Abstract
Bimatoprost, latanoprost, and unoprostone are prostaglandin F2α analogs (PGAs) and are used to lower intraocular pressure. We investigated the free acid effects of these three prostaglandin analogs: bimatoprost, latanoprost, and unoprostone on human matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMP) in the trabecular meshwork (TM) cells. Immunoblot results show that all three PGAs generally increased MMPs-1,9 and TIMPs-4. Additionally, bimatoprost and latanoprost both increased MMP-3 and TIMP-2, while unoprostone had an indeterminate effect on both. Zymography results show that all three PGAs except unoprostone increased intermediate MMP-1 activity while bimatoprost and latanoprost increased MMP-9 activity. Together, these data suggest that the balance between MMPs and TIMPs correlate to the relative intraocular pressure lowering effectiveness observed in clinical studies of these PGAs.
Keywords: trabecular meshwork endothelial cells, Prostaglandin analogs, transcriptional expression, matrix metalloproteinases, tissue inhibitors of metalloproteinases, Glaucoma, Open-angle glaucoma
1. Introduction
Glaucoma is an intraocular pressure (IOP)-associated optic neuropathy. The IOP is determined by the balance between aqueous humor secretion and drainage. The non-pigmented epithelium of the ciliary body (CB) secretes aqueous humor. The aqueous humor either flows through the conventional pathway (through the trabecular meshwork into Schlemm’s canal and aqueous veins) or the alternative pathway (through the ciliary body face and suprachoroidal space diffusing through the sclera into the vortex veins or choroidal vessels).
Lowering intraocular pressure by enhancing uveoscleral outflow is thought to be the principle action of prostaglandin F2α analogs (PGAs). Bimatoprost, latanoprost, and unoprostone are PGAs (Ota et al., 2005; Sharif et al., 2003a; Sharif et al., 2002, 2003b; Sharif et al., 2003c). Numerous studies show that latanoprost is more effective at lowering IOP than unoprostone (Aung et al., 2002; Jampel et al., 2002; Sponsel et al., 2002; Susanna et al., 2001; Susanna et al., 2004; Takahashi and Tanaka, 2004; Tsukamoto et al., 2002).Some studies show that bimatoprost is more effective at lowering IOP compared to latanoprost (Noecker et al., 2003; Simmons et al., 2004),but others found them to be equivalent (Oh et al., 2006b).
Outflow resistance through the uveoscleral tract can be modulated in the ciliary body stroma by both ciliary smooth muscle cell tone (Bill, 1967; Schenker et al., 1981; Townsend and Brubaker, 1980) and enhanced turnover of extracellular matrix by matrix metalloproteinases (MMPs) (Anthony et al., 2002; el-Shabrawi et al., 2000; Lindsey et al., 1996; Lutjen-Drecoll and Tamm, 1988, 1989; Ocklind, 1998; Oh et al., 2006a; Weinreb et al., 1997). We previously reported that bimatoprost, latanoprost, and unoprostone all increased MMP-1, −3, and −9 in ciliary body smooth muscle (CBSM) cells; unoprostone decreased MMP-2(Oh et al., 2006a).All three PGAs had increased TIMP-3, but only unoprostone increased TIMP-1 and −4. These coordinated trends in CBSM cells may mediate the alteration of the extracellular matrix (ECM) in the ciliary body. Thus, in CBSM cells, the balance between MMPs and TIMPs correlated to the relative IOP lowering efficacy of these three medications.
In the TM, extracellular matrix (ECM) turnover, mediated by MMPs and TIMPs, alters IOP and outflow resistance (Bradley et al., 1998). Brubaker et al., first reported that bimatoprost, known as AGN 192024, increased trabecular outflow(Brubaker et al., 2001); Lim et al. found that bimatoprost, latanoprost, and travoprost also increased trabecular outflow (Brubaker et al., 2001; Lim et al., 2008). In TM endothelial cells, we reported that latanoprost increased the mRNA of MMPs-1, −3, −12, −17, and −24 as well as TIMP-2, −3, and −4 (Numaga et al., 2005). In other human tissues, the balance of MMPs to TIMPs correlates to the rate of ECM turnover (Ahmed et al., 2005; Butler et al., 1998; Butler et al., 1997; Curran et al., 2004; Khasigov et al., 2003; Maatta et al., 2005; Pauschinger et al., 2004; Steinmetz et al., 2005; Zhou et al., 2004). We wished to examine the effect of bimatoprost, latanoprost, and unoprostone on MMP and TIMP protein levels and MMP activity. We hypothesized that MMP levels, MMP activity, and TIMP levels will affected differently by the administration of either of these PGAs.
2. Materials and Methods
2.1. Tissue and Explant Culture
Trabecular meshwork (TM) endothelial cells were cultured according to protocols we previously published in detail and recent consensus guideline recently published (Keller et al., 2018; Oh et al., 2006a; Oh et al., 2006b). Briefly, all TM tissue was dissected from corneoscleral rims of human donors from Massachusetts Eye and Ear Infirmary. TM explants were isolated from 7 separate individuals, ages 23 to 65 years, and were used to generate TM endothelial cell cultures. Cultures were maintained in Dulbecco’s modified Eagle’s media (DMEM) (Invitrogen, Carlsbad, CA) containing 20% fetal bovine serum, 1% L-glutamine (2 mM), and gentamicin (0.1 mg/ml) at 37°C in a 10% CO2 incubator.
2.2. Latanoprost, Bimatoprost, and Unoprostone Incubation
Bimatoprost, latanoprost, and unoprostone were prepared according to protocols we previously published (Oh et al., 2006a; Oh et al., 2006b). Briefly, the free acid forms of latanoprost, bimatoprost, and unoprostone (Cayman Chemical, Ann Arbor, MI) were solubilized in ethanol and diluted to experimental concentrations with serum-free media for treatment. TM endothelial cells were plated and grown with medium changes every 3 to 5 days. After the cells were preincubated with serum-free media for 24 hours, they were exposed to vehicle control (0.015% ethanol), 25.7 nM or 257 nM of bimatoprost free acid, 76.8 nM or 768 nM of latanoprost free acid, or 0.341 μM or 3.415 μM of unoprostone free acid, for 24 hours. Suprapharmacological concentrations, ten times the peak aqueous concentration, was used for confirmation of the trends seen at pharmacologic concentrations.
2.3. Conditioned Medium and Cell Lysate Preparation
The cells were prepared according to protocols we previously published (Oh et al., 2006a; Oh et al., 2006b). Briefly, the conditioned medium was collected 24 hours after incubation with vehicle control or with free acid forms of latanoprost, bimatoprost, and unoprostone. The medium was concentrated up to 30-fold with a centrifugal filter unit (Amicon Ultra-4, 10K; Millipore, Milford, MA). To standardize protein loading amount, protein concentrations were measured using the BioMate Protein Assay protocol (Thermo Spectronic, Rochester, NY). RIPA with inhibitors were used as a control and 5μl of each sample were used to compare to a previously established BSA curve at 655nm wavelength.
For the cell lysate preparation, cells were lysed in radioimmunoprecipitation assay (RIPA) buffer with protease inhibitors (25 mTIU/mL aprotinin, 0.05 mg/mL PMSF, 1 mM EGTA, 1 mM EDTA, 1 μg/mL leupeptin, and 1 mM sodium orthovanadate to a final volume of 1 mL with RIPA buffer). The cells were sheared with a 21-guage needle and 0.05 mg/mL of PMSF was added. Contents were then put on ice for incubation for 30–60 minutes and then centrifuged at 14,000 g for 20 minutes at 4˚C. The supernatant was collected as whole cell lysate.
2.4. Immunoblot Analysis
Immunoblot analysis was conducted according to protocols we previously published (Oh et al., 2006a; Oh et al., 2006b).Briefly, equal amounts of protein samples were obtained using a protein assay and were electrophoresed in 11% SDS-PAGE gel using XCell SureLock Mini-Cell (Invitrogen) and then transferred onto nitrocellulose membrane (Invitrogen). Membranes were incubated in blocking buffer (Rockland, Gilbertsville, PA) half diluted in 1x TBS for one hour followed by primary antibody (Table 1) incubation overnight at 4°C. The membranes were washed with TBS/T and then incubated with IRDye™ 800 conjugated affinity purified anti-Mouse or anti-Rabbit IgG (Rockland) diluted 1:10,000 for 1 hour. After washing with 1x TBS/T, the membranes were scanned using Odyssey Infrared Imaging System (Li-Cor, Lincoln, NE). The protein bands were quantified using the densitometric software on the Odyssey (version 1.2).
Table 1.
Primary antibodies and dilutions for immunoblotting.
| Antibodies | Manufacturer | Dilution | Identified Band size (s) |
|---|---|---|---|
| MMP-1 (M) | R&D Systems | 1:1,000 | 52 & 57 kDa |
| MMP-2 (M) | R&D Systems | 1:1,000 | 72 kDa |
| MMP-3 (P) | Chemicon | 1:1,000 | 57 & 59 kDa |
| MMP-9 (P) | Chemicon | 1:1,000 | 92 kDa |
| MMP-24 (P) | Chemicon | 1:1,000 | 64 kDa |
| TIMP-1 (P) | Chemicon | 1:20,000 | 28 kDa |
| TIMP-2 (P) | Chemicon | 1:2,000 | 21 kDa |
| TIMP-3 (M) | Calbiochem | 1:200 | 27 kDa |
| TIMP-4 (P) | R&D Systems | 1:7,000 | 29 kDa |
| GAPDH (P) | R&D Systems | 1:20–40K | 36 kDa |
P: polyclonal rabbit anti-human antibody; M: monoclonal mouse anti-human antibody.
2.5. Zymographic Analysis
Zymographic analysis was conducted accordingly to protocols we previously published (Oh et al., 2006a; Oh et al., 2006b). Briefly, polyacrylamide gels were made with either gelatin (0.1%) for MMPs-2 and −9 or β-casein (0.1%) for MMPs-1 and −3. Concentrated conditioned medium in serum free media was mixed with 2x Tris-glycine-SDS zymography and was loaded into 10% SDS-PAGE gels, then electrophoresed in tank buffer. The gels were incubated at room temperature with 2.5% Triton X-100 (renaturing buffer) and then transferred to enzyme assay buffer at 37 °C overnight. The gels were stained with 0.1% Coomassie Brilliant Blue G-250 (Bio-Rad, Hercules, CA) solution for 3 hours and then destained until clear bands were visible. Odyssey Infrared Imaging System was used to scan and analyze the relative band densities using the densitometric software (version 1.2).
2.6. Determination of Change/Statistical Analysis
It is commonly noted for patients and human tissue to have variability in response to PGAs. For patients, the response rate for IOP lowering to latanoprost is about 85%, but this response is variable (Sjoquist and Stjernschantz, 2002). Bimatoprost also demonstrates variability of response among individuals (Cantor et al., 2006). Similarly, our laboratory and others have found these minor inconsistencies in response to prostaglandins from cell lines among different donors (Oh et al., 2006a; Sjoquist and Stjernschantz, 2002; Weinreb et al., 1997). Based on these observations, we proceeded to follow protocols that we have previously published (Oh et al., 2006a; Oh et al., 2006b). Briefly, we determined any change greater than 10% was noted to be an elevation or decrease because the variability of our immunoblots revealed an average difference of 10.05 ± 9.45% (mean ± standard error). The ultimate determination of the effect of the PGAs was determined by the trend of the sample majority (>60%) (Oh et al., 2006a; Oh et al., 2006b; Weinreb et al., 1997; Weinreb and Lindsey, 2002). All statistical analysis comparing changes between the control group and the treated cells were completed using a two-tailed paired Student’s t-test.
3. Results
3.1. PGA effect on MMPs and TIMPs at pharmacologic levels
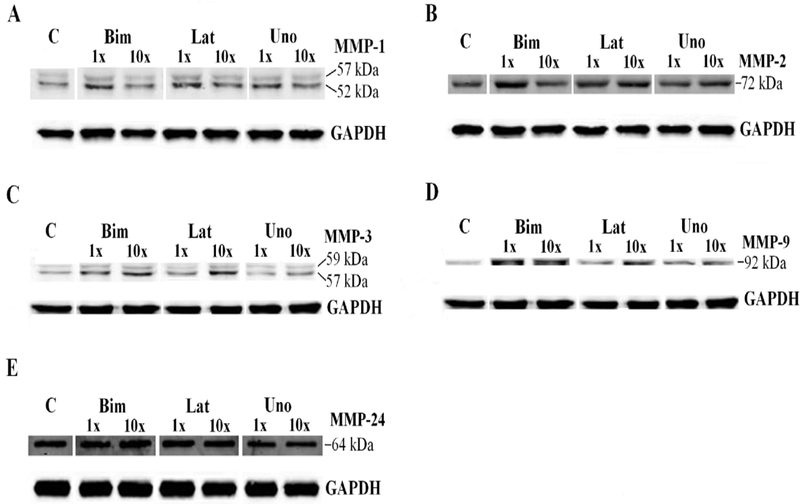
We examined the effects of the free acid forms of three PGAs: bimatoprost (BFA), latanoprost (LFA), and unoprostone (UFA) on MMPs-1, −2, −3, −9, −24 and TIMPs-1 – 4 in the TM. MMPs-1, −2, −3, −9 are secreted enzymes and were detected in conditioned media while MMP-24, which is membrane bound, was found in cell lysates.
The free acid moieties of bimatoprost (BFA), latanoprost (LFA), and unoprostone (UFA) all increased pro-MMPs −1 and −9 which translated into increased activity of MMPs-1 and −9 (Table 2). Although UFA increased pro-MMPs-1 and −9, there was no increase of enzymatic activity as was seen with LFA and BFA (Table 2C). BFA and LFA both increase pro-MMP-3 which UFA did not (Table 2A, 2C). None of the PGAs affected MMP-2 levels or activity (Table 2, Fig. 1B). BFA, LFA, and UFA did not alter MMP-24 at pharmacological levels (MT5-MMP) (Table 2 and Fig. 1E).
Table 2.
MMP and TIMP expression upon free acid PGA treatment of bimatoprost (A), latanoprost (B), and unoprostone (C) in conditioned media of human TM endothelial cells incubated at the pharmacologic concentrations of 0.01 μg/ml, 0.03 μg/ml, and 0.145 μg/ml respectively, for 24 hours.
| A. Bimatoprost Free Acid Pharmacologic Concentrations | ||||||
| MMPs/TIMP | ↑/↔ /↓ | Trend | % Change of Trend | % Change of All Samples | N | P-Value |
| MMPs | ||||||
| Pro-MMP-1 | 4/1/0 | ↑ | (42 ± 13%) | (32 ± 14%) | 5 | 0.0068 |
| Pro-MMP-2 | 2/3/0 | ↔ | (4 ± 5%) | (4 ± 5%) | 5 | 0.4468 |
| Pro-MMP-3 | 5/0/0 | ↑ | (44 ± 9%) | (44 ± 9%) | 5 | 0.0001 |
| Pro-MMP-9 | 4/1/0 | ↑ | (109 ± 36%) | (90 ± 34%) | 5 | 0.0031 |
| MMP-24(CL) | 1/3/1 | ↔ | (−2 ± 5%) | (−2 ± 5%) | 5 | 0.6996 |
| Active MMPs | ||||||
| Inter MMP-1 | 4/1/0 | ↑ | (15 ± 2%) | (14 ± 2%) | 5 | 0.0015 |
| Active MMP-2 | 0/5/0 | ↔ | (6 ± 2%) | (6 ± 2%) | 5 | 0.2248 |
| Active MMP-9 | 4/1/0 | ↑ | (17 ± 2%) | (15 ± 3%) | 5 | 0.0012 |
| TIMPs | ||||||
| TIMP-1 | 3/0/2 | ↑ | (88 ± 34%) | (35 ± 37%) | 5 | 0.1731 |
| TIMP-2 | 4/1/0 | ↑ | (83 ± 42%) | (65 ± 37%) | 5 | 0.0240 |
| TIMP-3 | 2/1/2 | Ind. | (−3 ± 10%) | (−3 ± 10%) | 5 | 0.6479 |
| TIMP-4 | 3/1/1 | ↑ | (200±133%) | (106 ± 93%) | 5 | 0.1091 |
| B. Latanoprost Free Acid Pharmacologic Concentrations | ||||||
| MMPs/TIMP | ↑/↔ /↓ | Trend | % Change of Trend | % Change of All Samples | N | P-Value |
| MMPs | ||||||
| Pro-MMP-1 | 4/1/0 | ↑ | (49 ± 11%) | (33 ± 12%) | 5 | 0.0025 |
| Pro-MMP-2 | 0/5/0 | ↔ | (3 ± 2%) | (3 ± 2%) | 5 | 0.5291 |
| Pro-MMP-3 | 3/2/0 | ↑ | (67 ± 47%) | (37 ± 31%) | 5 | 0.0958 |
| Pro-MMP-9 | 5/0/0 | ↑ | (37 ± 5%) | (37 ± 5%) | 5 | 0.0001 |
| MMP-24(CL) | 0/4/1 | ↔ | (−3 ± 4%) | (−3 ± 4%) | 5 | 0.5507 |
| Active MMPs | ||||||
| Inter MMP-1 | 5/0/0 | ↑ | (13 ± 1%) | (13 ± 1%) | 5 | 0.0201 |
| Active MMP-2 | 0/5/0 | ↔ | (5 ± 2%) | (5 ± 2%) | 5 | 0.3048 |
| Active MMP-9 | 4/1/0 | ↑ | (20 ± 2%) | (14 ± 4%) | 5 | 0.0197 |
| TIMPs | ||||||
| TIMP-1 | 2/0/3 | ↓ | (−42 ± 4%) | (−3 ± 25%) | 5 | 0.8542 |
| TIMP-2 | 5/0/0 | ↑ | (33 ± 7%) | (33 ± 7%) | 5 | 0.0003 |
| TIMP-3 | 1/4/0 | ↔ | (9 ± 7%) | (9 ± 7%) | 5 | 0.1378 |
| TIMP-4 | 4/0/1 | ↑ | (76 ± 37%) | (55 ± 35%) | 5 | 0.0378 |
| C. Unoprostone Free Acid Pharmacologic Concentrations | ||||||
| MMPs/TIMP | ↑/↔ /↓ | Trend | % Change of Trend | % Change of All Samples | N | P-Value |
| MMPs | ||||||
| Pro-MMP-1 | 4/1/0 | ↑ | (45 ± 21%) | (37 ± 18%) | 5 | 0.0117 |
| Pro-MMP-2 | 0/4/1 | ↔ | (−6 ± 2%) | (−6 ± 2%) | 5 | 0.2248 |
| Pro-MMP-3 | 2/2/1 | Ind. | (15 ± 27%) | (15 ± 27%) | 5 | 0.4053 |
| Pro-MMP-9 | 4/1/0 | ↑ | (58 ± 11%) | (41 ± 19%) | 5 | 0.0092 |
| MMP-24(CL) | 1/3/1 | ↔ | (−3 ± 8%) | (−3 ± 8%) | 5 | 0.6146 |
| Active MMPs | ||||||
| Inter MMP-1 | 2/3/0 | ↔ | (7 ± 2%) | (7 ± 2%) | 5 | 0.1634 |
| Active MMP-2 | 0/5/0 | ↔ | (1 ± 2%) | (1 ± 2%) | 5 | 0.8319 |
| Active MMP-9 | 2/2/1 | Ind. | (5 ± 5%) | (5 ± 5%) | 5 | 0.3466 |
| TIMPs | ||||||
| TIMP-1 | 2/1/2 | Ind. | (18 ± 27%) | (18 ± 27%) | 5 | 0.3226 |
| TIMP-2 | 2/1/2 | Ind. | (5 ± 17%) | (5 ± 17%) | 5 | 0.6543 |
| TIMP-3 | 1/0/4 | ↓ | (−19 ± 2%) | (−6 ± 13%) | 5 | 0.4864 |
| TIMP-4 | 5/0/0 | ↑ | (70 ± 28%) | (70 ± 28%) | 5 | 0.0042 |
For MMP-24, the protein extracted from cell lysate was used. Different cell lines were used for each experiment. The data represents determination of effect (↑, ↓, ↔, ind); # of samples with densitometry greater than 10% of the respective control / # of samples within 10% of the control/ and # of samples with densitometry lower than 10% of the control; (average % increase or decrease ± std error); ind is indeterminate. The % change of all samples refers to the mean of all samples tested. The % change of trend refers to the mean of the samples that were directionally consistent with the overall results.
Figure 1.
Representative immunoblots for MMP-1 (A), MMP-2 (B), MMP-3 (C), MMP-9 (D), and MMP-24 (E) of conditioned media in human TM endothelial cells treated with free acid moieties of bimatoprost, latanoprost, and unoprostone for 24 hours. C represents control, 1x and 10x represent samples incubated with the peak aqueous concentrations and ten times the peak aqueous concentration of these drugs, respectively, for 24 hours.
TIMP-4 was increased by all three PGAs (Table 2, Fig. 2D). TIMP-1 demonstrated great variability - increased by BFA, decreased by LFA and unchanged by UFA (Table 2, Fig. 2A). TIMP-2 was increased by BFA and LFA, but not UFA (Table 2, Fig. 2B). TIMP-3 was decreased by UFA, but not BFA or LFA (Table 2, Fig. 2C).
Figure 2.
Representative immunoblots of TIMP-1 (A), TIMP-2 (B), TIMP-3 (C), and TIMP-4 (D) from conditioned media in human TM endothelial cells incubated with free acid moieties of bimatoprost, latanoprost, and unoprostone for 24 hours. C represents control, 1x and 10x represent samples incubated with the peak aqueous concentrations and ten times 10 times the peak aqueous concentration of these drugs, respectively, for 24 hours.
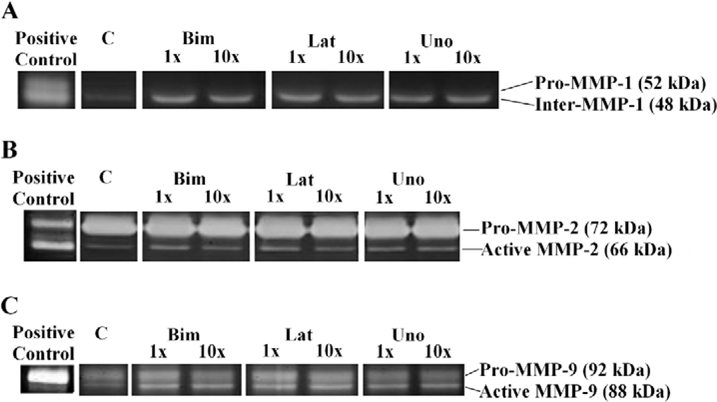
In summary, BFA and LFA increased intermediate MMP-1 activity and MMP-9 activity, but UFA did not change MMP-1 nor MMP-9 activity.
3.2. PGA effect on MMPs and TIMPS at suprapharmacologic levels
At suprapharmacologic concentrations, pro-MMP-1 increased in response to LFA and UFA, but did not change in response to BFA (Table 3 and Fig.1A). BFA, LFA, and UFA did not change pro-MMP-2 (gelatinase A) or MMP-24 levels (Table 3 and Fig. 1B). BFA and UFA increased pro-MMP-3, but LFA did not alter pro-MMP-3. Pro-MMP-9 (gelatinase B) increased in response to all three PGAs at suprapharmacologic concentrations (Table 3 and Fig. 1D). BFA and LFA both increased the activity of MMP-9 on zymography. LFA also increased the activity of MMP-1. None of the PGAs affected MMP-2 activity; furthermore, UFA did not alter the activity of any of the tested MMPs.
Table 3.
MMP and TIMP expression upon free acid PGA treatment of bimatoprost (A), latanoprost (B), and unoprostone (C) in conditioned media of human TM endothelial cells incubated at the suprapharmacologic concentrations of 0.1 μg/ml, 0.3 μg/ml, and 1.45 μg/ml respectively, for 24 hours.
| A. Bimatoprost Free Acid Suprapharmacologic Concentrations | ||||||
| MMPs/TIMP | ↑/↔ /↓ | Trend | % Change of Trend | % Change of All Samples | N | P-Value |
| MMPs | ||||||
| Pro-MMP-1 | 1/4/0 | ↔ | (12 ± 13%) | (15 ± 14%) | 5 | 0.1287 |
| Pro-MMP-2 | 0/5/0 | ↔ | (4 ± 5%) | (4 ± 5%) | 5 | 0.4468 |
| Pro-MMP-3 | 3/2/0 | ↑ | (41 ± 13%) | (34 ± 15%) | 5 | 0.0072 |
| Pro-MMP-9 | 3/2/0 | ↑ | (121 ± 38%) | (60 ± 45%) | 5 | 0.0680 |
| MMP-24(CL) | 2/2/1 | Ind. | (4 ± 8%) | (4 ± 8%) | 5 | 0.5047 |
| Active MMPs | ||||||
| Inter MMP-1 | 1/4/0 | ↔ | (3 ± 8%) | (4 ± 9%) | 5 | 0.5249 |
| Active MMP-2 | 0/5/0 | ↔ | (6 ± 8%) | (6 ± 8%) | 5 | 0.3254 |
| Active MMP-9 | 4/1/0 | ↑ | (14 ± 2%) | (15 ± 3%) | 5 | 0.0124 |
| TIMPs | ||||||
| TIMP-1 | 3/2/0 | ↑ | (60 ± 27%) | (35 ± 30%) | 5 | 0.1023 |
| TIMP-2 | 3/2/0 | ↑ | (38 ± 18%) | (25 ± 20%) | 5 | 0.0835 |
| TIMP-3 | 0/2/3 | ↓ | (−30 ± 4%) | (−3 ± 10%) | 5 | 0.6479 |
| TIMP-4 | 4/1/0 | ↑ | (90±13%) | (80 ± 17%) | 5 | 0.0001 |
| B. Latanoprost Free Acid Suprapharmacologic Concentrations | ||||||
| MMPs/TIMP | ↑/↔ /↓ | Trend | % Change of Trend | % Change of All Samples | N | P-Value |
| MMPs | ||||||
| Pro-MMP-1 | 4/1/0 | ↑ | (33 ± 12%) | (30 ± 15%) | 5 | 0.0134 |
| Pro-MMP-2 | 0/5/0 | ↔ | (3 ± 5%) | (3 ± 5%) | 5 | 0.5651 |
| Pro-MMP-3 | 2/3/0 | ↔ | (6 ± 13%) | (12 ± 13%) | 5 | 0.1826 |
| Pro-MMP-9 | 4/1/0 | ↑ | (60 ± 24%) | (37 ± 25%) | 5 | 0.0474 |
| MMP-24(CL) | 0/4/1 | ↔ | (−3 ± 4%) | (−5 ± 6%) | 5 | 0.3658 |
| Active MMPs | ||||||
| Inter MMP-1 | 4/1/0 | ↑ | (14 ± 1%) | (12 ± 2%) | 5 | 0.0301 |
| Active MMP-2 | 0/5/0 | ↔ | (5 ± 2%) | (5 ± 2%) | 5 | 0.3048 |
| Active MMP-9 | 3/2/0 | ↑ | (18 ± 4%) | (14 ± 6%) | 5 | 0.0277 |
| TIMPs | ||||||
| TIMP-1 | 0/2/3 | ↓ | (−29 ± 3%) | (−15 ± 10%) | 5 | 0.0451 |
| TIMP-2 | 3/2/0 | ↑ | (20 ± 5%) | (15 ± 7%) | 5 | 0.0251 |
| TIMP-3 | 2/1/2 | Ind. | (9 ± 7%) | (9 ± 7%) | 5 | 0.1378 |
| TIMP-4 | 4/1/0 | ↑ | (76 ± 37%) | (55 ± 35%) | 5 | 0.0378 |
| C. Unoprostone Free Acid Suprapharmacologic Concentrations | ||||||
| MMPs/TIMP | ↑/↔ /↓ | Trend | % Change of Trend | % Change of All Samples | N | P-Value |
| MMPs | ||||||
| Pro-MMP-1 | 3/2/0 | ↑ | (45 ± 26%) | (37 ± 28%) | 5 | 0.0701 |
| Pro-MMP-2 | 0/5/0 | ↔ | (−6 ± 2%) | (−6 ± 2%) | 5 | 0.2248 |
| Pro-MMP-3 | 3/2/0 | ↑ | (15 ± 27%) | (13 ± 25%) | 5 | 0.4348 |
| Pro-MMP-9 | 3/2/0 | ↑ | (86 ± 10%) | (41 ± 19%) | 5 | 0.0092 |
| MMP-24(CL) | 0/4/1 | ↔ | (−3 ± 8%) | (−5 ± 9%) | 5 | 0.4301 |
| Active MMPs | ||||||
| Inter MMP-1 | 2/3/0 | ↔ | (7 ± 2%) | (10 ± 5%) | 5 | 0.0805 |
| Active MMP-2 | 0/5/0 | ↔ | (1 ± 2%) | (1 ± 2%) | 5 | 0.8319 |
| Active MMP-9 | 0/5/0 | ↔ | (5 ± 5%) | (5 ± 5%) | 5 | 0.3466 |
| TIMPs | ||||||
| TIMP-1 | 3/2/0 | ↑ | (93 ± 32%) | (68 ± 53%) | 5 | 0.0770 |
| TIMP-2 | 3/2/0 | ↑ | (59 ± 33%) | (32 ± 37%) | 5 | 0.2087 |
| TIMP-3 | 3/0/2 | ↑ | (29 ± 14%) | (4 ± 33%) | 5 | 0.8528 |
| TIMP-4 | 3/2/0 | ↑ | (151 ± 70%) | (70 ± 58%) | 5 | 0.0928 |
For MMP-24, the protein extracted from cell lysate was used. Different cell lines were used for each experiment. The data represents determination of effect (↑, ↓, ↔, ind); # of samples with densitometry greater than 10% of the respective control / # of samples within 10% of the control/ and # of samples with densitometry lower than 10% of the control; (average % increase or decrease ± std error); ind is indeterminate. The % change of all samples refers to the mean of all samples tested. The % change of trend refers to the mean of the samples that were directionally consistent with the overall results.
At suprapharmacologic concentrations, BFA, UFA increased TIMP-1, but LFA decreased TIMP-1 (Table 3 and Fig. 2A). BFA, LFA, and UFA, increased TIMP-2. (Table 3 and Fig. 2B). TIMP-3 was unaffected by the different free acid moieties of prostaglandins. (Table 3 and Fig. 2C). TIMP-4 increased in response to BFA, LFA, and UFA. (Table 3 and Fig. 2D).
4. Discussion
Most notable in our data was that while BFA and LFA increased MMP-1 and MMP-9 activity, UFA had no effect on any MMP activity. All three PGAs lower IOP clinically; bimatoprost and latanoprost have an equivalent effect with regard to IOP lowering (Noecker et al., 2003; Parrish et al., 2003; Simmons et al., 2004). Latanoprost has been found to be more effective at lowering IOP than unoprostone in numerous studies (Aung et al., 2002; Jampel et al., 2002; Sponsel et al., 2002; Susanna et al., 2001; Susanna et al., 2004; Takahashi and Tanaka, 2004; Tsukamoto et al., 2002). Our findings may help explain why unoprostone may not be as affective in lowering IOP due to the lack of effect on TM and conventional outflow.
Our data shows that the three acid moieties of PGAs have different effects on TIMPs. However, these three acid moieties of PGAs generally increased pro-MMP levels. The differing response of TIMPs could cause a different ratio of MMPs and TIMPs leading to potential differences in enzymatic activity. We found direct evidence of this on zymography finding that BFA and LFA increased MMP-9 activity while UFA did not. In general, more MMP activity is associated with increased ECM turnover. Bradly et al. previously demonstrated that altering MMP enzymatic activity, via induction and conversely inhibition with TIMPs and small molecule inhibitors of MMPs, alters outflow through the trabecular meshwork in perfused human cadaveric organ culture (Bradley et al., 1998). The higher ratio of TIMPs to MMPs induced by UFA may explain why there is worse outflow facility through the conventional pathway as the increased TIMPs inhibit MMP activity.
MMPs are generally regulated at the level of transcription or kinetic enzymatic inhibition (Maatta et al., 2005; Oh et al., 2006a). Not surprisingly, we found a correlation between the protein levels of MMPs and TIMPs and our published mRNA levels in response to LFA. LFA increased the mRNA of MMPs-1, −3, and −24, and in these experiments, MMPs-1 and −3 were elevated (Oh et al., 2006b). MMP-24 (MT5-MMP) is membrane bound. In response to LFA, MMP-24 mRNA was very consistently elevated, but the protein levels were unchanged suggesting that the regulation of MMP-24 occurs beyond transcription. TIMPs-2, −3, and −4 mRNA levels were elevated by LFA, but only TIMPs-2 and −4 increased at the protein level. Although we were not previously able to detect the mRNA of MMP-9, we were able to detect protein levels and measure enzymatic activity consistent with other groups’ findings (Butler et al., 1997; Matsubara et al., 1991; Nagase and Woessner, 1999; Oh et al., 2006a; Parrish et al., 2003; Sponsel et al., 2002; Weinreb and Lindsey, 2002).
In the ciliary body smooth muscle cells, latanoprost increased MMPs-1.−2, and −9, as well as TIMP-3 (Oh et al., 2006a). In TM cells, we found that latanoprost increased the same MMPs, but the increase of TIMPs-1 and −4 could be the cause of the observed reduction of MMP activity and account for the greater movement of fluid through the uveoscleral pathway (Lim et al., 2008).
The increase in pro-MMPs-1, −3, −9 and active MMP-9 suggests the role of these MMPs in prostaglandin analogue-mediated ECM turnover (Oh et al., 2006a). Studies have also reported that latanoprost increases MMP-3 and −9 at transcriptional levels, protein levels and/or enzymatic activity (el-Shabrawi et al., 2000; Lindsey et al., 1996; Weinreb et al., 1997; Weinreb and Lindsey, 2002). MMP-9 is found in ciliary body (el-Shabrawi et al., 2000)and is involved in basement membrane degradation (Parrish et al., 2003). Although mRNA levels and enzymatic activity of MMP-2 have been reported to increase from latanoprost treatment by others, (Weinreb et al., 1997; Weinreb and Lindsey, 2002)MMP-2 was found to be unaffected by latanoprost or other prostaglandin analogues at transcription(Oh et al., 2006a), protein level, or enzymatic activity. The role of MMP-2 in latanoprost-mediated ECM equilibrium is unclear.
Patients and human tissue are known to have variability in response to PGAs, both in clinical settings in regards to lowering IOP, and in vitro in regards to effecting MMPs. For patients, the response rate for IOP lowering to latanoprost is about 85%, but this response is variable (Parrish et al., 2003). For example, Hedman and Camras43 found that approximately 75% of patients had great than 20 percent IOP reduction but approximately 30% had greater than 30 percent lowering of IOP. Bimatoprost also demonstrates variability of response among individuals (Matsubara et al., 1991). Similarly, our laboratory and others have found variability between cell lines of different donors in response to these prostaglandins (Camras et al., 2004; Cantor et al., 2006; Oh et al., 2006a; Weinreb et al., 1997).
In most tissues, the balance between MMPs and TIMPs determines the rate of ECM turnover (Butler et al., 1997; Steinmetz et al., 2005). Therefore, the decrease in MMP-2 and increases in TIMP-1 and −4 by unoprostone compared to latanoprost and bimatoprost in CBSM cells combined with the absence of MMP activity in TM cells may explain the lower clinical efficacy of unoprostone in lowering IOP compared to latanoprost and bimatoprost (Aung et al., 2002; Aung et al., 2001; Choplin et al., 2004; Dirks et al., 2006; Enoki et al., 2006; Jampel et al., 2002; Kobayashi et al., 2001; Noecker et al., 2003; Parrish et al., 2003; Sponsel et al., 2002; Susanna et al., 2001; Tsukamoto et al., 2002).
Although there is disagreement on the relative binding affinity strength between bimatoprost and latanoprost, studies show lower binding affinity of unoprostone to the FP receptor (Enoki et al., 2006; Sharif et al., 2002, 2003b; Sharif et al., 2003c; Steinmetz et al., 2005). A possible explanation for this is that free acid moieties of latanoprost and bimatoprost are full agonist at the FP-receptor while unoprostone free acid is a partial agonist at the FP-receptor. (Sharif et al., 2002) Perhaps it is this lower binding affinity that explains our results and helps explain why unoprostone isopropyl ester weakly lowers IOP. Our findings may benefit future drug development, as potential PGAs may be screened inexpensively by testing their effects on MMPs and TIMPs.
Figure 3.
Representative zymography for pro- and active forms of MMPs-1, −2, and −9 in human TM endothelial cells incubated with free acid moieties bimatoprost, latanoprost, and unoprostone for 24 hours. Active form of MMP-3 was not detected. C represents control, 1x and 10x represent samples incubated at the peak aqueous concentrations and ten times the peak aqueous concentration of these drugs, respectively, for 24 hours.
Highlights.
All three PGAs: Bimatoprost, latanoprost, and Unoprostone generally increased MMPs-1,9 and TIMPs-4.
Bimatoprost and latanoprost both increased MMP-3 and TIMP-2, while unoprostone had an indeterminate effect on both.
All three PGAs increased intermediate MMP-1 activity. Bimatoprost and latanoprost increased MMP-9 activity.
The balance between MMPs and TIMPs leading to zymographic activity differences correlates to the relative intraocular pressure lowering effectiveness observed in comparative clinical studies of these PGAs.
The worse IOP lowering effect of unoprostone may be due to its inability to affect MMP activity in TM.
Acknowledgments
Declaration of interest for Corresponding author:
Research Funding (to institution): Allergan, Glaukos, Ivantis
Consultant: Aerie, Alcon, Allergan, Bausch and Lomb, Ivantis, pH-Pharma
Speakers Bureau: Aerie, Bausch and Lomb, Ivantis
Scientific Advisory Board: Ocular Therapeutix
Funding: This work was supported by the National Institutes of Health Grant: NIH R01 EY 019654-02 (DJR), NIH P30 EY011373 (core), unrestricted funds from Research to Prevent Blindness (New York, NY) and Allergan Pharmaceuticals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References
- Ahmed Z, Dent RG, Leadbeater WE, Smith C, Berry M, Logan A, 2005. Matrix metalloproteases: degradation of the inhibitory environment of the transected optic nerve and the scar by regenerating axons. Mol Cell Neurosci 28, 64–78. [DOI] [PubMed] [Google Scholar]
- Anthony TL, Lindsey JD, Weinreb RN, 2002. Latanoprost’s effects on TIMP-1 and TIMP-2 expression in human ciliary muscle cells. Invest Ophthalmol Vis Sci 43, 3705–3711. [PubMed] [Google Scholar]
- Aung T, Chew PT, Oen FT, Chan YH, Thean LH, Yip L, Lim BA, Soh J, Seah SK, 2002. Additive effect of unoprostone and latanoprost in patients with elevated intraocular pressure. Br J Ophthalmol 86, 75–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung T, Chew PT, Yip CC, Chan YH, See JL, Khng CG, Hoh ST, Ng LH, Lee HM, 2001. A randomized double-masked crossover study comparing latanoprost 0.005% with unoprostone 0.12% in patients with primary open-angle glaucoma and ocular hypertension. Am J Ophthalmol 131, 636–642. [DOI] [PubMed] [Google Scholar]
- Bill A, 1967. Effects of atropine and pilocarpine on aqueous humour dynamics in cynomolgus monkeys (Macaca irus). Exp Eye Res 6, 120–125. [DOI] [PubMed] [Google Scholar]
- Bradley JM, Vranka J, Colvis CM, Conger DM, Alexander JP, Fisk AS, Samples JR, Acott TS, 1998. Effect of matrix metalloproteinases activity on outflow in perfused human organ culture. Invest Ophthalmol Vis Sci 39, 2649–2658. [PubMed] [Google Scholar]
- Brubaker RF, Schoff EO, Nau CB, Carpenter SP, Chen K, Vandenburgh AM, 2001. Effects of AGN 192024, a new ocular hypotensive agent, on aqueous dynamics. Am J Ophthalmol 131, 19–24. [DOI] [PubMed] [Google Scholar]
- Butler GS, Butler MJ, Atkinson SJ, Will H, Tamura T, Schade van Westrum S, Crabbe T, Clements J, d’Ortho MP, Murphy G, 1998. The TIMP2 membrane type 1 metalloproteinase “receptor” regulates the concentration and efficient activation of progelatinase A. A kinetic study. J Biol Chem 273, 871–880. [DOI] [PubMed] [Google Scholar]
- Butler GS, Will H, Atkinson SJ, Murphy G, 1997. Membrane-type-2 matrix metalloproteinase can initiate the processing of progelatinase A and is regulated by the tissue inhibitors of metalloproteinases. Eur J Biochem 244, 653–657. [DOI] [PubMed] [Google Scholar]
- Camras CB, Toris CB, Sjoquist B, Milleson M, Thorngren JO, Hejkal TW, Patel N, Barnett EM, Smolyak R, Hasan SF, Hellman C, Meza JL, Wax MB, Stjernschantz J, 2004. Detection of the free acid of bimatoprost in aqueous humor samples from human eyes treated with bimatoprost before cataract surgery. Ophthalmology 111, 2193–2198. [DOI] [PubMed] [Google Scholar]
- Cantor LB, Hoop J, Morgan L, Wudunn D, Catoira Y, Bimatoprost-Travoprost Study G, 2006. Intraocular pressure-lowering efficacy of bimatoprost 0.03% and travoprost 0.004% in patients with glaucoma or ocular hypertension. Br J Ophthalmol 90, 1370–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choplin N, Bernstein P, Batoosingh AL, Whitcup SM, Bimatoprost/Latanoprost Study, G., 2004. A randomized, investigator-masked comparison of diurnal responder rates with bimatoprost and latanoprost in the lowering of intraocular pressure. Surv Ophthalmol 49 Suppl 1, S19–25. [DOI] [PubMed] [Google Scholar]
- Curran S, Dundas SR, Buxton J, Leeman MF, Ramsay R, Murray GI, 2004. Matrix metalloproteinase/tissue inhibitors of matrix metalloproteinase phenotype identifies poor prognosis colorectal cancers. Clin Cancer Res 10, 8229–8234. [DOI] [PubMed] [Google Scholar]
- Dirks MS, Noecker RJ, Earl M, Roh S, Silverstein SM, Williams RD, 2006. A 3-month clinical trial comparing the IOP-lowering efficacy of bimatoprost and latanoprost in patients with normal-tension glaucoma. Adv Ther 23, 385–394. [DOI] [PubMed] [Google Scholar]
- el-Shabrawi Y, Eckhardt M, Berghold A, Faulborn J, Auboeck L, Mangge H, Ardjomand N, 2000. Synthesis pattern of matrix metalloproteinases (MMPs) and inhibitors (TIMPs) in human explant organ cultures after treatment with latanoprost and dexamethasone. Eye (Lond) 14 ( Pt 3A), 375–383. [DOI] [PubMed] [Google Scholar]
- Enoki M, Saito J, Hara M, Uchida T, Sagara T, Nishida T, 2006. Additional reduction in intraocular pressure achieved with latanoprost in normal-tension glaucoma patients previously treated with unoprostone. Jpn J Ophthalmol 50, 334–337. [DOI] [PubMed] [Google Scholar]
- Jampel HD, Bacharach J, Sheu WP, Wohl LG, Solish AM, Christie W, Latanoprost/Unoprostone Study, G., 2002. Randomized clinical trial of latanoprost and unoprostone in patients with elevated intraocular pressure. Am J Ophthalmol 134, 863–871. [DOI] [PubMed] [Google Scholar]
- Keller KE, Bhattacharya SK, Borras T, Brunner TM, Chansangpetch S, Clark AF, Dismuke WM, Du Y, Elliott MH, Ethier CR, Faralli JA, Freddo TF, Fuchshofer R, Giovingo M, Gong H, Gonzalez P, Huang A, Johnstone MA, Kaufman PL, Kelley MJ, Knepper PA, Kopczynski CC, Kuchtey JG, Kuchtey RW, Kuehn MH, Lieberman RL, Lin SC, Liton P, Liu Y, Lutjen-Drecoll E, Mao W, Masis-Solano M, McDonnell F, McDowell CM, Overby DR, Pattabiraman PP, Raghunathan VK, Rao PV, Rhee DJ, Chowdhury UR, Russell P, Samples JR, Schwartz D, Stubbs EB, Tamm ER, Tan JC, Toris CB, Torrejon KY, Vranka JA, Wirtz MK, Yorio T, Zhang J, Zode GS, Fautsch MP, Peters DM, Acott TS, Stamer WD, 2018. Consensus recommendations for trabecular meshwork cell isolation, characterization and culture. Exp Eye Res 171, 164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasigov PZ, Podobed OV, Gracheva TS, Salbiev KD, Grachev SV, Berezov TT, 2003. Role of matrix metalloproteinases and their inhibitors in tumor invasion and metastasis. Biochemistry (Mosc) 68, 711–717. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Kobayashi K, Okinami S, 2001. A comparison of intraocular pressure-lowering effect of prostaglandin F2 -alpha analogues, latanoprost, and unoprostone isopropyl. J Glaucoma 10, 487–492. [DOI] [PubMed] [Google Scholar]
- Lim KS, Nau CB, O’Byrne MM, Hodge DO, Toris CB, McLaren JW, Johnson DH, 2008. Mechanism of action of bimatoprost, latanoprost, and travoprost in healthy subjects. A crossover study. Ophthalmology 115, 790–795 e794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey JD, Kashiwagi K, Boyle D, Kashiwagi F, Firestein GS, Weinreb RN, 1996. Prostaglandins increase proMMP-1 and proMMP-3 secretion by human ciliary smooth muscle cells. Curr Eye Res 15, 869–875. [DOI] [PubMed] [Google Scholar]
- Lutjen-Drecoll E, Tamm E, 1988. Morphological study of the anterior segment of cynomolgus monkey eyes following treatment with prostaglandin F2 alpha. Exp Eye Res 47, 761–769. [DOI] [PubMed] [Google Scholar]
- Lutjen-Drecoll E, Tamm E, 1989. The effects of ocular hypotensive doses of PGF2 alpha-isopropylester on anterior segment morphology. Prog Clin Biol Res 312, 437–446. [PubMed] [Google Scholar]
- Maatta M, Tervahartiala T, Harju M, Airaksinen J, Autio-Harmainen H, Sorsa T, 2005. Matrix metalloproteinases and their tissue inhibitors in aqueous humor of patients with primary open-angle glaucoma, exfoliation syndrome, and exfoliation glaucoma. J Glaucoma 14, 64–69. [DOI] [PubMed] [Google Scholar]
- Matsubara M, Zieske JD, Fini ME, 1991. Mechanism of basement membrane dissolution preceding corneal ulceration. Invest Ophthalmol Vis Sci 32, 3221–3237. [PubMed] [Google Scholar]
- Nagase H, Woessner JF Jr., 1999. Matrix metalloproteinases. J Biol Chem 274, 21491–21494. [DOI] [PubMed] [Google Scholar]
- Noecker RS, Dirks MS, Choplin NT, Bernstein P, Batoosingh AL, Whitcup SM, Bimatoprost/Latanoprost Study, G., 2003. A six-month randomized clinical trial comparing the intraocular pressure-lowering efficacy of bimatoprost and latanoprost in patients with ocular hypertension or glaucoma. Am J Ophthalmol 135, 55–63. [DOI] [PubMed] [Google Scholar]
- Numaga J, Koseki N, Kaburaki T, Kawashima H, Tomita G, Araie M, 2005. Intraocular metabolites of isopropyl unoprostone. Curr Eye Res 30, 909–913. [DOI] [PubMed] [Google Scholar]
- Ocklind A, 1998. Effect of latanoprost on the extracellular matrix of the ciliary muscle. A study on cultured cells and tissue sections. Exp Eye Res 67, 179–191. [DOI] [PubMed] [Google Scholar]
- Oh DJ, Martin JL, Williams AJ, Peck RE, Pokorny C, Russell P, Birk DE, Rhee DJ, 2006a. Analysis of expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human ciliary body after latanoprost. Invest Ophthalmol Vis Sci 47, 953–963. [DOI] [PubMed] [Google Scholar]
- Oh DJ, Martin JL, Williams AJ, Russell P, Birk DE, Rhee DJ, 2006b. Effect of latanoprost on the expression of matrix metalloproteinases and their tissue inhibitors in human trabecular meshwork cells. Invest Ophthalmol Vis Sci 47, 3887–3895. [DOI] [PubMed] [Google Scholar]
- Ota T, Aihara M, Narumiya S, Araie M, 2005. The effects of prostaglandin analogues on IOP in prostanoid FP-receptor-deficient mice. Invest Ophthalmol Vis Sci 46, 4159–4163. [DOI] [PubMed] [Google Scholar]
- Parrish RK, Palmberg P, Sheu WP, Group XLTS, 2003. A comparison of latanoprost, bimatoprost, and travoprost in patients with elevated intraocular pressure: a 12-week, randomized, masked-evaluator multicenter study. Am J Ophthalmol 135, 688–703. [DOI] [PubMed] [Google Scholar]
- Pauschinger M, Chandrasekharan K, Schultheiss HP, 2004. Myocardial remodeling in viral heart disease: possible interactions between inflammatory mediators and MMP-TIMP system. Heart Fail Rev 9, 21–31. [DOI] [PubMed] [Google Scholar]
- Schenker HI, Yablonski ME, Podos SM, Linder L, 1981. Fluorophotometric study of epinephrine and timolol in human subjects. Arch Ophthalmol 99, 1212–1216. [DOI] [PubMed] [Google Scholar]
- Sharif NA, Crider JY, Husain S, Kaddour-Djebbar I, Ansari HR, Abdel-Latif AA, 2003a. Human ciliary muscle cell responses to FP-class prostaglandin analogs: phosphoinositide hydrolysis, intracellular Ca2+ mobilization and MAP kinase activation. J Ocul Pharmacol Ther 19, 437–455. [DOI] [PubMed] [Google Scholar]
- Sharif NA, Kelly CR, Crider JY, 2002. Agonist activity of bimatoprost, travoprost, latanoprost, unoprostone isopropyl ester and other prostaglandin analogs at the cloned human ciliary body FP prostaglandin receptor. J Ocul Pharmacol Ther 18, 313–324. [DOI] [PubMed] [Google Scholar]
- Sharif NA, Kelly CR, Crider JY, 2003b. Human trabecular meshwork cell responses induced by bimatoprost, travoprost, unoprostone, and other FP prostaglandin receptor agonist analogues. Invest Ophthalmol Vis Sci 44, 715–721. [DOI] [PubMed] [Google Scholar]
- Sharif NA, Kelly CR, Crider JY, Williams GW, Xu SX, 2003c. Ocular hypotensive FP prostaglandin (PG) analogs: PG receptor subtype binding affinities and selectivities, and agonist potencies at FP and other PG receptors in cultured cells. J Ocul Pharmacol Ther 19, 501–515. [DOI] [PubMed] [Google Scholar]
- Simmons ST, Dirks MS, Noecker RJ, 2004. Bimatoprost versus latanoprost in lowering intraocular pressure in glaucoma and ocular hypertension: results from parallelgroup comparison trials. Adv Ther 21, 247–262. [DOI] [PubMed] [Google Scholar]
- Sjoquist B, Stjernschantz J, 2002. Ocular and systemic pharmacokinetics of latanoprost in humans. Surv Ophthalmol 47 Suppl 1, S6–12. [DOI] [PubMed] [Google Scholar]
- Sponsel WE, Paris G, Trigo Y, Pena M, 2002. Comparative effects of latanoprost (Xalatan) and unoprostone (Rescula) in patients with open-angle glaucoma and suspected glaucoma. Am J Ophthalmol 134, 552–559. [DOI] [PubMed] [Google Scholar]
- Steinmetz EF, Buckley C, Shames ML, Ennis TL, Vanvickle-Chavez SJ, Mao D, Goeddel LA, Hawkins CJ, Thompson RW, 2005. Treatment with simvastatin suppresses the development of experimental abdominal aortic aneurysms in normal and hypercholesterolemic mice. Ann Surg 241, 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susanna R Jr., Giampani J Jr., Borges AS, Vessani RM, Jordao ML, 2001. A double-masked, randomized clinical trial comparing latanoprost with unoprostone in patients with open-angle glaucoma or ocular hypertension. Ophthalmology 108, 259–263. [DOI] [PubMed] [Google Scholar]
- Susanna R Jr., Medeiros FA, Vessani RM, Giampani J Jr., Borges AS, Jordao ML, 2004. Intraocular pressure fluctuations in response to the water-drinking provocative test in patients using latanoprost versus unoprostone. J Ocul Pharmacol Ther 20, 401–410. [DOI] [PubMed] [Google Scholar]
- Takahashi I, Tanaka M, 2004. Switching to latanoprost monotherapy for 24 weeks in glaucoma patients. Eur J Ophthalmol 14, 401–406. [DOI] [PubMed] [Google Scholar]
- Townsend DJ, Brubaker RF, 1980. Immediate effect of epinephrine on aqueous formation in the normal human eye as measured by fluorophotometry. Invest Ophthalmol Vis Sci 19, 256–266. [PubMed] [Google Scholar]
- Tsukamoto H, Mishima HK, Kitazawa Y, Araie M, Abe H, Negi A, Glaucoma Study G, 2002. A comparative clinical study of latanoprost and isopropyl unoprostone in Japanese patients with primary open-angle glaucoma and ocular hypertension. J Glaucoma 11, 497–501. [DOI] [PubMed] [Google Scholar]
- Weinreb RN, Kashiwagi K, Kashiwagi F, Tsukahara S, Lindsey JD, 1997. Prostaglandins increase matrix metalloproteinase release from human ciliary smooth muscle cells. Invest Ophthalmol Vis Sci 38, 2772–2780. [PubMed] [Google Scholar]
- Weinreb RN, Lindsey JD, 2002. Metalloproteinase gene transcription in human ciliary muscle cells with latanoprost. Invest Ophthalmol Vis Sci 43, 716–722. [PubMed] [Google Scholar]
- Zhou X, Hovell CJ, Pawley S, Hutchings MI, Arthur MJ, Iredale JP, Benyon RC, 2004. Expression of matrix metalloproteinase-2 and −14 persists during early resolution of experimental liver fibrosis and might contribute to fibrolysis. Liver Int 24, 492–501. [DOI] [PubMed] [Google Scholar]