Short abstract
Objective
To describe a new mini-invasive surgical technique for carpal tunnel release and to present clinical findings associated with using this technique.
Methods
Patients with idiopathic carpal tunnel syndrome without prior surgical treatment, who underwent a new minimally-invasive surgical technique using a specific surgical tool-kit developed by the author, were included. Prospective data were collected, including preoperative electrodiagnostic testing. The subjective condition of all patients was evaluated pre- and postoperatively with a five-level Likert-type scale (LS) and muscular strength was tested using a JAMAR dynamometer and pinch gauge.
Results
A total of 116 patients (157 hands/cases) underwent surgery performed by the author, and were followed for a mean of 40 months (range, 6 months–7 years). Of these, preoperative electrodiagnostic testing was performed in 112 patients (96.6%). No significant complications were reported. By three months, patients reported that they were satisfied or very satisfied in 147/149 cases (98.7%; LS grade I and II). Strength recovery at three months, based on the average of four measures, was 99.17% (range, 97.43–100.97%).
Conclusions
The described technique is minimally invasive, safe and simple to perform, and provides good results.
Keywords: Carpal tunnel, carpal tunnel release, endoscopic carpal tunnel release, transverse carpal ligament, flexor retinaculum, median neuropathy, insight-precision
Introduction
Carpal tunnel syndrome is the most common entrapment neuropathy.1–5 Carpal tunnel release is, therefore, a very common operation performed on the hand, with numbers in the USA alone varying up to 463 673 cases/year.5
Current available surgical techniques can be classified as open (classic or mini/limited) or endoscopic (Table 1).2,6–17 Unfortunately, many expectations generated by the new endoscopic techniques not only didn’t materialize, but introduced a series of new difficulties and problems,18,19 of which, undoubtably the most serious is the possibility of severe, potentially irreversible, neurological damage.5,13,19–23 Hence, the classic open technique continues to be considered by many as the paradigm (gold standard) surgical treatment of carpal tunnel syndrome.1,5,19
Table 1.
A summary of publications describing endoscopic and limited-open surgical techniques in the treatment of carpal tunnel syndrome.
| Technique | No. of skin incisions | Publication |
|---|---|---|
| Endoscopic | One portal proximal | 7Okutsu et al., 1987 |
| 2Agee et al., 1992 | ||
| 8Menon, 1994 | ||
| Distal palmar uniportal | 9Mirza et al., 1995 | |
| Two portals | 11Chow, 1989 | |
| 12Resnick and Miller, 1991 | ||
| 13Brown et al., 1993 | ||
| Limited-Open | One incision midpalmar | 14Backhouse et al., 1981 |
| 15Bromley, 1994 | ||
| Double incision | 16Biyani and Downes, 1993 | |
| 17Wilson, 1994 |
The present article describes a new technique for treating carpal tunnel syndrome, namely, the ‘Insight-Precision’ technique, in which the section of the transverse carpal ligament (TCL) is guided from beginning to end, proximal to distal. A single portal of entrance over the palmar distal wrist crease is used, avoiding any type of incision in the palm of the hand. The use of an arthroscope for ligament release is not required, however, it can be used at will at the end of the procedure for documentation or to confirm the complete section of the TCL; once the ligament is sectioned, introduction of the arthroscope is very easy. Therefore, the main objective in developing this new technique was to achieve a simple method for carpal tunnel release (without need for a steep learning curve) with a high degree of safety, minimizing the most frequent complication of carpal tunnel surgery: cicatricial and pericicatricial (pillar) pain; and the most alarming complication: iatrogenic neurovascular injury. Additional objectives were to improve the quality of the postoperative period and the cosmetic result of the intervention.
Patients and methods
Study population
This prospective case series included patients who underwent a new mini-invasive surgical technique for carpal tunnel release, performed by the author (JDC) at the Hospital de Santa Maria, Oporto, Portugal, between October 2008 and January 2016, using a specific set of surgical instruments developed by the author. Inclusion criteria comprised a diagnosis of idiopathic carpal tunnel syndrome, without previous surgical treatment, and without response to conservative treatment established for at least 6 months. The absolute exclusion criteria were: previous surgery of the flexor retinaculum area, inflammatory joint disease, and significant deformities of the wrist area due to any cause.
The study was reviewed and approved by the Ethics Committee of the Hospital de Santa Maria, Oporto, Portugal, and written informed consent was obtained from all participants, using a form based on the Declaration of Helsinki of 1975 (revised in 2008) and on the regulations of the institution.
The primary diagnosis was confirmed based on symptomatology, e.g. painful nocturnal paraesthesia with waking in the middle of the night, relieved by raising and shaking the hand, and classic tests for compression of the median nerve plus electrodiagnostic assessments. Patients with normal electrodiagnostic test results underwent surgery based on the clinical criteria,24 and patients received surgery either on one hand or on two hands simultaneously during the same surgery session.25,26
Outcome measures
The following parameters were evaluated in this study: (1) procedure duration, including surgery duration (skin to skin) and total duration (including tourniquet time); (2) postoperative pain, assessed by asking patients at the first postoperative visit and thereafter, about their use of analgesic medication following discharge from hospital;9 (3) resolution of symptoms according to patient’s subjective assessment using a five-level Likert-type scale (ranging from 1, not at all bothersome to 5, extremely bothersome), assessed at 12 weeks postoperatively; (4) specific complications, comprising those that have been previously reported in the literature (summarised in Table 2);1–5,8,13,18,19,21–23,25–32 (5) recovery of muscle strength at 12 weeks post-surgery, assessed by quantitative measurements of preoperative and postoperative grip and key, palmar and tip pinch strengths of the involved and uninvolved hands, recorded using a Jamar Dynamometer1,2 (Saehan Corporation, Masan, Korea) with the handle in the second notch, and a hydraulic pinch Gauge3 (Saehan Corporation, Masan, Korea). The mean of three measurements for each type of assessment was calculated; and (6) patient satisfaction, measured using a five-level Likert-type scale at 3-months postoperatively. In cases of bilateral surgery, a separate Likert-like response was obtained for each hand.
Table 2.
Incidence of surgical complications in 116 patients (comprising 157 operated hands) who underwent the new ‘Insight-Precision’ mini-invasive surgical technique for carpal tunnel release.
| Complication | Incidence (n) |
|---|---|
| Conversion to mini-open technique | 2 |
| Accidental skin laceration | – |
| Haematoma | – |
| Ecchymosis (palmar) hand/forearm | 6 |
| Infection | – |
| Suture dehiscence | – |
| Persistent cicatricial pain | 1 |
| Pillar pain (at one month) | 4 |
| Persistent ‘pillar pain’ | – |
| Decreased sensitivity | – |
| Paraesthesia not present preoperatively | – |
| Transient Neuropraxia | |
| Median nerve and branches | 1 |
| Cubital nerve and branches | 3 |
| Traction neuropathy | – |
| Extrusion (‘bowstring’) of flexor tendons | – |
| Iatrogenic nerve lesions | |
| Cutaneous palmar branch median nerve | – |
| Other median nerve branches including recurrent | – |
| Cubital nerve | – |
| Iatrogenic tendon lesions | – |
| Iatrogenic vascular lesions | |
| Cubital artery or branches | – |
| Superficial palmar arterial arch | – |
| Accidental release of Guyon’s canal | – |
| Incomplete section of carpal tunnel | – |
| Recurrence | – |
| Complex regional pain syndrome | – |
Quality of the postoperative period was also measured in a subgroup of patients who had received prior alternative surgical treatment to the contralateral hand. Patients were asked to compare postoperative quality between the alternative techniques and the new surgical technique described in the present study.
Instrument kit
Images of the specific surgical tool-kit developed by the author and used in the present study are shown in Figures 1–4, with tool-kit features described in the following text.
Figure 1.
Image of the surgical tool kit used for performing the new mini-invasive surgical technique for carpal tunnel release, showing: (1) flexible metal guide needle (0.6 × 200 mm); (2) straight fasciotome; (3) slotted fenestrated-tip guide cannula; (4) curved tip cannulated guide rod; and (5) 90° angled shaft fasciotome.
Figure 2.
Image of assembled instruments for the section of the transverse carpal ligament, showing: (1) flexible metal guide needle (0.6 × 200 mm); (2) straight fasciotome; (3) slotted fenestrated-tip guide cannula; (6) metal sphere; (7) blade portion; and (8) arch brake.
Figure 3.
Image showing the 90° angled shaft fasciotome tip, with: (6) metal spheres and (7) the blade portion.
Figure 4.
Image of the straight fasciotome tip, showing: (1) flexible metal guide needle (0.6 × 200 mm); (6) metal sphere; (7) blade portion; and (9) cannulated finger-like prong.
A flexible metal guide needle (Figure 1(1)) is used to guide progression of the straight fasciotome blade (Figure 2(7)) during the entire cutting action, eliminating the possibility of device deviation from the intended route into any wrong passageway. The straight fasciotome (Figure 1(2)) has a blade portion similar to the 90° angled shaft fasciotome (Figure 1(5) and Figure 3), but with the additional distinctive feature of a finger-like cannulated prong attached to the bottom surface of the inferior finger-like solid prong (Figure 4(9)), which allows passage of the flexible metal guide needle after this needle is placed below the under surface of the TCL with the help of the curved tip cannulated guide rod (Figure 1(4)). The slotted fenestrated-tip guide cannula (Figures 1(3) and 2(3)) has a closed but fenestrated distal end by means of a central hole that allows for the passage of the flexible guide needle (Figure 2) to lead the straight fasciotome across the TCL, placed first under the guidance of the curved tip cannulated guide rod. It provides additional protection to the underlying tissues and prevents unwanted forward progression of the fasciotome into the palm of the hand. The curved tip cannulated guide rod guides the passage of the flexible metal guide needle across the under surface of the TCL to a selected point in the palm of the hand. The purpose of the 90° angled shaft fasciotome is to cut blindly across the distal antebrachial fascia, with the right-angled bend making it particularly suitable for the task. Unintended proximal section of the antebrachial fascia is prevented by the abutment of the angled shaft against the skin at the level of the incision. The blade portion is bounded by two finger-like solid prongs that shield the blade, each of which has a 2-mm sphere at the end (Figure 3(6)). The purpose of these spheres is to increase the bluntness of the finger-like prongs, virtually eliminating the possibility of the blade deviating from the intended route, which could result in an incomplete cut or no cut of the flexor retinaculum, or a cut across the ligament towards the median nerve and tendons. Although very simple, the concept of the fasciotome blade being guided by a guide-wire by means of an attached cannulated rod (in a manner comparable to tracks of a railroad guiding the wheels of a locomotive), is key to this technique and has been verified to be very effective.
Surgical technique
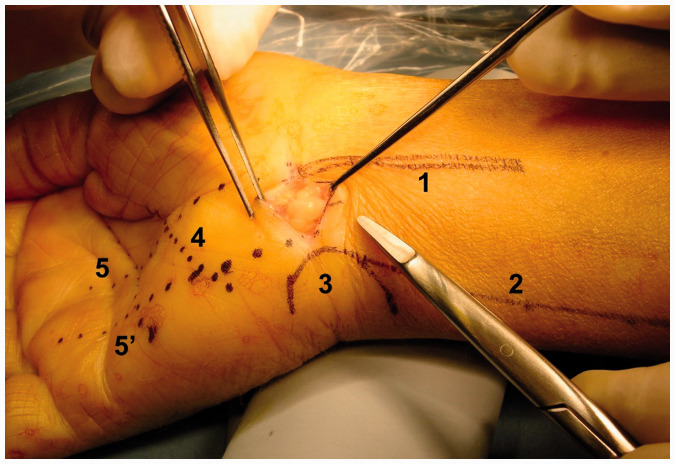
Surgical procedures were performed under axillary block, using a 10–20 cc single bolus of 0.5% ropivacaine hydrochloride (AstraZeneca, Cambridge, UK) into the neurovascular axillary sheath, or by general anaesthesia with an induction dose of 150–200 mg propofol, i.v. (Abbott Laboratories, Abbott Park, IL, USA) and maintenance with 0.8–1% sevoflurane (Baxter, Deerfield, IL, USA) and a mixture of nitrous oxide plus oxygen 40% (Air Liquide, Portugal), in cases of simultaneous bilateral surgery. With the patient lying supine on the operating table, and the affected extremity in 90° of abduction and placed on a hand table, the extremity was properly prepared and draped. A tourniquet was applied to the arm and inflated to 250 mmHg.4,27 Surgical loupes (3 × magnification) were used for the procedure. One goal of this technique is to avoid any type of palm incision, thus, a transverse incision was made over the palmar distal wrist crease, starting at a couple of mm radial to the medial border of the palmaris longus tendon32 and extending toward the ulna by 1 cm25 (Figures 5 and 6), however, any other surgical approach may be used.3,4,8,10
Figure 5.
Image of skin incision with exposed subcutaneous fat, showing: (1) PL tendon; (2) radial border of the flexor carpi ulnaris muscle; (3) pisiform; (4) Kaplan’s cardinal line; (5) line of continuation of the 3rd interdigital space; and (5′) line of continuation of the 4th interdigital space. The skin incision extends to width of 1 cm between the 5 and 5′ line.
Figure 6.
Image of skin incision with bulging subcutaneous fat removed exposing the underlying flexor retinaculum, showing: (1) PL tendon; (2) radial border of the flexor carpi ulnaris muscle; (3) pisiform; (4) Kaplan’s cardinal line; (5) line of continuation of the 3rd interdigital space; and (5′) line of continuation of the 4th interdigital space.
If the palmaris longus tendon was not detectable or out of its most usual anatomical position, then the line of continuation of the 3rd interdigital space was used as reference. Only the skin was cut sharply. Any deeper fat bulging into the operative field (Figure 5) was removed only to the extent necessary for clear visualization of the flexor retinaculum (Figure 6). With the wrist slightly extended, the area corresponding to the junction of the distal edge of the palmar carpal ligament and the proximal edge of the transverse carpal ligament was lifted with a pair of fine forceps and sectioned transversely and (optionally) also vertically, creating a diamond shaped defect, with an extension similar to the one made in the skin. Great caution was used in making this incision, with the fascia always under traction, so that it remained continuously away from the underlying tissues, as the median nerve may have been immediately below the cut being made. This was a crucial step to avoid iatrogenic injuries of the median nerve, as described previously in relation to the transverse palmar incision.23 A blunt curved dissector, e.g. Freer, was first passed proximally under the antebrachial fascia to separate any fascial adhesions, followed by a 4-mm diameter blunt rod, to further verify that a clear passage had been established. Once it was confirmed that the above-mentioned instruments could be passed freely into the proximal forearm, the 90° angle shaft fasciotome was brought into the operative field and its cutting edge positioned so that it straddled the distal palmar carpal ligament. The ligament was blindly cut for an extent of approximately 2 cm by pushing the knife proximally, parallel to the main axis of the forearm, with no requirement for visual control of the cut, as the two spheres at the tip of the finger-like prongs (Figure 3) prevent the knife from disengaging the ligament. The same manoeuvre was then repeated distally. After the free progress of the curved-tip cannulated guide rod under the deeper surface of the TCL was ensured, it was advanced until its tip was felt bulging under the skin of the palm of the hand. The depth of instrument penetration could be objectively calculated using laser marks present on the concave surface of the rod. Next, a blunt spatula was placed over the skin of the palm, pressing down just distal to the felt tip of the curved tip cannulated guide rod, and with the help of an assistant, the flexible metal needle (200 cm × 0.6 mm thick) was advanced through the lumen of the rod in a proximal-distal direction until the tip pierced the skin of the palm of the hand in the pre-determined area. The most indicated spot is as close as possible to the line of continuation of the 3rd interdigital space and no more than about 10 mm distal to Kaplan’s line, as initially described by Kaplan (Figures 7 and 8).33,34
Figure 7.
Image showing: (4) the curved tip cannulated guide rod introduced into the palm under the transverse carpal ligament; and (1) the flexible guide wire pierced through the palm of the skin ± 10 mm distal to Kaplan’s line.
Figure 8.
Image showing: (3) the slotted fenestrated-tip guide canula advanced into the palm under guidance of (1) the flexible guide wire.
Not going further distally prevents iatrogenic injury to the superficial arterial palmar arch.33,34 If the needle position was less than ideal, the surgeon retrieved the needle, repositioned the curved-tip cannulated guide rod, and tried again. Once a satisfactory needle position was obtained, the guide rod was retrieved and the flexible metal guide needle was fed through the fenestrated-tip of the slotted fenestrated-tip guide cannula (Figure 3). The cannula was then advanced along the wire until it abutted the under surface of the skin at the point of needle perforation (Figure 8).
The flexible metal guide needle was then fed through the lumen of the cannulated finger-like prong of the straight fasciotome, with the solid prongs of the fasciotome orientated so that the blade edge of the instrument straddled the proximal edge of the TCL. It was essential that the upper prong with its tip sphere was accurately placed, under direct vision, on top of the proximal edge of the ligament; with the lower part of the blade end also accurately placed, under direct vision, below the under surface of the ligament (Figure 9). This is a critical step in the operative technique. Once the surgeon was absolutely sure of the fasciotome blade position in relation to the edge of the TCL, with the help of an assistant, the flexible metal guide needle was put under tension and lifted upwards towards the under surface of the TCL. The hand was brought into neutral or slightly extended position and the fasciotome, over the longitudinal slot of the cannula and under the guidance of the guide needle, was slid all the way across the TCL, cutting it (Figure 10).
Figure 9.
Image showing the tip of the straight fasciotome blade portion straddling the proximal edge of the transverse carpal ligament (white structure).
Figure 10.
Image showing: (2) the straight fasciotome advanced under guidance of (1) the flexible guide needle over (3) the slotted fenestrated tip guide cannula, cutting the transverse carpal ligament.
There was no requirement for direct observation of the cutting action of the ligament, however, in all cases in this series, an endoscopic ‘inside-out’ observation of the intervened area was made before wound closure (Figure 11).
Figure 11.
‘Inside-out’ view illustrating complete division of the transverse carpal ligament. The underlying synovium over the flexor tendons and the median nerve is seen undisturbed.
In some cases, the tourniquet was also deflated for bleeding evaluation, and part of these observations were photographically documented. Only the skin was closed with two intradermal sutures (Vicryl Rapide 4-0 [Polyglactin 910], ETHICON; Figure 12), ending with the generous application of steri-strips. The next day, the dressing was replaced by a simple adhesive strip (e.g. band-aid) and a removable commercial short wrist splint was applied.1,5 Patients were advised to wear the splint for 24 h/day for the first 2 weeks, then at night only for an additional 2 weeks. Finger movement was encouraged from the first day. Extension of the fingers and dorsiflexion of the wrist (without the splint) was initiated at 48 h following surgery. No specialized rehabilitation (e.g. physiotherapy) was routinely used.
Figure 12.
Image showing the intradermal suture of the skin incision following the new mini-invasive surgical technique for carpal tunnel release.
Statistical analyses
Data are presented as mean (range), mean ± SD, or n (%) incidence. All data were analysed by an independent statistician using SPSS software, version 20.0 (IBM, Armonk, NY, USA) for Windows®. Student’s t-test was used for paired samples, when data followed a normal distribution, and Wilcoxon signed rank test (W statistic) was used when data did not follow a normal distribution. Statistical associations between categorical variables were analysed using χ2-test. A P value <0.05 was considered statistically significant.
Results
Data from 116 patients from the author’s clinical practice, comprising a total of 157 operated hands, were prospectively collected. Of this cohort, 98 (84.5%) were female and 18 (15.5%) were male, corresponding to a ratio of 5.44: 1. Mean age was 55 years (range, 29–96 years), and 68% of the patients were aged between 45 and 65 years. Mean time from symptom onset to surgery was 9 months (range, 6 months to 20 years) during which several types of conservative treatment were performed without practical results. All had a typical history of carpal tunnel syndrome, the most frequent (100%) being painful nocturnal paraesthesia, with night-waking, that was relieved by raising and shaking the hand. Preoperative electrodiagnostic testing was performed in 112/116 patients (96.6%). Four patients declined the testing due to economic reasons, and the preoperative electrodiagnostic testing results were reported as normal in six patients (5.2%). Mean follow-up duration was 40 months (range, 6 months to 7 years). Surgery was performed to the right hand in 85/157 cases (54.1%) and the left hand in 72 cases (45.9%), a statistically non-significant difference (P = 0.34). Single hand surgery was performed in 75 patients (64.7%) and simultaneous surgery on both hands was performed in the remaining 41 patients (35.3%), as described previously.25,26 Surgery on 115 hands (73%) was timed.
No patients were lost to follow-up for at least 6 months.
Duration of surgery
The mean duration of surgery from skin to skin was 11 min, with a minimum of 7 min (one case) and a maximum of 20 min (one case). The mean duration including total tourniquet time was 17 min, with a minimum of 12 min (one case) and a maximum of 27 min (one case).
Post-operative pain
Post-operative pain was uniformly described as minimal discomfort from the first postoperative visit onwards with no registered relapses over time. No patient reported to have used specific analgesic medication following discharge from hospital, however, the possibility that some patients may have taken minor analgesics at home (e.g. acetaminophen) cannot be ruled out.
Resolution of symptoms
All patients reported a sufficient decrease in nocturnal paraesthesia to enable uninterrupted sleep from the night following surgical intervention. The patient’s subjective assessment of symptoms at three months, showed a statistically significant decrease in the five-level Likert-type scale from a preoperative mean score of 3.823 to a 12-week postoperative mean score of 1.245 (P <0.001; Figure 13). No symptom relapses were registered during the entire follow-up period.
Figure 13.
Subjective assessment of symptoms using a five-level Likert-type scale (LS), in 116 patients (comprising 157 treated hands) who underwent the new mini-invasive surgical technique for carpal tunnel release; P < 0.001, preoperative versus postoperative scores.
Complications
No significant complications were reported in any of the 116 patients (157 hands) in this cohort, namely neuro, vascular, or tendon injuries, and there were no cases of complex regional pain syndrome or traction neuropathy (Table 2).
The most frequently reported minor complication was a granulomatous type of reaction at the level of the resorbable sutures that were used. A total of 15 possible such reactions (9.55%) were recorded, however the exact number is unknown with certainty, due to the fact that some patients reported the reaction by phone and where unwilling to attend the clinic for observation. Thus, this was a self-reported frequency that could not be verified. Others were: significant palmar ecchymosis in the hand and/or forearm, of spontaneous resolution, in six out of 157 cases (3.82%); persistent cicatricial pain in one case (0.64%); pillar pain at one month in four cases (2.55%); transient neuropraxia of the territory of the ulnar nerve in three cases (1.9%) and median nerve in one (0.64%). In two cases (1.27%) of the initial group of surgically treated patients, the technique was abandoned for safety reasons, and converted to a palmar mini-open technique. No cases of haematomas, suture dehiscence or infections were verified, although prophylactic antibiotics were not used. No cases of incomplete section of the ligament were observed. In cases where the tourniquet deflated before closure, there were no cases of sudden, intense haemorrhage, suspicious of an arterial palmar arch lesion. To date, and to the best of the author’s knowledge, there has been no case of condition recurrence.
Strength recovery
At 3 months, strength recovery was 99.17%, based on the average of the four different strength measures (range, 97.43% to 100.97%). The 3-month strength recovery results are summarized in Table 3.
Table 3.
Postoperative recovery of muscle strength at 12 weeks following the new ‘Insight-Precision’ mini-invasive surgical technique for carpal tunnel release.
| Strength measure |
% recovery |
|
|---|---|---|
| Mean | ± SD | |
| Dynamometer force recovery | 98.846 | 12.3159 |
| Key pinch force recovery | 97.430 | 8.6651 |
| Palmar pinch force recovery | 99.442 | 14.5345 |
| Tip pinch force recovery | 100.969 | 17.8697 |
Patient satisfaction
In six patients, totalling eight cases, the postoperative record of patient satisfaction at three months was missing for unknown reasons, therefore, those cases were not included in the analyses of patient satisfaction. Among 149 valid responses (from 110 patients) at three months, patients declared themselves satisfied (LS = 2) regarding 32 treated hands (21.5%) and very satisfied (LS = 1) regarding 115 treated hands (77.2%) with respect to the surgery (Figure 14).
Figure 14.
Subjective assessment of treatment satisfaction using a five-level Likert-type scale (LS) in 110 patients (comprising 149 treated hands), at 3-months following the new mini-invasive surgical technique for carpal tunnel release.
There were no WCA (Workmen’s Compensation Act) cases in this patient cohort.35 A total of 15 patients had previously received a different surgical treatment to the contralateral hand (Table 4). When asked to compare the quality of the postoperative period between the two different techniques, all were unanimous in stating that the postoperative period was better following the new ‘Insight-Precision’ technique.
Table 4.
Subjective assessment of the quality of the postoperative period between the new ‘Insight-Precision’ and an alternative technique, in a subgroup of 15 patients who had previously received alternative surgical treatment to the contralateral hand.
| Contralateral hand incision | No. Cases | Insight-Precision worse | Insight-Precision equal | Insight-Precision better | |
|---|---|---|---|---|---|
| Transverse over the distal palmar wrist crease (2 cm) | 1 | – | – | 1 | |
| Palmar with extension into the distal forearm | 3 | – | – | 3 | |
| Classic palmar ‘mini-open’ (3/4 cm) | 3 | – | – | 3 | |
| Limited-open palmar (<3 cm) | 4 | – | – | 4 | |
| Middle palmar | 4 | – | – | 4 |
Discussion
The ‘Insight-Precision’ technique is simple to perform, not requiring previous special training (e.g. cadaveric work) or special skills (e.g. endoscopic). In the present case series, the new technique was shown to be effective and very safe, minimizing the two major carpal tunnel surgery complications: scar and pillar pain/tenderness and neuro/vascular/tendinous iatrogenic injuries. Other serious complications, such as complex regional pain syndrome and traction neuropathy, due to perinervous and peritendinous adhesions,36 were not recorded. No reinterventions were performed. Pillar pain, defined by most authors simply as pain in the thenar or hypothenar eminences,13,26,29,37 is one of the most common postoperative complications following TCL release,2,5,8,9,13,30 and has been associated with both open and endoscopic surgical techniques.36,38 Its reported incidence has been inconsistent, varying from 5% to as high as 61%.8,36,39–42 Several authors report a decreased incidence of postoperative pillar pain with endoscopic or limited-open carpal tunnel-release techniques2,4,10,18,26 but others have found no difference13,36 and one study reports a higher incidence of pillar pain with an endoscopic technique.43 Its aetiology remains unclear, with different theories falling into four categories:38 ligamentous or muscular, alteration to the structure of the carpal arch, neurogenic and oedematous. The biomechanical consequences of splitting the transverse carpal ligament are not yet fully understood.36 Surgical causes, such as skin incision, type of procedure, technical practice and surgeon’s experience have all been implicated as possible causes.41 Sparing of the densely enervated palmar skin, subcutaneous tissue, and small sensory nerve branches in the area, are all plausible reasons for a decrease of pillar pain,4,5,8,44 and are the rationale behind the introduction of both limited-open and endoscopic surgeries. The standard therapy for postoperative pillar pain has been physiotherapy, bracing and rehabilitation, with inconclusive results.41 More recently, local anaesthetic injections40 and extracorporeal shock wave therapy41 have been reported, with promising results. The present study showed an incidence of postoperative persistent cicatricial pain of 0.64% (one case) and pillar pain at one month of 2.55% (four cases), which compares favourably with other reports (Table 5).8,9,39,40,42,44–48
Table 5.
Incidence of postoperative pillar pain in the present study compared with previously published reports.
| Publication | Incidence(%) | No.Cases |
|---|---|---|
| Present study | 2.55 | 157 |
| 44Ahčan, 2002* | 3 | 200 |
| 45Al-Sudani, 2015 | 3.5 | 113 |
| 9Mirza, 1995 | 3.9 | 280 |
| 46Serra, 1995 | 5 | 153 |
| 8Menon, 1994 | 7 | 100 |
| 44Ahčan, 2002** | 11 | 216 |
| 42Tse et al., 2003 | 28.6 | 1 200 |
| 47Elmaraghy, 1996 | 36 | 86 |
| 40Monacelli, 2008 | 38 | 84 |
| 39Feller et al., 2017 | 45 | 34 |
| 48Yung, 2005 | 48 | 58 |
Modified, nerve-sparing, open palmar incision.
Standard, open palmar incision.
After endoscopic carpal tunnel release was first reported by Okutsu in 19877,49 and in 1989 by Okutsu6 and Chow,11 several devices and surgical techniques emerged with the common goal of creating a small incision to decrease the incidence of the well-recognized complications of open carpal tunnel release,1,2,5,13,18,19,25–27 and improve patients’ quality of life. A steep learning curve10,45 increased the technical difficulties,13,21,28,32,33,50 and potentially devastating complications have been reported with endoscopic techniques.1,5,13,18,21,27,28,51 A variety of limited-open or mini-open techniques using multiple devices,4,26,52–55 or no special devices,56–59 also aimed to improve results of the classic open technique by decreasing the incision size, however, some of those devices have been withdrawn from the market. Reports concerning limited-open or mini-open techniques are far less numerous than those concerning endoscopic techniques. As might be expected, employing smaller incisions may lessen the problems related to open procedures, but don’t solve them entirely as, in many cases, the incision still invades the palmar skin. The reliability and comparability of results with these techniques are controversial as a result of heterogeneity between outcome-assessments and different surgical procedures. The present author agrees with the observation that limited-open procedures have not yet undergone the intense scrutiny given to more established endoscopic carpal tunnel-release techniques, and that complications and learning curves associated with these techniques should be studied further before they are put to wider use.10
In the present article, a new technique for carpal tunnel release is reported for the first time in a peer reviewed journal. This technique was developed with the low experience-level surgeon in mind. As stated previously, it does not have a steep learning curve, nor does it require previous cadaveric training or experience with endoscopic techniques. The technique allows surgery to be performed through a single incision over the distal palmar wrist crease, that compares to the smallest reported with uniportal techniques,8,60 albeit without the need of endoscopic guidance. Nonetheless, the surgeon may opt for whatever his preferred incision might be. The instruments used in the new technique are of a very low profile, occupying minimal space inside the tight carpal tunnel, removing the need for any dilating instruments. It is a ‘blind-over-guide’ technique: once the guide needle is in place and the cutting portion of the fasciotome straddles the proximal portion of the TCL, there is no need for further visualization of the ligament, as the cut is entirely guided from beginning to end. The slotted fenestrated-tip guide cannula and the tensioned guide needle fed through the cannulated finger-like prong present in the inferior surface of the straight fasciotome, prevent any type of instrument deviation in any direction, avoiding any iatrogenic injury caused by inadvertent deviation of the blade. The section of the TCL is performed with a single passage of the fasciotome. Its shielded blade of 4 mm in diameter ensures that only the TCL is cut, sparing all the other anterior structures of the hand. Patient satisfaction with the technique was also of high level, due to absence of significant postoperative pain and cosmetic reasons (Figure 15).
Figure 15.
Image showing the left hand of a 53-year-old female patient, at 6 months following the new mini-invasive surgical technique for carpal tunnel release.
It should be noted that results of the present study may be limited by the fact that postoperative pain was evaluated based on the postoperative use of analgesic medication. Although this parameter has been used before,9 a quantitative evaluation using a face rating scale or visual analogue scale would have been more suitable and persuasive.61 One of the main doubts present in independent comparative studies between endoscopic and open techniques is whether, by virtue of the medium and long-term results, the former are economically justifiable.30 The technique ‘Insight-Precision’ responds to this problem being relatively economic, although more expensive than a simple scalpel blade.
In conclusion, the ‘Insight-Precision’ technique provided remission of symptoms and was demonstrated to be simple and safe to perform, with a low complication rate. The author reiterates a former recommendation that carpal tunnel release, although apparently simple, should not be underestimated under any circumstances, regardless of the practiced technique.
Acknowledgements
The author thanks Mr. José Antão, owner of the factory of surgical instruments Manuel Nunes Antão Lda., for having turned his ideas into surgical instruments; Prof. Duarte Nunes Vieira, PhD, ex-President of the Portuguese Institute of Forensic Medicine and co-workers; Prof. Rui Jesus, PhD, for performing the statistical analyses of the data; and my surgical team.
Declaration of conflicting interest
The author declares that he has been granted a patent valid in 25 different countries, including the USA,62 concerning the instruments developed by him for the execution of the technique described in this article. He is currently involved in the project of manufacturing a commercially disposable kit, from which he is expecting to make a financial gain.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Mackinnon SE, Novak CV. Compression neuropathies In: Wolfe SW. (ed.) Green's operative hand surgery. 6th ed Philadelphia: Elsevier, 2011, pp. 985–991. [Google Scholar]
- 2.Agee JM, McCarroll HR, Jr, Tortosa RD, et al. Endoscopic release of the carpal tunnel: a randomized prospective multicenter study. J Hand Surg Am 1992; 17: 987–995. [DOI] [PubMed] [Google Scholar]
- 3.Márquez-Espriella C, Dávila-Díaz R, Aguilar-Cruz EI. Clinical outcome of carpal tunnel patients operated by either endoscopic or open carpal tunnel release. Cirugía Plástica 2009; 19: 23–28 [In Spanish]. [Google Scholar]
- 4.Fernandes CH, Nakachima LR, Hirakawa CK, et al. Carpal tunnel release using the Paine retinaculotome inserted through a palmar incision. Hand (NY) 2014; 9: 48–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Einhorn N, Leddy JP. Pitfalls of endoscopic carpal tunnel release. Orthop Clin North Am 1996; 27: 373–380. [PubMed] [Google Scholar]
- 6.Okutsu I, Ninomiya S, Takatori Y, et al. Endoscopic management of carpal tunnel syndrome. Arthroscopy 1989; 5: 11–18. [DOI] [PubMed] [Google Scholar]
- 7.Okutsu I, Ninomiya S, Natsuyama M, et al. Subcutaneous operation and examination under the universal endoscope. Nihon Seikeigeka Gakkai Zasshi 1987; 61: 491–498 [In Japanese, English abstract]. [PubMed] [Google Scholar]
- 8.Menon J. Endoscopic carpal tunnel release: preliminary report. Arthroscopy 1994; 10: 31–38. [DOI] [PubMed] [Google Scholar]
- 9.Mirza MA King ET JrandTanveer S.. Palmar uniportal extrabursal endoscopic carpal tunnel release. Arthroscopy 1995; 11: 82–90. [DOI] [PubMed] [Google Scholar]
- 10.Mirza MA, King ET., Jr. Newer techniques of carpal tunnel release. Orthop Clin North Am 1996; 27: 355–371. [PubMed] [Google Scholar]
- 11.Chow JC. Endoscopic release of the carpal ligament: a new technique for carpal tunnel syndrome. Arthroscopy 1989; 5: 19–24. [DOI] [PubMed] [Google Scholar]
- 12.Resnick CT, Miller BW. Endoscopic carpal tunnel release using the subligamentous two-portal technique. Contemp Orthop 1991; 22: 269–277. [PubMed] [Google Scholar]
- 13.Brown RA, Gelberman RH, Seiler JG, et al. Carpal tunnel release. A prospective, randomized assessment of open and endoscopic methods. J Bone Joint Surg Am 1993; 75: 1265–1275. [DOI] [PubMed] [Google Scholar]
- 14.Backhouse KM, Harrison SH, Hutchings RT. 4: Nerve and tenosynovial compressions and other conditions. In A Colour Atlas of Rheumatoid Hand Surgery London: Wolfe Medical Publications Ltd, 1981, pp. 35–36.
- 15.Bromley GS. Minimal-incision open carpal tunnel decompression. J Hand Surg Am 1994; 19: 119–120. [DOI] [PubMed] [Google Scholar]
- 16.Biyani A, Downes EM. An open twin incision technique of carpal tunnel decompression with reduced incidence of scar tenderness. J Hand Surg Br 1993; 18: 331–334. [DOI] [PubMed] [Google Scholar]
- 17.Wilson KM. Double incision open technique for carpal tunnel release: an alternative to endoscopic release. J Hand Surg Am 1994; 19: 907–912. [DOI] [PubMed] [Google Scholar]
- 18.Abrams R. Endoscopic versus open carpal tunnel release. J Hand Surg Am 2009; 34: 535–539. [DOI] [PubMed] [Google Scholar]
- 19.Benson LS, Bare AA, Nagle DJ, et al. Complications of endoscopic and open carpal tunnel release. Arthroscopy 2006; 22: 919–924.e2. [DOI] [PubMed] [Google Scholar]
- 20.Neto JE, Leite JAD, Bezerra MJC. Evaluation of surgical section of transverse carpal ligament comparing endoscopic and conventional techniques in human corpses. Acta Cir Bras 2003; 18: 116–124 [In Portuguese, English abstract]. [Google Scholar]
- 21.Murphy RX, Jr, Jennings JF, Wukich DK. Major neurovascular complications of endoscopic carpal tunnel release. J Hand Surg Am 1994; 19: 114–118. [DOI] [PubMed] [Google Scholar]
- 22.Macdermid JC, Richards RS, Roth JH, et al. Endoscopic versus open carpal tunnel release: a randomized trial. J Hand Surg Am 2003; 28: 475–480. [DOI] [PubMed] [Google Scholar]
- 23.Slattery PG. Median nerve injury and the transverse wrist crease incision in open carpal tunnel release. Aust N Z J Surg 1994; 64: 768–770. [DOI] [PubMed] [Google Scholar]
- 24.Ceruso M, Angeloni R, Lauri G, et al. Clinical diagnosis In: Luchetti R, Amadio P. (eds) Carpal tunnel syndrome. Berlin: Springer, 2007, pp. 63–68. [Google Scholar]
- 25.Tuzuner S, Sherman GM, Ozkaynak S, et al. Endoscopic carpal tunnel release: modification of Menon's technique and data from 191 cases. Arthroscopy 2004; 20: 721–727. [DOI] [PubMed] [Google Scholar]
- 26.Wong KC, Hung LK, Ho PC, et al. Carpal tunnel release. A prospective, randomised study of endoscopic versus limited-open methods. J Bone Joint Surg Br 2003; 85: 863–868. [PubMed] [Google Scholar]
- 27.Luallin SR, Toby EB. Incidental Guyon's canal release during attempted endoscopic carpal tunnel release: an anatomical study and report of two cases. Arthroscopy 1993; 9: 382–386. [DOI] [PubMed] [Google Scholar]
- 28.Feinstein PA. Endoscopic carpal tunnel release in a community-based series. J Hand Surg Am 1993; 18: 451–454. [DOI] [PubMed] [Google Scholar]
- 29.Trumble TE, Diao E, Abrams RA, et al. Single-portal endoscopic carpal tunnel release compared with open release: a prospective, randomized trial. J Bone Joint Surg Am 2002; 84: 1107–1115. [DOI] [PubMed] [Google Scholar]
- 30.Atroshi I, Larsson GU, Ornstein E, et al. Outcomes of endoscopic surgery compared with open surgery for carpal tunnel syndrome among employed patients: randomised controlled trial. BMJ 2006; 332: 1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chow JC. Endoscopic release of the carpal ligament for carpal tunnel syndrome: long-term results using the Chow technique. Arthroscopy 1999; 15: 417–421. [DOI] [PubMed] [Google Scholar]
- 32.Vasiliadis HS, Xenakis TA, Mitsionis G, et al. Endoscopic versus open carpal tunnel release. Arthroscopy 2010; 26: 26–33. [DOI] [PubMed] [Google Scholar]
- 33.Vasiliadis HS, Tokis AV, Andrikoula SI, et al. Microsurgical dissection of the carpal tunnel with respect to neurovascular structures at risk during endoscopic carpal tunnel release. Arthroscopy 2006; 22: 807–812. [DOI] [PubMed] [Google Scholar]
- 34.Kaplan EB. Surface anatomy of the hand and wrist In: Spinner E. (ed.) Functional and surgical anatomy of the hand. Philadelphia: J.B. Lippincott Co, 1953, pp. 227–231. [Google Scholar]
- 35.Olney JR, Quenzer DE, Makowsky M. Contested claims in carpal tunnel surgery: outcome study of worker's compensation factors. Iowa Orthop J 1999; 19: 111–121. [PMC free article] [PubMed] [Google Scholar]
- 36.Morrell NT, Harris A, Skjong C, et al. Carpal tunnel release: do we understand the biomechanical consequences? J Wrist Surg 2014; 3: 235–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bedeschi P. Carpal tunnel syndrome surgical complications In: Luchetti R, Amadio P. (eds) Carpal tunnel syndrome. Berlin: Springer, 2007, pp. 269–289. [Google Scholar]
- 38.Ludlow KS, Merla JL, Cox JA, et al. Pillar pain as a postoperative complication of carpal tunnel release: a review of the literature. J Hand Ther 1997; 10: 277–282. [DOI] [PubMed] [Google Scholar]
- 39.Feller RJ, Shah KN, Gil JA, et al. Prospective evaluation of patients undergoing carpal tunnel release and the development of pillar pain. In: ORS 2017 annual meeting, San Diego, California, 2017, poster no 2117.
- 40.Monacelli G, Rizzo MI, Spagnoli AM, et al. The pillar pain in the carpal tunnel's surgery. Neurogenic inflammation? A new therapeutic approach with local anaesthetic. J Neurosurg Sci 2008; 52: 11–15. [PubMed] [Google Scholar]
- 41.Romeo P, d'Agostino MC, Lazzerini A, et al. Extracorporeal shock wave therapy in pillar pain after carpal tunnel release: a preliminary study. Ultrasound Med Biol 2011; 37: 1603–1608. [DOI] [PubMed] [Google Scholar]
- 42.Tse RW, Hurst LN, Al-Yafi TA. Early major complications of endoscopic carpal tunnel release: a review of 1200 cases. Can J Plast Surg 2003; 11: 131–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katz JN, Gelberman RH, Wright EA, et al. A preliminary scoring system for assessing the outcome of carpal tunnel release. J Hand Surg Am 1994; 19: 531–538. [DOI] [PubMed] [Google Scholar]
- 44.Ahčan U, Arnež ZM, Bajrović F, Zorman P. Surgical technique to reduce scar discomfort after carpal tunnel surgery. J Hand Surg Am 2002; 27: 821–827. [DOI] [PubMed] [Google Scholar]
- 45.Al-Sudani AF, Al-Iedani MS. Proximal palmar mini-incision carpal tunnel release technique. AL-Kindy Col Med J 2015; 11: 43–49. [Google Scholar]
- 46.Serra JM, Benito JR, Monner J. Carpal tunnel release with short incision. Plast Reconstr Surg 1997; 99: 129–135. [DOI] [PubMed] [Google Scholar]
- 47.Elmaraghy MW, Hurst LN. Single-portal endoscopic carpal tunnel release: agee carpal tunnel release system. Ann Plast Surg 1996; 36: 286–291. [DOI] [PubMed] [Google Scholar]
- 48.Yung PS, Hung LK, Tong CW, et al. Carpal tunnel release with a limited palmar incision: clinical results and pillar pain at 18 months follow-up. Hand Surg 2005; 10: 29–35. [DOI] [PubMed] [Google Scholar]
- 49.Lam CH, Yeung SH, Wong TC. Endoscopic carpal tunnel release: experience of surgical outcome in a Chinese population. Hong Kong Med J 2010; 16: 126–131. [PubMed] [Google Scholar]
- 50.Zuo D, Zhou Z, Wang H, et al. Endoscopic versus open carpal tunnel release for idiopathic carpal tunnel syndrome: a meta-analysis of randomized controlled trials. J Orthop Surg Res 2015; 10: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Agee JM, Peimer CA, Pyrek JD, et al. Endoscopic carpal tunnel release: a prospective study of complications and surgical experience. J Hand Surg Am 1995; 20: 165–171. [DOI] [PubMed] [Google Scholar]
- 52.Di Giuseppe P. The mini-invasive technique for carpal tunnel release: open approach with converse fiberoptic light retractor In: Luchetti R, Amadio P. (eds) Carpal tunnel syndrome. Berlin: Springer, 2007, pp. 135–139. [Google Scholar]
- 53.Wilhelmi BJ, Lee WPA. The Indiana Tome for carpal tunnel release In: Luchetti R, Amadio P. (eds) Carpal tunnel syndrome. Berlin: Springer, 2007, pp. 140–146. [Google Scholar]
- 54.Mantovani A, De Cristofaro L, Ciaraldi A. Closed technique with Paine retinaculotome and modified retinaculotome MDC In: Luchetti R, Amadio P. (eds) Carpal tunnel syndrome. Berlin: Springer, 2007, pp. 200–210. [Google Scholar]
- 55.Atzei A, Putnam MD, Tognon S, et al. Closed carpal tunnel release technique with GRS In: Luchetti R, Amadio P. (eds) Carpal tunnel syndrome. Berlin: Springer, 2007, pp. 211–219. [Google Scholar]
- 56.Corradi M. Alternative techniques and variants: double approach – proximal and distal mini-incisions In: Luchetti R, Amadio P. (eds) Carpal tunnel syndrome. Berlin: Springer, 2007, pp. 147–150. [Google Scholar]
- 57.Panciera C, Panciera P. Carpal tunnel release with limited visualization In: Luchetti R, Amadio P. (eds) Carpal tunnel syndrome. Berlin: Springer, 2007, pp. 194–199. [Google Scholar]
- 58.Nathan PA. Open carpal tunnel release with a short palmar incision and no specialized instruments combined with a rehabilitation program for early return to activity In: Luchetti R, Amadio P. (eds) Carpal tunnel syndrome. Berlin: Springer, 2007, pp. 130–134. [Google Scholar]
- 59.De Tullio VP. Carpal tunnel syndrome release using the Chiena technique In: Luchetti R, Amadio P. (eds) Carpal tunnel syndrome. Berlin: Springer, 2007, pp. 220–225. [Google Scholar]
- 60.Klein RD, Kotsis SV, Chung KC. Open carpal tunnel release using a 1-centimeter incision: technique and outcomes for 104 patients. Plast Reconstr Surg 2003; 111: 1616–1622. [DOI] [PubMed] [Google Scholar]
- 61.Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain 2016; 17: 131–157. [DOI] [PubMed] [Google Scholar]
- 62.Carmo JD. Surgical set of instruments for precision cutting Patent US 9,782,192 B2, USA, 2017.