Abstract
Background: Rhazya stricta has been used as a folkloric medicinal herb for treating various diseases such as diabetes, inflammatory disorders, and sore throat. Several studies have revealed the potential of this plant as an important source of phytochemicals with anticancer properties. Objective: The present study was designed to isolate a novel anticancer compound from Rhazya stricta and elucidate its mechanism of action using genomics approach. Methods: Rhazya stricta leaves extract was prepared, and several alkaloids were purified and characterized. These alkaloids were screened for their anticancer potential. One of the alkaloids, termed as isopicrinine, showed efficient cytotoxicity against MCF7 breast cancer cell line and was selected for further analysis. RNA-Seq transcription profiling was conducted to identify the affected genes and cellular pathways in MCF7 cells after treatment with isopicrinine alkaloid. Results: In vitro studies revealed that newly identified isopicrinine alkaloid possess efficient anticancer activity. Exposure of MCF7 cells with isopicrinine affected the expression of various genes involved in p53 signaling pathway. One of the crucial proapoptotic genes, significantly upregulated in MCF7 after exposure to alkaloid, was PUMA (p53 upregulated modulator of apoptosis), which is involved in p53-dependent and -independent apoptosis. Moreover, exposure of sublethal dose of isopicrinine alkaloid in breast cancer cell line led to the downregulation of survivin, which is involved in negative regulation of apoptosis. Besides, several genes involved in mitosis and cell proliferation were significantly downregulated. Conclusion: In this article, we report the determination of a new alkaloid isopicrinine from the aerial parts of Rhazya stricta with anticancer property. This compound has the potential to be developed as a drug for curing cancer.
Keywords: Rhazya stricta, alkaloids, isopicrinine, anticancer, RNA-Seq, gene expression profile
Introduction
Rhazya stricta has been used traditionally for the treatment of various diseases in many Middle East and South Asian countries. R stricta is known to be a rich source of several potent compounds with novel structures and with wide variety of pharmaceutical applications for treatment of various diseases such as diabetes, inflammatory diseases, sore throat, helminthesis, arthritis, and cancer.1 R stricta produces a large number of indoloterpenoid alkaloids. More than 100 alkaloids with diverse structure and pharmacological properties have been purified and characterized from the leaves, root, stem, and fruits of R stricta.2-4
Plant alkaloids are a diverse group of chemical compounds that contain a ring structure and nitrogen atoms. More than 27 683 alkaloids have been identified from various natural resources as mentioned in the Dictionary of Natural products. However, only 53 alkaloids have been developed successfully as drugs for therapeutic applications ranging from cough suppressants to antimalarial agents. Vast majority of alkaloids from natural resources are yet to be explored and developed as potent drugs for treatment of various diseases in human. Alkaloids are known to have wide variety of pharmacological activities and have been used as therapeutics for treatment of various diseases such as malaria (eg, quinine), asthma (ephedrine, theobromine, theophylline), hypertension (rescinnamine, reserpine), and cancer (vinblastine, vincristine, homoharringtonine).
Rhazya stricta is a rich source of various alkaloids with diverse structure and function. Some of the alkaloids isolated from R stricta, such as akuammidine, rhazimanine, stemmadenine, strictanol, and tetrahydrosecaminediol, have shown potential antimicrobial activity against various bacterial pathogens. These alkaloids, which are predominantly present in the leaves of the plants, have shown potent antibacterial activity against Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, and Candida albicans. Several studies have demonstrated the anticancer, antioxidant, and free radical scavenging properties of the phytochemicals present in R stricta.2,4 Leaf extract of R stricta has been shown to induce apoptosis in various cancer cell lines including breast, brain, and lung cancer cell lines.5,6 Alkaloids such as sewarine, vallesiachotamine, tetrahydrosecamine, tetrahydrosecaminediol, and strictanol have been shown to possess anticarcinogenic properties.3,7 Rhazinilam has been shown to exert cytotoxic activity by inhibiting microtubule assembly and promoting the growth of abnormal tubulin spirals.8
The main objective of our research work is to evaluate the therapeutic potential of new alkaloid isopicrinine from R stricta for treatment of cancer and delineate its mechanism of action by analyzing the differential gene expression changes due to the drug action.
Materials and Methods
Extraction and Purification of Alkaloids From Rhazya stricta
The plant leaves were collected from the Bahrah region, 30 km southeast Jeddah, Saudi Arabia. The identity of the plant was authenticated by a senior botanist, and a sample was deposited at our herbarium repositories in the Department of Biological Sciences, King Abdulaziz University, Jeddah, Saudi Arabia.
Preparation of Alcoholic Extract of R stricta Leaves and Purification of Indole Alkaloids
Rhazya stricta leaves were dried in a dark room. After that, the sample (1 kg) was crushed and extracted by chloroform: ethanol (1:2, v/v) at room temperature for 3 times. The extract was concentrated under a reduced pressure to obtain a dark brown residue (102 g). The total extract was then acidified with a solution of 2% HCl and then extracted again by chloroform to remove the nonbasic compounds. The aqueous layer was basified by ammonium hydroxide solution and was then extracted by chloroform. The chloroform extract was concentrated and dried over sodium sulfate anhydrous to obtain 20.88 g of crude alkaloids.
Aluminum oxide column (800 g, 80 × 2.5 cm) was used to purify individual alkaloids. Crude alkaloids mixture (20 g) was homogenized with aluminum oxide (80 g) and poured onto the top of the column and was equilibrated with chloroform. The eluents were used successively (chloroform, chloroform:ethyl acetate, chloroform:methanol). Fractions were collected (50 mL), and thin-layer chromatography (TLC) was performed using silica gel plates, and Dragendorff’s reagent was used as spraying agent. If the material was not homogeneous, preparative TLC (PTLC) was applied using the appropriate solvent system.
Preparation of Aqueous Extract of Rhazya stricta and Purification of Indole Alkaloids
The plant leaves were soaked in 2% HCl solution for 3 days followed by filtration and extraction by chloroform. The aqueous layer was basified by NH4OH and then extracted by chloroform. The organic layer was evaporated under reduced pressure to obtain 15 g of crude alkaloids.
Aluminum oxide column (750 g, 75 × 2.5 cm) was used to purify indole alkaloids. The crude alkaloid extract (15 g) was homogenized with a suitable amount of aluminum oxide and poured onto the top of the column. The eluents were used sequentially, that is, chloroform, chloroform:ethyl acetate, chloroform:methanol. Fractions were collected (50 mL), and TLC was performed using silica gel plates, and Dragendorff’s reagent was used as a spraying agent. If the material was not homogeneous, PTLC was applied using the appropriate solvent system.
Characterization of Isopicrinine (1)
The fractions eluted with a mixture of chloroform: ethyl acetate (8.5:1.5, v/v) were collected and examined by TLC on silica gel plates and visualized by Dragendorff’s reagent (orange spot developed). PTLC was applied using chloroform: methanol (9.5:0.5, v/v) to give as a brown oily substance (1) (2 mg, Rf = 0.47). Ultraviolet (UV) λmax (MeOH) 234 and 286 nm; infrared (IR) νmax 3300 (NH, st), 3015(=CH, st), 2925(CH, st), 1734 (C=O, st) and 1610, 1464 (C=C, st) cm−1; ESI-MS (electrospray ionization–mass spectrometry) (350 eV), m/z (relative intensity): 339.1 (100) [M+1, C20H22N2O3-1], 321.0 (100) [M+-C20H21N2O2], 309.0 (45) [M+-C19H21N2O2], 289.0 (75), 279.1 (100), 216.1(25), 202.1(25), 184.1 (10), 144.0 (20), 102.1 (20), and 106.2 (45); 1H-NMR (Bruker WM 400 MHz) and 13C-NMR (100 MHz) in CDCl3:CD3OD (as shown in Table 1).
Table 1.
1H-NMR (400 MHz) and 13C-NMR (100 MHz) Spectral Data of Isopicrinine.
|
13C-NMR |
1H-NMR |
HMBC | |||
|---|---|---|---|---|---|
| No. | δC | Multiplet | δΗ | Multiplet (J Hz) | |
| 2 | 108.2 | s | — | — | 5, 3, 6a, 14b, 21b |
| 3 | 53 | d | 3.47 | d (4.0) | 5, 6a |
| 5 | 88.1 | d | 4.76 | d (2.8) | 21b, 6a, 6b |
| 6a 6b |
41.1 | t | 3.45 2.12 |
d (14.0) m |
OCH3, 16, 5 |
| 7 | 54.3 | s | — | — | 6a, 6b, 15, 21a, OCH3 |
| 8 | 134.2 | s | — | — | 12, 6a, 6b |
| 9 | 125.4 | d | 7.23 | dd (8.8, 1.2) | 11 |
| 10 | 120.9 | d | 6.7 | ddd (8.8, 8.8, 1.2) | 12 |
| 11 | 129 | d | 7.07 | ddd (8.8, 8.8, 1.2) | 9 |
| 12 | 111.8 | d | 6.74 | dd (8.8, 1.2) | 10 |
| 13 | 149.8 | s | — | — | 9, 11 |
| 14a 14b |
22.4 | t | 2.04 1.98 |
m m |
|
| 15 | 33 | d | 3.57 | br.s | 3, 19, 21a, 21b |
| 16 | 53.2 | d | 2.34 | d (3.6) | 15, 3, 21b |
| 17 | 174 | s | — | — | 16, OCH3 |
| 18 | 13.2 | q | 1.47 | dd (7.2, 2.4) | |
| 19 | 122 | d | 5.46 | q (7.2) | 18, 21b |
| 20 | 136.7 | s | — | — | 3, 16, 18, 21b |
| 21a 21b |
47.0 | t | 3.75 3.21 |
br.d (18.0) d (18.0) |
|
| CO2Me | 52.1 | s | 3.64 | s | 3, 15 |
Abbreviations: HMBC, heteronuclear multiple bond correlation; s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; br.s, broad singlet; br.d, broad doublet; dd, double doublets.
Cell Culture
MCF7, a breast cancer cell line, was procured from the American Type Culture Collection and propagated in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS), 100 µg/mL streptomycin 100 U/mL penicillin at 37°C in a humidified incubator with 5% CO2. Human pancreatic cancer cell lines, AsPC1 and PANC1, and normal human WISH cell line were obtained from the American Type Culture Collection. AsPC1 cell line was grown in RPMI-1640 medium supplemented with 10% (v/v) FBS, 100 µg/mL streptomycin and 100 U/mL penicillin. PANC1 cell line and normal human WISH cell line were cultured in DMEM supplemented with 10% (v/v) FBS, 100 µg/mL streptomycin, and 100 U/mL penicillin.
Cell Viability and Apoptosis Assay
MCF7 cells (2 × 104 cells/well) were subcultured in 24-well plate and incubated at 37°C in a humidified incubator with 5% CO2 for 20 hours. Cells were treated with 10 µg/mL and 100 µg/mL of around 34 purified alkaloid fractions (ie, 14 alkaloid fractions from alcoholic extract and 20 alkaloid fractions from aqueous extract) of R stricta and further incubated for 18 hours. After the drug treatment, cells were analyzed under phase-contrast microscope. Based on the preliminary cytotoxicity data, purified alkaloid isopicrinine was selected for further analysis. MCF7 cells (2 × 104 cells/well) were treated with varying doses of isopicrinine, that is, 3 µM, 30 µM, 300 µM, 600 µM, and 1500 μM, at 37°C in a humidified incubator with 5% CO2 for 20 hours. After the drug treatment, cells were analyzed under phase-contrast microscope. Cytotoxicity was detected at 300 µM concentration onward in all the samples. MCF7 cells (2 × 104 cells/well) were treated with varying doses of isopicrinine alkaloid in triplicates, that is, 3 µM, 30 µ, 60 µM, 120 µM, 180 µM, 240 µM, 300 µM, and 600 µM, at 37°C in a humidified incubator with 5% CO2 for 20 hours, and half maximal inhibitory concentration (IC50) was determined. The nuclear morphological changes, which are mainly associated with apoptosis, were analyzed by Hoechst 33342 staining. Briefly, MCF7 cells (2 × 104 cells/well) were grown on coverslips and treated with 150 µM of purified alkaloid for 24 hours. The cells were washed with Dulbecco’s phosphate-buffered saline (DPBS) and fixed with 100% methanol for 15 minutes at room temperature. Cells were washed with DPBS and stained with Hoechst 33342 for 15 minutes at room temperature. The cells were washed thrice with DPBS and analyzed under fluorescent microscope (Nikon, Japan).
Human pancreatic cancer cell lines, PANC1, AsPC1 cells, and normal human WISH cell line (1.3 × 104 cells/well) were subcultured in 96-well plates and incubated at 37°C in a humidified incubator with 5% CO2 for 24 hours. Varying concentrations of isopicrinine, that is, 0, 30 µM, 75 µM, 150 µM, and 300 µM were, added to the cultured cells and further incubated at 37oC in a humidified incubator with 5% CO2 for 24 hours. The cell viability was determined using MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay by following the manufacturer’s instructions. Briefly, 20 µL/well of MTT reagent was added to the cells, and plates were incubated for 4 hours. After the incubation, media along with the MTT reagent was removed and 150 µL/well of isopropanol was added and further incubated for 15 minutes on shaker incubator. Spectrophotometer readings were taken at 450 nm and 630 nm. This assay is based on the conversion of yellow tetrazolium salt MTT to purple formazan crystal by metabolically active cells. The amount of formazan produced is proportional to the number of viable cells.
RNA-Seq Analysis
MCF7 cells (1.6 × 105 cells) were treated with varying doses (sublethal) of purified isopicrinine alkaloid, that is, 30 µM and 150 µM, in triplicates, for 18 hours at 37°C. Total RNA was extracted using TRIzol reagent. Gene expression analyses of drug-treated MCF7 cells, in the presence of sublethal concentrations of the isopicrinine alkaloid, were performed using Illumina HiSeq. Each RNA was ribo-depleted (Ribo-Zero, Epicentre) prior to cDNA library preparation (ScriptSeq epicenter). Each cDNA was separately barcode tagged during library preparation and sequenced in multiplex generating approximately >40 million 100 bp reads per sample for the mammalian cell line. The Illumina reads were mapped to their respective assembled reference transcriptome sequence using the mapping software TopHat and Cufflinks.9 Illumina read abundances mapping to each reference contig was normalized using the RPKM calculation (reads per Kb per million reads). Differentially expressed transcripts were identified and functionally annotated using a variety of statistical analyses available via Bioconductor in R.10 Clusters of differentially expressed and cycling transcripts were annotated to identify overrepresented GO/KEGG pathways.11
Results
Purification and Characterization of Isopicrinine (1)
Recent studies have shown the potential of phytochemicals present in the leaves of R stricta plant for treatment of cancer. In order to isolate new compounds with anticancer property, total alkaloids extract (alcoholic and aqueous extract) was subjected to column chromatography using aluminum oxide column. A total of 34 individual alkaloids were thus purified and characterized. The purified alkaloids were screened for their cytotoxicity against MCF-7, a breast cancer cell line.
Compound isopicrinine was isolated as an oily material. ESI-MS spectrum of isopicrinine showed a base peak at m/z 339.1, analyzed for the molecular formula, C20H22N2O3. The UV spectrum showed absorption maxima at 234 and 286 nm, indicated the presence of indoline ring. The IR spectrum displayed bands at 3300 (NH, st), 3015(=CH, st), 2925(CH, st), 1734 (C=O, st), and 1610, 1463 (C=C, st) cm−1. 1H NMR spectrum exhibited signals for: 4 aromatic protons characteristic for O-disubstituted benzene ring resonating at δH 7.23 (dd, J = 8.8, 1.2 Hz, H-9), 6.70 (ddd, J= 8.8, 8.8, 1.2 Hz, H-10), 7.07 (ddd, J = 8.8, 8.8, 1.2 Hz, H-11), 6.74 (dd, J= 8.8, 1.2 Hz, H-12), an olefinic methyl group appeared at δH 1.47 (dd, J = 7.2, 2.4 Hz, Me-18), which was coupled to an olefinic proton signal resonating at δH 5.46 (q, J = 7.2 Hz, H-19) indicating an ethylidene side chain, and a singlet signal at δH 3.64 characteristic for a methyl ester group (Table 1).
The 13C NMR spectra of isopicrinine, showed resonances of 20 carbons, differentiated by DEPT NMR experiment into 2 methyl (δC 52.1 and 13.2), 3 methylene (δC 47.0, 41.1, and 22.4), 9 methine (δC 129.0, 125.4, 122.9, 122.0, 111.8, 88.1, 53.2, 53.0, and 32.2), and 6 quaternary (δC 174.0, 149.8, 134.2, 108.2, 54.3, and 52.1) carbons.
Resonances attributable to 9 carbons in the 13C NMR spectrum accounted for 4 carbon-carbon double bonds and 1 carbonyl group, implying that isopicrinine has a hexacyclic skeleton. The association of all unions’ carbons and protons were achieved from the heteronuclear single quantum coherence (HSQC) spectrum.
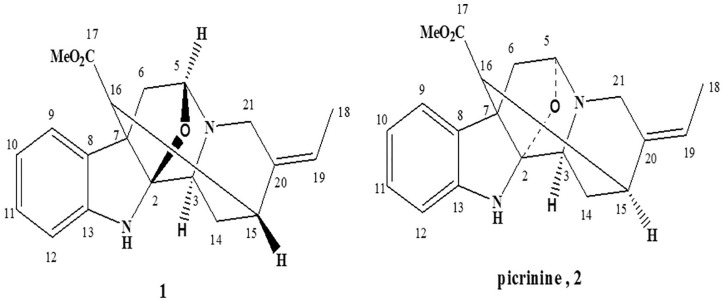
The 1H-1H COSY (correlated spectroscopy) spectrum supported the existence of 1, 2-disubstituted benzene ring through the 1H-1H spin system from H-9 to H-12, an ethylidene group through the correlation between the methine proton resonating at δH 5.46 and the methyl protons at δH 1.47. Furthermore, the 1H-1H COSY spectrum established 3 aliphatic proton sequence H-5-H2-6, H-3-H2-14-H-15-H-16, and an isolated methylene protons H2-21. From COSY and heteronuclear multiple bond correlation (HMBC) spectrum, the correlations of the proton H-3(δH 3.47) with C-2 (δC 108.2) and C-14 (δC 22.4) suggested the linkage C-2-CH-3-CH2-14-CH-15-CH-16. The correlations between H-16 with C-15; C-7 and H-15 with C-20; and C-21and H3-18 with C-20 establish the position of the ethylidene group and the attachments of C-15. The HMBC correlations of H-16 and Me of ester δH 3.64 to C-17 (δC 174.0) indicated the direct connection of H-16 and methyl ester to C-17, the correlation from H-5 to δC 108.2 supported the existence of a C2-O-C5 bond, which was confirmed by its 11 degrees of unsaturation consistent with the molecular formula of C20H22N2O3, as established by ESI-MS. The downfield NMR data of CH-5 allowed the attachment of an oxygen atom and nitrogen atom (N-4) to C-5. The HMBC correlation between δH 3.21 H-21 and C-5 revealed the connection of N-4 with C-21, correlation between H-21 and H-18 to C-20 δC 136.7. In NOSEY spectrum of compound isopicrinine (1), H-15 (δH 3.57) is correlated with H-16 (δH 2.34) and H-3 (δH 3.47) is correlated with H-5 (δH 4.76). Comparison between the above results and literature data, isopicrinine (1) is greatly similar to picrinine (2).12 The main differences are in the stereochemistry of C-5 and C-15, where H-3 and H-5 occupy α position while H-15 with H-16 have β position. So, (1) is a new compound and we have named it isopicrinine (Figure 1). The approximate yield of purified isopicrinine alkaloid was 2 mg after processing 1 kg of dry leaves of R stricta.
Figure 1.
Chemical structures of isopicrinine 1 and picrinine 2 alkaloids.
Anticancer Activity of Alkaloid Isopicrinine (1)
Compound 1, that is, isopicrinine, showed efficient cytotoxic activity with IC50 value of 240 µM. As depicted in Figure 2D-F, cell shrinkage, cellular detachment, and loss of adherent morphology was seen after treatment with high doses of isopicrinine alkaloid as compared with dimethyl sulfoxide (DMSO)-treated cells (Figure 2A), indicating its anticancer potential. No cytotoxicity was detected at low doses of the alkaloid (Figure 2B and C). Hoechst 33342 staining revealed the chromatin condensation and nuclear fragmentation in MCF7 cells after treatment with isopicrinine, which are important features of apoptotic cell death (Figure 3). Isopicrinine also showed efficient cytotoxic activity against pancreatic cancer cell lines AsPC1 and PANC1 as shown in Table 2. However, no significant cytotoxicity was observed in normal human WISH cells after treatment with isopicrinine (Table 2).
Figure 2.
MCF7 breast cancer cell line treated with varying doses of isopicrinine alkaloid. (A) Dimethyl sulfoxide (DMSO) control, (B) 30 µM, (C) 150 µM, (D) 300 µM, (E) 600 µM, and (F) 1500 µM. Cell shrinkage, cellular detachment, and loss of adherent morphology was seen after treatment with high doses of isopicrinine alkaloid.
Figure 3.
Fluorescent images from Hoechst 33342 staining reveal that isopicrinine alkaloid induced apoptotic cell death. (A) Control MCF7 cells treated with dimethyl sulfoxide (DMSO) alone (B) MCF7 cells treated with 150 µM of isopicrinine. Arrows indicate chromatin condensation and nuclear fragmentation.
Table 2.
MTT Assay to Determine the Cytotoxic Potential of Isopicrinine Alkaloid on Pancreatic Cancer Cell Lines, PANC1 and AsPC1, and Normal Human WISH Cell Line.
| No. | Isopicrinine Dose (µM) | PANC1 Cell Line % Cell Viability | AsPC1 Cell Line % Cell Viability | WISH Cell Line % Cell Viability |
|---|---|---|---|---|
| 1 | 0 | 100 | 100 | 100 |
| 2 | 30 | 74.52 | 87.71 | 100 |
| 3 | 75 | 70.75 | 55.90 | 100 |
| 4 | 150 | 69.62 | 53.49 | 98.91 |
| 5 | 300 | 64.6 | 42.41 | 81.55 |
Differential Gene Expression Profiling Using RNA-Seq
MCF7 cell line was treated with sublethal doses of 30 µM and 150 µM of isopicrinine alkaloid. No cytotoxicity was detected at these tested doses. RNA-Seq was performed for analyzing the transcriptome profile in alkaloid-treated MCF7 cells in order to evaluate the pharmacological effects of the new alkaloid isolated from R stricta. To identify the differentially expressed genes after treatment with isopicrinine alkaloid, RNA was extracted from alkaloid-treated MCF7 cells (30 µM and 150 µM doses) and DMSO-treated control cells, and RNA-Seq experiment was carried out. The sequencing data were compared and analyzed to identify the differentially expressed genes in mammalian cells after treatment with isopicrinine alkaloid. Compared with the DMSO-treated control cells, 148 genes were upregulated (fold change > 1; P < .05) and 64 genes were downregulated (fold change < −1; P < .05) in MCF7 cells treated with 30 µM of isopicrinine. Moreover, treatment of MCF7 cells with 150 µM of isopicrinine resulted in the upregulation of 36 genes (fold change > 1; P < .05) and downregulation of 59 genes (fold change < −1; P < .05), as compared with the DMSO-treated control cells (Tables 3 and 4; Figure 4).
Table 3.
Upregulated Genes in MCF7 Breast Cancer Cells After Exposure to Isopicrinine Alkaloid Derived From Leaves of Rhazya stricta.
| Functional Role | Gene | Log2 (Fold Change) | P | Significant |
|---|---|---|---|---|
| Induction of apoptosis | INHBA | 1.02564 | .0002 | Yes |
| MAGED1 | 0.895445 | 5.00E-05 | Yes | |
| BBC3 | 1.48064 | 5.00E-05 | Yes | |
| CDKN1A | 1.11896 | 5.00E-05 | Yes | |
| Response to DNA damage stimulus | GADD45A | 1.33298 | 5.00E-05 | Yes |
| BTG2 | 1.48468 | 5.00E-05 | Yes | |
| DDB2 | 1.19055 | 5.00E-05 | Yes | |
| CCND1 | 1.49047 | .00015 | Yes | |
| Regulation of transcription | MXI1 | 1.11765 | .00015 | Yes |
| EGR1 | 1.39018 | 5.00E-05 | Yes | |
| ATF3 | 1.3961 | .00025 | Yes | |
| FHL2 | 1.02376 | 5.00E-05 | Yes | |
| MDM2 | 1.72451 | 5.00E-05 | Yes | |
| NRG1 | 0.882487 | .00015 | Yes | |
| Regulation of cell growth | SPP1 | 1.07532 | 5.00E-05 | Yes |
| LTBP4 | 1.50385 | .0001 | Yes | |
| VGF | 0.947189 | 5.00E-05 | Yes | |
| Cytoskeleton | AGBL2 | 2.62717 | .0003 | Yes |
| KRT81 | 1.79689 | .0004 | Yes | |
| DLG2 | 2.26801 | 5.00E-05 | Yes | |
| PTP4A1 | 1.33147 | 5.00E-05 | Yes | |
| DRP2 | 2.04721 | 5.00E-05 | Yes |
Table 4.
Downregulated Genes in MCF7 Breast Cancer Cells After Exposure to Isopicrinine Alkaloid Derived From Leaves of Rhazya stricta..
| Functional Role | Gene | Log2 (Fold Change) | P | Significant |
|---|---|---|---|---|
| Cell division | CDC6 | −2.32211 | 5.00E-05 | Yes |
| CDK1 | −1.14933 | 5.00E-05 | Yes | |
| CDCA5 | −1.55071 | .0003 | Yes | |
| ASPM | −1.3045 | 5.00E-05 | Yes | |
| NUSAP1 | −1.53939 | 5.00E-05 | Yes | |
| KIF23 | −0.904965 | .00035 | Yes | |
| PRC1 | −1.52742 | .00045 | Yes | |
| BIRC5 | −0.957158 | .0002 | Yes | |
| AURKB | −1.64598 | 5.00E-05 | Yes | |
| NDC80 | −1.34275 | .0001 | Yes | |
| SGOL1 | −1.04359 | .0005 | Yes | |
| FBXO5 | −1.06667 | 5.00E-05 | Yes | |
| PTTG1 | −1.05005 | 5.00E-05 | Yes | |
| NCAPH | −1.01758 | .00015 | Yes | |
| NCAPG | −1.31394 | 5.00E-05 | Yes | |
| DNA replication | MCM3 | −1.64431 | 5.00E-05 | Yes |
| MCM4 | −1.52185 | 5.00E-05 | Yes | |
| MCM5 | −1.87773 | 5.00E-05 | Yes | |
| MCM6 | −1.42256 | 5.00E-05 | Yes | |
| MCM7 | −1.71581 | 5.00E-05 | Yes | |
| MCM8 | −1.01268 | .0001 | Yes | |
| MCM10 | −1.32104 | .0003 | Yes | |
| TOP2A | −1.66227 | 5.00E-05 | Yes | |
| CDC45 | −1.80081 | 5.00E-05 | Yes | |
| RFC3 | −1.14176 | .00045 | Yes | |
| RRM2 | −1.2797 | 5.00E-05 | Yes | |
| Chromosome organization | HIST2H4A | −2.13768 | 5.00E-05 | Yes |
| HIST2H2AA4,HIST2H2AC | −1.50511 | 5.00E-05 | Yes | |
| HIST2H3PS2 | −1.3321 | 5.00E-05 | Yes | |
| HIST2H2BF | −1.75681 | 5.00E-05 | Yes | |
| HIST2H3C | −2.71641 | .0001 | Yes | |
| HIST2H2AB | −2.14409 | 5.00E-05 | Yes | |
| HIST1H2BL | −2.03159 | .00025 | Yes | |
| HIST1H1B | −2.27766 | 5.00E-05 | Yes | |
| HIST1H2AM, HIST1H3J | −2.03436 | 5.00E-05 | Yes | |
| HIST1H2AJ, HIST1H2AK | −2.03436 | 5.00E-05 | Yes | |
| DLGAP5 | −1.26336 | 5.00E-05 | Yes | |
| ESPL1 | −1.9647 | .0003 | Yes | |
| H2AFZ | −0.95203 | 5.00E-05 | Yes | |
| DNA repair | EXO1 | −1.78374 | 5.00E-05 | Yes |
| BRCA1 | −1.07641 | .0002 | Yes | |
| UHRF1 | −1.1337 | .0001 | Yes | |
| RAD51AP1 | −1.65155 | 5.00E-05 | Yes | |
| Regulation of transcription | ATAD2 | −1.38776 | 5.00E-05 | Yes |
| MYBL2 | −1.23112 | 5.00E-05 | Yes | |
| BRIP1 | −1.41617 | 5.00E-05 | Yes | |
| ATAD5 | −1.62821 | 5.00E-05 | Yes |
Figure 4.
Cluster analysis of genes from MCF7 breast cancer cells after treatment with isopicrinine alkaloid compared with dimethyl sulfoxide (DMSO)-treated control cells. (A) Genes upregulated in MCF7 cells after treatment with 30 µM and 150 µM of alkaloid. (B) Genes downregulated in MCF7 cells after treatment with 30 µM and 150 µM of alkaloid. (C) Genes upregulated in MCF7 cells after treatment with 150 µM of alkaloid. (D) Genes downregulated in MCF7 cells after treatment with 150 µM of alkaloid.
Various genes that are involved in DNA replication, cell cycle, and cell proliferation were found to be downregulated in MCF7 cells after treatment with isopicrinine alkaloid (Table 4 and Figure 4). Genes that are involved in regulation of growth and cell signaling were found to be upregulated. One of the important proapoptotic gene, PUMA (p53 upregulated modulator of apoptosis), was upregulated in MCF7 breast cancer cell line after treatment with the alkaloid (Figure 5). Similarly, Mxi1 gene that acts as a tumor suppressor was found to be significantly upregulated in MCF7 cells. Downregulation of survivin, which is a member of IAP (inhibitor of apoptosis) family, was detected in MCF7 cells after treatment with sublethal dose of alkaloid. Survivin is known to play a key role in negative regulation of apoptosis. Several genes that are involved in cell cycle and cell proliferation, such as CDC6, CDK1, CDCA5, DLGAP5, NUSAP1,KIF23, PRC1, BIRC5, AURKB, NDC80, and CDC45, were significantly downregulated after the exposure with the sublethal dose of 150µM of isopicrinine alkaloid (Table 4; Figures 5 and 6). However, these genes were not affected at low concentration of 30 µM of the alkaloid.
Figure 5.
KEGG pathway analysis of differentially regulated genes in MCF7 cells after treatment with isopicrinine alkaloid, depicting the affected genes in p53 signaling pathway. Modulated genes are marked on top with red star.
Figure 6.
KEGG pathway analysis of differentially regulated genes in MCF7 cells after treatment with isopicrinine alkaloid, depicting the affected genes that play significant role in cell cycle and cell proliferation. Modulated genes are marked with red star.
Discussion
Phytochemical components of R stricta is known to contain more than 100 alkaloids with diverse and complex structures with wide range of biological activities. Some of these alkaloids have been shown to possess antibacterial, anticancer, or wound healing activities.2 We isolated a new alkaloid, termed as isopicrinine, from the leaves of R stricta. This purified alkaloid exhibited significant cytotoxic activity for MCF7 breast cancer cell line. RNA-Seq transcription profiling revealed several differentially regulated genes in MCF7 cells after exposure to sublethal dose of the alkaloid. Several genes that play important roles in DNA replication, mitosis, and cell proliferation were significantly downregulated, and some key proapoptotic genes were found to be upregulated, indicating the potential of new alkaloid isopicrinine as an antitumor agent.
One of the important proapoptotic genes, significantly upregulated in MCF7 cells after treatment with isopicrinine alkaloid, was BBC3 (Bcl-2-binding component 3). This gene, also known as PUMA, is a member of the Bcl-2 protein family and is involved in p53-dependent and -independent apoptosis.13,14 BBC3 expression is controlled by tumor suppressor p53, and on activation, it interacts with antiapoptotic Bcl-2 family members and hence releases Bax or Bak, which leads to mitochondrial outer membrane permeabilization. Following mitochondrial dysfunction, caspases are activated, which finally leads to apoptotic cell death.15,16 Induction of BBC3 proapoptotic gene after exposure to sublethal dose of the alkaloid clearly suggest its potential as a potential drug target for controlling tumor development.
Mxi1 gene, which encodes for Max-interacting protein 1, was found to be upregulated in MCF7 cells after treatment with the alkaloid. Mxi1 protein act as a tumor suppressor and negatively regulate c-Myc activity. Expression of the oncogenic c-Myc gene is very strongly controlled in normal cell; however, it is found to be deregulated in various types of human cancer. Myc protein interacts and dimerizes with Max transcription factor and regulates the expression of various genes involved in cell division and apoptosis. Mxi1 protein inhibit the activity of Myc by competing for Max protein, and hence prevent the tumor growth. Defects in Mxi1 gene are frequently detected in prostate cancer patients.17
Exposure of high dose of isopicrinine alkaloid in MCF7 cells led to the downregulation of BIRC5 gene, which encodes for survivin, also known as baculoviral IAP repeat-containing 5 (BIRC5). Survivin is a member of IAP family and play an important role in negative regulation of apoptosis by inhibiting the caspase activation.18 Tamm and colleagues have demonstrated that survivin inhibits both Bax- and Fas-induced apoptotic pathways.19 It has been observed that silencing of survivin expression leads to increase in apoptotic cell death and subsequent decrease in tumor growth. Moreover, expression of survivin protein is found to be highly upregulated in almost all types of human cancer and also in fetal tissue, but it is not expressed in normal adult cell, hence making it an attractive drug target for cancer therapy. Tamm and colleague reported the expression of survivin in all the 60 different human cancer cell lines that are being used in the National Cancer Institute’s cancer drug screening program, with the maximum level of expression in breast and lung cancer cell lines.19 The mechanism of survivin regulation are still not well characterized, but its regulation seems to be linked to p53 protein and is also a target gene of the Wnt pathway. Our gene expression profiling data suggest that new alkaloid from R stricta might be inducing apoptosis in MCF7 breast cancer cell line by downregulating the expression of survivin.
Several genes that play a crucial role in cell division and mitosis, such as PTTG1, DLGAP5, Cdc6, NDC80, KIF15, KIF23, PRC1, NUSAP1, Aurora B kinase, CDCA5, and ATAD2, were also found to be downregulated after the treatment of MCF7 cells with the high dose of the alkaloid. Cdc6 (cell division cycle 6) plays an important role in regulation of DNA replication and is required for stacking mini chromosome maintenance (MCM) proteins onto the DNA during the initiation of DNA synthesis.20 Cdc6 protein has been shown to possess proto-oncogenic activity as its overexpression leads to inhibition of INK4/ARF tumor suppressor genes. Moreover, Cdc6 is found to be highly upregulated in various cancers such as lung cancer, brain cancer, and cervical cancer.21 KIF-15 gene encodes for kinesin family member 15 protein, a motor protein that is involved in maintaining half spindle separation. KIF15 interacts with microtubules and actin filaments in dividing cells. This motor protein is found to be overexpressed in various tumors including breast cancer.22 Similarly KIF23 is involved in chromosomes movement during cell division. It has been demonstrated that KIF23 is involved in the formation and proliferation of gliomas in mice.23 DLGAP5 gene encodes for disks large-associated protein 5, also known as hepatoma upregulated protein (HURP). This protein is involved in stabilizing microtubules in proximity of chromosomes and controlling spindle dynamics during cell division. Studies have shown that overexpression of DLGAP5 led to hepatocellular carcinoma by promoting cell proliferation. RNAi-mediated silencing of DLGAP5 effectively inhibited the proliferation and metastasis of hepatocellular carcinoma cells.24 Another important gene found to be downregulated by high dose of isopicrinine alkaloid is ATAD2 (ATPase family, AAA domain containing 2). The protein encoded by this gene is involved in the estrogen-induced cell proliferation of breast cancer cells.25 PRC1 (protein regulator of cytokinesis 1) gene encodes for a protein that is key regulator of cytokinesis. It is required to recruit PLK1 and KIF14 to the central spindle. It has been shown to act as an oncogene for promoting cell proliferation, inhibition of apoptosis, and tumor progression in bladder cancer.26
An interesting gene found to be downregulated by sublethal dose of isopicrinine alkaloid is NUSAP1, which is involved in mitotic spindle assembly, chromosome segregation during cell division and cytokinesis. Recent study has revealed the role of NUSAP1 in BRCA1-regulated pathways of DNA repair and centrosome duplication, which is essential for maintaining genome stability.27 Several studies have demonstrated the overexpression of NUSAP1 in various cancer such as melanoma, prostate, glioblastoma, and liver carcinoma.27,28 It has been linked to enhanced disease aggression in meningiomas, increased risk in breast cancer patients, and development of resistance to chemotherapy in pancreatic cancer patients. It has now been identified as an important biomarker for breast ductal carcinoma and development of drug resistance.
Ndc80 gene, found to be downregulated by isopicrinine alkaloid, encodes for a kinetochore protein that is responsible for correct chromosome segregation during mitosis by ensuring the proper bipolar attachment of chromosome. Overexpression of Ndc80 has been shown to induce hepatitis B virus–related hepatocellular carcinoma. RNAi-mediated silencing of Ndc80 expression in hepatoma cell line HepG2 resulted in inhibition of cell proliferation and suppressed the replication of hepatitis B virus.29 CDCA5 gene encodes for sororin protein, which is required for sister chromatid cohesion in interphase. Phosphorylation and activation of sororin by ERK had been shown to play a key role in cell proliferation in lung cancer.30,31 Aurora B kinase is required for the attachment of mitotic spindle to the centromere during cell division. Overexpression of this kinase leads to unequal chromosomal separation, resulting in abnormal number of chromosomes in the daughter cells, thus causing cancer development.32,33 PTTG1 (pituitary tumor-transforming 1) gene found downregulated after exposure with isopicrinine alkaloid, encodes for a protein that inhibit separins from promoting sister chromatid separation. This protein has been shown to have tumorigenic activity in animal models and is also highly upregulated in several tumors.34,35 Our gene expression profiling data have revealed that novel alkaloid isopicrinine, isolated from medicinal herb R stricta, exert its anticancer activity by targeting the p53 pathway, and thus causing the inhibition of various genes involved in cell proliferation. Our earlier studies have also demonstrated that medicinal plant R stricta is an important resource for discovering and isolating novel compounds with potential anticancer activity.4,36
Conclusion
In conclusion, our data clearly indicate that exposure of MCF7 breast cancer cells with sublethal dose of isopicrinine alkaloid affected the expression of various genes involved in p53 signaling pathway. One of the crucial proapoptotic genes, significantly upregulated in MCF7 after exposure to alkaloid, was PUMA, which is a member of the Bcl-2 protein family, and is involved in p53-dependent and p53-independent apoptosis. Moreover, exposure of sublethal dose of isopicrinine alkaloid in breast cancer cell line led to the downregulation of survivin, which is involved in negative regulation of apoptosis. Besides, inhibition of several genes involved in mitosis and cell proliferation indicate the potential of isopicrinine alkaloid from R stricta leaves as a new cancer agent.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors gratefully acknowledge the financial support from KAU Vice President for Educational Affairs Prof Dr Abdulrahman O. Alyoubi, represented by the Unit of Strategic Technologies Research through the Project number (D 008/431) for the project titled “Identification and Isolation of Salt and Heat-Tolerance Genes of R stricta and Detection of Metabolites and their Therapeutic Effects via Cheminformatics.”
ORCID iD: Roop Singh Bora  https://orcid.org/0000-0002-5586-3829
https://orcid.org/0000-0002-5586-3829
References
- 1. Rahman AU, Qureshi MM, Zaman K, Malik S, Ali SS. The alkaloids of Rhazya stricta and R orientalis. A review. Fitoterapia. 1989;60:291-322. [Google Scholar]
- 2. Marwat SK, Rehman FU, Usman K, Shah SS, Anwar N, Khan IU. A review of phytochemistry, bioactivities and ethnomedicinal uses of Rhazya stricta Decsne. Afr J Microbiol Res. 2012;6:1629-1641. [Google Scholar]
- 3. Baeshen MN, Khan R, Bora RS, Baeshen NA. Therapeutic potential of the folkloric medicinal plant Rhazya stricta. Biol Syst Open Access. 2015;5:1. [Google Scholar]
- 4. Obaid AY, Voleti S, Bora RS, et al. Cheminformatics studies to analyze the therapeutic potential of phytochemicals from Rhazya stricta. Chem Cent J. 2017;11:11. doi:10.1186/s 13065-017-0240-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baeshen NA, Elkady AI, Abuzinadah OA, Mutwakil MH. Potential anticancer activity of the medicinal herb, Rhazya stricta, against human breast cancer. Afr J Biotechnol. 2012;11:8960-8972. [Google Scholar]
- 6. Elkady AI, Hussein RAH, Abu-Zinadah OA. Differential control of growth, apoptotic activity and gene expression in human colon cancer cells by extracts derived from medicinal herbs Rhazya stricta and Zingibar officinale and their combination. World J Gastroenterol. 2014;20:15275-15288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mukhopadhayay S, Handy GA, Funayama S, Cordell GA. Anticancer indole alkaloids of Rhazya stricta. J Nat Prod. 1981;44:696-700. [DOI] [PubMed] [Google Scholar]
- 8. Gu Z, Zakarian A. Total synthesis of rhazinilam: axial to point chirality transfer in an enantiospecific Pd-catalyzed transannular cyclization. Org Lett. 2010;12:4224-4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105-1111. doi:10.1093/ bioinformatics/btp120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Durinck S, Moreau Y, Kasprzyk A, et al. BioMart and Bioconductor: a powerful link between biological databases and microarray data analysis. Bioinformatics. 2005;21:3439-3440. doi: 10.1093/bioinformatics/bti525 [DOI] [PubMed] [Google Scholar]
- 11. Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012; 40(database issue):D109-D114. doi: 10.1093/nar/gkr988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fumiko A, Rong-Fuchen, Yamauchi T, Marubayashi N, Ueda I. Alschomine and isoalschomine, new alkaloids from the leaves of Alstonia scholaris. Chem Pharma Bull. 1989;37:887-890. [Google Scholar]
- 13. Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683-694. [DOI] [PubMed] [Google Scholar]
- 14. Zhang LN, Li JY, Xu W. A review of the role of Puma, Noxa and Bim in the tumorigenesis, therapy and drug resistance of chronic lymphocytes leukemia. Cancer Gene Ther. 2013;20:1-7. [DOI] [PubMed] [Google Scholar]
- 15. Jabbour AM, Heraud JE, Daunt CP, et al. Puma indirectly activates Bax to cause apoptosis in the absence of Bid and Bim. Cell Death Differ. 2009;16:555-563. [DOI] [PubMed] [Google Scholar]
- 16. Yee KS, Wilkinson S, James J, Ryan KM, Vousden KH. PUMA- and Bax-induced autophagy contributes to apoptosis. Cell Death Differ. 2009;16:1135-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Erichsen DA, Armstrong MB, Wechsler DS. Mxi1 and Mxi1-0 antagonize N-myc function and independently mediate apoptosis in neuroblastoma. Trans Oncol. 2015;8:65-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Varughese RK, Torp SH. Survivin and gliomas: a literature review. Oncol Lett. 2016;12:1679-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tamm I, Wang Y, Sausville E, et al. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998;58:5315-5320. [PubMed] [Google Scholar]
- 20. Petrakis TG, Vougas K, Gorgoulis VG. Cdc6: a multi-functional molecular switch with critical role in carcinogenesis. Transcription. 2012;3:124-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murphy N, Ring M, Heffron CC, et al. Quantitation of CDC6 and MCM5 mRNA in cervical intraepithelial neoplasia and invasive squamous cell carcinoma of the cervix. Mod Pathol. 2005;18:844-849. [DOI] [PubMed] [Google Scholar]
- 22. Rath O, Kozielski F. Kinesins and cancer. Nat Rev Cancer. 2012;12:527-539. [DOI] [PubMed] [Google Scholar]
- 23. Takahashi S, Fusaki N, Ohta S, et al. Downregulation of KIF23 suppresses glioma proliferation. J Neurooncol. 2012;106:519-529. [DOI] [PubMed] [Google Scholar]
- 24. Liao W, Liu W, Yuan Q, et al. Silencing of DLGAP5 by siRNA significantly inhibits the proliferation and invasion of hepatocellular carcinoma cells. PLoS One. 2013;8:e80789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zou JX, Revenko AS, Li LB, Gemo AT, Chen HW. ANCCA, an estrogen-regulated AAA+ ATPase coactivator for ERalpha, is required for coregulator occupancy and chromatin modification. Proc Natl Acad Sci U S A. 2007;104:18067-18072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shimo A, Nishidate T, Ohta T, Fukuda M, Nakamura Y, Katagiri T. Elevated expression of protein regulator of cytokinesis 1, involved in the growth of breast cancer cells. Cancer Sci. 2006;98:174-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kotian S, Banerjee T, Lockhart A, et al. NUSAP1 influences the DNA damage response by controlling BRCA1 protein levels. Cancer Bio Ther. 2014;15:533-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gulzar ZG, McKenney JK, Brooks JD. Increased expression of NuSAP in recurrent prostate cancer is mediated by E2F1. Oncogene. 2013;32:70-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu B, Yaoa Z, Hua K, et al. ShRNA-mediated silencing of the Ndc80 gene suppress cell proliferation and affected hepatitis B virus-related hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2016;40:297-303. [DOI] [PubMed] [Google Scholar]
- 30. Nguyen MH, Koinuma J, Ueda K, et al. Phosphorylation and activation of cell division cycle associated 5 by mitogen-activated protein kinase play a crucial role in human lung carcinogenesis. Cancer Res. 2010;70:5337-5347. [DOI] [PubMed] [Google Scholar]
- 31. Tokuzen N, Nakashiro K, Tanaka H, Iwamoto K, Hamakawa H. Therapeutic potential of targeting cell division cycle associated 5 for oral squamous cell carcinoma. Oncotarget. 2015;7:2343-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shannon KB, Salmon ED. Chromosome dynamics: new light on dispatch Aurora B kinase function. Curr Biol. 2002;12:R458-R460. [DOI] [PubMed] [Google Scholar]
- 33. Fu J, Bian M, Jiang Q, Zhang C. Roles of Aurora kinases in mitosis and tumorigenesis. Mol Cancer Res. 2007;5:1-10. [DOI] [PubMed] [Google Scholar]
- 34. Molina-Jimenez F, Benedicto I, Murata M, et al. Expression of pituitary tumor-transforming gene1 (PTTG1)/securin in hepatitis B virus (HBV)-associated liver diseases: evidence for an HBV X protein–mediated inhibition of PTTG1 ubiquitination and degradation. Hepatology. 2010;51:777-787. [DOI] [PubMed] [Google Scholar]
- 35. Chen DT, Nasir A, Culhane, et al. Proliferative genes dominate malignancy-risk gene signature in histologically-normal breast tissue. Breast Cancer Res Treat. 2010;119:335-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Iqbal W, Alkarim S, Kamal T, et al. Rhazyaminine from Rhazya stricta inhibits metastasis and induces apoptosis by downregulating Bcl-2 gene in MCF7 cell line [published online October 29, 2018]. Integr Cancer Ther. doi: 10.1177/1534735418809901 [DOI] [PMC free article] [PubMed] [Google Scholar]