TR model provides space for further studies.
The current overview of remotely monitored TR was designed for cardiology patients. However, it has potential for use in other groups of patients with different diseases (for example, cardio-oncology, cardiopulmonary, neurology, metabolic syndrome, and many others.). Also, these patients could benefit from this form in which the program is tailored to their individual needs and health condition.
CONCLUSION
According to this review, it is estimated that TR could (compared to traditional center-based program of cardiac rehabilitation) represent usable, effective, and safe alternative forms of rehabilitation for patients with heart disease. Most of the currently published works studied remotely monitored TR interventions offering a comprehensive approach, which indicates significant development and steps forward in this field of study.
Our research evidence supports the effectiveness of TR, which could positively influence barriers to participation in cardiac rehabilitation programs. TR seems to be a great possibility to facilitate taking part in programs for those individuals who cannot decide any other way. These encouraging findings should be supported by additional studies but already present a strong reason for implementing TR in existing secondary preventive therapies of cardiac rehabilitation.
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interest for this article.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: Czech Society of Cardiology.
Peer-review started: February 21, 2020
First decision: March 18, 2020
Article in press: April 28, 2020
Specialty type: Medicine, research and experimental
Country/Territory of origin: Czech Republic
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A, A
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Barik R, Boussuges A, Dai XM, Karatza AA, Kaypakli O, Kharlamov AN, Tzamaloukas AHH, Ueda H S-Editor: Zhang L L-Editor: A E-Editor: Zhang YL
Contributor Information
Ladislav Batalik, Department of Rehabilitation, University Hospital Brno, Brno 62500, Czech Republic; Department of Cardiology and Internal Medicine, University Hospital Brno, Faculty of Medicine, Masaryk University Brno, Brno 62500, Czech Republic. batalik.ladislav@fnbrno.cz.
Katerina Filakova, Department of Rehabilitation, University Hospital Brno, Brno 62500, Czech Republic.
Katerina Batalikova, Department of Rehabilitation, University Hospital Brno, Brno 62500, Czech Republic.
Filip Dosbaba, Department of Rehabilitation, University Hospital Brno, Brno 62500, Czech Republic.
References
- 1.World Health Organization. Health statistics and information systems - Global Health Estimates. Available from: https://www.who.int/healthinfo/global_burden_disease/estimates/en/index1.html. [Google Scholar]
- 2.Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corrà U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Løchen ML, Löllgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, Binno S ESC Scientific Document Group. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur Heart J. 2016;37:2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Association of Cardiovascular and Pulmonary Rehabilitation. Guidelines for Cardiac Rehabilitation and Secondary Prevention Programs. 5th ed, United States: Human Kinetics, 2013: 12-72. [Google Scholar]
- 4.Sandesara PB, Lambert CT, Gordon NF, Fletcher GF, Franklin BA, Wenger NK, Sperling L. Cardiac rehabilitation and risk reduction: time to "rebrand and reinvigorate". J Am Coll Cardiol. 2015;65:389–395. doi: 10.1016/j.jacc.2014.10.059. [DOI] [PubMed] [Google Scholar]
- 5.Hammill BG, Curtis LH, Schulman KA, Whellan DJ. Relationship between cardiac rehabilitation and long-term risks of death and myocardial infarction among elderly Medicare beneficiaries. Circulation. 2010;121:63–70. doi: 10.1161/CIRCULATIONAHA.109.876383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shanmugasegaram S, Oh P, Reid RD, McCumber T, Grace SL. Cardiac rehabilitation barriers by rurality and socioeconomic status: a cross-sectional study. Int J Equity Health. 2013;12:72. doi: 10.1186/1475-9276-12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunlay SM, Witt BJ, Allison TG, Hayes SN, Weston SA, Koepsell E, Roger VL. Barriers to participation in cardiac rehabilitation. Am Heart J. 2009;158:852–859. doi: 10.1016/j.ahj.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson L, Sharp GA, Norton RJ, Dalal H, Dean SG, Jolly K, Cowie A, Zawada A, Taylor RS. Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst Rev. 2017;6:CD007130. doi: 10.1002/14651858.CD007130.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piepoli MF, Corrà U, Benzer W, Bjarnason-Wehrens B, Dendale P, Gaita D, McGee H, Mendes M, Niebauer J, Zwisler AD, Schmid JP Cardiac Rehabilitation Section of the European Association of Cardiovascular Prevention and Rehabilitation. Secondary prevention through cardiac rehabilitation: from knowledge to implementation. A position paper from the Cardiac Rehabilitation Section of the European Association of Cardiovascular Prevention and Rehabilitation. Eur J Cardiovasc Prev Rehabil. 2010;17:1–17. doi: 10.1097/HJR.0b013e3283313592. [DOI] [PubMed] [Google Scholar]
- 10.Balady GJ, Ades PA, Bittner VA, Franklin BA, Gordon NF, Thomas RJ, Tomaselli GF, Yancy CW American Heart Association Science Advisory and Coordinating Committee. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond: a presidential advisory from the American Heart Association. Circulation. 2011;124:2951–2960. doi: 10.1161/CIR.0b013e31823b21e2. [DOI] [PubMed] [Google Scholar]
- 11.Jin K, Khonsari S, Gallagher R, Gallagher P, Clark AM, Freedman B, Briffa T, Bauman A, Redfern J, Neubeck L. Telehealth interventions for the secondary prevention of coronary heart disease: A systematic review and meta-analysis. Eur J Cardiovasc Nurs. 2019;18:260–271. doi: 10.1177/1474515119826510. [DOI] [PubMed] [Google Scholar]
- 12.Our Mobile planet: Understanding mobile consumer beavior. May 2012, In: Google services [Internet]. Available from: https://www.thinkwithgoogle.com/advertising-channels/mobile-marketing/our-mobile-planet-us-infographic/ [Google Scholar]
- 13.Sanou B. ICT Facts and Figures 2016. International Telecommunication Union Fact Sheet, 2016. Available from: https://www.itu.int/en/ITU-D/Statistics/Documents/facts/ICTFactsFigures2016.pdf Cited 17 January 2020. [Google Scholar]
- 14.Smart NA, Waldron M, Ismail H, Giallauria F, Vigorito C, Cornelissen V, Dieberg G. Validation of a new tool for the assessment of study quality and reporting in exercise training studies: TESTEX. Int J Evid Based Healthc. 2015;13:9–18. doi: 10.1097/XEB.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 15.Avila A, Claes J, Goetschalckx K, Buys R, Azzawi M, Vanhees L, Cornelissen V. Home-Based Rehabilitation With Telemonitoring Guidance for Patients With Coronary Artery Disease (Short-Term Results of the TRiCH Study): Randomized Controlled Trial. J Med Internet Res. 2018;20:e225. doi: 10.2196/jmir.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bravo-Escobar R, González-Represas A, Gómez-González AM, Montiel-Trujillo A, Aguilar-Jimenez R, Carrasco-Ruíz R, Salinas-Sánchez P. Effectiveness and safety of a home-based cardiac rehabilitation programme of mixed surveillance in patients with ischemic heart disease at moderate cardiovascular risk: A randomised, controlled clinical trial. BMC Cardiovasc Disord. 2017;17:66. doi: 10.1186/s12872-017-0499-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang J, Huang B, Xu D, Li J, Au WW. Innovative Application of a Home-Based and Remote Sensing Cardiac Rehabilitation Protocol in Chinese Patients After Percutaneous Coronary Intervention. Telemed J E Health. 2019;25:288–293. doi: 10.1089/tmj.2018.0064. [DOI] [PubMed] [Google Scholar]
- 18.Frederix I, Hansen D, Coninx K, Vandervoort P, Vandijck D, Hens N, Van Craenenbroeck E, Van Driessche N, Dendale P. Medium-Term Effectiveness of a Comprehensive Internet-Based and Patient-Specific Telerehabilitation Program With Text Messaging Support for Cardiac Patients: Randomized Controlled Trial. J Med Internet Res. 2015;17:e185. doi: 10.2196/jmir.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kraal JJ, Peek N, Van den Akker-Van Marle ME, Kemps HM. Effects of home-based training with telemonitoring guidance in low to moderate risk patients entering cardiac rehabilitation: short-term results of the FIT@Home study. Eur J Prev Cardiol. 2014;21:26–31. doi: 10.1177/2047487314552606. [DOI] [PubMed] [Google Scholar]
- 20.Lee YH, Hur SH, Sohn J, Lee HM, Park NH, Cho YK, Park HS, Yoon HJ, Kim H, Nam CW, Kim YN, Kim KB. Impact of home-based exercise training with wireless monitoring on patients with acute coronary syndrome undergoing percutaneous coronary intervention. J Korean Med Sci. 2013;28:564–568. doi: 10.3346/jkms.2013.28.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maddison R, Rawstorn JC, Stewart RAH, Benatar J, Whittaker R, Rolleston A, Jiang Y, Gao L, Moodie M, Warren I, Meads A, Gant N. Effects and costs of real-time cardiac telerehabilitation: randomised controlled non-inferiority trial. Heart. 2019;105:122–129. doi: 10.1136/heartjnl-2018-313189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skobel E, Knackstedt C, Martinez-Romero A, Salvi D, Vera-Munoz C, Napp A, Luprano J, Bover R, Glöggler S, Bjarnason-Wehrens B, Marx N, Rigby A, Cleland J. Internet-based training of coronary artery patients: the Heart Cycle Trial. Heart Vessels. 2017;32:408–418. doi: 10.1007/s00380-016-0897-8. [DOI] [PubMed] [Google Scholar]
- 23.Varnfield M, Karunanithi M, Lee CK, Honeyman E, Arnold D, Ding H, Smith C, Walters DL. Smartphone-based home care model improved use of cardiac rehabilitation in postmyocardial infarction patients: results from a randomised controlled trial. Heart. 2014;100:1770–1779. doi: 10.1136/heartjnl-2014-305783. [DOI] [PubMed] [Google Scholar]
- 24.Hwang R, Bruning J, Morris NR, Mandrusiak A, Russell T. Home-based telerehabilitation is not inferior to a centre-based program in patients with chronic heart failure: a randomised trial. J Physiother. 2017;63:101–107. doi: 10.1016/j.jphys.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 25.Peng X, Su Y, Hu Z, Sun X, Li X, Dolansky MA, Qu M, Hu X. Home-based telehealth exercise training program in Chinese patients with heart failure: A randomized controlled trial. Medicine (Baltimore) 2018;97:e12069. doi: 10.1097/MD.0000000000012069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piotrowicz E, Zieliński T, Bodalski R, Rywik T, Dobraszkiewicz-Wasilewska B, Sobieszczańska-Małek M, Stepnowska M, Przybylski A, Browarek A, Szumowski Ł, Piotrowski W, Piotrowicz R. Home-based telemonitored Nordic walking training is well accepted, safe, effective and has high adherence among heart failure patients, including those with cardiovascular implantable electronic devices: a randomised controlled study. Eur J Prev Cardiol. 2015;22:1368–1377. doi: 10.1177/2047487314551537. [DOI] [PubMed] [Google Scholar]
- 27.Buckingham SA, Taylor RS, Jolly K, Zawada A, Dean SG, Cowie A, Norton RJ, Dalal HM. Home-based versus centre-based cardiac rehabilitation: abridged Cochrane systematic review and meta-analysis. Open Heart. 2016;3:e000463. doi: 10.1136/openhrt-2016-000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clark AM, Haykowsky M, Kryworuchko J, MacClure T, Scott J, DesMeules M, Luo W, Liang Y, McAlister FA. A meta-analysis of randomized control trials of home-based secondary prevention programs for coronary artery disease. Eur J Cardiovasc Prev Rehabil. 2010;17:261–270. doi: 10.1097/HJR.0b013e32833090ef. [DOI] [PubMed] [Google Scholar]
- 29.Pardaens S, Willems AM, Clays E, Baert A, Vanderheyden M, Verstreken S, Du Bois I, Vervloet D, De Sutter J. The impact of drop-out in cardiac rehabilitation on outcome among coronary artery disease patients. Eur J Prev Cardiol. 2017;24:1490–1497. doi: 10.1177/2047487317724574. [DOI] [PubMed] [Google Scholar]
- 30.Yohannes AM, Yalfani A, Doherty P, Bundy C. Predictors of drop-out from an outpatient cardiac rehabilitation programme. Clin Rehabil. 2007;21:222–229. doi: 10.1177/0269215506070771. [DOI] [PubMed] [Google Scholar]
- 31.De Schutter A, Kachur S, Lavie CJ, Menezes A, Shum KK, Bangalore S, Arena R, Milani RV. Cardiac rehabilitation fitness changes and subsequent survival. Eur Heart J Qual Care Clin Outcomes. 2018;4:173–179. doi: 10.1093/ehjqcco/qcy018. [DOI] [PubMed] [Google Scholar]
- 32.Kachur S, Chongthammakun V, Lavie CJ, De Schutter A, Arena R, Milani RV, Franklin BA. Impact of cardiac rehabilitation and exercise training programs in coronary heart disease. Prog Cardiovasc Dis. 2017;60:103–114. doi: 10.1016/j.pcad.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Frederix I, Vanhees L, Dendale P, Goetschalckx K. A review of telerehabilitation for cardiac patients. J Telemed Telecare. 2015;21:45–53. doi: 10.1177/1357633X14562732. [DOI] [PubMed] [Google Scholar]
- 34.Balady GJ, Williams MA, Ades PA, Bittner V, Comoss P, Foody JM, Franklin B, Sanderson B, Southard D American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Nursing; American Heart Association Council on Epidemiology and Prevention; American Heart Association Council on Nutrition, Physical Activity, and Metabolism; American Association of Cardiovascular and Pulmonary Rehabilitation. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: a scientific statement from the American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; the Councils on Cardiovascular Nursing, Epidemiology and Prevention, and Nutrition, Physical Activity, and Metabolism; and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation. 2007;115:2675–2682. doi: 10.1161/CIRCULATIONAHA.106.180945. [DOI] [PubMed] [Google Scholar]
- 35.Buckley JP, Furze G, Doherty P, Speck L, Connolly S, Hinton S, Jones JL BACPR. BACPR scientific statement: British standards and core components for cardiovascular disease prevention and rehabilitation. Heart. 2013;99:1069–1071. doi: 10.1136/heartjnl-2012-303460. [DOI] [PubMed] [Google Scholar]
- 36.Piepoli MF, Corrà U, Adamopoulos S, Benzer W, Bjarnason-Wehrens B, Cupples M, Dendale P, Doherty P, Gaita D, Höfer S, McGee H, Mendes M, Niebauer J, Pogosova N, Garcia-Porrero E, Rauch B, Schmid JP, Giannuzzi P. Secondary prevention in the clinical management of patients with cardiovascular diseases. Core components, standards and outcome measures for referral and delivery: a policy statement from the cardiac rehabilitation section of the European Association for Cardiovascular Prevention & Rehabilitation. Endorsed by the Committee for Practice Guidelines of the European Society of Cardiology. Eur J Prev Cardiol. 2014;21:664–681. doi: 10.1177/2047487312449597. [DOI] [PubMed] [Google Scholar]
- 37.Frederix I, Vandenberk T, Janssen L, Geurden A, Vandervoort P, Dendale P. eEduHeart I: A Multicenter, Randomized, Controlled Trial Investigating the Effectiveness of a Cardiac Web-Based eLearning Platform - Rationale and Study Design. Cardiology. 2017;136:157–163. doi: 10.1159/000448393. [DOI] [PubMed] [Google Scholar]
- 38.Piotrowicz E, Piotrowicz R, Opolski G, Pencina M, Banach M, Zaręba W. Hybrid comprehensive telerehabilitation in heart failure patients (TELEREH-HF): A randomized, multicenter, prospective, open-label, parallel group controlled trial-Study design and description of the intervention. Am Heart J. 2019;217:148–158. doi: 10.1016/j.ahj.2019.08.015. [DOI] [PubMed] [Google Scholar]
- 39.Kraal JJ, Van den Akker-Van Marle ME, Abu-Hanna A, Stut W, Peek N, Kemps HM. Clinical and cost-effectiveness of home-based cardiac rehabilitation compared to conventional, centre-based cardiac rehabilitation: Results of the FIT@Home study. Eur J Prev Cardiol. 2017;24:1260–1273. doi: 10.1177/2047487317710803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frederix I, Vandijck D, Hens N, De Sutter J, Dendale P. Economic and social impact of increased cardiac rehabilitation uptake and cardiac telerehabilitation in Belgium - a cost-benefit analysis. Acta Cardiol. 2018;73:222–229. doi: 10.1080/00015385.2017.1361892. [DOI] [PubMed] [Google Scholar]
- 41.Avila A, Claes J, Buys R, Azzawi M, Vanhees L, Cornelissen V. Home-based exercise with telemonitoring guidance in patients with coronary artery disease: Does it improve long-term physical fitness? Eur J Prev Cardiol. 2020;27:367–377. doi: 10.1177/2047487319892201. [DOI] [PubMed] [Google Scholar]
- 42.Ruano-Ravina A, Pena-Gil C, Abu-Assi E, Raposeiras S, van 't Hof A, Meindersma E, Bossano Prescott EI, González-Juanatey JR. Participation and adherence to cardiac rehabilitation programs. A systematic review. Int J Cardiol. 2016;223:436–443. doi: 10.1016/j.ijcard.2016.08.120. [DOI] [PubMed] [Google Scholar]
- 43.Neubeck L, Freedman SB, Clark AM, Briffa T, Bauman A, Redfern J. Participating in cardiac rehabilitation: a systematic review and meta-synthesis of qualitative data. Eur J Prev Cardiol. 2012;19:494–503. doi: 10.1177/1741826711409326. [DOI] [PubMed] [Google Scholar]
- 44.Grace SL, Leung YW, Reid R, Oh P, Wu G, Alter DA, CRCARE Investigators. The role of systematic inpatient cardiac rehabilitation referral in increasing equitable access and utilization. J Cardiopulm Rehabil Prev. 2012;32:41–47. doi: 10.1097/HCR.0b013e31823be13b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grace SL, Russell KL, Reid RD, Oh P, Anand S, Rush J, Williamson K, Gupta M, Alter DA, Stewart DE Cardiac Rehabilitation Care Continuity Through Automatic Referral Evaluation (CRCARE) Investigators. Effect of cardiac rehabilitation referral strategies on utilization rates: a prospective, controlled study. Arch Intern Med. 2011;171:235–241. doi: 10.1001/archinternmed.2010.501. [DOI] [PubMed] [Google Scholar]
- 46.Tang LH, Kikkenborg Berg S, Christensen J, Lawaetz J, Doherty P, Taylor RS, Langberg H, Zwisler AD. Patients' preference for exercise setting and its influence on the health benefits gained from exercise-based cardiac rehabilitation. Int J Cardiol. 2017;232:33–39. doi: 10.1016/j.ijcard.2017.01.126. [DOI] [PubMed] [Google Scholar]
- 47.Munro J, Angus N, Leslie SJ. Patient focused Internet-based approaches to cardiovascular rehabilitation--a systematic review. J Telemed Telecare. 2013;19:347–353. doi: 10.1177/1357633X13501763. [DOI] [PubMed] [Google Scholar]
- 48.Coppetti T, Brauchlin A, Müggler S, Attinger-Toller A, Templin C, Schönrath F, Hellermann J, Lüscher TF, Biaggi P, Wyss CA. Accuracy of smartphone apps for heart rate measurement. Eur J Prev Cardiol. 2017;24:1287–1293. doi: 10.1177/2047487317702044. [DOI] [PubMed] [Google Scholar]
- 49.Sartor F, Gelissen J, van Dinther R, Roovers D, Papini GB, Coppola G. Wrist-worn optical and chest strap heart rate comparison in a heterogeneous sample of healthy individuals and in coronary artery disease patients. BMC Sports Sci Med Rehabil. 2018;10:10. doi: 10.1186/s13102-018-0098-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Batalik L, Dosbaba F, Hartman M, Batalikova K, Spinar J. Rationale and design of randomized controlled trial protocol of cardiovascular rehabilitation based on the use of telemedicine technology in the Czech Republic (CR-GPS) Medicine (Baltimore) 2018;97:e12385. doi: 10.1097/MD.0000000000012385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vysoký R, Fiala J, Dosbaba F, Bat'alik L, Nehyba S, Ludka O. Preventive training programme for patients after acute coronary event-- correlation between selected parameters and age groups. Cent Eur J Public Health. 2015;23:208–213. doi: 10.21101/cejph.a4125. [DOI] [PubMed] [Google Scholar]
- 52.Aamot IL, Forbord SH, Gustad K, Løckra V, Stensen A, Berg AT, Dalen H, Karlsen T, Støylen A. Home-based versus hospital-based high-intensity interval training in cardiac rehabilitation: a randomized study. Eur J Prev Cardiol. 2014;21:1070–1078. doi: 10.1177/2047487313488299. [DOI] [PubMed] [Google Scholar]