Abstract
Objectives
Tracheostomy utilization has dramatically increased recently. Large gaps exist between expected and actual outcomes resulting in significant decisional conflict and regret. We determined 1-year patient outcomes and healthcare utilization following tracheostomy to aid in decision-making and resource allocation.
Design
Retrospective cohort study.
Setting
All California hospital discharges from 2012 to 2013 with follow-up through 2014.
Patients
Nonsurgical patients who received a tracheostomy for acute respiratory failure.
Interventions
None.
Measurements and Main Results
Our primary outcome was 30-day, 90-day, and 1-year mortality. We also determined hospitals readmissions rates and healthcare utilization in the first year following tracheostomy. We identified 8,343 tracheostomies during the study period. One-year mortality following tracheostomy was high, 46.5%. Older adults (≥ 65 yr) had significantly higher mortality compared with younger patients (< 65 yr) (54.7% vs 36.5%; p < 0.0001). Median survival for older adults was 175 days (95% CI, 150–202 d) compared with greater than 1 year for younger adults (adjusted hazard ratio, 1.25; 95% CI, 1.14–1.36). Within 1 year of tracheostomy, 60.3% of patients required hospital readmission. Older adults were more likely to be readmitted in the first year after tracheostomy compared with younger adults (66.1% vs 55.2%; adjusted hazard ratio, 1.19; 95% CI, 1.09–1.29). Total short-term acute care hospital costs (index and readmissions) in the first year after tracheostomy were high (mean, $215,369; SD, $160,874).
Conclusions
Long-term outcomes following tracheostomy are extremely poor with high mortality, morbidity, and healthcare resource utilization especially among older patients. Some subsets of younger patients may have better outcomes compared with the general tracheostomy population. Short-term acute care costs were extremely high in the first year following tracheostomy. If extended to the entire U.S. population, total short-term acute care hospital costs approach $11 billion dollars per year for tracheostomy-related to acute respiratory failure. These findings may aid families and surrogates in the decision-making process.
Keywords: health services, hospital readmission, mechanical ventilation, mortality, respiratory failure, tracheostomy
More than 100,000 adults receive a tracheostomy in the United States each year, and more than half are performed to facilitate prolonged mechanical ventilation (MV) for acute respiratory failure (1, 2). These patients account for a disproportionate amount of the $263 billion spent annually on critical illness (3, 4). Utilization of MV and tracheostomy among both surgical and nonsurgical patients has risen dramatically in the United States in the past 2 decades with the growth of tracheostomy outpacing that of MV (2, 5). Simultaneously, there has been a trend toward earlier tracheostomies, shorter hospital length of stay, decreasing in-hospital mortality, and an increase in discharges to long-term care facilities as opposed to home (2). Some have questioned whether these hospital-based changes in outcomes have actually translated into improvements in long-term outcomes or if mortality and high healthcare utilization have been shifted to into settings other than short-term acute care hospitals without meaningful changes in actual patient outcomes (6).
Prognostication of long-term outcomes following tracheostomy is an important part of the decision-making process for patients and families. However, current tools to support decision-making have limitations. The ProVENT score can predict mortality at 14 days and 21 days of MV but the majority of tracheostomies occur well before these time points, making the score less informative for tracheostomy-related decisions (7–10). A decision-aid for prolonged MV (not tracheostomy) also based its outcome predictions on a small sample of patients. Finally, the majority of investigations into tracheostomy outcomes focus on mortality, but many patients view multiple states (such as being attached to machines or living in a hospital) as “worse than death” (11). Unfortunately, the lack of information with which to guide patients contributes to patients and families feeling significant decisional conflict and regret, an overly optimistic view of potential outcomes, and sense of poor communication from the care team (12–15).
We conducted an epidemiologic study to investigate 1-year outcomes including mortality, hospital readmission, days in a short-term acute care hospital, and total costs of short-term acute care hospitalization using a population-level database.
MATERIALS AND METHODS
See Online Supplemental Methods (Supplemental Digital Content 1, http://links.lww.com/CCM/E857) for full details.
We conducted a retrospective cohort study using the California Office of Statewide Health Planning and Developing Patient Discharge Database with linkage to the State’s Vital Statistics File (PDD-VSF) (16, 17). The PDD-VSF is an administrative discharge database with 100% of discharge records from nonfederal hospitals within California that allows for longitudinal analyses with linkage to state death data. It is one of the largest and most diverse population-level databases capable of longitudinal tracking patients of all ages. We analyzed hospital discharges between January 1, 2012, and December 31, 2013, with follow-up of readmissions through December 31, 2014. The PDD-VSF typically has a 3- to 4-year delay in release after data are collected, and 2013 is the last year for which linked death information is available.
Patients
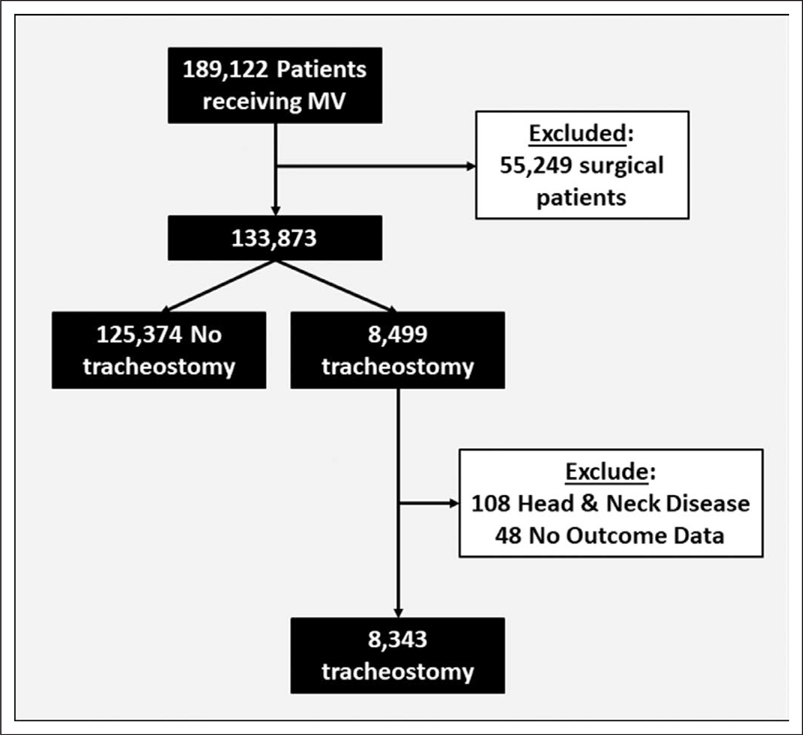
In line with previous studies, we identified adult patients (≥ 18 yr) who received MV with International Classification of Diseases, 9th Edition, Clinical Modification billing codes 96.7x and tracheostomy with codes 31.x and 32.x (5, 18). We excluded all patients with a major therapeutic operating room procedure other than tracheostomy (surgical patients) at any time during the hospitalization, as defined by the Healthcare Cost and Utilization Project’s procedure classification system (19). We excluded individuals with tracheostomy related to head and neck disease (diagnosis-related group 011, 012, and 013) and non-California residents (Fig. 1).
Figure 1.
Study design: we identified 189,122 patients who received mechanical ventilation (MV) during the study period. After excluding patients with a major therapeutic surgical procedure other than tracheostomy, patients with head and neck disease, and patients who did not have outcome data, we identified 8,343 patients who received tracheostomy during a short-term acute care hospitalization and were discharged between January 1, 2012, and December 31, 2013.
Outcomes
The primary outcome was 1-year mortality after the day of tracheostomy. We also determined mortality at hospital discharge, 30 days, and 90 days after tracheostomy. Secondary outcomes included discharge destination for hospital survivors, all-cause readmissions for survivors following hospital discharge, and healthcare utilization following tracheostomy. We stratified our analyses by age (< 65 yr and ≥ 65 yr).
Healthcare utilization was assessed with days in a short-term acute care hospital and total hospital costs. Given the competing risks of cumulative hospital days and death, we measured short-term acute care hospital utilization in several ways: 1) days in a short-term acute care hospital, 2) percent of days alive in a short-term acute care hospital in the first year following tracheostomy, and 3) percent of patients who spent 50% or more of their days alive in a short-term acute care hospital.
We also assessed healthcare utilization in terms of costs associated with short-term acute care hospitalization in the first year after tracheostomy including the index hospitalization and all readmissions (20). Cost data on postacute care (e.g., long-term facilities, nursing facilities, outpatient care) were not available. We excluded patients with hospitalizations with missing cost data (11.6%) from the financial analysis.
Statistical Analysis
Continuous and categorical variables are reported as means, medians, SDs, interquartile ranges (IQRs), and percentages as appropriate. We report patient demographics, comorbidities, severity of illness at admission, and acute illnesses for patients with tracheostomy with patients who received MV but did not have a tracheostomy as a reference point to describe tracheostomy utilization. We used Student t test, Wilcoxon rank-sum test, and chi-square test for univariate comparisons as appropriate. We report the outcomes for all patients with tracheostomy and also stratified by age (≤ 65 vs > 65 yr). We used multivariable generalized estimating equations with a compound symmetry covariance structure to account for correlation within hospitals to identify factors associated with 1-year outcomes (21, 22). We generated Kaplan-Meier curves for survival and hospital readmission (censoring for death). We compared survival curves by age category with the log-rank test and used multivariable Cox proportional hazard models to compare age groups. Multivariable models were adjusted for patient demographics, comorbidities, acute organ failures at admission, and causes of respiratory failure for nonsurgical patients (Tables E1 and E2, Supplemental Digital Content 1, http://links.lww.com/CCM/E857) (23–25).
Exploratory Analyses
It is unknown if there is a survival benefit for extremely old adults (≥ 85 yr). As such, we stratified age into three categories (< 65, 65–85, and ≥ 85 yr old) as outcomes may differ for the extremely old.
Additionally, our previous work has demonstrated different outcomes and temporal trends based on patient type (e.g., surgical vs nonsurgical patients) (2, 7). Therefore, we conducted an exploratory analysis in which we considered the broader population of patients who received a tracheostomy only excluding patients with a tracheostomy for head and neck disease and not all surgical patients. We evaluated 1-year mortality, readmissions, and resource utilization following tracheostomy for patients with a primary neurologic diagnosis (e.g., stroke, cerebral hemorrhage) (Table E3, Supplemental Digital Content 1, http://links.lww.com/CCM/E857), surgical patients, and nonneurologic nonsurgical patients (i.e., medical patients). We used similar methods to our primary analysis with comparisons by age group (< 65 vs ≥ 65 yr).
The study was approved by the California Committee for the Protection of Human Subjects and deemed exempt by the National Jewish Health Institutional Review Board. All statistical analyses were two-tailed with α equals to 0.05 and used SAS v9.4 (SAS Institute, Cary, NC).
RESULTS
Patients
During the study period, we identified 133,765 cases of nonsurgical MV of which 8,343 resulted in tracheostomy. Patient characteristics are presented in Table 1. Patients who received a tracheostomy tended to be slightly older with more chronic comorbidities than individuals who did not receive a tracheostomy. Patients who required MV but did not have a tracheostomy performed were more acutely ill. Table 2 presents differences in patients who receive a tracheostomy by age category (≥ 65 vs < 65 yr). Overall, patients greater than or equal to 65 years were more chronically ill. Differences in rates of acute organ failures and acute illness such as pneumonia and sepsis were also present but to a smaller magnitude.
TABLE 1.
Characteristics of Patients Who Received Tracheostomy Compared to Mechanically Ventilated Patients Who Did Not Receive Tracheostomy
| Variable | Tracheostomy (n = 8,391) | Mechanical Ventilation Without Tracheostomy (n = 125,374) | p |
|---|---|---|---|
| Mean age (SD), yr | 65.2 (16.0) | 64.8 (17.3) | < 0.03 |
| Female (%) | 45.5 | 46.0 | 0.33 |
| Race/ethnicity (%) | < 0.0001 | ||
| White | 48.4 | 53.0 | |
| Black | 13.9 | 11.5 | |
| Hispanic | 22.0 | 21.7 | |
| Asian | 11.7 | 9.8 | |
| Other | 3.9 | 4.0 | |
| Primary payer (%) | < 0.0001 | ||
| Medicare | 60.7 | 60.2 | |
| Medicaid | 19.8 | 17.0 | |
| Private insurance | 15.2 | 15.0 | |
| Self-pay | 1.4 | 3.5 | |
| Other | 2.8 | 4.3 | |
| Median Elixhauser comorbidity score (IQR)a | 14.0 (8.0–20.0) | 10.0 (4.0–16.0) | < 0.0001 |
| Early do not resuscitate order (within 24 hr of admission) (%) | 5.4 | 12.8 | < 0.0001 |
| Organ failures present at admission (%) | |||
| Shock | 26.5 | 28.4 | 0.0002 |
| Renal failure | 31.6 | 32.4 | 0.13 |
| Neurologic failure | 26.7 | 24.2 | < 0.0001 |
| Hematologic failure | 14.0 | 13.9 | 0.76 |
| Hepatic failure | 3.9 | 6.1 | < 0.0001 |
| Metabolic acidosis | 15.6 | 18.7 | < 0.0001 |
| Common causes of respiratory failure (%)b | |||
| Pneumonia | 79.4 | 54.0 | < 0.0001 |
| Severe sepsis | 56.0 | 42.3 | < 0.0001 |
| Acute exacerbation of chronic obstructive pulmonary disease | 14.0 | 10.8 | < 0.0001 |
| Acute exacerbation of asthma | 3.3 | 3.4 | 0.62 |
| Acute exacerbation of heart failure | 12.2 | 10.5 | < 0.0001 |
| Median time to tracheostomy (IQR) (d) | 11.0 (7.0–16.0) | NA | NA |
| Early tracheostomy (%)c | 27.9 | NA | NA |
IQR = interquartile range, NA = not applicable.
Calculated without arrhythmia comorbidity. Higher score indicates more chronically ill.
Diagnoses present during hospitalization. More than one diagnosis possible.
Early tracheostomy defined as tracheostomies performed on day 7 of mechanical ventilation or before.
TABLE 2.
Characteristics of Patients Who Received Tracheostomy by Age
| Variable | Age < 65 yr (n = 3,757) | Age ≥ 65 yr (n = 4,586) | p |
|---|---|---|---|
| Mean age (SD), yr | 51.0 (11.2) | 76.8 (7.9) | < 0.0001 |
| Female (%) | 42.0 | 48.4 | < 0.0001 |
| Race/ethnicity (%) | < 0.0001 | ||
| White | 47.4 | 49.3 | |
| Black | 16.0 | 12.3 | |
| Hispanic | 24.8 | 18.8 | |
| Asian | 8.3 | 14.6 | |
| Other | 3.7 | 4.0 | |
| Primary payer (%) | < 0.0001 | ||
| Medicare | 28.2 | 87.3 | |
| Medicaid | 36.9 | 5.8 | |
| Private insurance | 26.2 | 6.4 | |
| Self-pay | 3.1 | 0.2 | |
| Other | 5.7 | 0.5 | |
| Median Elixhauser comorbidity score (IQR)a | 12.0 (5.0–18.0) | 15.0 (10.0–21.0) | < 0.0001 |
| Early do not resuscitate order (within 24 hr of admission) (%) | 3.7 | 6.7 | < 0.0001 |
| Organ failures present at admission (%) | |||
| Shock | 24.7 | 28.0 | 0.0007 |
| Renal failure | 28.7 | 34.2 | < 0.0001 |
| Neurologic failure | 25.9 | 27.1 | 0.22 |
| Hematologic failure | 14.5 | 13.7 | 0.28 |
| Hepatic failure | 5.6 | 2.5 | < 0.0001 |
| Metabolic acidosis | 15.8 | 15.3 | 0.51 |
| Common causes of respiratory failure (%)b | |||
| Pneumonia | 75.8 | 82.1 | < 0.0001 |
| Severe sepsis | 51.8 | 59.3 | < 0.0001 |
| Acute exacerbation of chronic obstructive pulmonary disease | 9.5 | 17.6 | < 0.0001 |
| Acute exacerbation of asthma | 3.7 | 3.0 | 0.06 |
| Acute exacerbation of heart failure | 8.5 | 15.1 | < 0.0001 |
| Median time to tracheostomy (IQR) (d) | 11.0 (6.0–16.0) | 12.0 (7.0–17.0) | < 0.0001 |
| Early tracheostomy (%)c | 30.8 | 25.2 | < 0.0001 |
IQR = interquartile range.
Calculated without arrhythmia comorbidity. Higher score indicates more chronically ill.
Diagnoses present during hospitalization. More than one diagnosis possible.
Early tracheostomy defined as tracheostomies performed on day 7 of mechanical ventilation or before.
Mortality
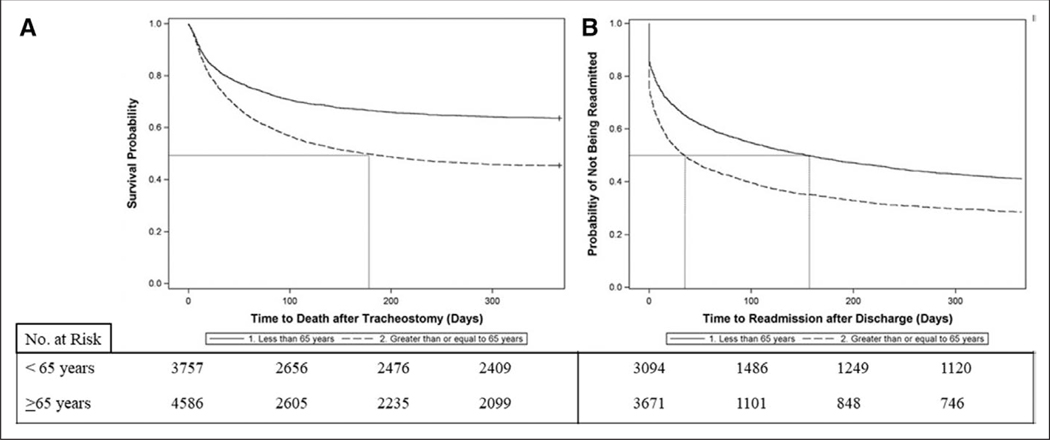
In-hospital mortality for patients with tracheostomy was 18.9% (95% CI, 18.1–19.8). Thirty-day mortality after tracheostomy was 22.1% (95% CI, 21.2–23.0%), increasing to 42.8% (95% CI, 41.7–43.8%) at 6 months and 46.5% (95% CI, 45.4–47.5%) at 1-year following tracheostomy (Table 3). Individuals greater than or equal to 65 years had significantly higher mortality than those less than 65 years at each time point with an absolute mortality difference of 18.2% (36.5% vs 54.7%; p < 0.0001) at 1 year (Fig. 2A). Median survival for those less than 65 years was greater than 1 year. Median survival for those greater than or equal to 65 years was 175 days (95% CI, 150–202 d), significantly lower than individuals less than 65 years (p < 0.0001 for difference between age strata). Patients greater than or equal to 65 years had a 25% higher hazard of death at any time point in the first year following tracheostomy (adjusted hazard ratio [aHR], 1.25; 95% CI, 1.14– 1.36). Among patients with tracheostomy, sepsis and chronic comorbidities (e.g., heart failure, chronic renal insufficiency, and cancer) were more strongly associated with death at 1 year than severity of illness (Table E4, Supplemental Digital Content 1, http://links.lww.com/CCM/E857). Neurologic comorbidities appeared to have some protective effect over other comorbidities.
TABLE 3.
Outcomes for Patients With Tracheostomy
| Outcome | Overall (n = 8.343) | Age < 65 yr (n = 3,757) | Age ≥ 65 yr (n = 4,586) | p |
|---|---|---|---|---|
| Hospital discharge destination, n (%)a | < 0.0001 | |||
| Home | 758 (11.2) | 545 (17.6) | 213 (5.8) | |
| Long-term facility | 5,825 (86.1) | 2,476 (80.0) | 3,349 (91.2) | |
| Other | 182 (2.7) | 73 (2.4) | 109 (3.0) | |
| Mortality, n (%)b | ||||
| Hospital | 1,578 (18.9) | 663 (17.7) | 915 (20.0) | 0.008 |
| 30-d | 1,846 (22.1) | 695 (18.5) | 1,151 (25.1) | < 0.0001 |
| 90-d | 2,987 (35.8) | 1,071 (28.5) | 1,916 (41.8) | < 0.0001 |
| 1-yr | 3,877 (46.5) | 1,370 (36.5) | 2,507 (54.7) | < 0.0001 |
| All cause hospital readmission, n (%)c | ||||
| 30-d | 2,361 (40.2) | 922 (32.6) | 1,439 (47.1) | < 0.0001 |
| 90-d | 2,464 (48.4) | 1,070 (41.6) | 1,394 (55.4) | < 0.0001 |
| 1-yr | 2,648 (60.3) | 1,299 (55.2) | 1,349 (66.1) | < 0.0001 |
| Median hospital days following tracheostomy for survivors (interquartile range)d | ||||
| Within 30 d | 26 (12–30) | 24 (13–30) | 27 (12–30) | 0.03 |
| Within 90 d | 28 (13–50) | 27 (14–47) | 30 (13–53) | 0.006 |
| Within 1 yr | 30 (15–55) | 29 (15–52) | 32 (14–57) | 0.11 |
| Percent of patients that spent ≥ 50% of days alive in hospital, n (%) | 3,015 (36.3) | 1,075 (28.7) | 1,940 (42.5) | < 0.0001 |
| Total hospital costs (U.S. dollars), mean (SD)e | $215,369 (160,874) | $215,570 (166,124) | $215,138 (154,658) | 0.93 |
Among survivors of index hospitalization.
30-d, 90-d, and 1 yr mortality is calculated from date of tracheostomy procedure.
All cause readmission rates are calculated from date of discharge for patients that survive the specified time-period (survive 30 d, 90 d, and 1 yr post discharge).
Acute care hospital days were calculated among individuals who survived the specified period.
Indicates total acute care hospital costs in the first year following tracheostomy for individuals who survive the first year. Costs do not include postacute care.
Figure 2.
Kaplan-Meier survival and readmission curves for patients with tracheostomy. A, Survival curves. Median survival was greater than 1 yr for patients less than 65 yr old and 175 d for patients greater than or equal to 65 yr. Older patients had higher risk of death at any time point (adjusted hazard ratio [aHR], 1.25; 95% CI, 1.14–1.36). Number at risk indicates the number of patients alive at each time point. B, Readmission curves. Patients were censored at the time of death (as they were no longer eligible for readmission) or at 1 yr. Median time to readmission for older patients was 35 d versus 154 d for younger patients. Older patients had a higher risk of readmission throughout the first year (aHR, 1.19; 95% CI, 1.09–1.29). Number at risk indicates the number of patients alive who have not been readmitted at each time point.
Discharge Destination
Survivors of the index hospitalization overwhelmingly required care in a long-term facility (Table 2). There were significant differences by age with 91.2% of patients greater than or equal to 65 years being discharged to a long-term facility compared with 80.0% of patients less than 65 years (p < 0.0001).
Hospitalization
Patients with tracheostomy had high likelihood of being readmitted (Table 3). Among patients who survived for 30 days after hospital discharge, 40.2% (95% CI, 38.9–414.4) required readmission within 30 days. One year after discharge, 60.3% (95% CI, 58.8–61.7%) of survivors required readmission. Patients greater than or equal to 65 years had significantly higher 1-year readmission rates (66.1% vs 55.2%; p < 0.0001). Median time to readmission was 72 days (95% CI, 63–82 d). However, adults greater than or equal to 65 years had significantly shorter median time to readmission compared with adults less than 65 years (35 d [95% CI, 29–40 d] vs 154 d [95% CI, 132–181 d]; p < 0.0001) (Fig. 2B). After adjusting for patient and disease characteristics, adults greater than or equal to 65 years had a 19% increased risk of readmission during the first year following tracheostomy (aHR, 1.19; 95% CI, 1.09–1.29).
The majority of patients who survived the first month following tracheostomy spent almost the entire month in a short-term acute care hospital (Table 3) and adults greater than or equal to 65 years spent more days in the hospital compared with adults less than 65 years. There was little increase in short-term acute care hospital days for individuals who survived the entire first year following tracheostomy. However, the variation between survivors broadened during this time. At 30 and 90 days, patients greater than or equal to 65 years spent more time in a short-term acute care hospital. At 1 year, there was a trend toward more hospital days for adults greater than or equal to 65 years (median, 32 d; IQR, 14–57) compared with adults less than 65 years (median, 29 d; IQR, 15–52) (p = 0.10). One out of five adults less than 65 years and 23.5% of adults greater than or equal to 65 years spent more than 60 days in a short-term acute care hospital setting (p = 0.006). More than one-third of patients spent greater than 50% of days alive in a short-term acute care hospital with higher rates for patients greater than or equal to 65 years (28.7% vs 42.5%; p < 0.0001) (Table 2 and Table E5, Supplemental Digital Content 1, http://links.lww.com/CCM/E857).
We summed hospital costs across all short-term acute care hospitalizations in the first year following tracheostomy. Mean total hospital costs for individuals who survived the first year were $215,369 (SD, $160,874). There was no significant difference in mean costs between age groups (Table 2). For all patients (including those who died) mean total hospital costs in the first year was $204,306 (SD, $154,442) with slightly lower costs for adults greater than or equal to 65 years (Table E5, Supplemental Digital Content 1, http://links.lww.com/CCM/E857).
Sensitivity Analyses
Extremely old patients (≥ 85 yr) experienced worse outcomes than younger patients (65–85 and ≤ 65 yr) without evidence of a survivor benefit (Tables E6 and E7 and Fig. E2, Supplemental Digital Content 1, http://links.lww.com/CCM/E857). Additionally, we conducted an exploratory analysis including all patients with tracheostomy except those who had a tracheostomy for head and neck disease. Medical patients were older and sicker when compared with neurologic and surgical patients (Table E8, Supplemental Digital Content 1, http://links.lww.com/CCM/E857). One-year outcomes were poor for all patients but worse for medical patients compared with neurologic and surgical patients (Table E9 and Figs. E3 and E4, Supplemental Digital Content 1, http://links.lww.com/CCM/E857). Age differences persisted across all groups with much worse survival and higher risk of readmission for adults greater than or equal to 65 years (Tables E10–E12 and Figs. E5 and E6, Supplemental Digital Content 1, http://links.lww.com/CCM/E857).
DISCUSSION
We investigated long-term patient outcomes and healthcare resource utilization following tracheostomy for acute respiratory failure in a large population-level claims database. We observed high 30-day, 90-day, and 1-year mortality, hospital readmission, and healthcare utilization following tracheostomy with large differences between age groups (5, 26). Our analysis is unique in that it uses more recent data than previous studies, provides time to event analyses to provide information about outcome trajectories, and goes well beyond mortality to create a picture of morbidity and healthcare utilization following tracheostomy. Our findings can play a significant role in tracheostomy decision-making.
Our mortality findings extend prior works. Engoren et al (27, 28) used data from 429 patients from a single center from 1998 to 2000 to identify a 36% 1-year mortality following tracheostomy. Cox et al (14) and Unroe et al (29) studied 126 patients with a tracheostomy hospitalized in 2008 and observed a 44% 1-year mortality rate. Cox et al (30, 31) also developed a decision-aid for prolonged MV based on the outcomes data from his earlier study. However, these studies were based on a small sample of patients and did not provide significant details about outcome trajectories. The ProVENT studies created a scoring system to predict 1-year mortality at 14 days and 21 days of MV. However, they are unable to inform tracheostomy decision-making as the majority of tracheostomies happen well before day 14 (8–10).
To our knowledge, our findings represent the first description of long-term tracheostomy outcomes using a large population-level database. The overall 1-year mortality we observed was slightly higher than that which has been previously reported. Our previous work showed that in-hospital tracheostomy mortality has dramatically decreased in the last 20 years (2). Given trends toward earlier tracheostomies, shorter hospital length of stay, and increases in discharges to long-term care facilities versus home, our findings indicate that long-term mortality has not really changed over time but rather that the location of death has been shifted to the postacute care locations. Not surprisingly, older adults had a 50% much higher mortality compared with younger adults.
We also provide a broader understanding of survival with time to event analyses than has not previously been described. Younger patients had a median survival of greater than 1 year suggesting that a subgroup of younger patients may do well following tracheostomy. However, half of older adults died within 6 months of tracheostomy. In the context of other chronic conditions, our findings suggest that older adults who receive a tracheostomy have worse survival than patients with advanced lung and pancreatic cancer (32). However, for both age groups, mortality essentially plateaued 6 months after tracheostomy. Further studies investigating the events related to mortality in the first 6 months after tracheostomy may provide insight toward reducing tracheostomy related mortality.
The exploratory analyses suggest that poor outcomes persist across additional age categorizations and patient subtypes. No survivor benefit was observed for individuals greater than or equal to 85 years. Individuals greater than or equal to 85 years suffered worse outcomes compared with younger patients. Furthermore, when we included all tracheostomy patients but categorized them as neurologic, surgical, or medical, we observed similarly poor outcomes especially for adults greater than or equal to 65 years. Although neurologic and surgical patients had slightly better outcomes compared with medical patients, the differences were relatively small especially when subcategorized by age. Age alone may have a larger impact on outcomes compared with the whether the patient’s primary issue was neurologic, surgical, or medical.
As outcomes other than mortality are equally important to many patients and families, we investigated healthcare utilization and costs following tracheostomy (11). A large percentage of patients required readmission following tracheostomy with older adults having a much shorter time out of hospital. The competing risks between long-term healthcare utilization (e.g., days in hospital, costs) and death necessitated multiple different views of time spent in the hospital following tracheostomy. In terms of days in the hospital, most patients spent around 1 month in the hospital, although there was wide variation. However, this number likely underrepresents the true burden of hospitalization as individuals who died spent more time in the hospital but were not included in the calculation given the competing risks between death and hospital days. We did observe that a high percentage of patients spent more than 50% of their days alive in a short-term acute care setting. These multiple measures highlight the many issues looking at long-term outcomes for conditions with high mortality. When dealing with individual patients/families, it is important to translate this information into terms that they need and understand. We are unaware of previous population-level descriptions of healthcare utilization following tracheostomy.
We also describe total short-term acute care hospitalization costs in the first year following tracheostomy. Costs approached one-quarter of a million dollars regardless of survivor status. Interestingly costs did not differ significantly by age for individuals who survived the first year. However, when all patients (included those who died) were included in the cost analysis, older patients had a slightly lower total cost likely because of higher mortality. With half of tracheostomies being performed for nonsurgical patients, our findings suggest that annualized national short-term acute care costs for tracheostomy approach $11 billion dollars (2). This estimate does not include postacute care (e.g., long-term care/nursing facilities, outpatient care), lost productivity, or costs incurred by family members. These findings have significant societal implications especially given an aging population for whom postacute care utilization is higher.
We stratified our analyses by age based on preliminary qualitative data on how tracheostomy decisions are made and the strong association between age and poor outcomes for other critical care conditions (26, 33–35). As anticipated, we found far worse outcomes for older adults. However, when investigating factors associated with 1-year mortality, we observed several baseline comorbidities and causes of respiratory failure that were as strongly associated with 1-year mortality as age. Similarly, when investigating healthcare utilization, we found multiple subgroups of patients with different utilization profiles even within age groups. Some patients only spent 1 month in a short-term acute care hospital while others spent a large percentage of days alive hospitalized. These findings highlight the potential of studies utilizing deep-learning algorithms to determine combinations of age groups, comorbidities, and acute illness that can predict different outcome trajectories. A broader understanding of different patient trajectories following tracheostomy may help families with complex decision-making.
Taken together, these findings have the potential to greatly affect the decision-making process. Older patients tend to do far worse compared with younger patients. However, patients with primary neurologic comorbidities may have better outcomes compared with individuals with cancer or cardiovascular disease. Although preliminary, these findings may help families find value-concordant decisions or have less decisional conflict given a broader understanding of potential outcomes.
Our study has several important limitations. The use of California state data may limit the generalizability of our conclusions. However, the PDD-VSF provides one of the largest and most diverse population-based datasets inclusive of all ages and payers with longitudinal tracking and linkage to state death data. Additionally, nationally representative databases that have information about long-term outcomes such as Medicare databases would ignore the large percentage of tracheostomies performed for younger patients. We speculate that outcomes in other regions of the United States may be significantly worse owing to higher rates of chronic comorbidities. Billing codes in administrative datasets used to identify tracheostomy and medical diagnoses can be subject to misclassification. Our approach was to use high fidelity codes that are well represented in the literature. Billing codes also only partially capture severity of illness. Although previous studies have shown that risk-adjustment with administrative data approaches more physiologic scores (e.g., Acute Physiology and Chronic Health Evaluation IV or Simplified Acute Physiology Score 3), we recognize the inherent limitations of using administrative for gauging severity of illness. We were also unable to determine the total financial costs associated with tracheostomy, as we did not have postacute care costs. Finally, we were unable to measure quality of life in either a qualitative or a quantitative way (e.g., quality-adjusted life years). These measures are likely of key importance to decision-makers and should be included in future prospective studies. Despite these limitations, this study provides novel and in-depth insight into mortality and healthcare utilization following tracheostomy not previously described at the population-level.
CONCLUSIONS
Our findings of high mortality, low median survival for older patients, high readmission rates, potentially burdensome cost, and informative outcome trajectories provide significant insight into long-term outcomes following tracheostomy. Outcomes for older adults remain poor but certain subsets of younger patients and patients with few comorbidities may have better survival and lower risk of readmission. As comorbidities play a crucial role in long-term outcomes, future studies should attempt to identify different patient trajectories based on a combination of age and comorbidities. A key part of this discontent has been the lack of robust outcomes data to ground expectations. Our results provide some highly informative data to improve the decision-making process, providing families with a greater sense of what to expect following tracheostomy, a major current limitation (14, 30). Additionally, our findings highlight the significant impact on healthcare utilization and cost associated with prolonged MV. Future studies are needed to investigate long-term outcomes of other more granular patient-centered outcomes and how this information can be used to improve decision-making.
Supplementary Material
Acknowledgments
This work was performed at National Jewish Health.
Dr. Mehta was involved in study design, data analysis, interpretation, and article preparation; he takes full responsibility for the content of the article, data analysis, and data interpretation; he had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the analysis; and he was responsible for drafting of the article. Dr. Walkey involved in data interpretation and article preparation. Dr. Curran-Everett involved in statistical and data interpretation. Dr. Douglas involved in study design, data interpretation, and article preparation. Drs. Mehta and Douglas were responsible for the study design. Drs. Mehta and Curran-Everett were responsible for the statistical analysis.
Drs. Mehta, Curran-Everett, and Douglas received support for article research from the National Institutes of Health (NIH) (K12HL137862, RHL089897B, and R01NR016459). Dr. Walkey’s institution received funding from the NIH (R01HL136660), and he received funding from UptoDate.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
REFERENCES
- 1.HCUPnet: Healthcare Cost and Utilization Project. 2017. Available at: http://hcupnet.ahrq.gov/ Accessed October 25, 2017 [Google Scholar]
- 2.Mehta AB, Syeda SN, Bajpayee L, et al. : Trends in tracheostomy for mechanically ventilated patients in the United States, 1993–2012. Am J Respir Crit Care Med 2015; 192:446–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carson SS, Bach PB: The epidemiology and costs of chronic critical illness. Crit Care Clin 2002; 18:461–476 [DOI] [PubMed] [Google Scholar]
- 4.Coopersmith CM, Wunsch H, Fink MP, et al. : A comparison of critical care research funding and the financial burden of critical illness in the United States. Crit Care Med 2012; 40:1072–1079 [DOI] [PubMed] [Google Scholar]
- 5.Mehta AB, Syeda SN, Wiener RS, et al. : Epidemiological trends in invasive mechanical ventilation in the United States: A population-based study. J Crit Care 2015; 30:1217–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scales DC: The implications of a tracheostomy for discharge destination. Am J Respir Crit Care Med 2015; 192:404–405 [DOI] [PubMed] [Google Scholar]
- 7.Mehta AB, Cooke CR, Wiener RS, et al. : Hospital variation in early tracheostomy in the United States: A population-based study. Crit Care Med 2016; 44:1506–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carson SS, Kahn JM, Hough CL, et al. ; ProVent Investigators: A multicenter mortality prediction model for patients receiving prolonged mechanical ventilation. Crit Care Med 2012; 40:1171–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hough CL, Caldwell ES, Cox CE, et al. ; ProVent Investigators and the National Heart Lung and Blood Institute’s Acute Respiratory Distress Syndrome Network: Development and validation of a mortality prediction model for patients receiving 14 days of mechanical ventilation. Crit Care Med 2015; 43:2339–2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leroy G, Devos P, Lambiotte F, et al. : One-year mortality in patients requiring prolonged mechanical ventilation: Multicenter evaluation of the ProVent score. Crit Care 2014; 18:R155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubin EB, Buehler AE, Halpern SD: States worse than death among hospitalized patients with serious illnesses. JAMA Intern Med 2016; 176:1557–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frost DW, Cook DJ, Heyland DK, et al. : Patient and healthcare professional factors influencing end-of-life decision-making during critical illness: A systematic review. Crit Care Med 2011; 39:1174–1189 [DOI] [PubMed] [Google Scholar]
- 13.Thompson BT, Cox PN, Antonelli M, et al. : Challenges in end-of-life care in the ICU: Statement of the 5th International Consensus Conference in Critical Care: Brussels, Belgium, April 2003: Executive summary. Crit Care Med 2004; 32:1781–1784 [DOI] [PubMed] [Google Scholar]
- 14.Cox CE, Martinu T, Sathy SJ, et al. : Expectations and outcomes of prolonged mechanical ventilation. Crit Care Med 2009; 37:2888–2894; quiz 2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azoulay E, Chevret S, Leleu G, et al. : Half the families of intensive care unit patients experience inadequate communication with physicians. Crit Care Med 2000; 28:3044–3049 [DOI] [PubMed] [Google Scholar]
- 16.California Office of Statewide Health Planning and Development: California Office of Statewide Health Planning and Development: Patient Discharge Data. Last updated: May 26, 2017. Available at: https://www.oshpd.ca.gov/HID/Patient-Discharge-Data.html Accessed May 29, 2017
- 17.California Office of Statewide Health Planning and Development: Healthcare Information Division: Types of Data. Last updated: January 27, 2017. Available at: http://www.oshpd.ca.gov/HID/Data_Request_Center/Types_of_Data.html Accessed February 3, 2017
- 18.Quan H, Parsons GA, Ghali WAs: Validity of procedure codes in International Classification of Diseases, 9th revision, clinical modification administrative data. Med Care 2004; 42:801–809 [DOI] [PubMed] [Google Scholar]
- 19.Healthcare Cost and Utilization Project (HCUP): Procedure Classes 2015. Rockville, MD, Agency for Healthcare Research and Quality; 2016. Available at: http://www.hcup-us.ahrq.gov/toolssoftware/procedure/procedure.jsp Accessed June 1, 2016 [Google Scholar]
- 20.California Office of Statewide Health Planning and Development: Hospital Annual Financial Data. 2018. Available at: https://data.chhs.ca.gov/dataset/hospital-annual-financial-data-selected-data-pivot-tables Accessed October 11, 2018
- 21.Liang KY, Zeger SL: Longitudinal data analysis using generalized linear models. Biometrika 1986; 73:13–22 [Google Scholar]
- 22.SAS Institute: PROC GENMOD: Generalized Estimating Equations. 2018. Available at: https://support.sas.com/documentation/cdl/en/statug/63347/HTML/default/viewer.htm#statug_genmod_sect008.htm Accessed July 18, 2018
- 23.Quan H, Sundararajan V, Halfon P, et al. : Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43:1130–1139 [DOI] [PubMed] [Google Scholar]
- 24.Elixhauser A, Steiner C, Harris DR, et al. : Comorbidity measures for use with administrative data. Med Care 1998; 36:8–27 [DOI] [PubMed] [Google Scholar]
- 25.Martin GS, Mannino DM, Eaton S, et al. : The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003; 348:1546–1554 [DOI] [PubMed] [Google Scholar]
- 26.Wunsch H, Linde-Zwirble WT, Angus DC, et al. : The epidemiology of mechanical ventilation use in the United States. Crit Care Med 2010; 38:1947–1953 [DOI] [PubMed] [Google Scholar]
- 27.Engoren M, Arslanian-Engoren C, Fenn-Buderer N: Hospital and long-term outcome after tracheostomy for respiratory failure. Chest 2004; 125:220–227 [DOI] [PubMed] [Google Scholar]
- 28.Engoren MC, Arslanian-Engoren CM: Outcome after tracheostomy for respiratory failure in the elderly. J Intensive Care Med 2005; 20:104–110 [DOI] [PubMed] [Google Scholar]
- 29.Unroe M, Kahn JM, Carson SS, et al. : One-year trajectories of care and resource utilization for recipients of prolonged mechanical ventilation: A cohort study. Ann Intern Med 2010; 153:167–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cox CE, Lewis CL, Hanson LC, et al. : Development and pilot testing of a decision aid for surrogates of patients with prolonged mechanical ventilation. Crit Care Med 2012; 40:2327–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cox CE, Wysham NG, Walton B, et al. : Development and usability testing of a web-based decision aid for families of patients receiving prolonged mechanical ventilation. Ann Intensive Care 2015; 5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noone AM, Howlader N, Krapcho M, et al. ; National Cancer Institute: SEER Cancer Statistics Review, 1975–2015. Last updated: April 28, 2018. Available at: https://seer.cancer.gov/csr/1975_2015/ Accessed April 30, 2018 [Google Scholar]
- 33.Carson SS: The epidemiology of critical illness in the elderly. Crit Care Clin 2003; 19:605–617, v [DOI] [PubMed] [Google Scholar]
- 34.Carson SS, Cox CE, Holmes GM, et al. : The changing epidemiology of mechanical ventilation: A population-based study. J Intensive Care Med 2006; 21:173–182 [DOI] [PubMed] [Google Scholar]
- 35.Wunsch H, Guerra C, Barnato AE, et al. : Three-year outcomes for Medicare beneficiaries who survive intensive care. JAMA 2010; 303:849–856 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.