Abstract
A series of hybrid nanoparticle-based nicotine nanovaccines (NanoNicVac) were engineered in this work by conjugating potent carrier protein candidates (Keyhole limpet hemocyanin (KLH) multimer, KLH subunit, cross-reactive material 197 (CRM197), or tetanus toxoid (TT)) for enhanced immunological efficacy. NanoNicVac with CRM197 or TT were processed by dendritic cells more efficiently than that with KLH multimer or subunit. NanoNicVac carrying CRM197 or TT exhibited a significantly higher immunogenicity against nicotine and a considerably lower immunogenicity against carrier proteins than NanoNicVac carrying KLH multimer or subunit in mice. The in vivo results revealed that NanoNicVac with CRM197 or TT resulted in lower levels of nicotine in the brain of mice after nicotine challenge. All findings suggest that an enhanced immunological efficacy of NanoNicVac can be achieved by using CRM197 or TT instead of KLH or KLH subunit as carrier proteins, making NanoNicVac a promising next-generation immunotherapeutic candidate against nicotine addiction.
Keywords: Nicotine addiction, Nicotine vaccine, Hybrid nanoparticle, Carrier protein, Anti-nicotine antibody
Tobacco smoking continues to be the leading preventable cause of disease, disability, and death worldwide.1 Every year in the United States alone, more than 480,000 people die from tobacco smoking.2 Current pharmacological medications for smoking cessation are only partially successful and associated with the risk of serious side effects.3 Nicotine vaccines that can elicit the production of nicotine-specific antibodies capable of sequestering nicotine in serum and reducing nicotine entering the brain have shown to be a promising approach to treating nicotine addiction.4–9 Several conjugate nicotine vaccines have reached various stages of clinical trials.10,11 Despite the prominent results in preclinical and early-stage clinical trials, no conjugate nicotine vaccines have proven overall to enhance smoking cessation rate, mainly due to the insufficient and highly-variable antibody titers.5,12,13
In our previous work, we devised the next-generation nanoparticle-based nicotine vaccines to achieve improved immunogenicity over conjugate nicotine vaccines.14–17 These next-generation nanoparticle-based nicotine nanovaccines have many unique properties, such as high bioavailability, enhanced recognition and uptake by immune cells, long immune persistence, high specificity, and ease of incorporation with adjuvants. In particular, a lipid-polymeric hybrid nanoparticle-based nicotine nanovaccine (NanoNicVac) was demonstrated to result in significantly higher immunological efficacy than the conjugate nicotine vaccine.16 In addition, we previously demonstrated that the immunogenicity of NanoNicVac could be improved by modulating nanoparticle size,16 hapten density,18 hapten localization,19 and molecular adjuvants.20 In this present work, we attempted to study the effect of another significant factor, carrier protein, on the immunological efficacy of NanoNicVac.
Immunologically, although T cell help is not absolutely required for anti-nicotine antibody production, T cell help can promote the generation of an effective humoral immune response against nicotine.21,22 The maturation of nicotine-specific B cells to antibody-secreting cells requires two pivotal T cell-dependent processes. The two processes are the formation of T helper cells and the interaction between T-helper cells and B cells, both of which only occur via presentation of peptidic antigens on the major histocompatibility complex (MHC) of antigen presenting cells.5,23 Basically, effective T cell help makes the humoral immune response against nicotine specific, intense, and long-lasting.9 Therefore, a carrier protein that provides peptidic antigens is a necessity for a nanoparticle-based nicotine nanovaccine.24 Incorporation of different carrier proteins into a nanoparticle-based nicotine nanovaccine may cause differential effectiveness of T cell immunity, thus leading to different immunological efficacy.
In this study, potent carrier proteins were incorporated into NanoNicVac to boost its immunological efficacy. Specifically, four carrier protein candidates, including keyhole limpet hemocyanin (KLH) multimer,25 KLH subunit (KS),26 cross-reactive material 197 (CRM197),27 and tetanus toxoid (TT),28 all of which have been reported to be highly-immunogenic and widely used as carrier proteins, were conjugated to NanoNicVac to study the impact of carrier proteins on its immunogenicity and pharmacokinetic efficacy. NanoNicVac with different carrier proteins (Figure 1, A) were prepared and characterized. The cellular uptake and processing of NanoNicVac particles were studied in dendritic cells. The immunogenicity and pharmacokinetic efficacy of NanoNicVac were tested in mice.
Figure 1.
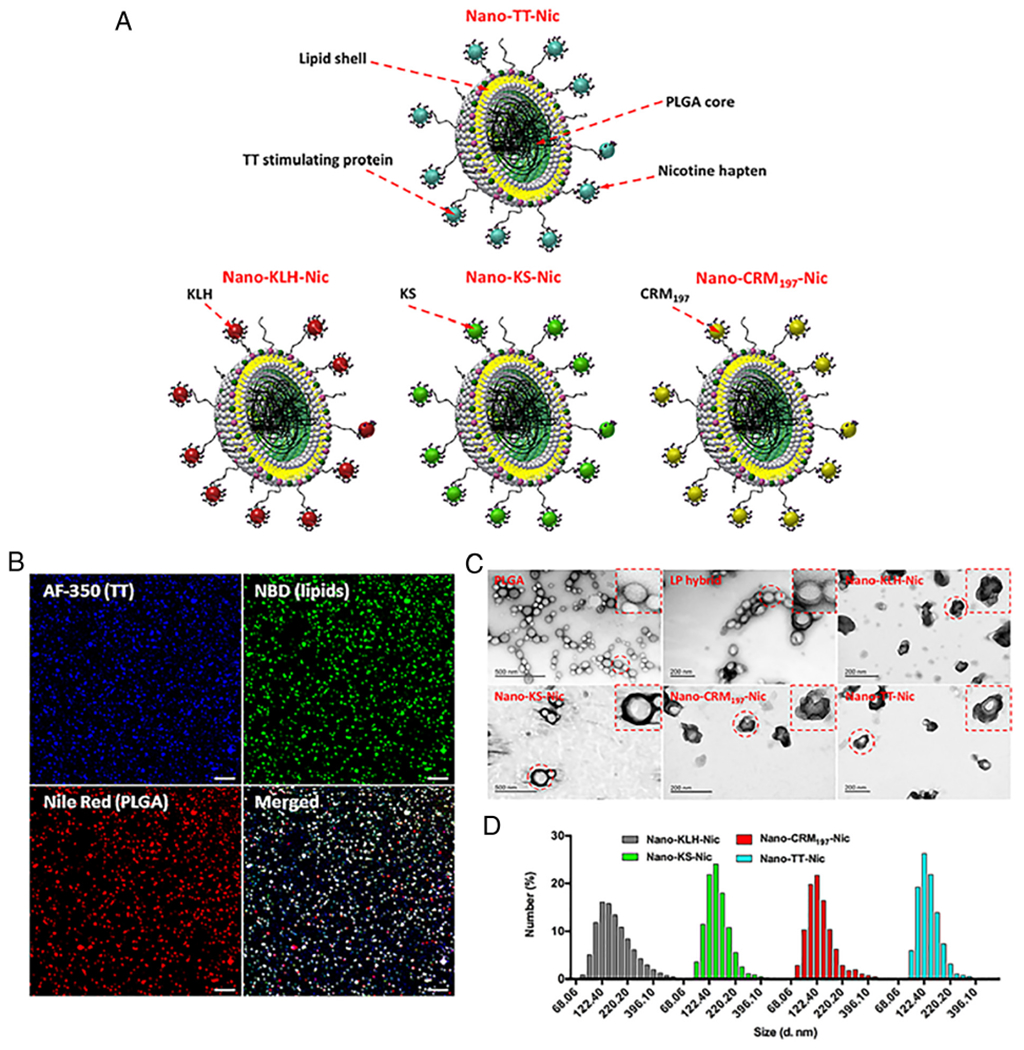
Synthesis and characterization of NanoNicVac. (A) Schematic illustration of NanoNicVac carrying different carrier proteins. (B) CLSM images showing the co-localization of TT carrier protein, lipid shell, and PLGA core, which were labeled by AF-350, NBD, and Nile Red, respectively. Scale bars represent 10 μm. (C) TEM images showing the morphological characteristics of NanoNicVac nanoparticles. (D) Dynamic size distribution of NanoNicVac nanoparticles.
Methods
Synthesis and characterization of Nicotine (Nic)-carrier protein conjugates
Nic-carrier protein conjugates (Nic-KLH, Nic-KS, Nic-CRM197, and Nic-TT) were synthesized using a 1-Ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC)/ N-hydroxysulfosuccinimide (Sulfo-NHS)-mediated reaction.18 In brief, an appropriate amount of Nic-haptens was dissolved in 0.5 mL activation buffer (0.1 M 2-(N-morpholino)ethanesulfonic acid, 0.5 M NaCl, pH 6.0). EDC and NHS (EDC: NHS: Nic-hapten = 10: 10: 1) were subsequently added. The mixture was incubated at room temperature for 30 min to activate Nic-haptens. Ten mg of carrier proteins that were dissolved in 3 mL of a coupling buffer (0.1 M phosphate buffer saline (PBS), pH 7.4) were mixed with the activated Nic-haptens. The reaction was allowed to proceed for 10 h, and unconjugated Nic-haptens were eliminated by dialysis. The Nic-hapten loading in Nic-carrier protein conjugates was estimated by a 2,4,6-trinitrobenzene sulfonic acid (TNBSA)-based method.15 In brief, carrier proteins and Nic-carrier protein conjugates were prepared at a concentration of 1 mg/mL. Two hundred μL of the protein solution was mixed with 200 μL of 4% NaHCO3 solution. Two hundred μL of 0.1% TNBSA solution was added to the mixture and incubated at 37 °C for 1 h. Two hundred and fifty μL of 10% sodium dodecyl sulfate and 100 μL of 1 N HCl were added to stop the reaction, and the absorbance was read at 335 nm. Glycine was used as an amino standard. Carrier proteins onto which no Nic hapten was conjugated were used as a control. Hapten density was calculated from the differences between the O.D. of the control and the conjugates.
Assembly of NanoNicVac particles
NanoNicVac particles were assembled by a thiol-maleimide-mediated reaction.16 In brief, lipid-poly(lactic-co-glycolic acid) (PLGA) nanoparticles were fabricated according to a method described in Supplementary Information. An appropriate amount of Traut’s reagent was added to 6 mg of Nic-carrier protein conjugates that were dissolved in 2 mL of 0.01 M PBS. The mixture was incubated at room temperature for 1 h to form thiolated Nic-carrier protein conjugates. The activated conjugates were added to 75 mg of lipid-PLGA hybrid nanoparticles and incubated for 2 h. NanoNicVac nanoparticles were separated by centrifugation at 10,000 g, 4 °C for 30 min. Unconjugated Nic-carrier protein conjugates in the supernatants were quantified by the bicinchoninic acid assay.
In vivo study of the immunogenicity and efficacy of NanoNicVac in mice
Animal studies were carried out following the National Institutes of Health guidelines for animal care and use. Animal protocols were approved by the Institutional Animal Care and Use Committee at Virginia Tech. Animal studies were conducted once in this study with one batch of vaccine formulations. Female Balb/c mice (6–7 weeks, 5–6 per group) were immunized with nicotine vaccines or PBS on days 0, 14, and 28. For NanoNicVac groups, mice were injected with 200 μL of nanovaccines (Nano-KLH-Nic, Nano-KS-Nic, Nano-CRM197-Nic, or Nano-TT-Nic) containing 25 μg of protein antigens. For the Nic-TT conjugate group, mice were immunized with a mixture of Nic-TT equivalent to 25 μg of TT and 40 μg Alum that was dissolved in 200 μL of PBS. For the control group, mice were injected with 200 μL of sterilized PBS. Blood samples were collected on days 0, 12, 26, and 40.
Titers of anti-nicotine antibody, anti-nicotine IgG subclass antibody (IgG1, IgG2a, IgG2b, and IgG3), and anti-carrier protein antibody in the serum were assayed by an enzyme-linked immunosorbent assay (ELISA) using a method reported previously.16 Antibody titer was defined as the dilution factor at which absorbance at 450 nm dropped to half maximal.
The affinity and specificity of anti-nicotine antibodies induced by nicotine vaccines were estimated by a competition ELISA method. In brief, serum samples were diluted to a factor at which the absorbance at 450 nm was around 1. Inhibitors (nicotine, cotinine, nornicotine, nicotine-N-oxide, and acetylcholine) with concentrations of 10−2 to 10−6 M were serially prepared. Inhibitor samples were added to plates that were coated with Nic-BSA, and serum samples were subsequently added. The following steps were the same as in measuring anti-nicotine antibody titers. Percent inhibition was calculated at each inhibitor concentration, and the concentration at which 50% inhibition was achieved (IC50) was determined. Pooled serum samples were used for specificity estimation.
The ability of nicotine nanovaccines to reduce nicotine in the brain of mice was examined using a method reported previously.16 Female Balb/c mice (6–7 weeks, 5–6 per group) were immunized as described in the previous context. On day 42, mice were dosed 0.06 mg/kg of nicotine subcutaneously. After 3 min, mice were sacrificed, and the brain and blood samples were collected. The nicotine challenge study was conducted for 3 min because: 1) this time is sufficient for subcutaneously administered nicotine to reach to blood; 2) within this time, nicotine has not been significantly metabolized to other nicotine metabolites; 3) this time mimics the real smoking condition in which nicotine is rapidly transported into blood after exposure to lungs. The nicotine levels in the brain and serum samples were measured using a GC/MS method as reported previously.29
Statistical analyses
Data were expressed as means ± standard error of the mean (SEM) unless otherwise specified. Comparisons among multiple groups were conducted with one-way ANOVA followed by Tukey’s HSD test. Differences were considered significant when p-values were less than 0.05.
Results
Morphological and physicochemical properties of NanoNicVac conjugated with different carrier proteins
Confocal laser scanning microscopy (CLSM) was used to characterize the structure of NanoNicVac nanoparticles conjugated with different carrier proteins. The PLGA core, lipid shell, and carrier proteins were labeled by Nile Red, 7-nitro-2–1,3-benzoxadiazol-4-yl (NBD), and Alexa Fluor® 350 (AF-350), respectively. The co-localization of red, green, and blue fluorescence on most of the particles (Figure 1, B) suggested the successful and efficient assembly of NanoNicVac particles. The morphology of nanoparticles was characterized by transmission electron microscopy (TEM). As shown in Figure 1, C, a “core-shell” structure was shown on lipid-polymeric (LP) hybrid nanoparticles. Upon conjugation of Nic-carrier protein conjugates, a dark layer, which was formed by protein antigens, was observed on all four NanoNicVac nanoparticles. This further verified the efficient conjugation of protein antigens to hybrid nanoparticle surface.
The physicochemical properties of NanoNicVac were also characterized. As shown in Figure 1, D, all four NanoNicVac nanoparticles exhibited narrow size distributions. This narrow size distribution is in concordance with the uniform size shown in the TEM images (Figure 1, C) and the low PDI indexes (Table 1). Specifically, the average size of Nano-KLH-Nic (167.2 nm) and Nano-KS-Nic (153.2 nm) was slightly larger than that of Nano-CRM197-Nic (125.2 nm) and Nano-TT-Nic (136.6 nm) (Table 1). The four NanoNicVac nanoparticles, regardless of carrier proteins, were negatively charged (indicated by the negative zeta-potentials shown in Table 1), which was probably caused by the conjugation of negatively-charged Nic-carrier protein conjugates. The conjugation efficiency of Nic-carrier protein conjugates was 87.6 ± 7.9%, 83.2 ± 11.3%, 90.0 ± 7.6%, and 84.3 ± 9.4% for Nano-KLH-Nic, Nano-KS-Nic, Nano-CRM197-Nic, and Nano-TT-Nic, respectively (Table 1). Meanwhile, the loading contents of Nic-haptens on NanoNicVac particles were 0.88 ± 0.07, 0.93 ± 0.12, 0.84 ± 0.07, and 0.81 ± 0.09 μg Nic/mg nanoparticle, respectively. This suggested that the four NanoNicVac nanoparticles had similar hapten loading contents.
Table 1.
Physicochemical properties of NanoNicVac nanoparticles conjugated with different carrier proteins.
| NanoNicVac | Size (d. nm) | Zeta-potential (mV) | PDI | Conjugation efficiency (%) | Hapten loading (μg Nic/mg NP) |
|---|---|---|---|---|---|
| Nano-KLH-Nic | 167.2 ± 9.7 | −11.00 ± 0.98 | 0.256 ± 0.067 | 87.6 ± 7.9 | 0.88 ± 0.07 |
| Nano-KS-Nic | 153.2 ± 10.2 | −11.80 ± 0.93 | 0.271 ± 0.076 | 83.2 ± 11.3 | 0.91 ± 0.12 |
| Nano-CRM197-Nic | 125.2 ± 13.5 | −12.50 ± 0.75 | 0.230 ± 0.066 | 90.0 ± 7.6 | 0.86 ± 0.07 |
| Nano-TT-Nic | 136.6 ± 7.4 | −11.20 ± 2.07 | 0.218 ± 0.045 | 84.3 ± 9.4 | 0.81 ± 0.09 |
Data were expressed as means ± standard deviation (n = 3).
Cellular uptake and processing of NanoNicVac by dendritic cells
The uptake efficiency of NanoNicVac nanoparticles by dendritic cells were studied by flow cytometry. As shown in Figure 2, A, > 95.3% of the studied cells had taken up nanoparticles in all four NanoNicVac groups after being incubated with nanoparticles for 10 min. This revealed that NanoNicVac nanoparticles could be internalized by dendritic cells efficiently in a short period of time. As shown in Figure 2, B, indicated by the significantly increased mean fluorescence intensity (M. F. I.) of CM-6, NanoNicVac nanoparticles were continuously internalized from 10 to 90 min. However, the M. F. I. of CM-6 at 240 min was similar to that at 90 min, suggesting that the uptake of NanoNicVac was saturated after 90 min. Meanwhile, all four NanoNicVac nanoparticles, regardless of carrier proteins, had a similar cellular uptake efficiency, as they exhibited comparable M. F. I. of CM-6 at all the studied time points.
Figure 2.
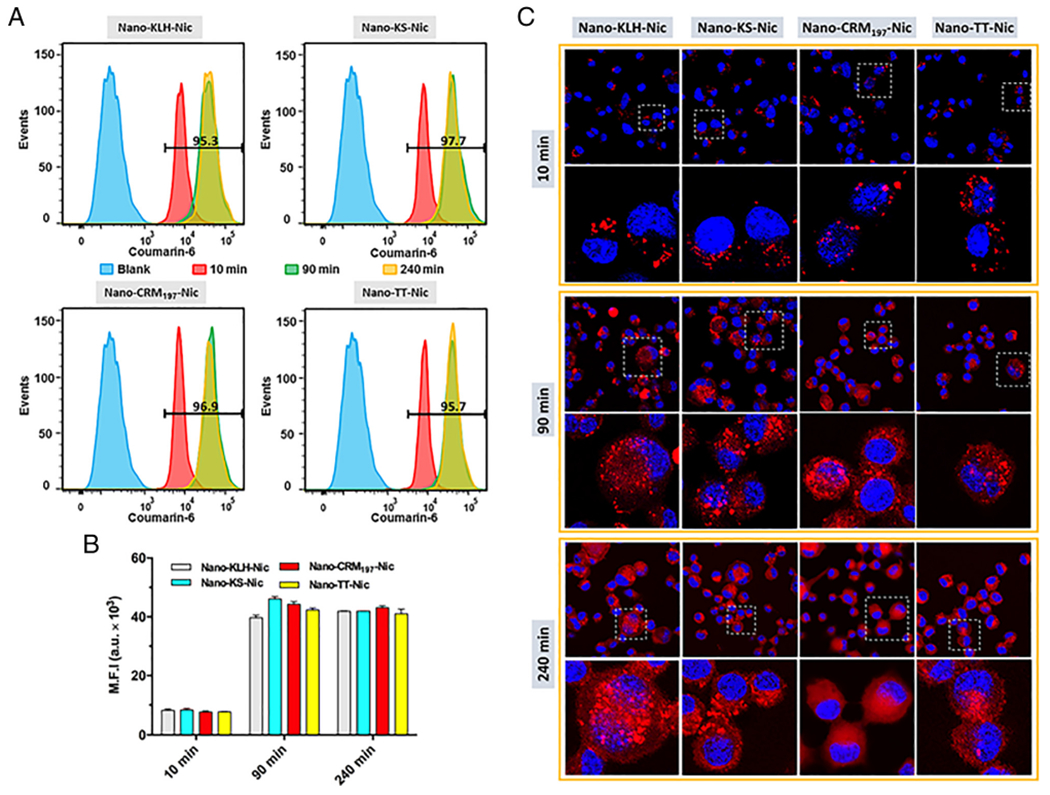
Cellular uptake and processing of NanoNicVac conjugated with different carrier proteins. (A) Intensity distribution and (B) M.F.I. of CM-6 fluorescence in cells treated with CM-6 labeled NanoNicVac nanoparticles for 10, 90, or 240 min. Blank represents non-treated cells. The numbers shown in (A) denote the percentage of CM-6 positive cells that had taken up nanoparticles after incubation for 10 min. (C) Processing of protein antigens carried by NanoNicVac particles. Protein antigens on NanoNicVac particles were labeled by AF647. Cells were treated with NanoNicVac particles for 10 or 90 min. The medium containing particles were replaced with fresh medium at 90 min, and cells were continuously incubated until 240 min.
The processing of carrier proteins carried by NanoNicVac was studied using CLSM (Figure 2, C). The carrier proteins on NanoNicVac particles were labeled by Alexa Fluor® 647 (AF647). At 10 min, the AF647 fluorescence displayed as individual dots in cells, revealing that the carrier proteins had not been processed. At 90 min, a substantial amount of AF647 fluorescence was found to spread throughout the cells. This suggested that the carrier proteins began to be processed to small peptidic antigens. At 240 min, a substantial percentage of AF647 fluorescence was still observed to display as individual dots in the Nano-KLH-Nic and Nano-KS-Nic groups, indicating KLH and KS carrier proteins had not been completely processed. In contrast, less red individual dots were found in cells treated with Nano-CRM197-Nic and Nano-TT-Nic, suggesting that CRM197 and TT carrier proteins were efficiently processed to small peptidic antigens. NanoNicVac conjugated with CRM197 or TT appeared to be processed more efficiently than that conjugated with KLH or KS.
Immunogenicity of NanoNicVac conjugated with different carrier proteins against nicotine
The immunogenicity of NanoNicVac against nicotine was tested in female Balb/c mice. As shown in Figure 3, A, comparable anti-nicotine antibody titers were found in all nicotine vaccine groups 12 days after the primary immunization (on day 12). The anti-nicotine antibody levels significantly increased in all vaccine groups 12 days after the first booster immunization (on day 26). Twelve days after the second booster immunization (on day 40), the anti-nicotine antibody titers increased by 7.5 × 10,3 5.6 × 10,3 26.3 × 10,3 17.5 × 10,3 and 4.8 × 103 in Nano-KLH-Nic, Nano-KS-Nic, Nano-CRM197-Nic, Nano-TT-Nic, and Nic-TT + alum groups, respectively, compared to that on day 26. The second booster immunization boosted antibody titers in the groups of Nano-CRM197-Nic and Nano-TT-Nic more significantly than in the other groups. The end-point anti-nicotine antibody titers of individual mice on day 40 are shown in Figure 3, B. Compared to TT-Nic + alum, Nano-TT-Nic induced a significantly higher anti-nicotine antibody titer (p < 0.05). This suggests that conjugating hapten-protein conjugates to hybrid nanoparticles would enhance the immunogenicity of the conjugate nicotine vaccine. The titers of Nano-CRM197-Nic and Nano-TT-Nic were comparable (p > 0.91), and were significantly higher than that of Nano-KLH-Nic and Nano-KS-Nic (p < 0.05). These indicate NanoNicVac conjugated with CRM197 or TT had an enhanced immunogenicity against nicotine when compared to NanoNicVac carrying KLH or KS.
Figure 3.
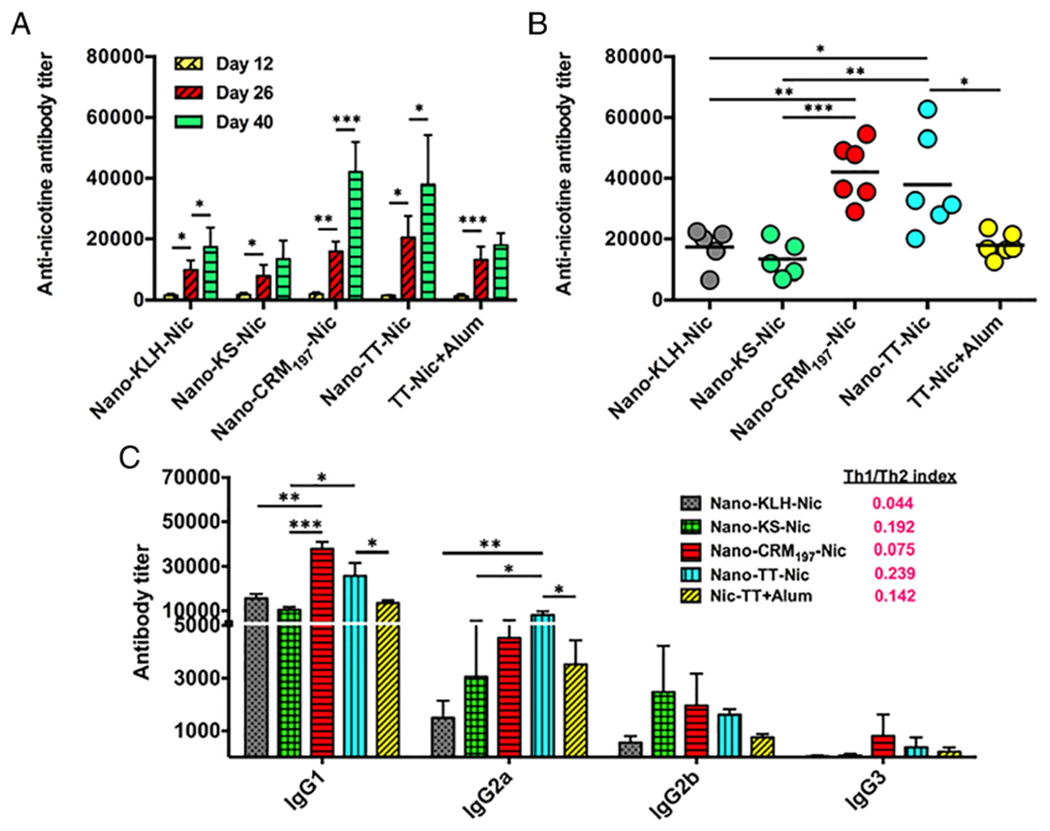
Immunogenicity of NanoNicVac conjugated with different carrier proteins against nicotine. (A) Time-course of the anti-nicotine antibody titers induced by NanoNicVac. Bars are shown as means ± standard deviation. (B) End-point anti-nicotine antibody titers of individual mice on day 40. (C) Titers of anti-nicotine IgG subclass antibodies and Th1/Th2 indexes induced by NanoNicVac on day 40. Significantly different: * p < 0.05, ** p < 0.01, *** p < 0.001.
Subclass distribution of anti-nicotine IgG antibodies elicited by NanoNicVac
The titers of anti-nicotine IgG subclass antibodies on day 40 were assayed and presented in Figure 3, C. For all vaccine groups, IgG1 and IgG3 were the most and least dominant subtype, respectively. Compared to Nic-TT conjugate vaccine, Nano-TT-Nic resulted in higher titers of all four IgG subtypes, especially IgG1 and IgG2a, which are consistent with our previous report.16 Nano-CRM197-Nic and Nano-TT-Nic induced higher levels of IgG1, IgG2a, and IgG3 than Nano-KLH-Nic and Nano-KS-Nic. Specifically, Nano-CRM197-Nic generated the highest IgG1 titer among the four NanoNicVac vaccines. The IgG1 titer of Nano-CRM197-Nic was significantly higher than that of Nano-KLH-Nic and Nano-KS-Nic (p < 0.01). Nano-TT-Nic induced the highest IgG2a titer among the four NanoNicVac vaccines, and the IgG2a titer of Nano-TT-Nic was significantly higher than that of Nano-KLH-Nic and Nano-KS-Nic (p < 0.05). Interestingly, although the overall IgG titer of Nano-KLH-Nic is slightly higher than that of Nano-KS-Nic (Figure 3, B), Nano-KLH-Nic had a higher IgG1 titer but lower IgG2a and IgG2b titers compared to Nano-KS-Nic. The Th1/Th2 indexes were 0.044, 0.192, 0.075, 0.239, and 0.142 for Nano-KLH-Nic, Nano-KS-Nic, Nano-CRM197-Nic, Nano-TT-Nic, and Nic-TT + alum, respectively. All the values were considerably less than 1, indicating that the immune responses induced by all nicotine vaccines were skewed toward Th2 (humoral response).
Anti-carrier protein antibody levels induced by NanoNicVac carrying different carrier proteins
Anti-carrier protein antibody titers were assayed and shown in Figure 4. Similar to anti-nicotine antibody titers, the anti-carrier protein antibody titers increased after each injection. On day 12, the anti-carrier protein antibody titers were (1.8 ± 0.1) × 10,3 (1.9 ± 0.2) × 10,3 (0.5 ± 0.1) italic 10,3 (1.9 ± 0.1) × 10,3 and (3.3 ± 0.1) × 10,3 for Nano-KLH-Nic, Nano-KS-Nic, Nano-CRM197-Nic, Nano-TT-Nic, and Nic-TT + alum, respectively. On day 26, the titers increased to (35.3 ± 2.2) × 10,3 (35.2 ± 2.5) × 10,3 (16.0 ± 6.0) × 10,3 (23.5 ± 12.8) × 10,3 and (42.2 ± 4.2) × 10,3 respectively. On day 40, the titers further increased to (46.2 ± 1.8) × 10,3 (50.9 ± 4.6) × 10,3 (27.5 ± 2.9) × 103, (36.6 ± 2.5) × 10,3 and (51.4 ± 4.0) × 10,3 respectively. On all the studied days, Nano-TT-Nic induced significantly lower anti-carrier protein antibody titers compared to Nic-TT + alum (p < 0.05). Among the four NanoNicVac carrying different carrier proteins, Nano-CRM197-Nic and Nano-TT-Nic elicited considerably lower anti-carrier protein antibody levels than Nano-KLH-Nic and Nano-KS-Nic, especially on days 26 and 40.
Figure 4.
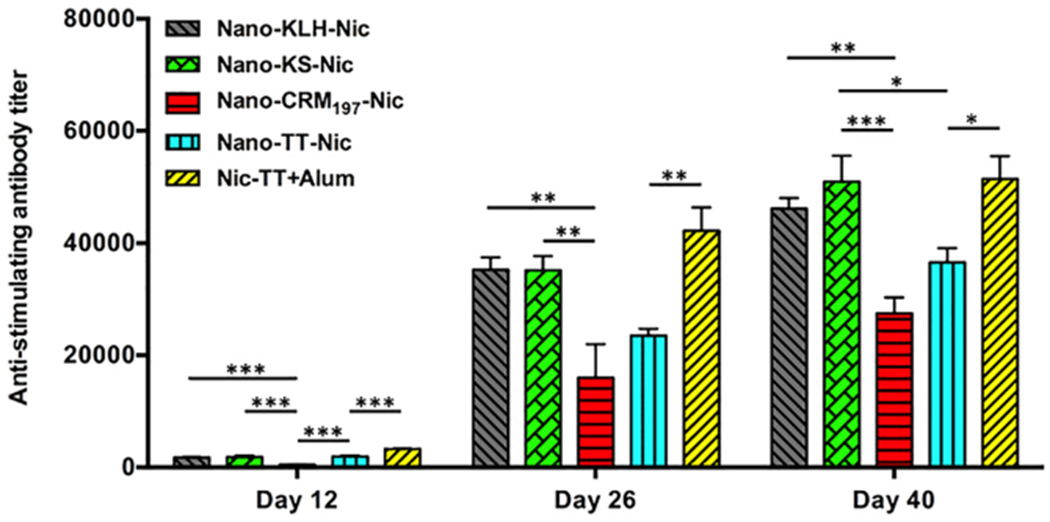
Time-course of anti-carrier protein antibody titers induced by NanoNicVac with different carrier proteins. Significantly different: * p < 0.05, ** p < 0.01, *** p < 0.001.
Affinity of anti-nicotine antibodies generated by NanoNicVac
The affinity of anti-nicotine antibodies elicited by NanoNicVac carrying different carrier proteins was estimated by competition ELISA on days 12, 26, and 40 (Figure 5). The affinity of antibodies increased after each immunization in all nicotine vaccine groups, except the Nano-KLH-Nic group, in which the antibody affinity slightly decreased after the second booster immunization. On day 40, the IC50 of nicotine was 96 ± 35, 137 ± 92, 167 ± 78, 212 ± 103, and 277 ± 199 μM for Nano-KLH-Nic, Nano-KS-Nic, Nano-CRM197-Nic, Nano-TT-Nic, and Nic-TT + alum, respectively. The antibodies induced by Nano-TT-Nic had a comparable affinity to that elicited by Nic-TT + alum (p > 0.99). Nano-KLH-Nic resulted in the highest average antibody affinity, but the differences among the four NanoNicVac were not significant (p > 0.92). Interestingly, the maturation of anti-nicotine antibody affinity exhibited different patterns in the four NanoNicVac groups. Specifically, the maturation of antibody affinity in the Nano-KLH-Nic and Nano-KS-Nic groups was significantly completed after the first booster immunization, and the second booster immunization did not remarkably enhance the antibody affinity. In contrast, the anti-nicotine antibody affinity gradually matured in the Nano-CRM197-Nic and Nano-TT-Nic groups. Both the first and second booster immunizations remarkably promoted the affinity maturation.
Figure 5.
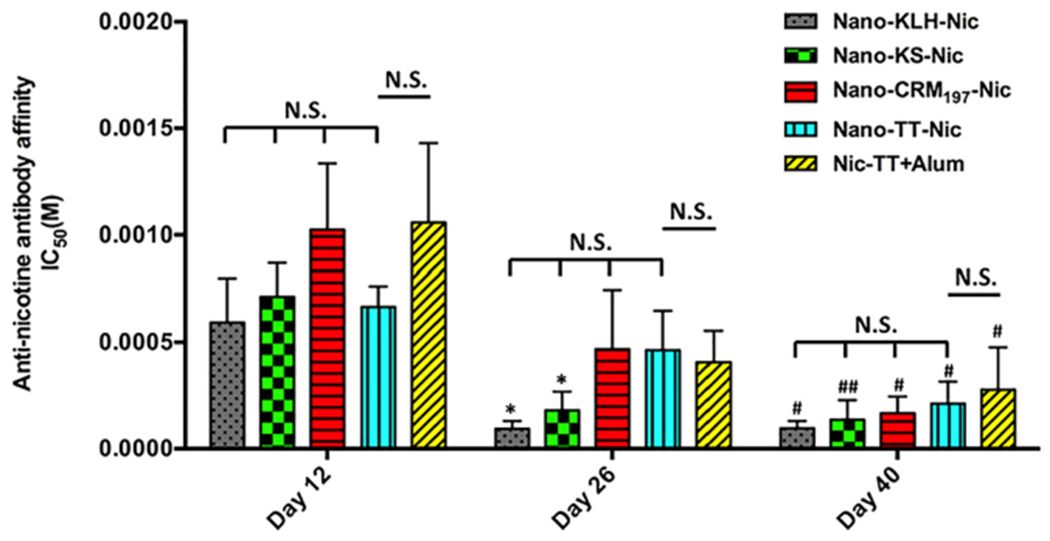
Affinity of anti-nicotine antibodies induced by nicotine vaccines estimated by competition ELISA. N.S. indicates no significant differences were found among groups (p > 0.55). Significantly different compared to the previous studied day: * p < 0.05. Significantly different compared to day 12: # p < 0.05, ## p < 0.01.
Specificity of anti-nicotine antibodies elicited by NanoNicVac
The specificity of anti-nicotine antibodies on day 40 was assayed by competition ELISA. The dose-dependent inhibitions of nicotine binding by nicotine metabolites (cotinine, nornicotine, and nicotine-N-oxide) and endogenous nicotine receptor ligand (acetylcholine) are shown in Figure 6. As shown in Figure 6, A–E, in all nicotine vaccine groups, anti-nicotine antibodies had the highest relative affinity to nicotine. A somewhat lower affinity was detected to the inactive nicotine metabolite (cotinine) and active but minor nicotine metabolite (nornicotine) in all nicotine vaccine groups. Specifically, the cross-reactivity between nicotine and cotinine was less than 2%, and that between nicotine and nornicotine was less than 7%, in all groups (Figure 6, F). Meanwhile, antibodies elicited by all nicotine vaccines had little affinity for the inactive nicotine metabolite (nicotine-N-oxide) and endogenous nicotine receptor ligand (acetylcholine). The cross-reactivity between nicotine and nicotine-N-oxide/acetylcholine was less than 1% in all groups (Figure 6, F). The anti-nicotine antibodies generated by NanoNicVac, regardless of carrier proteins, exhibited high specificity for nicotine.
Figure 6.
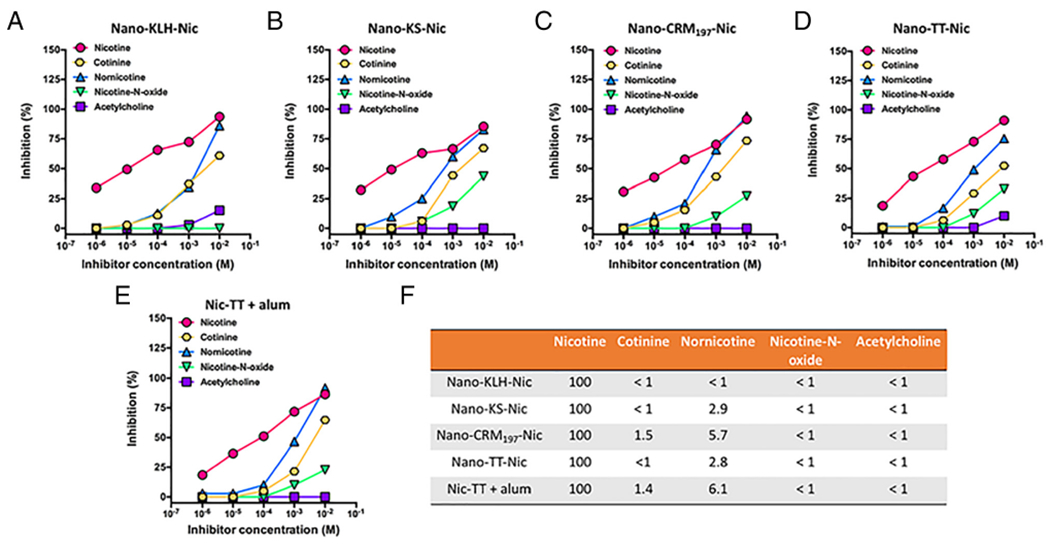
Specificity of anti-nicotine antibodies induced by NanoNicVac conjugated with different carrier proteins. Dose-dependent inhibitions of nicotine binding by various inhibitors in groups of (A) Nano-KLH-Nic, (B) Nano-KS-Nic, (C) Nano-CRM197-Nic, (D) Nano-TT-Nic, and (E) Nic-TT + alum were estimated by competition ELISA. (F) Percent ligand cross-reactivity defined as (IC50 of nicotine/IC50 of inhibitors).
Pharmacokinetic efficacy of NanoNicVac conjugated with different carrier proteins
The ability of NanoNicVac to retain nicotine in serum and reduce nicotine in the brain of mice was evaluated. Figure 7, A shows the serum nicotine levels of mice 3 min after being challenged with 0.06 mg/kg nicotine subcutaneously. More nicotine was retained in serum after immunization with NanoNicVac, regardless of the carrier proteins used. Nano-CRM197-Nic and Nano-TT-Nic exhibited considerably better abilities for sequestering nicotine in the serum of mice than Nano-KLH-Nic and Nano-KS-Nic. The brain nicotine levels of mice after being challenged with nicotine are shown in Figure 7, B. All NanoNicVac groups, regardless of the carrier proteins used, had significantly lower brain nicotine concentrations than the PBS-treated group (p < 0.001). Specifically, the brain nicotine levels were lowered by 48.5%, 45.9%, 65.2%, and 63.1% after treatment with Nano-KLH-Nic, Nano-KS-Nic, Nano-CRM197-Nic, and Nano-TT-Nic groups, compared to nicotine levels in the PBS-treated group. Nano-CRM197-Nic and Nano-TT-Nic had a significantly better capability for reducing nicotine in the brain of mice than Nano-KS-Nic (p < 0.05). Meanwhile, Nano-CRM197-Nic and Nano-TT-Nic also exhibited a considerably better ability in reducing the brain nicotine concentrations than Nano-KLH-Nic. Overall, NanoNicVac conjugated with CRM197 or TT had an enhanced efficacy in sequestering nicotine in serum and reducing nicotine levels in the brain than NanoNicVac conjugated with KLH or KS.
Figure 7.
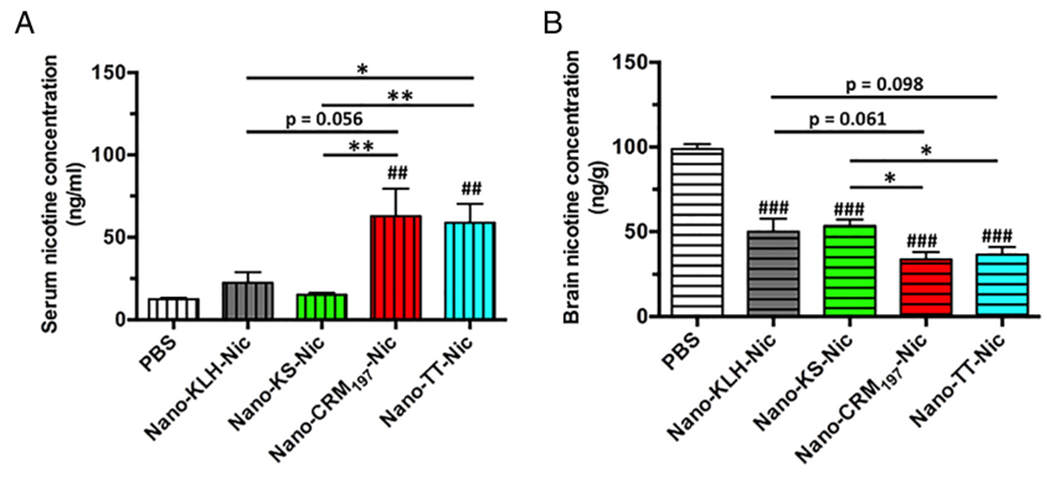
Pharmacokinetic efficacy of NanoNicVac conjugated with different carrier proteins. The nicotine levels in the serum and brain of mice were analyzed 3 min after challenging the mice with 0.06 mg/kg nicotine subcutaneously. Significantly different compared to the PBS-treated group: ## p < 0.01, ### p < 0.001. Significantly different: * p < 0.05, ** p < 0.01.
Discussion
Conventional hapten-protein conjugate nicotine vaccines tested in human clinical trials have not proven to enhance overall smoking cessation rate so far.5,10,11 In our previous work, we suggested a novel strategy to improve the immunological efficacy of conjugate nicotine vaccines by using biodegradable lipid-polymeric hybrid nanoparticles as delivery vehicles.16,17 The hybrid nanoparticle-based nicotine nanovaccine (NanoNicVac) was demonstrated to have a significantly higher immunogenicity than the conjugate nicotine vaccine. In addition, we proved that the immunogenicity of NanoNicVac could be enhanced by modulating the particle size,16 hapten density,18 and hapten localization.19 In this study, we developed a series of NanoNicVac in which various potent carrier proteins were conjugated, and systemically studied their physicochemical properties, cellular uptake and processing by immune cells, immunogenicity, and pharmacokinetic efficacy. We demonstrated in this current work that enhanced immunological efficacy could be achieved by using CRM197 or TT instead of KLH or KLH subunit as carrier proteins, making NanoNicVac be a promising next-generation nanoparticle-based immunotherapeutic against nicotine addiction.
The ELISA results demonstrated that NanoNicVac conjugated with TT (Nano-TT-Nic) exhibited a significantly higher immunogenicity against nicotine over Nic-TT even in the absence of alum adjuvant. This result is in agreement with our previous report that Nano-KLH-Nic was more immunogenic against nicotine than the Nic-KLH conjugate.16 Also, the result strengthened our hypothesis that the use of hybrid nanoparticles as delivery vehicles improves the immunogenicity of conjugate nicotine vaccines. The higher immunogenicity of Nano-TT-Nic over Nic-TT may be attributed to the better recognition and internalization by immune cells. The conjugation of multiple TT-Nic to one hybrid nanoparticle may increase the availability of antigens for uptake, thus contributing to an enhanced antigen internalization. Meanwhile, the immune system prefers to recognize and take up particulate pathogens (such as bacteria and virus) and is relatively invisible to small soluble protein antigens.30–32 The stable and spherical lipid-polymeric hybrid nanoparticles33–38 endowed Nano-TT-Nic with a particulate property that mimics that of particulate pathogens. This particulate nature together with the optimal particle size (~100 nm) is beneficial for improved recognition and uptake by immune cells.16,39
Efficient uptake and processing of NanoNicVac by antigen presenting cells (such as dendritic cells and macrophages) are prerequisites for the generation of a potent immune response.5,40,41 The in vitro data demonstrated that NanoNicVac conjugated with different carrier proteins were similarly taken up but differently processed by dendritic cells. All NanoNicVac developed in this study, regardless of the carrier proteins used, were found to be internalized rapidly and efficiently. The rapid and efficient internalization of vaccine particles may provide sufficient amounts of antigens for processing, and thus contributes to the generation of a quick immune response. The CLSM data suggested that Nano-CRM197-Nic and Nano-TT-Nic, especially Nano-CRM197-Nic, were processed more efficiently than Nano-KLH-Nic and Nano-KS-Nic. This higher effectiveness of antigen processing may be attributed to the smaller size and lower structural complexity of CRM197 and TT as carrier proteins. KS has a molecular weight of ~400 kDa, and KLH multimer is an assembled form of multiple KS.42 Both of them have a relatively high structural complexity due to the large size. In contrast, CRM197 and TT have a molecular weight of ~150 kDa and ~59 kDa, respectively. The relatively small size makes them have a relatively low structural complexity.43,44 Immunologically, the generation of an effective humoral immune response requires two T cell-dependent processes, the formation of T helper cells and the interaction between B cells and T-helper cells, both of which only occur via the presentation of peptidic antigens on MHC of antigen presenting cells.23,45 Thus, the efficient processing of protein antigens to peptidic antigens may enhance the T cell-dependent processes, subsequently leading to a potent humoral immune response.
The immunogenicity data demonstrated that Nano-CRM197-Nic and Nano-TT-Nic could induce significantly higher anti-nicotine antibody titers and considerably lower anti-carrier protein antibody titers than Nano-KLH-Nic and Nano-KS-Nic. The lower antibody titers against carrier proteins induced by Nano-CRM197-Nic and Nano-TT-Nic may be caused by the relatively smaller size of CRM197 and TT. Compared with larger KS and KLH multimer, smaller CRM197 and TT had fewer immunogenic epitopes available for B cells, thus producing fewer anti-carrier protein antibodies. A lower anti-carrier protein antibody level is desirable in nicotine vaccine design, as the anti-carrier protein antibodies may neutralize the vaccine particles that are injected during booster immunizations. This neutralization may cause wastages and impair the efficacy of nicotine vaccines.17,46 Noticeably, the levels of anti-nicotine antibodies induced by NanoNicVac were in concordance with the effectiveness of antigen processing by dendritic cells. As discussed in the earlier context, the efficient processing of protein antigens that were carried by Nano-CRM197-Nic and Nano-TT-Nic would result in a potent T-cell immunity and contribute to an enhanced immunogenicity against nicotine. Interestingly, the second booster immunization boosted the anti-nicotine antibody titers in Nano-CRM197-TT and Nano-TT-Nic groups more remarkably than in Nano-KLH-Nic and Nano-KS-Nic groups. Although we do not have direct evidences to show the mechanism, the following may explain the finding. On one hand, the higher effectiveness of Nano-CRM197-Nic and Nano-TT-Nic in generating a T-cell immunity may enhance the humoral immune response, resulting in more anti-nicotine antibodies to be generated. On the other hand, Nano-CRM197-Nic and Nano-TT-Nic had lower anti-carrier protein antibody titers than Nano-KLH-Nic and Nano-KS-Nic after the first booster immunization. The lower anti-carrier protein antibody levels may neutralize fewer vaccine particles administered in the second booster immunization, and thus leave more vaccine particles available for inducing the production of anti-nicotine antibodies. In agreement with the data of anti-nicotine antibody titer, affinity, and specificity, the pharmacokinetic data showed that NanoNicVac conjugated with CRM197 or TT exhibited better capability to sequester nicotine in serum and reduce nicotine entering the brain than NanoNicVac conjugated with KLH or KS. It should be noted that the difference in immunological efficacy among NanoNicVac conjugated with different carrier proteins may also result from differential levels of antigen presentation, variable proteolytic susceptibility of different carrier proteins and a host of other facts in vivo. The exact mechanism needs to be further illustrated in future study.
In summary, a series of hybrid nanoparticle-based nicotine nanovaccines (NanoNicVac) were developed in this study by conjugating potent carrier proteins (KLH, KS, CRM197, and TT) to the nanoparticle surface. Although all four NanoNicVac were taken up by dendritic cells efficiently, NanoNicVac conjugated with CRM197 or TT were processed more efficiently than that conjugated with KLH or KS. In addition, compared to NanoNicVac carrying KLH or KS, NanoNicVac conjugated with CRM197 or TT induced remarkably higher anti-nicotine antibody titers and considerably lower anti-carrier protein antibody levels. Meanwhile, the anti-nicotine antibodies induced by all four NanoNicVac, regardless of the carrier proteins used, exhibited high affinity and specificity to nicotine. Also, NanoNicVac conjugated with CRM197 or TT had better capability to reduce nicotine in the brain of mice than NanoNicVac conjugated with KLH or KS. This study illustrated the necessity of selecting potent carrier proteins in maximizing the immunological efficacy of nicotine nanovaccine. The findings can potentially be applied in the development of other drug abuse and nanoparticle-based vaccines. Furthermore, NanoNicVac with boosted immunological efficacy could be a promising candidate for treating nicotine addiction.
Acknowledgements
We thank Dr. Andrew Lees from Fina Biosolutions for providing us with CRM197.
Financial support: This work was financially supported by the National Institute of Health (National Institute on Drug Abuse) through grant number U01DA036850.
Footnotes
Conflict of interest: All authors declare that there are no conflicts of interest.
References
- 1.Benowitz NL. Nicotine addiction. N Engl J Med 2010;362:2295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prochaska JJ, Benowitz NL. The past, present, and future of nicotine addiction therapy. Annu Rev Med 2016;67:467–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polosa P, Benowitz NL. Treatment of nicotine addiction: present therapeutic options and pipeline developments. Trends Pharmacol Sci 2011;32:281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreno AY, Janda KD. Immunopharmacotherapy: vaccination strategies as a treatment for drug abuse and dependence. Pharmacol Biochem Behav 2009;92:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pentel PR, LeSage MG. New directions in nicotine vaccine design and use. Emerging Targets & Therapeutics in the Treatment of Psychostimulant Abuse. 2014;69:553–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeigler DF, Roque R, Clegg CH. Construction of an enantiopure bivalent nicotine vaccine using synthetic peptides. PLoS One 2017;12e0178835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fraleigh NL, Boudreau J, Bhardwaj N, Eng NF, Murad Y, Lafrenie R, et al. Evaluating the immunogenicity of an intranasal vaccine against nicotine in mice using the adjuvant Finlay Proteoliposome (AFPL1). Heliyon 2016;2e00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lockner WJ, Lively JM, Collins KC, Vendruscolo JCM, Azar MR, Janda KD. A conjugate vaccine using enantiopure hapten imparts superior nicotine-binding capacity. J Med Chem 2015;58:1005–11. [DOI] [PubMed] [Google Scholar]
- 9.Jacob NT, Lockner JW, Schlosburg JE, Ellis BA, Eubanks LM, Janda KD. Investigations of enantiopure nicotine haptens using an adjuvanting carrier in anti-nicotine vaccine development. J Med Chem 2016;59:2523–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornuz J, Zwahlen S, Jungi WF, Osterwalder J, Klingler K, van Melle G, et al. A vaccine against nicotine for smoking cessation: a randomized controlled trial. PLoS One 2008;3e2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatsukami DK, Jorenby DE, Gonzales D, Rigotti NA, Glover ED, Oncken CA, et al. Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clin Pharmacol Ther 2011;89:392–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goniewicz ML, Delijewski M. Nicotine vaccines to treat tobacco dependence. Hum Vaccin Immunother 2013;9:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raupach T, Hoogsteder PH, Onno van Schayck CP. Nicotine vaccines to assist with smoking cessation: current status of research. Drugs 2012;72: e1–e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu Y, Zheng H, Huang W, Zhang C. A novel and efficient nicotine vaccine using nano-lipoplex as a delivery vehicle. Hum Vaccin Immunother 2014;10:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng H, Hu Y, Huang W, de Villiers S, Pentel P, Zhang JF, et al. Negatively charged carbon nanohorn supported cationic liposome nanoparticles: a novel delivery vehicle for anti-nicotine vaccine. J Biomed Nanotechnol 2015;11:2197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao Z, Hu Y, Hoerle R, Devine M, Raleigh M, Pentel P, et al. A nanoparticle-based nicotine vaccine and the influence of particle size on its immunogenicity and efficacy. Nanomedicine 2016;13:443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu Y, Smith D, Frazier E, Hoerle R, Ehrich M, Zhang C. The next-generation nicotine vaccine: a novel and potent hybrid nanoparticle-based nicotine vaccine. Biomaterials 2016;106:228–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Z, Powers K, Hu Y, Raleigh M, Pentel P, Zhang C. Engineering of a hybrid nanoparticle-based nicotine nanovaccine as a next-generation immunotherapeutic strategy againstnicotine addiction: a focus on hapten density. Biomaterials 2017;123:107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Z, Hu Y, Harmon T, Pentel P, Ehrich M, Zhang C. Rationalization of a nanoparticle-based nicotine nanovaccine as an effective next-generation nicotine vaccine: a focus on hapten localization. Biomaterials 2017;138:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Z, Harris B, Hu Y, Harmon T, Pentel PR, Ehrich M, et al. Rational incorporation of molecular adjuvants into a hybrid nanoparticle-based nicotine vaccine for immunotherapy against nicotine addiction. Biomaterials 2018;155:165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crotty S. A brief history of T cell help to B cells. Nat Rev Immunol 2015;15:185–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature 1996;383:787–93. [DOI] [PubMed] [Google Scholar]
- 23.Collins KC, Janda KD. Investigating hapten clustering as a strategy to enhance vaccines against drugs of abuse. Bioconjug Chem 2014;25:593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fraser CC, Altreuter DH, Ilyinskii P, Pittet L, LaMothe RA, Keegan M, et al. Generation of a universal CD4 memory T cell recall peptide effective in humans, mice and non-human primates. Vaccine 2014;32:2896–903. [DOI] [PubMed] [Google Scholar]
- 25.Bi SG, Bailey W, Brisson C. Performance of keyhole limpethemocyanin (KLH) as an antigen carrier for protein antigens depends on KLH property and conjugation route. J Immunol 2016;196. [Google Scholar]
- 26.Zhong TY, Arancibia S, Born R, Tampe R, Villar J, Del Campo M, et al. Hemocyanins stimulate innate immunity by inducing different temporal patterns of proinflammatory cytokine expression in macrophages. J Immunol 2016;196:4650–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCluskie MJ, Thorn J, Gervais DP, Stead DR, Zhang NL, Benoit M, et al. Anti-nicotine vaccines: comparison of adjuvanted CRM197 and Qb-VLP conjugate formulations for immunogenicity and function in non-human primates. Int Immunopharmacol 2015;29:663–71. [DOI] [PubMed] [Google Scholar]
- 28.Haile CN, Kosten TA, Shen XY, O’Malley PW, Winoske KJ, Kinsey BM, et al. Altered methamphetamine place conditioning in mice vaccinated with a succinyl-methamphetamine-tetanus-toxoid vaccine. Am J Addict 2015;24:748–55. [DOI] [PubMed] [Google Scholar]
- 29.de Villiers SHL, Cornish KE, Troska AJ, Pravetoni M, Pentel PR. Increased efficacy of a trivalent nicotine vaccine compared to a dose-matched monovalent vaccine when formulated with alum. Vaccine 2013;31:6185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Storni T, Kundig TM, Senti G, Johansen P. Immunity in response to particulate antigen-delivery systems. Adv Drug Deliv Rev 2005;57:333–55. [DOI] [PubMed] [Google Scholar]
- 31.Benne N, van Duijn J, Kuiper J, Jiskoot W, Slutter B. Orchestrating immune responses: how size, shape and rigidity affect the immunogenicity of particulate vaccines. J Control Release 2016;234:124–34. [DOI] [PubMed] [Google Scholar]
- 32.De Temmerman ML, Rejman J, Demeester J, Irvine DJ, Gander B, De Smedt SC. Particulate vaccines: on the quest for optimal delivery and immune response. Drug Discov Today 2011;16:569–82. [DOI] [PubMed] [Google Scholar]
- 33.Zheng MB, Yue CX, Ma YF, Gong P, Zhao PF, Zheng CF, et al. Singlestep assembly of DOX/ICG loaded lipid-polymer nanoparticles for highly effective chemo-photothermal combination therapy. ACS Nano 2013;7:2056–67. [DOI] [PubMed] [Google Scholar]
- 34.Zhang LF, Chan JM, Gu FX, Rhee JW, Wang AZ, Radovic-Moreno AF, et al. Self-assembled lipid-polymer hybrid nanoparticles: a robust drug delivery platform. ACS Nano 2008;2:1696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu Y, Hoerle R, Ehrich M, Zhang C. Engineeringthe lipid layer of lipid-PLGA hybrid nanoparticles for enhanced in vitro cellular uptake and improved stability. Acta Biomater 2015;28:149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu Y, Zhao Z, Ehrich M, Fuhrman K, Zhang C. In vitro controlled release of antigen in dendritic cells using pH-sensitive liposomepolymeric hybrid nanoparticles. Polymer 2015;80:171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hadinoto K, Sundaresan A, Cheow WS. Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review. Eur J Pharm Biopharm 2013;85:427–43. [DOI] [PubMed] [Google Scholar]
- 38.Mandal B, Bhattacharjee H, Mittal N, Sah H, Balabathula P, Thoma LA, et al. Core-shell-type lipid-polymer hybrid nanoparticles as a drug delivery platform. Nanomedicine 2013;9:474–91. [DOI] [PubMed] [Google Scholar]
- 39.Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol 2010;10:787–96. [DOI] [PubMed] [Google Scholar]
- 40.Metlay JP, Pure E, Steinman RM. Control of the immune-response at the level of antigen-presenting cells - a comparison of the function of dendritic cells and lymphocytes-B. Adv Immunol 1989;47:45–116. [DOI] [PubMed] [Google Scholar]
- 41.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998;392:245–52. [DOI] [PubMed] [Google Scholar]
- 42.Harris JR, Markl J. Keyhole limpet hemocyanin (KLH): a biomedical review. Micron 1999;30:597–623. [DOI] [PubMed] [Google Scholar]
- 43.Broker M, Costantino P, DeTora L, McIntosh ED, Rappuoli R. Biochemical and biological characteristics of cross-reacting material 197 (CRM197), a non-toxic mutant of diphtheria toxin: use as a conjugation protein in vaccines and other potential clinical applications. Biologicals 2011;39:195–204. [DOI] [PubMed] [Google Scholar]
- 44.Pichichero ME. Protein carriers of conjugate vaccines: characteristics, development, and clinical trials. Hum Vaccin Immunother 2013;9:2505–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu XW, Xu Y, Yu T, Clifford C, Liu Y, Yan H, et al. A DNA nanostructure platform for directed sssembly of synthetic vaccines. Nano Lett 2012;12:4254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skolnick P. Biologic approaches to treat substance-use disorders. Trends Pharmacol Sci 2015;36:628–35. [DOI] [PMC free article] [PubMed] [Google Scholar]


