Abstract
Hematopoietic stem cell transplantation has become a curative choice of many hematopoietic malignancy, but graft-vs-host disease (GVHD) has limited the survival quality and overall survival of hematopoietic stem cell transplantation. Understanding of the immune cells’ reaction in pathophysiology of GVHD has improved, but a review on the role of macrophages in GVHD is still absent. Studies have observed that macrophage infiltration is associated with GVHD occurrence and development. In this review, we summarize and analyze the role of macrophages in GVHD based on pathophysiology of acute and chronic GVHD, focusing on the macrophage recruitment and infiltration, macrophage polarization, macrophage secretion, and especially interaction of macrophages with other immune cells. We could conclude that macrophage recruitment and infiltration contribute to both acute and chronic GVHD. Based on distinguishing pathology of acute and chronic GVHD, macrophages tend to show a higher M1/M2 ratio in acute GVHD and a lower M1/M2 ratio in chronic GVHD. However, the influence of dominant cytokines in GVHD is controversial and inconsistent with macrophage polarization. In addition, interaction of macrophages with alloreactive T cells plays an important role in acute GVHD. Meanwhile, the interaction among macrophages, B cells, fibroblasts, and CD4+ T cells participates in chronic GVHD development.
Keywords: Macrophage, Graft-vs-host disease, Hematopoietic stem cell transplantation, Polarization, Cytokine, Regulation
Core tip: Macrophages tend to show a higher M1/M2 ratio in acute graft-vs-host disease (GVHD) and a lower M1/M2 ratio in chronic GVHD. Influence of cytokines on GVHD is controversial. Macrophages interact with alloreactive T cells in acute GVHD, and the interaction among macrophages, B cells, fibroblasts, and CD4+ T cells participates in chronic GVHD development.
INTRODUCTION
As a therapy to cure hematopoietic malignancy, allogeneic hematopoietic stem cell transplantation has greatly improved the survival rate of many malignant hematologic diseases. However, graft-vs-host disease (GVHD) can occur after transplantation as a major complication and cause non-relapse mortality principally[1]. GVHD is classified into acute GVHD and chronic GVHD. It is not the temporal relationship to transplantation, but clinical features that should be considered to identify acute or chronic GVHD syndromes[2]. Three classic target organs of acute GVHD, including skin, gastrointestinal (GI) tract and liver, and eight essential organs of chronic GVHD, including skin, mouth, eyes, GI tract, liver, lung, joint and fascia, and genital tract, are recommended to calculate the score of chronic GVHD based on the global scoring system evaluating targeted organs and general status. GVHD severity is described as mild, moderate, and severe[3,4].
The mechanism of GVHD is not clearly understood. Tissue damage caused by conditioning regimens, chemotherapy, and total body irradiation is essential in GVHD biology[5]. Recipient human leukocyte antigen mismatching is a great risk of GVHD[6-8] because it can prime the alloreactive T cell reaction with the help of antigen presenting cells. Alloreactive T cells recognize the recipient as non-self, attack the target organs of recipients, and initiate GVHD[9]. Antigen presentation, naive T cell differentiation, evoked cytolytic machinery, and cytokine regulation network establish the process of acute GVHD[10-13]. The final effect of these mechanisms in acute GVHD is apoptosis caused by cytolytic effector and cytokine storms from adaptive and innate immune cells, whereas end-organ fibrosis is a prominent feature of chronic GVHD[12]. Characteristic chronic GVHD is caused by impaired thymic damage, reaction of pathogenic germinal center (GC) B cells and macrophages, unbalanced T cells differentiation with accumulation of Th17/Tc17 and T follicular helper (Tfh) cells and suppression of T regulatory (Treg) cells, antibody disposition and concomitant cytokine production (e.g., increased transforming growth factor (TGF)-β, interleukin (IL)-17 from Th17, IL-21 produced by Tfh driving GC B cell formation and antibody secretion)[14,15]. Therapies have worked via targeting T cells or B cells, infusing immune regulatory cells, and using cytokine antagonists[11,16-18]. The process of GVHD manifests an aberrant homeostasis of immune response.
Studies have reviewed the mission of many adaptive and innate immune cells, such as B cells, Treg cells, natural killer T cells, dendritic cells, and innate lymphoid cells in GVHD, but the role of macrophages in GVHD has not been reported before[19-23]. In this review, we outlined the role of macrophages in GVHD, focusing on the macrophage infiltration, cytokine production, and their interaction with other cells.
FUNCTIONS OF MACROPHAGES IN IMMUNE RESPONSE
Macrophages show great heterogeneity and plasticity that it is able to activate and polarize to different phenotypes through the stimulation of multiple signaling molecules in the same or different microenvironment[24-26]. Tissue-resident macrophages participate in many pathologies, such as microglia in neurodegeneration, osteoclasts and macrophages in osteoporosis, cardiac or vasculature macrophages in atherosclerosis, Kupffer cells in liver disease, alveolar macrophages in pulmonary disease and so on[27,28]. Macrophages can be categorized as classically activated macrophages with microbicidal activity, wound-healing macrophages with tissue repair function, and regulatory macrophages with anti-inflammatory activity[29]. Another traditional classification divides macrophages into M1 macrophages and M2 macrophages[25]. Notably, reciprocal switch between M1 macrophages to M2 macrophages can be induced[30]. Macrophage-targeted therapies were used in clinical trials, based on macrophage functions, such as self-renewal, phagocytosis, chemotaxis, inflammatory response, pro-tumor response, and therapeutic protein secretion[31,32].
INFILTRATION OF MACROPHAGES CONTRIBUTES TO GVHD
Studies about the relationship between macrophages and GVHD in recent years were summarized and presented in Table 1. We found that macrophage infiltration is an important feature in GVHD pathogenesis.
Table 1.
Studies about macrophages in graft-vs-host disease
| Function of macrophage | Macrophage in GVHD | Ref. |
| Infiltration | Macrophage infiltration contributes to GVHD | [33-36,39,40,44-50,54,56-58,64,65,86,115-118] |
| Polarization | M1/M2 ratio is increased in acute GVHD | [37,39,61] |
| M1/M2 ratio is decreased in chronic GVHD | [45,49,50,86] | |
| M2 macrophage infiltration contributes to steroid refractory acute GVHD | [40,44] | |
| Recruitment; migration | Recruitment of macrophage to target organs contributes to GVHD | [36,39,48,49,54,117-119] |
| Cytokine secretion | Macrophage activation with an upregulated expression of cytokines contributes to GVHD | [46,49,55-60,114,116] |
| Interaction with T cells | Interaction between macrophages and T cells regulates GVHD directly or indirectly | [56,57,60,61,63-66] |
| Participating in fibrosis | Macrophages attribute to fibrosis in chronic GVHD | [46-50,54,60,86] |
GVHD: Graft-vs-host disease; M2: M2 macrophage; M1/M2 ratio: The ratio of M1 macrophage and M2 macrophage.
Macrophage infiltration is a biomarker for GVHD occurrence and development. Both free and clustered macrophages are important in GVHD pathogenesis. In the study by Nissen et al[33], an increased number of macrophages was detected in 11 out of 30 patients. Ten of these eleven patients developed GVHD. A remarkable difference was noted between the 10 out of 14 patients who showed the macrophage pattern before bone marrow transplantation, and there was only one patient among the 19 without GVHD. Also, Terakura et al[34] indicated that heavier macrophage infiltration is correlated with a higher severity of cutaneous GVHD. Piérard et al[35] also illustrated that biopsies from the liver, gut, and skin of patients with lethal GVHD showed a striking preponderance of CD68+ macrophages in the inflammatory infiltration. These findings showed that macrophage infiltration is positively correlated with the occurrence and development of GVHD. Furthermore, macrophages polarize to different populations and infiltrate in different target organs (Table 1), and the dominant macrophage population in acute GVHD differs from that in chronic or refractory GVHD.
Taken together, macrophage regulation in GVHD can be considered from the following directions, including macrophage polarization, regulation of cytokines and interaction with other cells such as T cells, B cells, and mesenchymal stem cells, and fibrosis.
MACROPHAGE POLARIZATION IN GVHD
As mentioned above, macrophage infiltration contributes to GVHD, but macrophage populations vary in different phases, tissues, and conditions of GVHD. Macrophages infiltrating in acute GVHD tend to be pro-inflammatory M1 macrophages, whereas it is M2 macrophages that are predominant in chronic and refractory acute GVHD. Studies demonstrated that the recruitment of macrophages is one of hallmarks in the initiation of acute GVHD, and a higher ratio of M1 macrophage/M2 macrophage (M1/M2) correlates to a higher incidence of grade 2-4 acute GVHD[36,37].
During acute GVHD pathogenesis, except in immune responses, there is a cytostatic effect to inhibit cellular proliferation via releasing iron from target cells induced by macrophage-producing nitric oxide (NO)[38]. Infiltration of inducible NO synthase (iNOS)‐positive M1 macrophages was found in oral mucosal acute GVHD[39]. It means that M1 macrophage polarization can modulate acute GVHD by producing NO.
Although the association between M1 macrophages and acute GVHD have been reported, Holtan et al[40] observed more CD4+ activated memory T cells and M0 macrophages in onset GI acute GVHD, increased M1 macrophages in onset and steroid-refractory acute GVHD but higher M2 macrophages in steroid-refractory GI acute GVHD. For the diversity between macrophage polarization in acute GVHD and refractory GI acute GVHD, it might be due to the phases and complicated mechanism of steroid-refractory GVHD that refractory GVHD was more associated with thrombotic system[41,42]. In addition, as a scavenger receptor, CD163 is mostly expressed on M2 macrophages[43]. Nishiwaki et al[44] also demonstrated that CD163 macrophage infiltration was the only predictor for refractory acute GVHD when the number of CD163(+) macrophages, CD8(+) T cells, and CD1a(+) dendritic cells was counted. Meanwhile, a higher plasma soluble CD163 concentration at day 80 is related to the incidence of de novo-onset chronic GVHD[45]. Donor-derived M2 macrophage phenotype contributes to chronic GVHD, not only manifesting CD163+ macrophage population, but also CD11b+ monocyte/macrophages and F4/80+CSF-1R+CD206+iNOS- populations[46-50]. Therefore, we could conclude that M1 macrophage polarization is predominant in acute GVHD, while M2 macrophage polarization is dominant in refractory acute GVHD and chronic GVHD.
RECRUITMENT OF MACROPHAGES
Chemokines regulate macrophage infiltration in GVHD. On the basis of cysteine residues, four subfamilies of chemokines, including C, CC, CXC, and CX3C are defined, which are able to bind to XCR, CCR, CXCR and CX3CR, respectively, with the ability to regulate recruitment of leukocytes[51,52]. CXCL2 is also known as macrophage inflammatory protein-2. CXCL2 played an important role in recruitment of macrophages and T cells to target organs in GVHD, and the severity of GVHD was decreased by blocking CXCL2 and its receptor CXCR2[53]. In addition, by binding to CC chemokine receptor 2, monocyte chemoattractant protein (MCP)-1 can also regulate the recruitment of monocytes/macrophages, T cells, and other target cells and engage in the inflammatory response. An accumulation of iNOS‐positive M1 macrophages was found in oral mucosal acute GVHD via both laminin/CD29 β1 intern and MCP-1/CC chemokine receptor 2 pathways[39]. Macrophage migration is mediated by laminin/CD29 β1 intern, meanwhile, macrophage-derived matrix metalloproteinase-2 contributed to basement membrane degradation and activated macrophages interacted with oral epithelium via the MCP‐1/CC chemokine receptor 2 adhesive pathway directly[39].
On the other hand, in chronic GVHD, Du et al[54] indicated that CCL9 showed a biological relevance for chronic GVHD by promoting macrophage infiltration, increasing lung immunoglobulin deposition, and upregulating splenic GC B cells and Tfh cells and the Tfh/T follicular regulatory cells ratio. They also observed that the mouse homolog of human CCL15 was a prognostic and diagnostic biomarker for chronic GVHD in clinical cohorts. In brief, previous studies showed that macrophage recruitment could be regulated by chemokines and results in modulation of GVHD severity. Notably, most chemokines or chemokine inhibitors are not professional, but pleiotropic.
MACROPHAGE-RELATED CYTOKINES IN GVHD
Cytokines secreted by macrophages and receptors play an important role in GVHD. The research of Hyvärinen et al[55] focused on gene expression related to GVHD. They found that genes regulating IL-1β, interferon (IFN)-γ, and IL-6 responses were associated to GVHD; moreover, IL-1, IL-23R, TLR9, TNF, and NOD2 genes were associated to the immunological response by monocytes/macrophages that can precede GVHD in intestinal lesions. In other words, macrophages could regulate GVHD by secreting cytokines. Here, we focus on several cytokines.
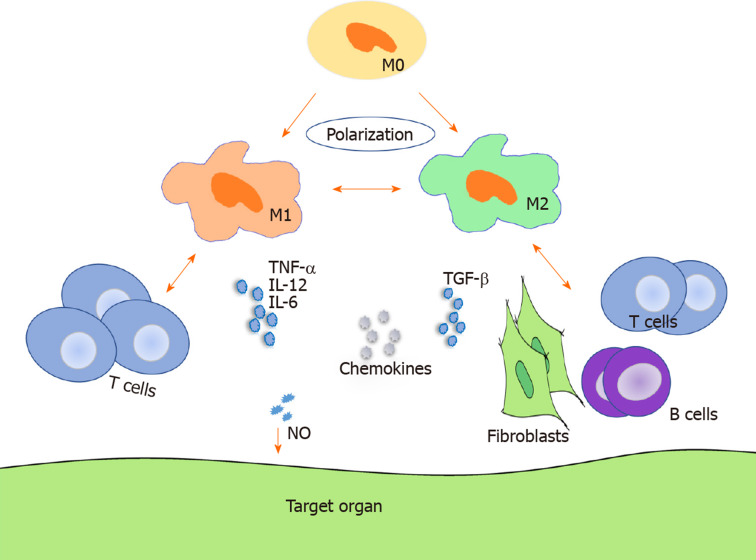
As shown in Figure 1, TNF-α, IL-12, and IL-6 increased in acute GVHD, whereas TGF-β and IL-6 were upregulated in chronic GVHD[56-58]. By analyzing forty-seven consecutive patients, Hueso et al[59] found that IL-10 (reflects monocyte-derived macrophage reactivity), citrulline, and myeloablative conditioning are independent factors of acute GVHD development and that IL-10 was increased in acute GVHD. A preponderance of macrophage infiltration with production of TNF-α was observed in acute GVHD[58]. Using a human IL-6 transgenic humanized mouse model, Ono et al[60] demonstrated that elevated human IL-12p40, IL-18, M-CSF, and IFN-α2 produced by monocytes/macrophages might facilitate GVHD in chronic GVHD humanized mice. However, most cytokines are not professional but pleiotropic and may present an inverse effect on GVHD in different states.
Figure 1.
Macrophage regulation of graft-vs-host disease. Macrophages can polarize to M1 macrophages and M2 macrophages and regulate both acute and chronic graft-vs-host disease through migration, cytokine secretion, interaction with T cells in acute graft-vs-host disease, and interaction with T cells, B cells, and fibroblasts in chronic graft-vs-host disease. M0: M0 macrophage; M1: M1 macrophage; M2: M2 macrophage; NO: Nitric oxide.
It is worth noting that macrophages in GVHD can be regulated by cytokines. Th17-production of IL-17 participates in GVHD by modulating the interaction between macrophages and CD4+ T cells. IL-17 can reduce macrophage infiltration, downregulate IL-12 and IFN-γ production, repress Th1 responses, and alleviate acute GVHD[56]. Reducing infiltration of macrophages that migrated to MCP-1 and IL-17A and TGF-β production could be therapeutic targets for GVHD[49]. Also, M1 macrophage polarization and effector T cell infiltration can be suppressed by expanded Tregs by using IL-33 for acute GVHD[61]. IL-33 also has a paradoxical effect because administration of IL-33 after allogeneic hematopoietic stem cell transplantation aggravated acute GVHD by engaging in the augmentation of donor T cells[62]. The opposite effect may be due to the complicated cytokine network, which shows a balance of synergetic and antergic effect.
Notably, via MHC class II molecules, intestinal epithelium cells could present antigen, activate CD4+ T cells, and initiate lethal gut GVHD, and macrophages could interact with intestinal epithelium cells and regulate MHC class II expression on intestinal epithelium cells via IL-12-IFN-γ cytokine axis in addition to the required microbiota[63]. Overall, macrophage-produced cytokines participate in interaction between macrophages and other cells, and this role will be described further in the following sections.
INTERACTION BETWEEN MACROPHAGES AND T CELLS
In GVHD pathogenesis, the alloreactive T cell response is essential. It is demonstrated in Table 1 and Figure 1 that infiltration of both T cells and macrophages correlate to GVHD.
T cells, primarily T helper cells, regulate macrophage activation. Using a humanized model and in vitro experiment, an infiltration of F4/80+ macrophages and human effector memory T helper cells was observed in lymphatic tissues and skin GVHD. Their interaction revealed that macrophages in humanized mice were activated by human T helper 2-type inflammatory cytokines[64]. Meanwhile, macrophages influenced the alloreactive T cell response to host antigen. In the study by Haniffa et al[65], persistent recipient dermal CD1a-/CD14+/FXIIIa+ macrophages were detected in GVHD lesions and further showed the ability to influence allogeneic CD8+ T cells on proliferation, secretion of cytokines, and expression of activation antigens. Furthermore, low macrophage infiltration reduced the percentage of Th1 and Tc1 lineages but upregulated the percentages of Th2, Tc2, and Treg lineages that can suppress effector T cell infiltration and eventually alleviate acute GVHD[37,56,60,61]. Additionally, chronic lung GVHD is IL-17 and CSF-1/CSF-1R dependent[49]. Activated macrophages can induce donor T cells to polarize toward Th17 with an elevation of IL-6, IL-1β, and IL-23. Finally, accumulated IL-17–producing CCR6+/CCR4+ Th17 cells exacerbate lung GVHD[66]. This means that macrophages contribute to T cell differentiation and affect the homeostasis of the immune response. Interestingly, host macrophages are opposite to donor macrophage in terms of their interaction with donor T cells (i.e. host macrophages enable the inhibition of donor T cell expansion). Hashimoto et al[67] observed that reduced host macrophage pool can increase donor T cell expansion and aggravate GVHD mortality via a CD47-dependent manner, whereas persisting host macrophages can engulf donor allogeneic T cells and inhibit proliferation[67].
Further work to understand the interaction between macrophages and T cells in GVHD models is limited, but complicated interactions between macrophages and T cells have been reported in other models. M1 macrophages can enhance cytolytic and immunomodulatory functions of CD107a+/CD8+ T cells in healthy individuals through a contact-dependent manner with cytokine- and antigen-independent induction[68]. In contrast, Liu et al[69] observed that M1 macrophage polarization and macrophage-derived CXCL10 cannot recruit CD4+ and CD8+ T cells, but DCs are recruited, which then promote CD4+ T cells migration. Therefore, macrophages can also regulate T cells indirectly with the help of other immune cells[69]. However, macrophage-derived CXCL10 contributed to T cell recruitment. Petty et al[70] demonstrated that M2 macrophage polarization can mediate immunosuppression through tumor-associated macrophages, which mostly exhibit an M2-like phonotype. These macrophages suppress CD8+ T cell recruitment by inhibiting chemokines of CD8+ T cells produced by macrophages, such as CXCL9 and CXCL10[70]. It is noteworthy that cytokine efficacy differs in these two studies may be explained with the different macrophage subsets interacting with T cells.
These results provide a clue that M1 macrophages tend to prime CD8+ T cells in the immune response while M2 macrophages keep the balance by suppressing CD8+ T cells infiltration. Bouchlaka et al[71] also demonstrated that mesenchymal stem cell-educated anti-inflammatory immunophenotype macrophages, presenting increased CD206, CD163, IL-6, TGF-β, arginase-1, etc expression and decreased IL-12 and TNF-α expression, can attenuate GVHD with the help of reduced human T cell proliferation and enhanced fibroblast proliferation[71]. Notably, T cells can reciprocally regulate macrophage polarization. T cell Ig mucin-3 on activated T effector cells promotes differentiation of M2 macrophage[72-74]. In addition, M2 macrophages may be converted into M1 macrophages and contribute to T cell function. Stimulated by low-dose irradiation, M2-like phonotype macrophages can differentiate towards an iNOS+/M1 phenotype macrophage, produce NO, and promote infiltration of CD3+, CD8+, and CD4+ intratumoral T cells with an increased expression of Th1 cytokines[75,76]. But it is still incompletely consistent in the interaction between macrophages and T cells. A study by Wood et al[77] indicated that increased macrophage-derived nitrite production could suppress peritoneal cavity T cells.
Macrophages can interact with T cells and subsequently influence T cell activation and function via macrophage polarization, cytokine release, and activating antigen presentation[28,78,79]. However, macrophages may promote immunological tolerance via attenuating effector T cell activation and promoting regulatory T cell differentiation using macrophage-derived complement receptor of immunoglobulin family (an immune checkpoint molecule)[80]. Also, granulin derived from macrophages contributes to the exclusion of cytotoxic CD8+ T cells through its resistance to inhibition of immune checkpoints, which may provide new strategies to treat GVHD[81]. Notably, binding of the Fcγ-receptor expressed by macrophages to Fc domain glycan of drugs that target alloreactive T cells may be considered for GVHD therapy[82]. Other molecules expressed by macrophages can also be alternative targets, such as scavenger receptor MARCO[83].
MACROPHAGES ATTRIBUTE TO FIBROSIS IN CHRONIC GVHD
Autoantibody production, immunoglobulin deposition, and fibrosis are characteristic features of chronic GVHD[84,85]. CD4+ T cells, fibroblasts, and B cells interact with macrophages and this interaction plays an important role in chronic GVHD. Reduced infiltration of CD4+ T cells and CD11b+ monocytes/macrophages and suppressed fibroblast proliferation attenuate severity and fibrosis of chronic cutaneous sclerodermatous GVHD[47,48]. Du et al[49] also indicated that treatment with pirfenidone can reduce infiltration of macrophages and TGF-β production, impair GC reaction, and inhibit antibody production of B cells and fibrosis, thus attenuating chronic GVHD. In brief, macrophage infiltration, macrophage-production of TGF-β, B cell reactivity, fibroblast proliferation, and CD4+ T cell infiltration contribute to chronic GVHD development.
Another effort to ameliorate fibrosis in chronic GVHD is to use 4-phenylbutyric acid. There are elevated endoplasmic reticulum stress markers in chronic GVHD. Chronic GVHD-elicited endoplasmic reticulum stress in macrophages could be mitigated by administrating 4-phenylbutyric acid, leading to M1 macrophage differentiation and dysfunctional fibroblasts[86]. In other words, M2 macrophages and fibroblasts contribute to fibrosis in GVHD. As mentioned before, CCL9 works in chronic GVHD by regulating macrophage infiltration, immunoglobulin deposition, splenic GC B cell reaction, and CD4+ T cell polarization[54]. In other words, the alloreactivity and interaction of macrophages, B cells, and T cells are regulated by cytokines. Therefore, we conclude that macrophage infiltration, interactions between macrophages and other cells, and the cytokine network should be considered in chronic GVHD.
Macrophage infiltration has been observed in many fibrotic diseases. Meanwhile, apoptosis and autophagy of macrophages attenuates fibrosis[87-89]. Membrane molecules expressed on macrophages and cytokines produced by macrophages participate in this fibrogenesis process. CD14, which is a co-receptor of Toll-like receptor 4 and is expressed on macrophages, may be stimulated by Toll-like receptor exposure and activate macrophages with an induction of TGF-β production, resulting in a profibrotic effect via a myeloid differentiation factor 88-dependent manner in systemic sclerosis[90]. Macrophage receptors with collagenous structure containing arginine residues is another scavenger receptor expressed on macrophages, and it can induce the polarization of macrophages to a profibrotic M2 subtype and contribute to fibrosis[91]. As for cytokines, increased TGF-β signaling promotes fibrosis[92-94].
Macrophage polarization is another factor that can be modulated to reduce fibrosis. M2 macrophages are infiltrated in fibrosis predominately following TGF-β secretion[95-98]. Also, myofibroblasts transited from M2 macrophages could be a source of interstitial fibrosis in chronic allograft rejection[99]. Furthermore, macrophages are able to drive fibroblast recruitment and contribute to fibroblast activation, which is also associated with the development of fibrosis[100,101]. Macrophage polarization to alternative macrophage subgroups stimulates fibroblasts and attributes to fibrosis. Meanwhile, macrophage polarization to M2 macrophages in fibrosis can be mediated by fibroblasts and cytokines, such as IL-4 and TGF-β1[102,103]. Interestingly, the M2c macrophage subset may reduce lung fibrosis by increasing IL-10 levels[104]. Regulatory macrophages can inhibit alternative macrophage activation and regulate alternative macrophage-mediated fibrosis[105]. Therefore, the balance between M1 macrophages and M2 macrophages and the balance among subgroups of M2 macrophages and regulatory macrophages as well as the dominant effect of the complicated cytokine network should be taken into account in terms of the role of macrophage polarization in fibrosis.
B cell activation regulated by B cell receptor–associated pathways and B cell activating factor plays an important role in the pathology of chronic GVHD[22]. Targeting the B cell reaction, especially by blocking some B cell receptor–associated pathways, such as BTK, ITK, and JAK1/2, has been an effective treatment mechanism for chronic GVHD[106].As an important feature of chronic GVHD, the deposition of antibodies from donor B cells augments cutaneous chronic GVHD by damaging the thymus and interacting with CD4+ T cells, especially the pathogenic Th17 and Th2 cells differentiated from Tfh cells[84,107]. The deposition of antibodies also plays an important role in fibroblast activity and the pathogenesis of systemic sclerosis[108]. In chronic GVHD, which is similar to fibrosis disease, macrophages play an important role to regulate B cells. Fantastic reciprocity was found in the relationship between macrophages and B cells. On one hand, CD19-/- donor B cells could augment GVHD severity and fibrosis by increasing splenic IL-6–producing monocyte/macrophage expansion during the early stage of the disease and by increasing TGF-β–producing monocyte/macrophage infiltration in the later stage of chronic sclerodermatous GVHD[46]. On the other hand, increased macrophage density is related to antibody‐mediated rejection[109]. Macrophages can modulate antibody production, and macrophage depletion inhibits anti-graft antibody production[110,111]. Also, macrophages increase B cell autoantibody production in autoantibody-dependent systemic autoimmune disease[112]. Reciprocally, recipient B cells and MHC class II–reactive donor-specific antibodies promote macrophage infiltration[113]. Interestingly, macrophage-depletion decreased TGF-β levels and worsened GVHD but increased B cell infiltration, while B cell-depletion led to higher levels of TGF-β and less severe GVHD, especially liver fibrosis[114].A potential explanation is that depleting macrophages can lead to the misbalance of homeostasis in the immune response, whereas the regulation of B cells, which are primary actors, lead to direct and explicit results.
CONCLUSION
Despite advances in GVHD pathogenesis and therapy, GVHD is still a threat to limit administration of hematopoietic stem cell transplantation. Essentially, it is immune cell responses that contribute to GVHD. Macrophage infiltration correlates to GVHD occurrence and development with a functional regulation of macrophage polarization, production of cytokines, and interaction with other cells. Therapies targeting macrophages to regulate macrophage infiltration have been reported.
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interests for this article.
Manuscript source: Invited manuscript
Peer-review started: January 1, 2020
First decision: February 20, 2020
Article in press: April 22, 2020
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mizuguchi T S-Editor: Dou Y L-Editor: Filipodia E-Editor: Liu JH
Contributor Information
Ya-Qun Hong, Fujian Institute of Hematology, Fujian Provincial Key Laboratory on Hematology, Department of Hematology, Fujian Medical University Union Hospital, Fuzhou 350000, Fujian Province, China.
Bo Wan, Faculty of Life Sciences and Medicine, King’s College London, London WC1N 3BG, United Kingdom.
Xiao-Fan Li, Fujian Institute of Hematology, Fujian Provincial Key Laboratory on Hematology, Department of Hematology, Fujian Medical University Union Hospital, Fuzhou 350000, Fujian Province, China; INSERM U1160, Hospital Saint Louis, Université Paris Diderot, Paris 94430, France. morningshiplee@sina.com.
References
- 1.Pidala J, Kim J, Anasetti C, Nishihori T, Betts B, Field T, Perkins J. The global severity of chronic graft-versus-host disease, determined by National Institutes of Health consensus criteria, is associated with overall survival and non-relapse mortality. Haematologica. 2011;96:1678–1684. doi: 10.3324/haematol.2011.049841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, Martin P, Chien J, Przepiorka D, Couriel D, Cowen EW, Dinndorf P, Farrell A, Hartzman R, Henslee-Downey J, Jacobsohn D, McDonald G, Mittleman B, Rizzo JD, Robinson M, Schubert M, Schultz K, Shulman H, Turner M, Vogelsang G, Flowers ME. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, Palmer J, Weisdorf D, Treister NS, Cheng GS, Kerr H, Stratton P, Duarte RF, McDonald GB, Inamoto Y, Vigorito A, Arai S, Datiles MB, Jacobsohn D, Heller T, Kitko CL, Mitchell SA, Martin PJ, Shulman H, Wu RS, Cutler CS, Vogelsang GB, Lee SJ, Pavletic SZ, Flowers ME. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21:389–401.e1. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeiser R, Blazar BR. Acute Graft-versus-Host Disease - Biologic Process, Prevention, and Therapy. N Engl J Med. 2017;377:2167–2179. doi: 10.1056/NEJMra1609337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gyurkocza B, Sandmaier BM. Conditioning regimens for hematopoietic cell transplantation: one size does not fit all. Blood. 2014;124:344–353. doi: 10.1182/blood-2014-02-514778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flowers ME, Inamoto Y, Carpenter PA, Lee SJ, Kiem HP, Petersdorf EW, Pereira SE, Nash RA, Mielcarek M, Fero ML, Warren EH, Sanders JE, Storb RF, Appelbaum FR, Storer BE, Martin PJ. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood. 2011;117:3214–3219. doi: 10.1182/blood-2010-08-302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersdorf EW. Genetics of graft-versus-host disease: the major histocompatibility complex. Blood Rev. 2013;27:1–12. doi: 10.1016/j.blre.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petersdorf EW. The major histocompatibility complex: a model for understanding graft-versus-host disease. Blood. 2013;122:1863–1872. doi: 10.1182/blood-2013-05-355982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7:340–352. doi: 10.1038/nri2000. [DOI] [PubMed] [Google Scholar]
- 10.Henden AS, Hill GR. Cytokines in Graft-versus-Host Disease. J Immunol. 2015;194:4604–4612. doi: 10.4049/jimmunol.1500117. [DOI] [PubMed] [Google Scholar]
- 11.Holtan SG, Pasquini M, Weisdorf DJ. Acute graft-versus-host disease: a bench-to-bedside update. Blood. 2014;124:363–373. doi: 10.1182/blood-2014-01-514786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markey KA, MacDonald KP, Hill GR. The biology of graft-versus-host disease: experimental systems instructing clinical practice. Blood. 2014;124:354–362. doi: 10.1182/blood-2014-02-514745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacDonald KP, Blazar BR, Hill GR. Cytokine mediators of chronic graft-versus-host disease. J Clin Invest. 2017;127:2452–2463. doi: 10.1172/JCI90593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flynn R, Du J, Veenstra RG, Reichenbach DK, Panoskaltsis-Mortari A, Taylor PA, Freeman GJ, Serody JS, Murphy WJ, Munn DH, Sarantopoulos S, Luznik L, Maillard I, Koreth J, Cutler C, Soiffer RJ, Antin JH, Ritz J, Dubovsky JA, Byrd JC, MacDonald KP, Hill GR, Blazar BR. Increased T follicular helper cells and germinal center B cells are required for cGVHD and bronchiolitis obliterans. Blood. 2014;123:3988–3998. doi: 10.1182/blood-2014-03-562231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi SW, Reddy P. Current and emerging strategies for the prevention of graft-versus-host disease. Nat Rev Clin Oncol. 2014;11:536–547. doi: 10.1038/nrclinonc.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blazar BR, MacDonald KPA, Hill GR. Immune regulatory cell infusion for graft-versus-host disease prevention and therapy. Blood. 2018;131:2651–2660. doi: 10.1182/blood-2017-11-785865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cutler CS, Koreth J, Ritz J. Mechanistic approaches for the prevention and treatment of chronic GVHD. Blood. 2017;129:22–29. doi: 10.1182/blood-2016-08-686659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneidawind D, Pierini A, Negrin RS. Regulatory T cells and natural killer T cells for modulation of GVHD following allogeneic hematopoietic cell transplantation. Blood. 2013;122:3116–3121. doi: 10.1182/blood-2013-08-453126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu H, Tian Y, Wang Y, Mineishi S, Zhang Y. Dendritic Cell Regulation of Graft-Vs.-Host Disease: Immunostimulation and Tolerance. Front Immunol. 2019;10:93. doi: 10.3389/fimmu.2019.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shao L, Pan S, Zhang QP, Jamal M, Chen LH, Yin Q, Wu YJ, Xiong J, Xiao RJ, Kwong YL, Zhou FL, Lie AKW. An Essential Role of Innate Lymphoid Cells in the Pathophysiology of Graft-vs.-Host Disease. Front Immunol. 2019;10:1233. doi: 10.3389/fimmu.2019.01233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarantopoulos S, Ritz J. Aberrant B-cell homeostasis in chronic GVHD. Blood. 2015;125:1703–1707. doi: 10.1182/blood-2014-12-567834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konya V, Mjösberg J. Innate lymphoid cells in graft-versus-host disease. Am J Transplant. 2015;15:2795–2801. doi: 10.1111/ajt.13394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Porta C, Riboldi E, Ippolito A, Sica A. Molecular and epigenetic basis of macrophage polarized activation. Semin Immunol. 2015;27:237–248. doi: 10.1016/j.smim.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonnardel J, Guilliams M. Developmental control of macrophage function. Curr Opin Immunol. 2018;50:64–74. doi: 10.1016/j.coi.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lavin Y, Mortha A, Rahman A, Merad M. Regulation of macrophage development and function in peripheral tissues. Nat Rev Immunol. 2015;15:731–744. doi: 10.1038/nri3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pelegrin P, Surprenant A. Dynamics of macrophage polarization reveal new mechanism to inhibit IL-1beta release through pyrophosphates. EMBO J. 2009;28:2114–2127. doi: 10.1038/emboj.2009.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee S, Kivimäe S, Dolor A, Szoka FC. Macrophage-based cell therapies: The long and winding road. J Control Release. 2016;240:527–540. doi: 10.1016/j.jconrel.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peterson KR, Cottam MA, Kennedy AJ, Hasty AH. Macrophage-Targeted Therapeutics for Metabolic Disease. Trends Pharmacol Sci. 2018;39:536–546. doi: 10.1016/j.tips.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nissen C, Gratwohl A, Tichelli A, Speck B. Abundant macrophage growth in culture from patients with chronic myelogenous leukemia: a risk factor for graft-versus-host disease after bone marrow transplantation. Experientia. 1988;44:167–169. doi: 10.1007/BF01952204. [DOI] [PubMed] [Google Scholar]
- 34.Terakura S, Martin PJ, Shulman HM, Storer BE. Cutaneous macrophage infiltration in acute GvHD. Bone Marrow Transplant. 2015;50:1135–1137. doi: 10.1038/bmt.2015.114. [DOI] [PubMed] [Google Scholar]
- 35.Piérard GE, Hermanns-Lê T, Paquet P, Rousseau AF, Delvenne P, Piérard-Franchimont C. Toxic Epidermal Necrolysis and Graft-versus-Host Reaction: Revisiting a Puzzling Similarity. ISRN Dermatol. 2013;2013:651590. doi: 10.1155/2013/651590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng Q, Ma S, Lin D, Mei Y, Gong H, Lei L, Chen Y, Zhao Y, Hu B, Wu Y, Yu X, Zhao L, Liu H. The S1P1 receptor-selective agonist CYM-5442 reduces the severity of acute GVHD by inhibiting macrophage recruitment. Cell Mol Immunol. 2015;12:681–691. doi: 10.1038/cmi.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wen Q, Kong Y, Zhao HY, Zhang YY, Han TT, Wang Y, Xu LP, Zhang XH, Huang XJ. G-CSF-induced macrophage polarization and mobilization may prevent acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2019;54:1419–1433. doi: 10.1038/s41409-019-0449-9. [DOI] [PubMed] [Google Scholar]
- 38.Nestel FP, Greene RN, Kichian K, Ponka P, Lapp WS. Activation of macrophage cytostatic effector mechanisms during acute graft-versus-host disease: release of intracellular iron and nitric oxide-mediated cytostasis. Blood. 2000;96:1836–1843. [PubMed] [Google Scholar]
- 39.Seno K, Yasunaga M, Kajiya H, Izaki-Hagio K, Morita H, Yoneda M, Hirofuji T, Ohno J. Dynamics of M1 macrophages in oral mucosal lesions during the development of acute graft-versus-host disease in rats. Clin Exp Immunol. 2017;190:315–327. doi: 10.1111/cei.13043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holtan SG, Shabaneh A, Betts BC, Rashidi A, MacMillan ML, Ustun C, Amin K, Vaughn BP, Howard J, Khoruts A, Arora M, DeFor TE, Johnson D, Blazar BR, Weisdorf DJ, Wang J. Stress Responses, M2 Macrophages, and a Distinct Microbial Signature in Fatal Intestinal Acute Graft-Versus-Host Disease. JCI Insight. 2019;5:e129762. doi: 10.1172/jci.insight.129762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wall SA, Zhao Q, Yearsley M, Blower L, Agyeman A, Ranganathan P, Yang S, Wu H, Bostic M, Jaglowski S, Brammer JE, William B, Choe H, Mims AS, Penza S, Efebera Y, Devine S, Cataland S, Davies SM, Vasu S. Complement-mediated thrombotic microangiopathy as a link between endothelial damage and steroid-refractory GVHD. Blood Adv. 2018;2:2619–2628. doi: 10.1182/bloodadvances.2018020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luft T, Dietrich S, Falk C, Conzelmann M, Hess M, Benner A, Neumann F, Isermann B, Hegenbart U, Ho AD, Dreger P. Steroid-refractory GVHD: T-cell attack within a vulnerable endothelial system. Blood. 2011;118:1685–1692. doi: 10.1182/blood-2011-02-334821. [DOI] [PubMed] [Google Scholar]
- 43.Law SK, Micklem KJ, Shaw JM, Zhang XP, Dong Y, Willis AC, Mason DY. A new macrophage differentiation antigen which is a member of the scavenger receptor superfamily. Eur J Immunol. 1993;23:2320–2325. doi: 10.1002/eji.1830230940. [DOI] [PubMed] [Google Scholar]
- 44.Nishiwaki S, Terakura S, Ito M, Goto T, Seto A, Watanabe K, Yanagisawa M, Imahashi N, Tsukamoto S, Shimba M, Ozawa Y, Miyamura K. Impact of macrophage infiltration of skin lesions on survival after allogeneic stem cell transplantation: a clue to refractory graft-versus-host disease. Blood. 2009;114:3113–3116. doi: 10.1182/blood-2009-03-209635. [DOI] [PubMed] [Google Scholar]
- 45.Inamoto Y, Martin PJ, Paczesny S, Tabellini L, Momin AA, Mumaw CL, Flowers MED, Lee SJ, Carpenter PA, Storer BE, Hanash S, Hansen JA. Association of Plasma CD163 Concentration with De Novo-Onset Chronic Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2017;23:1250–1256. doi: 10.1016/j.bbmt.2017.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Le Huu D, Matsushita T, Jin G, Hamaguchi Y, Hasegawa M, Takehara K, Tedder TF, Fujimoto M. Donor-derived regulatory B cells are important for suppression of murine sclerodermatous chronic graft-versus-host disease. Blood. 2013;121:3274–3283. doi: 10.1182/blood-2012-11-465658. [DOI] [PubMed] [Google Scholar]
- 47.Huu DL, Matsushita T, Jin G, Hamaguchi Y, Hasegawa M, Takehara K, Fujimoto M. FTY720 ameliorates murine sclerodermatous chronic graft-versus-host disease by promoting expansion of splenic regulatory cells and inhibiting immune cell infiltration into skin. Arthritis Rheum. 2013;65:1624–1635. doi: 10.1002/art.37933. [DOI] [PubMed] [Google Scholar]
- 48.Lim JY, Ryu DB, Lee SE, Park G, Min CK. Mesenchymal Stem Cells (MSCs) Attenuate Cutaneous Sclerodermatous Graft-Versus-Host Disease (Scl-GVHD) through Inhibition of Immune Cell Infiltration in a Mouse Model. J Invest Dermatol. 2017;137:1895–1904. doi: 10.1016/j.jid.2017.02.986. [DOI] [PubMed] [Google Scholar]
- 49.Du J, Paz K, Flynn R, Vulic A, Robinson TM, Lineburg KE, Alexander KA, Meng J, Roy S, Panoskaltsis-Mortari A, Loschi M, Hill GR, Serody JS, Maillard I, Miklos D, Koreth J, Cutler CS, Antin JH, Ritz J, MacDonald KP, Schacker TW, Luznik L, Blazar BR. Pirfenidone ameliorates murine chronic GVHD through inhibition of macrophage infiltration and TGF-β production. Blood. 2017;129:2570–2580. doi: 10.1182/blood-2017-01-758854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alexander KA, Flynn R, Lineburg KE, Kuns RD, Teal BE, Olver SD, Lor M, Raffelt NC, Koyama M, Leveque L, Le Texier L, Melino M, Markey KA, Varelias A, Engwerda C, Serody JS, Janela B, Ginhoux F, Clouston AD, Blazar BR, Hill GR, MacDonald KP. CSF-1-dependant donor-derived macrophages mediate chronic graft-versus-host disease. J Clin Invest. 2014;124:4266–4280. doi: 10.1172/JCI75935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Argyle D, Kitamura T. Targeting Macrophage-Recruiting Chemokines as a Novel Therapeutic Strategy to Prevent the Progression of Solid Tumors. Front Immunol. 2018;9:2629. doi: 10.3389/fimmu.2018.02629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruytinx P, Proost P, Van Damme J, Struyf S. Chemokine-Induced Macrophage Polarization in Inflammatory Conditions. Front Immunol. 2018;9:1930. doi: 10.3389/fimmu.2018.01930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cho KA, Woo SY, Park YS, Park MH, Ryu KH. Macrophage inflammatory protein-2 (MIP-2)/CXCR2 blockade attenuates acute graft-versus-host disease while preserving graft-versus-leukemia activity. Biochem Biophys Res Commun. 2012;426:558–564. doi: 10.1016/j.bbrc.2012.08.126. [DOI] [PubMed] [Google Scholar]
- 54.Du J, Flynn R, Paz K, Ren HG, Ogata Y, Zhang Q, Gafken PR, Storer BE, Roy NH, Burkhardt JK, Mathews W, Tolar J, Lee SJ, Blazar BR, Paczesny S. Murine chronic graft-versus-host disease proteome profiling discovers CCL15 as a novel biomarker in patients. Blood. 2018;131:1743–1754. doi: 10.1182/blood-2017-08-800623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hyvärinen K, Ritari J, Koskela S, Niittyvuopio R, Nihtinen A, Volin L, Gallardo D, Partanen J. Genetic polymorphism related to monocyte-macrophage function is associated with graft-versus-host disease. Sci Rep. 2017;7:15666. doi: 10.1038/s41598-017-15915-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cai Y, Ma S, Liu Y, Gong H, Cheng Q, Hu B, Wu Y, Yu X, Dong C, Sun K, Wu D, Liu H. Adoptively transferred donor IL-17-producing CD4+ T cells augment, but IL-17 alleviates, acute graft-versus-host disease. Cell Mol Immunol. 2018;15:233–245. doi: 10.1038/cmi.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Herretes S, Ross DB, Duffort S, Barreras H, Yaohong T, Saeed AM, Murillo JC, Komanduri KV, Levy RB, Perez VL. Recruitment of Donor T Cells to the Eyes During Ocular GVHD in Recipients of MHC-Matched Allogeneic Hematopoietic Stem Cell Transplants. Invest Ophthalmol Vis Sci. 2015;56:2348–2357. doi: 10.1167/iovs.14-15630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Facon T, Jouet JP, Noel-Walter MP, Bloget F, Bauters F, Janin A. Involvement of TNF-alpha secreting macrophages in lethal forms of human graft-versus-host disease. Bone Marrow Transplant. 1997;20:511–515. doi: 10.1038/sj.bmt.1700912. [DOI] [PubMed] [Google Scholar]
- 59.Hueso T, Coiteux V, Joncquel Chevalier Curt M, Labreuche J, Jouault T, Yakoub-Agha I, Seguy D. Citrulline and Monocyte-Derived Macrophage Reactivity before Conditioning Predict Acute Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2017;23:913–921. doi: 10.1016/j.bbmt.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 60.Ono R, Watanabe T, Kawakami E, Iwasaki M, Tomizawa-Murasawa M, Matsuda M, Najima Y, Takagi S, Fujiki S, Sato R, Mochizuki Y, Yoshida H, Sato K, Yabe H, Kato S, Saito Y, Taniguchi S, Shultz LD, Ohara O, Amagai M, Koseki H, Ishikawa F. Co-activation of macrophages and T cells contribute to chronic GVHD in human IL-6 transgenic humanised mouse model. EBioMedicine. 2019;41:584–596. doi: 10.1016/j.ebiom.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matta BM, Reichenbach DK, Zhang X, Mathews L, Koehn BH, Dwyer GK, Lott JM, Uhl FM, Pfeifer D, Feser CJ, Smith MJ, Liu Q, Zeiser R, Blazar BR, Turnquist HR. Peri-alloHCT IL-33 administration expands recipient T-regulatory cells that protect mice against acute GVHD. Blood. 2016;128:427–439. doi: 10.1182/blood-2015-12-684142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reichenbach DK, Schwarze V, Matta BM, Tkachev V, Lieberknecht E, Liu Q, Koehn BH, Pfeifer D, Taylor PA, Prinz G, Dierbach H, Stickel N, Beck Y, Warncke M, Junt T, Schmitt-Graeff A, Nakae S, Follo M, Wertheimer T, Schwab L, Devlin J, Watkins SC, Duyster J, Ferrara JL, Turnquist HR, Zeiser R, Blazar BR. The IL-33/ST2 axis augments effector T-cell responses during acute GVHD. Blood. 2015;125:3183–3192. doi: 10.1182/blood-2014-10-606830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koyama M, Mukhopadhyay P, Schuster IS, Henden AS, Hülsdünker J, Varelias A, Vetizou M, Kuns RD, Robb RJ, Zhang P, Blazar BR, Thomas R, Begun J, Waddell N, Trinchieri G, Zeiser R, Clouston AD, Degli-Esposti MA, Hill GR. MHC Class II Antigen Presentation by the Intestinal Epithelium Initiates Graft-versus-Host Disease and Is Influenced by the Microbiota. Immunity. 2019;51:885–898.e7. doi: 10.1016/j.immuni.2019.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sundarasetty B, Volk V, Theobald SJ, Rittinghausen S, Schaudien D, Neuhaus V, Figueiredo C, Schneider A, Gerasch L, Mucci A, Moritz T, von Kaisenberg C, Spineli LM, Sewald K, Braun A, Weigt H, Ganser A, Stripecke R. Human Effector Memory T Helper Cells Engage with Mouse Macrophages and Cause Graft-versus-Host-Like Pathology in Skin of Humanized Mice Used in a Nonclinical Immunization Study. Am J Pathol. 2017;187:1380–1398. doi: 10.1016/j.ajpath.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 65.Haniffa M, Ginhoux F, Wang XN, Bigley V, Abel M, Dimmick I, Bullock S, Grisotto M, Booth T, Taub P, Hilkens C, Merad M, Collin M. Differential rates of replacement of human dermal dendritic cells and macrophages during hematopoietic stem cell transplantation. J Exp Med. 2009;206:371–385. doi: 10.1084/jem.20081633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Uryu H, Hashimoto D, Kato K, Hayase E, Matsuoka S, Ogasawara R, Takahashi S, Maeda Y, Iwasaki H, Miyamoto T, Saijo S, Iwakura Y, Hill GR, Akashi K, Teshima T. α-Mannan induces Th17-mediated pulmonary graft-versus-host disease in mice. Blood. 2015;125:3014–3023. doi: 10.1182/blood-2014-12-615781. [DOI] [PubMed] [Google Scholar]
- 67.Hashimoto D, Chow A, Greter M, Saenger Y, Kwan WH, Leboeuf M, Ginhoux F, Ochando JC, Kunisaki Y, van Rooijen N, Liu C, Teshima T, Heeger PS, Stanley ER, Frenette PS, Merad M. Pretransplant CSF-1 therapy expands recipient macrophages and ameliorates GVHD after allogeneic hematopoietic cell transplantation. J Exp Med. 2011;208:1069–1082. doi: 10.1084/jem.20101709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ahmed F, Ibrahim A, Cooper CL, Kumar A, Crawley AM. Chronic Hepatitis C Virus Infection Impairs M1 Macrophage Differentiation and Contributes to CD8+ T-Cell Dysfunction. Cells. 2019;8 doi: 10.3390/cells8040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu P, Jia S, Lou Y, He K, Xu LX. Cryo-thermal therapy inducing MI macrophage polarization created CXCL10 and IL-6-rich pro-inflammatory environment for CD4+ T cell-mediated anti-tumor immunity. Int J Hyperthermia. 2019;36:408–420. doi: 10.1080/02656736.2019.1579373. [DOI] [PubMed] [Google Scholar]
- 70.Petty AJ, Li A, Wang X, Dai R, Heyman B, Hsu D, Huang X, Yang Y. Hedgehog signaling promotes tumor-associated macrophage polarization to suppress intratumoral CD8+ T cell recruitment. J Clin Invest. 2019;129:5151–5162. doi: 10.1172/JCI128644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bouchlaka MN, Moffitt AB, Kim J, Kink JA, Bloom DD, Love C, Dave S, Hematti P, Capitini CM. Human Mesenchymal Stem Cell-Educated Macrophages Are a Distinct High IL-6-Producing Subset that Confer Protection in Graft-versus-Host-Disease and Radiation Injury Models. Biol Blood Marrow Transplant. 2017;23:897–905. doi: 10.1016/j.bbmt.2017.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang X, Zhou T, Xiao Y, Yu J, Dou S, Chen G, Wang R, Xiao H, Hou C, Wang W, Shi Q, Feng J, Ma Y, Shen B, Li Y, Han G. Tim-3 promotes tumor-promoting M2 macrophage polarization by binding to STAT1 and suppressing the STAT1-miR-155 signaling axis. Oncoimmunology. 2016;5:e1211219. doi: 10.1080/2162402X.2016.1211219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 74.Liang S, Cai J, Li Y, Yang R. 1,25‑Dihydroxy‑Vitamin D3 induces macrophage polarization to M2 by upregulating T‑cell Ig‑mucin‑3 expression. Mol Med Rep. 2019;19:3707–3713. doi: 10.3892/mmr.2019.10047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, Pfirschke C, Voss RH, Timke C, Umansky L, Klapproth K, Schäkel K, Garbi N, Jäger D, Weitz J, Schmitz-Winnenthal H, Hämmerling GJ, Beckhove P. Low-dose irradiation programs macrophage differentiation to an iNOS⁺/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24:589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 76.Sektioglu IM, Carretero R, Bender N, Bogdan C, Garbi N, Umansky V, Umansky L, Urban K, von Knebel-Döberitz M, Somasundaram V, Wink D, Beckhove P, Hämmerling GJ. Macrophage-derived nitric oxide initiates T-cell diapedesis and tumor rejection. Oncoimmunology. 2016;5:e1204506. doi: 10.1080/2162402X.2016.1204506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wood MA, Goldman N, DePierri K, Somerville J, Riggs JE. Erythropoietin increases macrophage-mediated T cell suppression. Cell Immunol. 2016;306-307:17–24. doi: 10.1016/j.cellimm.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tashiro-Yamaji J, Maeda S, Ikawa M, Okabe M, Kubota T, Yoshida R. Macrophage MHC and T-cell receptors essential for rejection of allografted skin and lymphoma. Transplantation. 2013;96:251–257. doi: 10.1097/TP.0b013e3182985527. [DOI] [PubMed] [Google Scholar]
- 79.Marro BS, Legrain S, Ware BC, Oldstone MB. Macrophage IFN-I Signaling Promotes Autoreactive T Cell Infiltration Into Islets in Type 1 Diabetes Model. JCI Insight. 2019;4:e125067. doi: 10.1172/jci.insight.125067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yuan X, Yang BH, Dong Y, Yamamura A, Fu W. CRIg, a Tissue-Resident Macrophage Specific Immune Checkpoint Molecule, Promotes Immunological Tolerance in NOD Mice, via a Dual Role in Effector and Regulatory T Cells. Elife. 2017;6:e29540. doi: 10.7554/eLife.29540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Quaranta V, Rainer C, Nielsen SR, Raymant ML, Ahmed MS, Engle DD, Taylor A, Murray T, Campbell F, Palmer DH, Tuveson DA, Mielgo A, Schmid MC. Macrophage-Derived Granulin Drives Resistance to Immune Checkpoint Inhibition in Metastatic Pancreatic Cancer. Cancer Res. 2018;78:4253–4269. doi: 10.1158/0008-5472.CAN-17-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arlauckas SP, Garris CS, Kohler RH, Kitaoka M, Cuccarese MF, Yang KS, Miller MA, Carlson JC, Freeman GJ, Anthony RM, Weissleder R, Pittet MJ. In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy. Sci Transl Med. 2017;9:eaal3604. doi: 10.1126/scitranslmed.aal3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.La Fleur L, Boura VF, Alexeyenko A, Berglund A, Pontén V, Mattsson JSM, Djureinovic D, Persson J, Brunnström H, Isaksson J, Brandén E, Koyi H, Micke P, Karlsson MCI, Botling J. Expression of scavenger receptor MARCO defines a targetable tumor-associated macrophage subset in non-small cell lung cancer. Int J Cancer. 2018;143:1741–1752. doi: 10.1002/ijc.31545. [DOI] [PubMed] [Google Scholar]
- 84.Li X, Gao Q, Feng Y, Zhang X. Developing role of B cells in the pathogenesis and treatment of chronic GVHD. Br J Haematol. 2019;184:323–336. doi: 10.1111/bjh.15719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schroeder MA, DiPersio JF. Mouse models of graft-versus-host disease: advances and limitations. Dis Model Mech. 2011;4:318–333. doi: 10.1242/dmm.006668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mukai S, Ogawa Y, Urano F, Kudo-Saito C, Kawakami Y, Tsubota K. Novel Treatment of Chronic Graft-Versus-Host Disease in Mice Using the ER Stress Reducer 4-Phenylbutyric Acid. Sci Rep. 2017;7:41939. doi: 10.1038/srep41939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ayaub EA, Kolb PS, Mohammed-Ali Z, Tat V, Murphy J, Bellaye PS, Shimbori C, Boivin FJ, Lai R, Lynn EG, Lhoták Š, Bridgewater D, Kolb MR, Inman MD, Dickhout JG, Austin RC, Ask K. GRP78 and CHOP modulate macrophage apoptosis and the development of bleomycin-induced pulmonary fibrosis. J Pathol. 2016;239:411–425. doi: 10.1002/path.4738. [DOI] [PubMed] [Google Scholar]
- 88.Lodder J, Denaës T, Chobert MN, Wan J, El-Benna J, Pawlotsky JM, Lotersztajn S, Teixeira-Clerc F. Macrophage autophagy protects against liver fibrosis in mice. Autophagy. 2015;11:1280–1292. doi: 10.1080/15548627.2015.1058473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Du S, Li C, Lu Y, Lei X, Zhang Y, Li S, Liu F, Chen Y, Weng D, Chen J. Dioscin Alleviates Crystalline Silica-Induced Pulmonary Inflammation and Fibrosis through Promoting Alveolar Macrophage Autophagy. Theranostics. 2019;9:1878–1892. doi: 10.7150/thno.29682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stifano G, Affandi AJ, Mathes AL, Rice LM, Nakerakanti S, Nazari B, Lee J, Christmann RB, Lafyatis R. Chronic Toll-like receptor 4 stimulation in skin induces inflammation, macrophage activation, transforming growth factor beta signature gene expression, and fibrosis. Arthritis Res Ther. 2014;16:R136. doi: 10.1186/ar4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Murthy S, Larson-Casey JL, Ryan AJ, He C, Kobzik L, Carter AB. Alternative activation of macrophages and pulmonary fibrosis are modulated by scavenger receptor, macrophage receptor with collagenous structure. FASEB J. 2015;29:3527–3536. doi: 10.1096/fj.15-271304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shivshankar P, Halade GV, Calhoun C, Escobar GP, Mehr AJ, Jimenez F, Martinez C, Bhatnagar H, Mjaatvedt CH, Lindsey ML, Le Saux CJ. Caveolin-1 deletion exacerbates cardiac interstitial fibrosis by promoting M2 macrophage activation in mice after myocardial infarction. J Mol Cell Cardiol. 2014;76:84–93. doi: 10.1016/j.yjmcc.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Larson-Casey JL, Deshane JS, Ryan AJ, Thannickal VJ, Carter AB. Macrophage Akt1 Kinase-Mediated Mitophagy Modulates Apoptosis Resistance and Pulmonary Fibrosis. Immunity. 2016;44:582–596. doi: 10.1016/j.immuni.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chung S, Overstreet JM, Li Y, Wang Y, Niu A, Wang S, Fan X, Sasaki K, Jin GN, Khodo SN, Gewin L, Zhang MZ, Harris RC. TGF-β promotes fibrosis after severe acute kidney injury by enhancing renal macrophage infiltration. JCI Insight. 2018;3:e123563. doi: 10.1172/jci.insight.123563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yao Y, Wang Y, Zhang Z, He L, Zhu J, Zhang M, He X, Cheng Z, Ao Q, Cao Y, Yang P, Su Y, Zhao J, Zhang S, Yu Q, Ning Q, Xiang X, Xiong W, Wang CY, Xu Y. Chop Deficiency Protects Mice Against Bleomycin-induced Pulmonary Fibrosis by Attenuating M2 Macrophage Production. Mol Ther. 2016;24:915–925. doi: 10.1038/mt.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pan B, Liu G, Jiang Z, Zheng D. Regulation of renal fibrosis by macrophage polarization. Cell Physiol Biochem. 2015;35:1062–1069. doi: 10.1159/000373932. [DOI] [PubMed] [Google Scholar]
- 97.Steiger S, Kumar SV, Honarpisheh M, Lorenz G, Günthner R, Romoli S, Gröbmayr R, Susanti HE, Potempa J, Koziel J, Lech M. Immunomodulatory Molecule IRAK-M Balances Macrophage Polarization and Determines Macrophage Responses during Renal Fibrosis. J Immunol. 2017;199:1440–1452. doi: 10.4049/jimmunol.1601982. [DOI] [PubMed] [Google Scholar]
- 98.Nouno T, Okamoto M, Ohnishi K, Kaieda S, Tominaga M, Zaizen Y, Ichiki M, Momosaki S, Nakamura M, Fujimoto K, Fukuoka J, Shimizu S, Komohara Y, Hoshino T. Elevation of pulmonary CD163+ and CD204+ macrophages is associated with the clinical course of idiopathic pulmonary fibrosis patients. J Thorac Dis. 2019;11:4005–4017. doi: 10.21037/jtd.2019.09.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang YY, Jiang H, Pan J, Huang XR, Wang YC, Huang HF, To KF, Nikolic-Paterson DJ, Lan HY, Chen JH. Macrophage-to-Myofibroblast Transition Contributes to Interstitial Fibrosis in Chronic Renal Allograft Injury. J Am Soc Nephrol. 2017;28:2053–2067. doi: 10.1681/ASN.2016050573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Caires HR, Barros da Silva P, Barbosa MA, Almeida CR. A co-culture system with three different primary human cell populations reveals that biomaterials and MSC modulate macrophage-driven fibroblast recruitment. J Tissue Eng Regen Med. 2018;12:e1433–e1440. doi: 10.1002/term.2560. [DOI] [PubMed] [Google Scholar]
- 101.Ueshima E, Fujimori M, Kodama H, Felsen D, Chen J, Durack JC, Solomon SB, Coleman JA, Srimathveeravalli G. Macrophage-secreted TGF-β1 contributes to fibroblast activation and ureteral stricture after ablation injury. Am J Physiol Renal Physiol. 2019;317:F52–F64. doi: 10.1152/ajprenal.00260.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guo X, Li T, Xu Y, Xu X, Zhu Z, Zhang Y, Xu J, Xu K, Cheng H, Zhang X, Ke Y. Increased levels of Gab1 and Gab2 adaptor proteins skew interleukin-4 (IL-4) signaling toward M2 macrophage-driven pulmonary fibrosis in mice. J Biol Chem. 2017;292:14003–14015. doi: 10.1074/jbc.M117.802066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Humeres C, Vivar R, Boza P, Muñoz C, Bolivar S, Anfossi R, Osorio JM, Olivares-Silva F, García L, Díaz-Araya G. Cardiac fibroblast cytokine profiles induced by proinflammatory or profibrotic stimuli promote monocyte recruitment and modulate macrophage M1/M2 balance in vitro. J Mol Cell Cardiol. 2016:S0022–2828(16)30392-3. doi: 10.1016/j.yjmcc.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 104.Tang L, Zhang H, Wang C, Li H, Zhang Q, Bai J. M2A and M2C Macrophage Subsets Ameliorate Inflammation and Fibroproliferation in Acute Lung Injury Through Interleukin 10 Pathway. Shock. 2017;48:119–129. doi: 10.1097/SHK.0000000000000820. [DOI] [PubMed] [Google Scholar]
- 105.Chandrasekaran P, Izadjoo S, Stimely J, Palaniyandi S, Zhu X, Tafuri W, Mosser DM. Regulatory Macrophages Inhibit Alternative Macrophage Activation and Attenuate Pathology Associated with Fibrosis. J Immunol. 2019;203:2130–2140. doi: 10.4049/jimmunol.1900270. [DOI] [PubMed] [Google Scholar]
- 106.Zeiser R, Sarantopoulos S, Blazar BR. B-cell targeting in chronic graft-versus-host disease. Blood. 2018;131:1399–1405. doi: 10.1182/blood-2017-11-784017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jin H, Ni X, Deng R, Song Q, Young J, Cassady K, Zhang M, Forman S, Martin PJ, Liu Q, Zeng D. Antibodies from donor B cells perpetuate cutaneous chronic graft-versus-host disease in mice. Blood. 2016;127:2249–2260. doi: 10.1182/blood-2015-09-668145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Baroni SS, Santillo M, Bevilacqua F, Luchetti M, Spadoni T, Mancini M, Fraticelli P, Sambo P, Funaro A, Kazlauskas A, Avvedimento EV, Gabrielli A. Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N Engl J Med. 2006;354:2667–2676. doi: 10.1056/NEJMoa052955. [DOI] [PubMed] [Google Scholar]
- 109.Xu L, Collins J, Drachenberg C, Kukuruga D, Burke A. Increased macrophage density of cardiac allograft biopsies is associated with antibody-mediated rejection and alloantibodies to HLA antigens. Clin Transplant. 2014;28:554–560. doi: 10.1111/ctr.12348. [DOI] [PubMed] [Google Scholar]
- 110.Apicella C, Custidiano A, Miranda S, Novoa L, Dokmetjian J, Gentile T. Differential macrophage modulation of asymmetric IgG antibody synthesis by soluble or particulate stimuli. Immunol Lett. 2006;103:177–185. doi: 10.1016/j.imlet.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 111.Koyamada N, Sato A, Takayama J, Usuda M, Kawagishi N, Doi H, Fujimori K, Satomi S. Macrophage depletion prevents anti-graft antibody production and results in long-term survival in xenotransplantation. Transplant Proc. 2005;37:514–515. doi: 10.1016/j.transproceed.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 112.Haasken S, Auger JL, Taylor JJ, Hobday PM, Goudy BD, Titcombe PJ, Mueller DL, Binstadt BA. Macrophage scavenger receptor 1 (Msr1, SR-A) influences B cell autoimmunity by regulating soluble autoantigen concentration. J Immunol. 2013;191:1055–1062. doi: 10.4049/jimmunol.1201680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gorbacheva V, Fan R, Beavers A, Fairchild RL, Baldwin WM, 3rd, Valujskikh A. Anti-donor MHC Class II Alloantibody Induces Glomerular Injury in Mouse Renal Allografts Subjected to Prolonged Cold Ischemia. J Am Soc Nephrol. 2019;30:2413–2425. doi: 10.1681/ASN.2018111169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hogenes MCH, van Dorp S, van Kuik J, Monteiro FRP, Ter Hoeve N, Guedes L, van Dijk MR, Martens AC, de Weger RA. Modifying Graft-versus-Host Disease in a Humanized Mouse Model by Targeting Macrophages or B-Cells. J Immunol Res. 2019;2019:3538963. doi: 10.1155/2019/3538963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Facon T, Janin A, Noel MP, Jouet JP. Involvement of macrophages in lethal forms of graft-versus-host disease. Lancet. 1995;345:392. doi: 10.1016/s0140-6736(95)90382-8. [DOI] [PubMed] [Google Scholar]
- 116.Nishiwaki S, Nakayama T, Murata M, Nishida T, Terakura S, Saito S, Kato T, Mizuno H, Imahashi N, Seto A, Ozawa Y, Miyamura K, Ito M, Takeshita K, Kato H, Toyokuni S, Nagao K, Ueda R, Naoe T. Dexamethasone palmitate ameliorates macrophages-rich graft-versus-host disease by inhibiting macrophage functions. PLoS One. 2014;9:e96252. doi: 10.1371/journal.pone.0096252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Toubai T, Tanaka J, Nishihira J, Ohkawara T, Hirate D, Kondo N, Tone S, Shono Y, Ibata M, Sugita J, Kato N, Miura Y, Iwao N, Ota S, Imamura M. Effect of macrophage migration inhibitory factor (MIF) on acute graft-versus-host disease in a murine model of allogeneic stem cell transplantation. Transpl Immunol. 2006;16:117–124. doi: 10.1016/j.trim.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 118.Lo JW, Leung AY, Huang XR, Lie AK, Metz C, Bucala R, Liang R, Lan HY. Macrophage migratory inhibitory factor (MIF) expression in acute graft-versus-host disease (GVHD) in allogeneic hemopoietic stem cell transplant recipients. Bone Marrow Transplant. 2002;30:375–380. doi: 10.1038/sj.bmt.1703639. [DOI] [PubMed] [Google Scholar]
- 119.Castor MG, Rezende B, Resende CB, Alessandri AL, Fagundes CT, Sousa LP, Arantes RM, Souza DG, Silva TA, Proudfoot AE, Teixeira MM, Pinho V. The CCL3/macrophage inflammatory protein-1alpha-binding protein evasin-1 protects from graft-versus-host disease but does not modify graft-versus-leukemia in mice. J Immunol. 2010;184:2646–2654. doi: 10.4049/jimmunol.0902614. [DOI] [PubMed] [Google Scholar]