Short abstract
Objective
The p48 is a low-profile, intermediate-porosity flow diverter. phenox GmbH-trademarked hydrophilic polymer coating (pHPC) is a hydrophilic coating that has been shown in vitro to reduce the thrombogenicity of nitinol device surfaces. We performed the present study to determine whether the p48_HPC can be implanted using prasugrel alone.
Methods
We retrospectively identified all patients who were treated with the p48_HPC from January 2017 to December 2018 (n = 5) and underwent single antiplatelet therapy (SAPT) with prasugrel. P2Y12 inhibition was confirmed by the VerifyNow assay. The occurrence of thromboembolic and haemorrhagic complications was recorded alongside the occlusion rates of the treated aneurysms.
Results
All patients achieved adequate occlusion (Raymond–Roy Occlusion Classification I or II) during the follow-up period. No thromboembolic complications occurred. One patient developed a contained haematoma within the Sylvian fissure from the treated aneurysm 2 weeks postoperatively without clinical sequelae.
Conclusions
In this small series, no thromboembolic complications occurred in patients treated with the surface modified p48_HPC flow diverter and SAPT. Further studies with longer follow-up periods and larger cohorts should be performed.
Keywords: Aneurysm, flow diverter, surface modification, hydrophilic polymer coating, p48_HPC, single antiplatelet therapy
Abbreviations
- DAPT
dual antiplatelet therapy
- DFT
drawn filled tube
- FDS
flow-diverting stent
- HPC
hydrophilic polymer coating
- pHPC
phenox GmbH-trademarked hydrophilic polymer coating
- RROC
Raymond–Roy Occlusion Classification
- SAPT
single antiplatelet therapy
Introduction
The introduction of flow-diverting technology represents a paradigm shift in the way intracranial aneurysms can be treated. These new stents were originally used in previously uncoilable aneurysms or those in which previous endovascular treatments had failed.1 These devices were originally indicated only for unruptured aneurysms because of the need for dual antiplatelet therapy (DAPT). The exact mechanism of action of aneurysm exclusion is not completely understood; however, it likely involves several factors that occur in conjunction with one another and allow eventual healing of the underlying parent vessel.2 All of the various devices that are commercially available share common design features such as a braided wire design and higher metal coverage than conventional stents.3,4
A variety of different flow-diverting stents (FDSs) are currently available, including the Pipeline Embolisation Device (PED; Medtronic, Minneapolis, MN, USA), Silk (Balt Extrusion, Montmorency, France), Surpass (Stryker Neurovascular, Kalamazoo, MI, USA), p64 (phenox GmbH, Bochum, Germany), and the Flow Re-direction Endoluminal Device (FRED) (MicroVention, Aliso Viejo, CA, USA). Newer devices, such as the PED Shield, have also entered the market promising lower thrombogenicity. In addition to the similarity in the design of many of these devices, most FDSs require the concomitant use of DAPT to prevent thromboembolic complications. The PED Shield, a surface modified device, has been used off-label with single antiplatelet therapy (SAPT).5,6
We herein present an initial series of a newly developed flow diverter, the p48 (phenox GmbH), with its trademarked glycan-based surface hydrophilic polymer coating (pHPC). pHPC has been found to significantly reduce the thrombogenicity of the concerning nitinol device.7
Materials and methods
The p48_HPC
The p48_HPC flow diverter is constructed from 48 braided strands. Each strand is made from drawn filled tubes (DFTs), with each DFT strand constructed from a platinum-filled nitinol tube. Each of the 48 DFT strands is coated with the newly developed glycan-based multilayer pHPC. The p48 flow diverter is compatible with 0.021-inch inner diameter microcatheters. Unlike the p64 device, the p48 and p48_HPC are not mechanically detached. A proximal radiopaque marker on the insertion wire identifies the point at which the device can still be resheathed. Dependent upon the size and length of the device, it can be resheathed up to 80%. There is a central, independently moveable wire. The movable inner wire is made of nitinol and has an atraumatic distal tip to prevent rupture of small distal vessels and perforator branches. The p48 is currently available with a nominal diameter of 2 or 3 mm and is designed to treat vessels of 1.75 to 3 mm. The unrestrained diameters are 2.8 and 3.8 mm, respectively.
Patient population
We performed a retrospective analysis of our prospectively maintained database to identify all patients treated with the p48_HPC flow diverter at Clinica La Sagrada Familia, Buenos Aires, Argentina. Patients with saccular, dissecting, and fusiform aneurysms, both ruptured and unruptured, were included. We searched our database from January 2017 to December 2018.
We recorded each patient’s demographic data, clinical presentation, location of the aneurysm, size and location of the p48_HPC flow diverter, clinical and radiological follow-up information, and complications. Written informed consent was obtained from all patients before the procedure. Institutional Review Board approval was granted for this study (SF2017-003).
Endovascular treatment
All treatments were performed under general anaesthesia. All patients received SAPT (prasugrel at 10 mg/day) for at least 5 days prior to the treatment. The effectiveness of the antiplatelet regimen was tested using the VerifyNow assay (Accumetrics, Bedford, MA, USA). After the procedure, all patients were planned to remain on prasugrel for 1 year, followed by 100 mg of aspirin daily for life.
All procedures were performed via the right common femoral artery using an 8-Fr access and tri-axial system consisting of an 8-Fr Shuttle (Cook Medical, Bloomington, IN, USA), Neuron MAX (Penumbra, Almeida, CA, USA), and Headway 21 (MicroVention, Tustin, CA, USA). All procedures were performed under heparin anticoagulation with a 5,000-IU bolus dose at the start of the procedure and subsequent 1,000-IU bolus doses (if required) every hour to maintain the activated clotting time at 2.0 to 2.5 times the baseline level.
Procedural assessment and follow-up
The patency and flow characteristics within the parent vessel and any covered cortical branches or perforators were angiographically assessed immediately after placement of the FDS and during follow-up. Procedural follow-up was performed by digital subtraction angiography initially at 3 to 6 months and again at 9 to 12 months. Standard angiographic projections were used to assess the patency of the vessels and the aneurysms in addition to angiographic projections that repeated those used during the treatment. Aneurysm occlusion was graded using the 3-point Raymond–Roy Occlusion Classification (RROC).8 All patients underwent magnetic resonance imaging as part of the standard imaging follow-up. This was performed at 3 to 6 months postoperatively and included standard axial T1-weighted imaging, T2-weighted imaging, diffusion-weighted imaging, fluid-attenuated inversion recovery sequences, and gradient echo sequences.
Neurological examinations were performed to identify potential ischaemic or haemorrhagic complications in the post operative period (<24 hours post-procedure) and at each subsequent follow-up.
Statistical analysis
Descriptive statistics are reported as mean ± standard deviation (range) and frequency where appropriate.
Results
Baseline demographics and aneurysm characteristics
We identified five women with an average age of 61.2 ± 19.5 years (range, 30–83 years). Four of the aneurysms (80%) were located in the anterior circulation and one in the posterior circulation, and three of the five aneurysms (60%) were located on the right side. Two aneurysms were located at the middle cerebral artery bifurcation, one on an M2 branch, and one on the pericallosal artery, and one aneurysm arose from the posterior inferior cerebellar artery. The angiographic findings of each aneurysm prior to treatment is provided in Figure 1.
Figure 1.
Angiographic images of each of the patients’ aneurysms. (a) Patient 1. (b) Patient 2. (c) Patient 3. (d) Patient 4. (e) Patient 5.
Four of the aneurysms were classified as saccular, and the remaining aneurysm was thought to be dissecting in nature. The mean aneurysm dome size was 2.88 ± 2.2 mm (range, 1.4–6.8 mm), and the mean neck size was 2.1 ± 0.7 mm (range, 1.2–2.8 mm).
All patients received prasugrel at 10 mg/day. Patient 3 was prescribed apixaban for known atrial fibrillation. Patient 5 began taking aspirin at 75 mg/day of her own volition 2 weeks after the procedure (Table 1).
Table 1.
Baseline demographics, aneurysm characteristics, and perioperative findings.
| Patient No. | Demographics |
Aneurysm characteristics |
Antiplatelet therapy |
FDS size and positioning |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (y) | Sex | Location | Laterality | Morphology | Neck (mm) | Maximum dome dimension (mm) | Ruptured | Daily antiplatelet/anticoagulation | PRU | p48_HPC size | Position of the FDS | Side branch covered | Fate of covered side branch | |
| 1 | 59 | F | MCA Bif. | R | Saccular | 2.6 | 1.7 | No | Prasugrel 10 mg | 45 | 3 × 15 mm | M1-2 | Yes | Patent |
| 2 | 68 | F | Pericallosal | L | Saccular | 1.2 | 2.0 | No | Prasugrel 10 mg | 8 | 2 × 15 mm | A2-3 | Yes | Patent |
| 3 | 66 | F | PICA | L | Saccular | 1.5 | 2.5 | No | Prasugrel 10 mg, Apixaban 5 mg | 68 | 2 × 12 mm | PICA | No | NA |
| 4 | 83 | F | M2 | R | Dissecting | 2.8 | 6.8 | No | Prasugrel 10 mg | 9 | 2 × 15 mm | M2 | No | NA |
| 5 | 30 | F | MCA Bif. | R | Saccular | 2.5 | 1.4 | No | Prasugrel 10 mg | 80 | 3 × 12 mm | M1-2 | Yes | Patent |
F, female; MCA Bif., middle cerebral artery bifurcation; PICA, posterior inferior cerebellar artery; R, right; L, left; PRU, platelet reactivity units; FDS, flow-diverting stent; NA, not available.
Radiological follow-up
The data from the early (3-month) angiographic follow-up were available in four patients. Two of the aneurysms were graded as RROC I (complete occlusion), one was graded as II (minor filling at the aneurysm neck) (Figure 2), and one was graded as III (persistent filling of the dome), resulting in adequate occlusion in three aneurysms (75%).
Figure 2.
Patient 2. (a) This patient presented in 2016 with acute subarachnoid haemorrhage that was thought to be secondary to a ruptured right posterior communicating artery aneurysm. (b) In addition to the culprit aneurysm, three other aneurysms were identified, including a left pericallosal aneurysm. (c, d) A p48_HPC was implanted in the callosomarginal artery, covering the origin of the pericallosal artery. (e) Minimal opacification of the aneurysm neck was seen on the 3-month follow-up angiogram. (f) On delayed follow-up, the aneurysm was completely occluded and the covered pericallosal artery was reduced in calibre but remained patent with anterograde flow.
In three patients, side branches were covered by the flow diverter; however, these remained patent with anterograde flow on follow-up angiography. There was no clinical evidence of ischaemic stroke and no new lesions within the treated vascular territory on the follow-up cross-sectional imaging in any of the patients.
Delayed angiographic follow-up was available in four patients at an average of 8.5 months postoperatively (range, 6–12 months). In three patients, the aneurysm was completely occluded, and in the remaining patient, a small neck remnant was present (RROC II).
Overall, including the early and intermediate delayed imaging examinations, four aneurysms were graded as RROC I and one aneurysm was graded as RROC II, resulting in adequate occlusion of all aneurysms. An illustrative case is shown in Figure 2.
Clinical follow-up
No intraoperative complications occurred, and all patients had a modified Rankin scale score of 0 at their most recent clinical follow-up (Table 2).
Table 2.
Follow-up data.
| Initial follow-up (months) | Follow-up | MRI/CT | Delayed follow-up (months) | RROC | mRS score pre-intervention | mRS score post-intervention (30 days) | Comments |
|---|---|---|---|---|---|---|---|
| 3 | III | No new lesions | 9 | II | 0 | 0 | |
| 3 | II | No new lesions | 12 | I | 0 | 0 | |
| 3 | I | No new lesions | 6 | I | 0 | 0 | Known atrial fibrillation for which the patient was already taking apixaban |
| 3 | I | No new lesions | NA | NA | 0 | 0 | Contained haematoma after implantation of flow-diverting stent |
| NA | NA | No new lesions | 7 | I | 0 | 0 | Patient started taking aspirin of her own volition |
NA, not available; MRI, magnetic resonance imaging; CT, computed tomography; RROC, Raymond–Roy Occlusion Classification; mRS, modified Rankin scale.
Complications
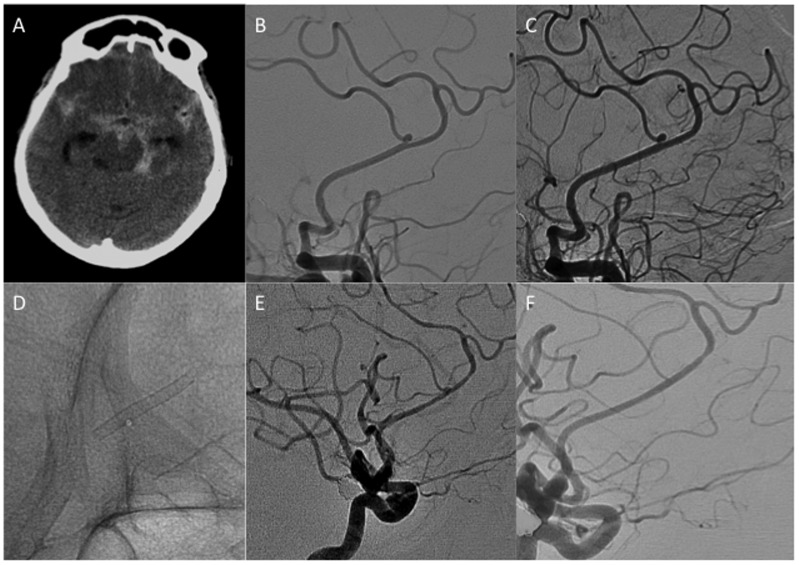
No intraoperative complications occurred. Patient 4 developed an acute thunderclap headache and was found to have a partially thrombosed aneurysm of an M2 branch on the right that was thought to be dissecting in nature. Two weeks postoperatively, she developed an acute severe headache that was unresponsive to analgesic medications; however, she had no acute neurological signs or symptoms and no definite features of meningism. A computed tomography scan demonstrated a localised haematoma in the right Sylvian fissure. The patient was managed conservatively and remained at her baseline neurological status. This case is summarised in Figure 3.
Figure 3.
Patient 4. (a, b) This patient was undergoing investigations for a headache and was found to have a partially thrombosed aneurysm arising from an M2 branch of the middle cerebral artery on the right side. This was thought to be dissecting in nature, and after discussion among the multidisciplinary team, treatment with flow diversion was offered. (c) After implantation of the p48_HPC, significant stagnation of contrast could be seen within the aneurysm. No intraoperative complications occurred, and the patient remained at her baseline neurological status. Two weeks postoperatively the patient returned to our clinic with a severe headache that was nonresponsive to standard analgesia. (d) Computed tomography imaging revealed a localised haematoma within the right Sylvian fissure. The patient was managed conservatively. (e, f) Delayed angiography showed complete exclusion of the aneurysm from the circulation.
Discussion
Since their initial introduction into the neurovascular space almost a decade ago, FDSs have become a mainstay of treatment for aneurysms of various morphologies. The Pipeline for Uncoilable or Failed Aneurysms (PUFS) trial published its initial results in 2013 and demonstrated that 73.6% of aneurysms were occluded at 6 months.1 More recently, the 5-year results of the PUFS trial were published, and of the 74 patients (76 aneurysms) examined by catheter angiography, 71 (93.4%) aneurysms were occluded with a 95.2% rate of progressive aneurysm occlusion (60/63) at the 5-year follow-up.11 To safely prevent thromboembolic complications, there is a need for DAPT when using FDSs and neurovascular stents in general. This can cause issues in certain circumstances, such as in patients with acute subarachnoid haemorrhage or in patients with pre-existing conditions such as gastric ulcers. Similarly, there are potential drug interactions that may interfere with the effectiveness of antiplatelet therapy.12 One way to negate these potential issues has been to develop surface-coated flow diverters and stents. pHPC is designed to replicate the glycocalyx of the arterial wall and is extremely hydrophilic. The mechanism of action of HPC is related to these properties rather than being a pharmacological mechanism of action. The thickness of the surface coatings ranges from 10 to 20 nm as determined by X-ray photoelectron spectroscopy analysis. The thin nature of the coating has no influence on the surface texture or physical properties of the stents.
Our initial in vitro studies of pHPC showed significantly less adherence of immunofluorescent CD61-positive platelets on coated than uncoated nitinol after incubation with whole blood (1.12% ± 0.4% vs. 48.61% ± 7.3%, respectively; p ≤ 0.001). Analysis of coated versus uncoated p48 flow diverters using scanning electron microscopy demonstrated minimal adherent platelets on the coated flow diverters compared with a thick layer of adherent platelets on uncoated devices.7 Individual thrombocytes tended to adhere in the vicinity of defects on the wire surface, but large cell agglomerates were not detected.
Further studies comparing the p48 with the p48_HPC in a flow model have also been performed. In this study, the thrombogenicity of the new polymer-coated p48_HPC flow diverter was compared with that of the uncoated p48 device in an in vitro flow model (Chandler loop). In each individual experiment, three loops were run simultaneously: one loop with a p48, one loop with a p48_HPC, and one loop without an implant as negative control. Thrombocyte adhesion and activation were assessed in five individual experiments after 120 minutes. Whole blood from five healthy donors was used in this study. After circulation in the Chandler loop for 120 minutes, the platelet count of the uncoated p48 (71% ± 8%) was significantly lower than that of the p48_HPC (87% ± 5%; p ≤ 0.05). Fluorescent microscopy showed a thick layer of adherent fluorescent cells embedded in a thick fibrin clot on the uncoated p48, whereas the p48_HPC fluorescence was much lower. Minimal fluorescent CD42a+ thrombocytes were detected on the p48_HPC, with no clot formation visible under fluorescence microscopy. Similarly, platelets in contact with the uncoated p48 flow diverters were significantly more activated than those incubated with the p48_HPC (73% ± 9% vs. 65% ± 6%, respectively; p < 0.05) and released significantly more microparticles (1.8 ± 0.5 vs. 1.4 ± 0.4, respectively; p < 0.05), which are markers of platelet activation.13
In vivo studies have shown that the pHPC coating is biocompatible after implantation in both dogs and rabbits.14,15 Furthermore, there is no evidence that pHPC elicits either an acute or chronic inflammatory reaction.14 The ability to inhibit thrombogenicity is naturally advantageous; however, this may not be the case if it simultaneously inhibits endothelialisation and hence exclusion of the treated aneurysm from the circulation. Our initial in vivo studies comparing coated and uncoated pCONUS devices (phenox GmbH), which are laser-cut nitinol stents used for the treatment of wide-necked bifurcation aneurysms, did not show a difference in the rate of neo-endothelialisation. In this study, both devices were virtually completely covered in neo-endothelium, with <1% of the stent struts being non-endothelialised at 30 days (0.6 ± 1.57 vs. 0.932 ± 0.78).14 Similar results have also been seen when comparing the uncoated p48 and coated p48_HPC (unpublished data). These results imply that pHPC has the ability to lower thrombogenicity but does not inhibit endothelialisation when compared with bare nitinol.
Our initial in vivo study (unpublished data) compared the p48 and p48_HPC in rabbits (n = 8). In this study, a p48 device was implanted into the left common carotid artery of each rabbit and a p48_HPC was implanted into the right common carotid artery of each rabbit. All animals received acetylsalicylic acid (10 mg/kg/day) and clopidogrel (10 mg/kg/day) via their drinking water. Angiography at 30 days showed fully expanded devices bilaterally without evidence of thrombus formation. Histopathological analysis did not demonstrate a significant difference in the degree of inflammation within the arterial wall between the two devices. The endothelial coverage was near complete for both devices. These results corroborate the findings in the analysis of the pCONUS HPC and suggest pHPC neither elicits an acute inflammatory response nor interferes with endothelialisation when applied to flow diverters.
Aspirin has variable platelet activity when assessed by different methods and is dependent upon the testing method used. From 5% to 57% of patients tested in different studies were judged to have suboptimal antiplatelet effects during aspirin treatment.16 Additionally, previous post-hoc analyses have shown an increased risk of cardiovascular events among such patients.16–18 Less than 5% residual capacity to generate thromboxane A2 is sufficient to fully sustain thromboxane-dependent platelet aggregation, and full aggregation was achieved in vitro with only 2.5% of the platelet population being free of aspirin.19,20 Thus, virtually complete inhibition of platelet COX is required for aspirin to provide an adequate antithrombotic effect. COX activity has been seen to recover by approximately 10% per day as a function of platelet turnover, and this may vary both within and between patients. Phasic increases in thromboxane synthesis have been seen in patients with unstable angina and other conditions associated with increased platelet turnover, such as diabetes mellitus, pre-eclampsia, and advanced coronary artery disease.21–23 Furthermore, the concept of enhanced platelet recovery was recently discussed in relation to the PED Shield.6 Although aspirin could be used in conjunction with coated devices, such as the p48_HPC, the potential for inadequate antiplatelet activity was considered too great especially because these were the first cases evaluated in human patients. For these reasons, aspirin is not currently considered to be the optimal antiplatelet agent in these patients. Ticagrelor and cangrelor, two cyclopentyl-triazolo-pyrimidines, are also not felt to be suitable. These drugs, unlike thienopyridines, bind reversibly to P2Y12 receptors on platelets, but they bind directly without biotransformation. The antiplatelet action of both of these drugs is reversible within hours of discontinuation of therapy; therefore, missing a single dose can result in complete loss of antiplatelet activity.24 Because of these concerns, clopidogrel (a second-generation thienopyridine) and prasugrel (a third-generation thienopyridine) are considered the optimal choices. Prasugrel is approximately 10- and 100-fold more potent in inhibiting platelet function in vivo than clopidogrel and ticlopidine, respectively.25 A 60-mg loading dose of prasugrel followed by a 10-mg daily maintenance dose resulted in greater inhibition of platelet aggregation at 4 hours compared with a 300-mg loading dose of clopidogrel with a 75-mg daily maintenance dose (68.4% vs. 30.0%, respectively). In addition, there were fewer pharmacologic nonresponders with prasugrel than clopidogrel (3% vs. 52%, respectively).26 For these reasons, we felt that our first experience with the p48_HPC should be in conjunction with prasugrel.
A single complication occurred in our series: delayed rupture of a dissecting aneurysm. The haematoma was believed to be secondary to local degeneration of the arterial wall by proteolytic enzymes released by the intra-aneurysmal thrombus, as previously postulated.9,10
Our study has the same limitations inherent to all small retrospective studies, and the results were self-adjudicated. All treated patients were female, and no ruptured aneurysms were treated with the p48_HPC. The safety of SAPT in the context of p48_HPC implantation requires further evaluation. Prospective registries using SAPT and randomised controlled trials comparing DAPT and SAPT are in preparation.
Conclusion
The p48_HPC flow diverter is a novel surface-modified flow diverter that can be used with SAPT (prasugrel) in selected patients with unruptured aneurysms. Although the initial results are promising, larger registries and randomised trials addressing safety and efficacy with longer clinical and angiographic follow-up are required.
Authors’ contributions
PB, JG, CB – data gathering, manuscript preparation
IL, NP, HH – review, editing
PL – guarantor
Declaration of conflicting interest
PB and PL have a consulting agreement with phenox GmbH.
HH is cofounder and shareholder of phenox GmbH.
The other authors declare that they have no competing interests.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Ethics statement
Informed consent was obtained from all patients. The retrospective data retrieval, analysis, and publication were approved by the institutional ethics committee (protocol number SF17-012). All procedures were in accordance with the 1964 Declaration of Helsinki and its later amendments. Data handling respected the privacy of the patients.
References
- 1.Becske T, Kallmes DF, Saatci I, et al. Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology 2013; 267: 858–868. [DOI] [PubMed] [Google Scholar]
- 2.Kadirvel R, Ding Y-H, Dai D, et al. Cellular mechanisms of aneurysm occlusion after treatment with a flow diverter. Radiology 2014; 270: 394–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Augsburger L, Farhat M, Reymond P, et al. Effect of flow diverter porosity on intraaneurysmal blood flow. Klin Neuroradiol 2009; 19: 204–214. [DOI] [PubMed] [Google Scholar]
- 4.Lieber BB, Stancampiano AP, Wakhloo AK. Alteration of hemodynamics in aneurysm models by stenting: influence of stent porosity. Ann Biomed Eng 1997; 25: 460–469. [DOI] [PubMed] [Google Scholar]
- 5.Hanel RA, Aguilar-Salinas P, Brasiliense LB, et al. First US experience with pipeline flex with shield technology using aspirin as antiplatelet monotherapy. BMJ Case Rep 2017; 2017: bcr-2017-219406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manning NW, Cheung A, Phillips TJ, et al. Pipeline shield with single antiplatelet therapy in aneurysmal subarachnoid haemorrhage: multicentre experience. J Neurointerv Surg 2018; 11: 694–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lenz-Habijan T, Bhogal P, Peters M, et al. Hydrophilic stent coating inhibits platelet adhesion on stent surfaces: initial results in vitro. Cardiovasc Intervent Radiol 2018; 41:1779–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke 2001; 32: 1998–2004. [DOI] [PubMed] [Google Scholar]
- 9.Thielen E, McClure M, Rouchaud A, et al. Concomitant coiling reduces metalloproteinase levels in flow diverter-treated aneurysms but anti-inflammatory treatment has no effect. J Neurointerventional Surg 2017; 9: 307–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulcsár Z, Houdart E, Bonafé A, et al. Intra-aneurysmal thrombosis as a possible cause of delayed aneurysm rupture after flow-diversion treatment. AJNR Am J Neuroradiol 2011; 32: 20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Becske T, Brinjikji W, Potts MB, et al. Long-term clinical and Angiographic outcomes following pipeline embolization device treatment of complex internal Carotid Artery Aneurysms: five-year results of the pipeline for uncoilable or failed aneurysms trial. Neurosurgery 2017; 80: 40–48. [DOI] [PubMed] [Google Scholar]
- 12.Martinez-Moreno R, Aguilar M, Wendl C, et al. Fatal thrombosis of a flow diverter due to ibuprofen-related antagonization of Acetylsalicylic acid. Clin Neuroradiol 2016; 26: 355–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lenz-Habijan T, Brodde M, Kehrel BE, et al. Comparison of the thrombogenicity of a bare and antithrombogenic coated flow diverter in an in vitro flow model. Cardiovasc Intervent Radiol 2019; doi: 10.1007/s00270-019-02307-5. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 14.Bhogal P, Lenz-Habijan T, Bannewitz C, et al. The pCONUS HPC: 30-day and 180-day in vivo biocompatibility results. Cardiovasc Intervent Radiol 2019; 42: 1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martínez Moreno R, Bhogal P, Lenz-Habijan T, et al. In vivo canine study of three different coatings applied to p64 flow-diverter stents: initial biocompatibility study. Eur Radiol Exp 2019; 3: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanderson S, Emery J, Baglin T, et al. Narrative review: aspirin resistance and its clinical implications. Ann Intern Med 2005; 142: 370–380. [DOI] [PubMed] [Google Scholar]
- 17.Cattaneo M. Aspirin and clopidogrel: efficacy, safety, and the issue of drug resistance. Arterioscler Thromb Vasc Biol 2004; 24: 1980–1987. [DOI] [PubMed] [Google Scholar]
- 18.Patrono C, Coller B, FitzGerald GA, et al. Platelet-active drugs: the relationships among dose, effectiveness, and side effects: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126: 234S–264S. [DOI] [PubMed] [Google Scholar]
- 19.Di Minno G, Silver MJ, Murphy S. Monitoring the entry of new platelets into the circulation after ingestion of aspirin. Blood 1983; 61: 1081–1085. [PubMed] [Google Scholar]
- 20.Reilly IA. and, FitzGerald GA. Inhibition of thromboxane formation in vivo and ex vivo: implications for therapy with platelet inhibitory drugs. Blood 1987; 69: 180–186. [PubMed] [Google Scholar]
- 21.Tschoepe D. The activated megakaryocyte-platelet-system in vascular disease: focus on diabetes. Semin Thromb Hemost 1995; 21: 152–160. [DOI] [PubMed] [Google Scholar]
- 22.Hamsten A, Svensson J, Walldius G, et al. Shortened megakaryocyte-platelet regeneration time in young survivors of myocardial infarction. Am Heart J 1985; 110: 1154–1160. [DOI] [PubMed] [Google Scholar]
- 23.Wallenburg HC, van Kessel PH. Platelet life span in pregnancies resulting in small-for-gestational age infants. Am J Obstet Gynecol 1979; 134: 739–742. [DOI] [PubMed] [Google Scholar]
- 24.Angiolillo DJ, Bhatt DL, Gurbel PA, et al. Advances in antiplatelet therapy: agents in clinical development. Am J Cardiol 2009; 103: 40A–51A. [DOI] [PubMed] [Google Scholar]
- 25.Sugidachi A, Ogawa T, Kurihara A, et al. The greater in vivo antiplatelet effects of prasugrel as compared to clopidogrel reflect more efficient generation of its active metabolite with similar antiplatelet activity to that of clopidogrel’s active metabolite. J Thromb Haemost JTH 2007; 5: 1545–1551. [DOI] [PubMed] [Google Scholar]
- 26.Jernberg T, Payne CD, Winters KJ, et al. Prasugrel achieves greater inhibition of platelet aggregation and a lower rate of non-responders compared with clopidogrel in aspirin-treated patients with stable coronary artery disease. Eur Heart J 2006; 27: 1166–1173. [DOI] [PubMed] [Google Scholar]