Abstract
Pulmonary complications after hematopoietic cell transplantation (HCT) can lead to significant morbidity and mortality. Limited evaluation of the true incidence of these complications in children and subsequent outcomes of these complications has not been recently evaluated. In April of 2018, the National Heart, Lung, and Blood Institute (NHLBI), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Cancer Institute (NCI) co-sponsored a meeting of experts to describe the status of pulmonary complications in children after HCT, to identify critical gaps in knowledge, to explore avenues for research, advance care and optimize outcomes. The Center for International Blood and Marrow Transplant Research (CIBMTR) was used to evaluate the cumulative incidence of pulmonary complications in children and their respective survival. 5,022 children received allogeneic HCT from 2010-2016 were included in this analysis and 606 developed pulmonary complications within the first year after HCT. Pneumonitis occurred in 388 patients, 125 patients developed pulmonary hemorrhage, and 200 patients developed lung graft-versus-host disease (GVHD). For those developing pulmonary complications within one year, overall survival 100 days after diagnosis of pulmonary complications was 49% (95% CI 43-54%) for patients with pneumonitis, 23% (95% CI 16-31%) in patients with pulmonary hemorrhage, and 87% (95% CI 81-91%) in patients with pulmonary GVHD. This study demonstrates the approximate incidence of these complications, their significant effects on survival and can serve as baseline for future research.
Keywords: Pneumonitis, Lung GVHD, Pulmonary hemorrhage, Pediatrics
Introduction
Hematopoietic cell transplantation (HCT) is a potentially curative therapy for pediatric patients with high risk malignancies and non-malignant diseases with approximately 1,600 children receiving HCT in the U.S. each year1. Outcomes for pediatric patients have continued to improve in recent years with 5-year overall survival (OS) of 40-64%2, 3 for children with hematologic malignancies and 66-97% for children with non-malignant diseases4-8. However, the effectiveness of this therapy is often limited by toxicities leading to morbidity and mortality. Pulmonary complications are a significant cause of post-HCT complications and include infectious as well as non-infectious etiologies such as idiopathic pneumonia syndrome (IPS), diffuse alveolar hemorrhage (DAH), and chronic graft versus host disease (GVHD) / bronchiolitis obliterans syndrome (BOS).
Historically, 40-60% of adult patients experience pulmonary complications after HCT and 30% of post-HCT deaths are attributed to pulmonary causes9, 10. There are few data on the incidence and outcomes of pulmonary complications in pediatric patients, however. A single center study from the 1990s described pulmonary complications in 25% of pediatric patients undergoing HCT and this translated into a significantly increased risk of death11. Introduction of reduced intensity regimens, improved supportive care, and targeted treatments for pulmonary complications have likely altered these statistics. However, the incidence and outcomes of pulmonary complications in pediatric patients have not been recently evaluated.
In April 2018, pediatric pulmonologists, intensivists, hematopoietic cell transplant physicians, and research scientists participated in a workshop sponsored by NHLBI, HICHD, and NCI on pulmonary complications in children after allogeneic HCT. The goals of this workshop were to identify critical gaps in existing knowledge of pulmonary complications in children after allogeneic HCT, to explore avenues for research to address these knowledge gaps to advance care and optimize outcomes. The lack of a multicenter description of the extent and impact of pulmonary complications in pediatric patients has limited the research efforts to further improve outcomes for these patients. Here, we present data from the Center for International Blood and Marrow Transplant Research (CIBMTR) database to help describe the incidence and outcomes of pediatric patients who develop pulmonary complications after allogeneic HCT. This knowledge will help guide future research into prevention and implementation of novel treatment strategies for these complications.
Materials and Methods
The CIBMTR is a research affiliation of the Medical College of Wisconsin and the National Marrow Donor Program/Be the Match and collects longitudinal outcome data on HCTs from more than 450 centers worldwide. In the US, a federal mandate requires all allogeneic HCTs to be reported to the Stem Cell Transplant Outcomes Database which is managed by the CIBMTR. Data quality procedures are implemented at all phases of data collection, processing and analysis. Transplant centers are encouraged to keep the data reporting up to date and onsite data audits assists in minimizing errors. International allogeneic HCTs are reported on a voluntary basis but data are also subject to audit. Observational studies are performed using the CIBMTR database, in compliance with all applicable federal regulations pertaining to the protection of human research participants. Protected health information is collected and maintained in the CIBMTR’s capacity as a Public Health Authority under the Health Insurance Portability and Accountability Act Privacy Rule.
All patients reported to the CIBMTR have transplant essential data (TED) forms completed that describe the indication for transplant and patient and transplant characteristics. In 2007, pre-transplant comorbidities, as defined by the Hematopoietic Cell Transplantation Comorbidity Index (HCT-CI), were added to the TED forms; this included reporting on pulmonary function with pulmonary dysfunction classified as moderate (DLCO or FEV1 66-80% of normal) or severe (DLCO or FEV1 ≤65% of normal, or requiring oxygen supplementation)12. If patients did not fit these categories, they were considered to have normal pulmonary function. The assessment of pulmonary function was made by the reporting institution based on their evaluation of pulmonary symptoms and function.
A weighted algorithm is used to select approximately 25% of patients for additional data reporting, collected on comprehensive report forms (CRF). CRFs supply more granular data on post-transplant complications including data on whether a patient experienced non-infectious pneumonitis, bronchiolitis obliterans, cryptogenic organizing pneumonia, diffuse alveolar hemorrhage, or chronic graft versus host disease (GVHD) and, if present, the date of onset. The diagnosis of these pulmonary complications was based on the report from the individual centers. Follow-up data are collected at 100 days, 6 months, and yearly after HCT.
Patients were included in this study if they underwent first allogeneic HCT between 2010-2016 for any indication, were under the age of 21 years (y) at time of HCT, and had CRF data reported to the CIBMTR. Patients who received a transplant from a syngeneic donor were excluded.
Statistical Analysis
Patient demographics, disease indications, HCT characteristics, and comorbidities were described using frequencies and percentages for discrete variables and median (range) for continuous variables. Cumulative incidences of pulmonary complications occurring in the first year after HCT were calculated, including pneumonitis (infection, idiopathic, or not otherwise specified), pulmonary hemorrhage, and lung GVHD (cryptogenic organizing pneumonia, bronchiolitis obliterans, both, or other). Death without a pulmonary event was considered a competing risk. Patients were censored at the time of a subsequent HCT. Survival rates were calculated using Kaplan-Meier estimates. For landmark analysis, time started at 12 months post transplant and history of pulmonary toxicity determined which group patients were assigned. Patients were followed until death or time of last follow-up. The Chi-squared test was used to compare pre-transplant pulmonary disease with post-transplant pulmonary outcomes. Analysis of cause of death after transplant was descriptive. All p-values are two-sided with a significance level defined as p<0.05. SAS 9.4 (SAS Inc., Cary, NC) was used for all analyses.
Results
Patient Characteristics
5,022 children who received allogeneic HCT from 2010-2016 were included in the study. The median age at HCT was 8y (<1-21y) and 51% underwent HCT for non-malignant diseases. Patient and transplant characteristics are noted in Table 1. Moderate pulmonary disease was present in 6% of children and severe pulmonary disease in 4% (Table 1).
Table 1.
Patient and Transplant Characteristics
| Patient characteristics | N (%) |
|---|---|
| Number of patients | 5022 |
| Number of centers | 183 |
| Age, median (range), years | 8 (<1-21) |
| Age group (years, y) | |
| <1 | 623 (12) |
| 1-<2 | 382 (8) |
| 2-<5 | 816 (16) |
| 5-<10 | 1183 (24) |
| 10-<21 | 2018 (40) |
| Indication for transplant | |
| Malignancies | |
| AML/MDS | 1248 (25) |
| ALL | 949 (19) |
| Other leukemia | 128 (3) |
| Lymphomas | 117 (2) |
| Other malignancies | 10 (<1) |
| Non-malignant diseases | |
| Aplastic Anemia | 449 (9) |
| Erythrocyte disorders | 869 (17) |
| SCID & other immune deficiencies | 752 (15) |
| Metabolic diseases | 295 (6) |
| Histiocytic disorders | 171 (3) |
| Other non-malignantα | 34 (<1) |
| Conditioning intensity | |
| MAC | 3552 (71) |
| RIC | 579 (11) |
| NMA | 566 (11) |
| No conditioning (non-malignant only) | 209 (4) |
| Missing | 118 (2) |
| TBI used in conditioning | |
| Yes | 1662 (33) |
| No | 3246 (65) |
| Missing | 114 (2) |
| Donor type | |
| HLA-identical sibling | 973 (19) |
| Other related | 662 (13) |
| Well-matched unrelated | 860 (17) |
| Partially-matched unrelated (partial and mis-matched) | 353 (7) |
| Unrelated match unknown | 83 (2) |
| Cord blood | 2089 (42) |
| Missing | 2 (<1) |
| Graft type | |
| Bone marrow | 2070 (41) |
| Peripheral blood | 863 (17) |
| Umbilical cord blood | 2089 (42) |
| Donor/recipient CMV serostatus | |
| +/+ | 1171 (23) |
| +/− | 384 (8) |
| −/+ | 655 (13) |
| −/− | 614 (12) |
| CB - recipient + | 1128 (22) |
| CB - recipient − | 909 (18) |
| CB - recipient CMV unknown | 52 (1) |
| Missing | 109 (2) |
| Pulmonary comorbidity | |
| No pulmonary disease | 4436 (88) |
| Moderate pulmonary disease | 308 (6) |
| Patient characteristics | N (%) |
| Severe pulmonary disease | 217 (4) |
| Missing | 61 (1) |
| Median follow-up of survivors (range), months | 31 (1-96) |
Other non-malignant: Platelet disorder: n=24, autoimmune disorder: n=10
606 children reported pulmonary complications after HCT of which 84% had no prior history of pulmonary disease, 8% had moderate pulmonary disease, 6% had severe pulmonary disease, and 1% did not report the pre-HCT pulmonary disease status (Table 2). 509 patients had a single pulmonary event, whereas 97 patients had 2 or more pulmonary events.
Table 2.
Pulmonary history and post-HCT pulmonary event comparison
| Characteristic | No pulmonary event |
Pulmonary event |
Not reported p-value |
|---|---|---|---|
| Pre-HCT Pulmonary comorbidity | 0.0006 | ||
| No pulmonary disease | 3922 (89) | 507 (84) | 7 (88) |
| Moderate pulmonary disease | 256 (6) | 51 (8) | 1 (13) |
| Severe pulmonary disease | 178 (4) | 39 (6) | 0 |
| Not reported | 52 (1) | 9 (1) | 0 |
Pneumonitis
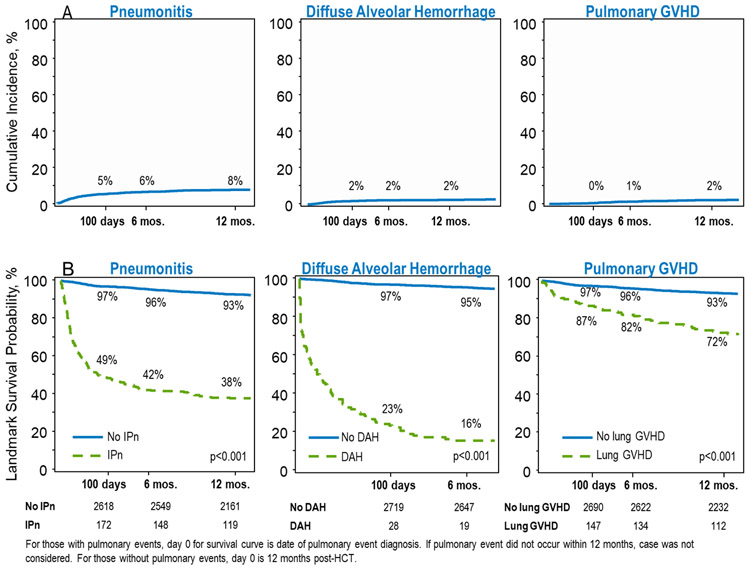
Pneumonitis occurred in 388 patients with an incidence of 8% 1yr post-HCT (Figure 1A). The median time to onset was 1.6 months (range 0-29 months). In these 388 patients, pneumonitis was described as idiopathic in 55%, infectious in 38%, and not otherwise specified in 7%. Survival after pneumonitis was poor with OS of 49% (95% CI 43-54%) at 100 days after diagnosis of pneumonitis and 38% (95% CI 33-43%) at 1y after diagnosis (Figure 1B). Pneumonitis was more frequent in patients transplanted for malignant diseases (p<0.001), in patients who received myeloablative conditioning (p<0.001), and in patients who received cord blood grafts (p<0.001), Table 3a. Pneumonitis was more common in infants <1y (p=0.04, Table 3b).
Figure 1. Post-HCT Pulmonary Complications.
A) Cumulative Incidence of pneumonitis, diffuse alveolar hemorrhage, and lung GVHD diagnosed by 1 year post-HCT. By 1 year after HCT, the incidence of pneumonitis was 8%, diffuse alveolar hemorrhage was 2%, and lung GVHD was 2%. B) 12-month landmark survival analysis at 100 days, 6 months, and 1 year.
Table 3a.
Pulmonary toxicity incidences
| N eval | Prob (95% CI) | N eval | Prob (95% CI) | p-value | |||
|---|---|---|---|---|---|---|---|
| Disease type | Malignant (N = 2452) | Non-malignant (N = 2570) | |||||
| Pneumonitis | 2375 | 2508 | <0.001 | ||||
| 100-day | 6 (5-7)% | 5 (4-5)% | |||||
| 6 months | 8 (7-9)% | 5 (5-6)% | |||||
| 1-year | 9 (8-10)% | 6 (5-7)% | |||||
| Diffuse alveolar hemorrhage | 2377 | 2509 | 0.39 | ||||
| 100-day | 2 (1-2)% | 2 (1-2)% | |||||
| 6 months | 2 (2-3)% | 2 (1-2)% | |||||
| 1-year | 3 (2-3)% | 2 (2-3)% | |||||
| Pulmonary GVHD | 2394 | 2541 | <0.001 | ||||
| 100-day | 1 (0-1)% | 0 (0-0)% | |||||
| 6 months | 2 (2-3)% | 1 (0-1)% | |||||
| 1-year | 3 (3-4)% | 1 (1-2)% | |||||
| Conditioning intensity | MAC (N = 3554) | RIC/NMA (N = 1143) | |||||
| Pneumonitis | 3533 | 1136 | <0.001 | ||||
| 100-day | 6 (5-7)% | 3 (2-5)% | |||||
| 6 months | 7 (7-8)% | 4 (3-5)% | |||||
| 1-year | 9 (8-10)% | 5 (3-6)% | |||||
| Diffuse alveolar hemorrhage | 3535 | 1137 | 0.14 | ||||
| 100-day | 2 (2-3)% | 1 (1-2)% | |||||
| 6 months | 2 (2-3)% | 1 (1-2)% | |||||
| 1-year | 3 (2-3)% | 2 (1-3)% | |||||
| Pulmonary GVHD | 3493 | 1123 | 0.002 | ||||
| 100-day | 1 (0-1)% | 0 (0-1)% | |||||
| 6 months | 2 (1-2)% | 1 (0-1)% | |||||
| 1-year | 3 (2-3)% | 1 (1-2)% | |||||
| Graft source | BM/PBSC graft (N = 2933) | Cord blood graft (N = 2089) | |||||
| Pneumonitis | 2885 | 1998 | <0.001 | ||||
| 100-day | 4 (3-5)% | 7 (6-8)% | |||||
| 6 months | 5 (4-6)% | 9 (7-10)% | |||||
| 1-year | 6 (5-7)% | 10 (9-11)% | |||||
| Diffuse alveolar hemorrhage | 2887 | 1999 | <0.001 | ||||
| 100-day | 1 (1-1)% | 3 (2-4)% | |||||
| 6 months | 1 (1-2)% | 3 (3-4)% | |||||
| 1-year | 1 (1-2)% | 4 (3-5)% | |||||
| Pulmonary GVHD | 2869 | 2063 | 0.48 | ||||
| 100-day | 0 (0-0)% | 1 (0-1)% | |||||
| 6 months | 1 (1-2)% | 2 (1-2)% | |||||
| 1-year | 2 (2-3)% | 2 (2-3)% | |||||
| Age, years | <1 (N = 623) | 1 - <10 (N = 2381) | 10 - <21 (N = 2018) | ||||
| Pneumonitis | 612 | 2307 | 1964 | 0.02 | |||
| 100-day | 8 (6-10)% | 4 (4-5)% | 6 (5-7)% | ||||
| 6 months | 8 (6-10)% | 6 (5-7)% | 7 (6-8)% | ||||
| 1-year | 9 (7-12)% | 7 (6-8)% | 8 (7-9)% | ||||
| Diffuse alveolar hemorrhage | 613 | 2309 | 1964 | <0.001 | |||
| 100-day | 4 (3-6)% | 1 (1-2)% | 2 (1-2)% | ||||
| 6 months | 4 (3-6)% | 2 (1-2)% | 2 (1-3)% | ||||
| 1-year | 5 (3-7)% | 2 (1-2)% | 2 (2-3)% | ||||
| Pulmonary GVHD | 617 | 2358 | 1957 | <0.001 | |||
| 100-day | 0 (0-1)% | 0 (0-1)% | 1 (0-1)% | ||||
| 6 months | 1 (0-1)% | 1 (1-2)% | 2 (1-2)% | ||||
| 1-year | 1 (0-2)% | 2 (1-2)% | 3 (2-4)% | ||||
Table 3b.
Incidence of pulmonary toxicity
| Outcomes | N eval | Prob (95% CI) | N eval | Prob (95% CI) | N eval | Prob (95% CI) |
N eval | Prob (95% CI) |
N eval | Prob (95% CI) |
p-value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Less than 1 (N = 623) | 1 year (N = 382) | 2-4 years (N = 816) | 5-9 years (N = 1183) | 10-20 years (N = 2018) | |||||||
| Pneumonitis | 612 | 364 | 785 | 1158 | 1964 | ||||||
| 100-day | 8 (6-10)% | 6 (4-9)% | 4 (3-5)% | 4 (3-5)% | 6 (5-7)% | 0.04 | |||||
| 6 months | 8 (6-10)% | 7 (5-10)% | 6 (4-7)% | 5 (4-6)% | 7 (6-8)% | ||||||
| 1-year | 9 (7-12)% | 9 (6-12)% | 6 (5-8)% | 6 (5-8)% | 8 (7-9)% | ||||||
| Diffuse alveolar hemorrhage | 613 | 364 | 786 | 1159 | 1964 | ||||||
| 100-day | 4 (3-6)% | 2 (1-4)% | 1 (1-2)% | 1 (0-2)% | 2 (1-2)% | 0.001 | |||||
| 6 months | 4 (3-6)% | 2 (1-4)% | 2 (1-3)% | 1 (1-2)% | 2 (1-3)% | ||||||
| 1-year | 5 (3-7)% | 3 (1-5)% | 2 (1-3)% | 1 (1-2)% | 2 (2-3)% | ||||||
| Pulmonary GVHD | 617 | 379 | 808 | 1171 | 1957 | ||||||
| 100-day | 0 (0-1)% | 0 (0-1)% | 0 (0-0)% | 1 (0-1)% | 1 (0-1)% | <0.001 | |||||
| 6 months | 1 (0-1)% | 1 (0-3)% | 1 (0-1)% | 2 (1-3)% | 2 (1-2)% | ||||||
| 1-year | 1 (0-2)% | 2 (1-4)% | 1 (0-2)% | 2 (2-3)% | 3 (2-4)% | ||||||
DAH, diffuse alveolar hemorrhage; GVHD, graft-versus-host disease.
Diffuse Alveolar Hemorrhage
Pulmonary hemorrhage occurred early after HCT in 125 children, an incidence of 2% at day 100 after HCT (Table 3, Figure 1 A). The median time to onset was 1.7 months (range 0-25 months). Outcomes for these patients were particularly poor, with only 23% (95% CI 16-31%) surviving 100 days after developing DAH and only 16% (95% CI 10-23%) surviving to 6 months (Figure 1). The Incidence of diffuse alveolar hemorrhage was more frequent in patients who received cord blood grafts (p<0.001) but was similar in patients with malignant and non-malignant diseases (p=0.39) and in patients with myeloablative and reduced intensity conditioning (p=0.14), Table 3a. Pulmonary hemorrhage was more frequent in infants <1y (p=0.001, Table 3b).
Lung GVHD
200 patients developed lung GVHD after HCT, or 4% of the cohort. Two percent of children had lung GVHD reported within 1 year (Figure 1 A). The median time to onset was 5.7 months (range 1-68 months). Lung GVHD was described as bronchiolitis obliterans in 52%, cryptogenic organizing pneumonia in 7%, both in 5%, and not specified in 36%. Outcomes are also poor for patients with chronic lung GVHD, but less so than for those with pneumonitis or DAH – by 100 days after diagnosis of chronic lung GVHD, OS declines to 87% (95% CI 81-91%) and decreases further to 72% (95% CI 65-79%) OS by 1 year after diagnosis (Figure 1B). Pulmonary GVHD were more frequent in patients transplanted for malignant diseases (p<0.001) and in patients who received myeloablative conditioning (p=0.002), but was similar among all graft types (p=0.48), Table 3a. The incidence of pulmonary GVHD increased with age (p<0.001, Table 3b). Twenty-four patients who were diagnosed with pneumonitis and 9 patients who were diagnosed with pulmonary hemorrhaged survived and later developed chronic GVHD.
Cause of Death
1,385 deaths occurred in this cohort. The most common causes of death were disease recurrence (33%) and infection (19%). Pulmonary disease, including lung GVHD, pulmonary failure, ARDS, pneumonitis, and diffuse alveolar hemorrhage, were noted as the primary cause of death in 13% of deaths from related donors, and 19% of deaths from unrelated donors. Survival declined in the year following diagnosis of each pulmonary complication to 38% (95% CI 33-43%) for patients with pneumonitis, 16% (95% CI 10-23%) in patients with pulmonary hemorrhage, and 72% (95% CI 65-79%) in patients with pulmonary GVHD (Figure 1B).
Discussion
Outcomes for pediatric patients who develop pulmonary complications after allogeneic HCT have not been well described. We used the CIBMTR database to define the incidence and the outcomes of pediatric patients that developed pulmonary complications after allogeneic HCT. These analyses demonstrated that pulmonary complications are not infrequent and are associated with significant mortality.
The incidence of pneumonitis was 8% by 1 year after transplant. Previous reports have described Idiopathic Pneumonia Syndrome after HCT in 2-12% of children13 and results presented here are consistent with this estimation. The introduction of etanercept as a targeted treatment for patients with IPS, decreased early mortality from 50-80% to less than 20% in a multi-institutional, single-arm trial13. In addition, reduced intensity conditioning regimens have been introduced for patients with non-malignant diseases and those unable to tolerate myeloablative conditioning, in an attempt to decrease toxic complications14. CMV and other viral prophylaxis measures have also decreased the incidence of CMV pneumonitis15. Mortality from pneumonitis was high in the current analysis, with survival of only 49% 100 days after diagnosis. Although etanercept may improve early outcomes, the long-term outcomes as demonstrated by our analysis and others suggest that these patients continue to have high rates of mortality after 1 year. It is possible that these patients have suffered pulmonary or other organ damage that makes them more susceptible to other complications16 . This highlights that the degree of long-term morbidity from pneumonitis is unknown and further research and interventions are needed to improve outcomes for these patients.
Diffuse alveolar hemorrhage affected only 2% of pediatric patients but was associated with a dismal prognosis with less than 20% of children surviving 6 months after diagnosis of pulmonary hemorrhage. Therapies such as high dose steroids, cyclophosphamide, recombinant factor VII, and extracorporeal membrane oxygenation have been attempted as treatments for pulmonary hemorrhage1, 17-19. Although case studies have described successes for a few patients, no treatment has reliably been shown to improve survival for patients with this complication.
Pulmonary chronic GVHD presented in 2% of patients before 1-yr post-HCT. Other single institution studies have estimated an incidence of 8%20. The lower incidence found in this analysis is likely related to the limitation in assessment of pulmonary complications within the first year after HCT. Moreover, the mainstay of diagnosis is spirometry which cannot be performed in young children and can be difficult even in teenagers who are unwell or uncooperative, leading to likely late diagnosis and under-diagnosis of this important complication. It is likely that incidence continues to increase over time. This analysis demonstrates that by 1 year after diagnosis of pulmonary GVHD, survival declines to 72%, highlighting a need for novel treatment approaches.
One might presume that patients with pulmonary dysfunction pre-HCT would be at increased risk of post-HCT pulmonary complications. However, only 17% of patients with evidence of pre-HCT pulmonary disease later developed post-HCT pulmonary complications. The number of patients with pre-HCT pulmonary disease is likely an underestimation; this may be at least partly due to the inability of many young patients to cooperate with standard pulmonary function testing. This highlights further that innovative approaching to assess pulmonary function in children are needed and that we need to better understand factors that affect a patient’s risk for developing pulmonary complications.
We acknowledge the limitations in this analysis. The data rely on the diagnosis and report of pulmonary complications from individual centers, where variable methods of assessment of pulmonary function are used and these methods or their results were reported. An important strength of this analysis is the large dataset, and includes centers worldwide, adding to the generalizability of the findings.
The recent NHLBI, NICHD, and NCI sponsored workshop on pulmonary complications highlighted the need for a coordinated approach to post-HCT pulmonary complications in children. In addition, the results of these analysis emphasize the burden of pulmonary complications, which limits the success of HCT in pediatric patients. Moving forward, a dedicated research effort is needed to understand the mechanisms contributing to pulmonary complications, to design innovative approaches to pulmonary assessment in children, to identify patients most at risk, and to develop novel therapeutic approaches. A multidisciplinary approach to post-HCT pulmonary complications in children is needed to help decrease mortality and improve outcomes for these patients.
Highlights:
Pulmonary complications occurred in 12% of pediatric and adolescent patients.
1-yr incidences of pneumonitis (8%), pulmonary hemorrhage (2%), and lung GVHD (2%).
Pulmonary complications led to decreased survival in the year following diagnosis.
Pulmonary disease accounted for 16% of deaths after HCT.
Multidisciplinary research approaches to improve pulmonary complications is needed.
Acknowledgements
This data was prepared and presented at The Workshop on Pulmonary Complications of Pediatric Hematopoietic Stem Cell Transplantation that was co-sponsored by the NHLBI, NICHD, and NCI and organized by Dr. Kenneth R Cooke MD, Samuel B Goldfarb MD, Ashok Srinivasan MD, James S Hagood MD, and Dennis C Stokes MD MPH. We also acknowledge the support of the lead program directors from the NHLBI, NICHD, and NCI, and in particular Aruna Natarajan, MD PhD and Robert Tamburro, MD MSc.
Funding
The CIBMTR is supported primarily by Public Health Service Grant/Cooperative Agreement 5U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 1U24HL138660 from NHLBI and NCI; a contract HHSH250201700006C with Health Resources and Services Administration (HRSA/DHHS); Grants N00014-17-1-2388, N00014-17-1-2850 and N00014-18-1-2045 from the Office of Naval Research HHSH250201700006C; and grants from Adaptive Biotechnologies; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; Atara Biotherapeutics, Inc.; Be the Match Foundation; *bluebird bio, Inc.; *Bristol Myers Squibb Oncology; *Celgene Corporation; *Chimerix, Inc.; *CytoSen Therapeutics, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Gilead Sciences, Inc.; HistoGenetics, Inc.; Immucor; *Incyte Corporation; Janssen Scientific Affairs, LLC; *Jazz Pharmaceuticals, Inc.; Karius, Inc.; Karyopharm Therapeutics, Inc.; *Kite Pharma, Inc.; Medac, GmbH; *Mediware; The Medical College of Wisconsin; *Merck & Co, Inc.; *Mesoblast; MesoScale Diagnostics, Inc.; Millennium, the Takeda Oncology Co.; *Miltenyi Biotec, Inc.; Mundipharma EDO; National Marrow Donor Program; Novartis Pharmaceuticals Corporation; PCORI; *Pfizer, Inc; *Pharmacyclics, LLC; PIRCHE AG; *Sanofi Genzyme; *Seattle Genetics; Shire; Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; Swedish Orphan Biovitrum, Inc.; *Takeda Oncology; and University of Minnesota. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
*Corporate Members
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure Statement: The authors have no financial conflicts of interest to disclose.
References
- 1.Rathi NK, Tanner AR, Dinh A, et al. Low-, medium- and high-dose steroids with or without aminocaproic acid in adult hematopoietic SCT patients with diffuse alveolar hemorrhage. Bone marrow transplantation. 2015;50:420–426. [DOI] [PubMed] [Google Scholar]
- 2.Majhail NS, Brazauskas R, Hassebroek A, et al. Outcomes of allogeneic hematopoietic cell transplantation for adolescent and young adults compared with children and older adults with acute myeloid leukemia. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012;18:861–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wood WA, Lee SJ, Brazauskas R, et al. Survival improvements in adolescents and young adults after myeloablative allogeneic transplantation for acute lymphoblastic leukemia. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2014;20:829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bacigalupo A, Socie G, Hamladji RM, et al. Current outcome of HLA identical sibling versus unrelated donor transplants in severe aplastic anemia: an EBMT analysis. Haematologica. 2015;100:696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergsten E, Horne A, Arico M, et al. Confirmed efficacy of etoposide and dexamethasone in HLH treatment: long-term results of the cooperative HLH-2004 study. Blood. 2017;130:2728–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pai SY, Logan BR, Griffith LM, et al. Transplantation outcomes for severe combined immunodeficiency, 2000-2009. The New England journal of medicine. 2014;371:434–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panepinto JA, Walters MC, Carreras J, et al. Matched-related donor transplantation for sickle cell disease: report from the Center for International Blood and Transplant Research. British journal of haematology. 2007;137:479–485. [DOI] [PubMed] [Google Scholar]
- 8.Shenoy S, Walters MC, Ngwube A, et al. Unrelated Donor Transplantation in Children with Thalassemia using Reduced-Intensity Conditioning: The URTH Trial. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2018;24:1216–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breuer R, Lossos IS, Berkman N, Or R. Pulmonary complications of bone marrow transplantation. Respiratory medicine. 1993;87:571–579. [DOI] [PubMed] [Google Scholar]
- 10.Krowka MJ, Rosenow EC 3rd, Hoagland HC. Pulmonary complications of bone marrow transplantation. Chest. 1985;87:237–246. [DOI] [PubMed] [Google Scholar]
- 11.Eikenberry M, Bartakova H, Defor T, et al. Natural history of pulmonary complications in children after bone marrow transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2005;11:56–64. [DOI] [PubMed] [Google Scholar]
- 12.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yanik GA, Grupp SA, Pulsipher MA, et al. TNF-receptor inhibitor therapy for the treatment of children with idiopathic pneumonia syndrome. A joint Pediatric Blood and Marrow Transplant Consortium and Children's Oncology Group Study (ASCT0521). Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2015;21:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al Mulla N, Kahn JM, Jin Z, et al. Survival Impact of Early Post-Transplant Toxicities in Pediatric and Adolescent Patients Undergoing Allogeneic Hematopoietic Cell Transplantation for Malignant and Nonmalignant Diseases: Recognizing Risks and Optimizing Outcomes. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2016;22:1525–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erard V, Guthrie KA, Seo S, et al. Reduced Mortality of Cytomegalovirus Pneumonia After Hematopoietic Cell Transplantation Due to Antiviral Therapy and Changes in Transplantation Practices. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015;61:31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frangoul H, Koyama T, Domm J. Etanercept for treatment of idiopathic pneumonia syndrome after allogeneic hematopoietic stem cell transplantation. Blood. 2009;113:2868–2869; author reply 2869. [DOI] [PubMed] [Google Scholar]
- 17.Elinoff JM, Bagci U, Moriyama B, et al. Recombinant human factor VIIa for alveolar hemorrhage following allogeneic stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2014;20:969–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koh H, Nakamae H, Koh KR, et al. Serum cytokine profiles at the onset of severe, diffuse alveolar hemorrhage complicating allogeneic hematopoietic stem cell transplantation, treated successfully with pulse intravenous cyclophosphamide. Acta haematologica. 2010;124:171–175. [DOI] [PubMed] [Google Scholar]
- 19.Morris SH, Haight AE, Kamat P, Fortenberry JD. Successful use of extracorporeal life support in a hematopoietic stem cell transplant patient with diffuse alveolar hemorrhage. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2010;11:e4–7. [DOI] [PubMed] [Google Scholar]
- 20.Duncan CN, Buonanno MR, Barry EV, Myers K, Peritz D, Lehmann L. Bronchiolitis obliterans following pediatric allogeneic hematopoietic stem cell transplantation. Bone marrow transplantation. 2008;41:971–975. [DOI] [PubMed] [Google Scholar]