Abstract
Background
Immune checkpoint inhibitors (ICIs) have greatly improved the prognosis and overall management of non‐small cell lung cancer (NSCLC) patients, but in the long term less than 20% of patients benefit from treatment with ICIs. Therefore, it is necessary to guide the choice of immunotherapy population through biomarkers in order to maximize the benefit for NSCLC patients. This article mainly explores the relationship between the efficacy of immunotherapy and specific tumor mutation gene characteristics in an NSCLC population.
Methods
This was a prospective analysis of patients with advanced NSCLC who visited the Department of Respiratory Medicine of Peking Union Medical College Hospital from March 2018 to June 2019 and were instructed to use PD‐1 inhibitors. The follow‐up deadline was 31 December 2019. The tumor pathological tissues were tested for tumor mutation genes, and the patients were evaluated for efficacy according to RECIST 1.1. The patients were divided into the durable benefit group (DCB) and the nonsustainable benefit group (NDB). DCB/NDB was used as the outcome variable. Various statistics methods were used to explore the independent predictors of long‐term benefits associated with immunotherapy and to draw a progression‐free survival curve for the relevant predictors.
Results
A total of 44 patients were examined for tumor mutation genes in pathological tissues; 20 in the DCB group and 24 in the NDB group. Specific gene mutations occurred in TP53 38.64%, KRAS 31.82%, EGFR 20.45%, BRCA 20.45%, ERBB (excluding EGFR) 18.18%, PTEN 15.91%, CDK4/6 13.64%, POLE 11.36%, MET 11.36%, PIK3CA 9.10%, FGFR 9.10%, BRAF 9.10%, JAK 9.10%, ALK 6.82%, POLD1 4.55%, BLM 4.55%. Chi‐square test results showed that there were statistically significant differences between DCB and NDB groups with eight mutations such as KRAS. Logistic regression showed that the KRAS mutation was statistically significant (P < 0.001). Two accuracy indicators, Random Forest Classification of Mean Decrease Gini and Mean Decrease Accuracy, evaluated the importance of the impact of different gene mutations on the outcome. Under two different measures, the variables were all KRAS mutations. It is suggested that the mutation of the KRAS gene is an independent predictor of the long‐term benefit of immunotherapy.
Conclusions
The mutation of KRAS gene in tumor tissues is an independent predictor of the long‐term benefit of immunotherapy, and the predictive ability is better.
Keywords: Immune checkpoint inhibitor, KRAS, non‐small cell lung cancer, PD‐1, PD‐L1
Introduction
Non‐small cell lung cancer (NSCLC) accounts for 85% of diagnosed lung cancers. About 50% of NSCLC patients are diagnosed when they are already in stage IV, and their five‐year survival rate is less than 10%.1 The emergence of immune checkpoint inhibitors (ICIs) targeting programmed cell death 1 (PD‐1) or its ligand (PD‐L1) has significantly changed the treatment and management of locally advanced and advanced NSCLC. Multiple randomized controlled trials (RCTs) have shown that ICIs are superior to docetaxel2, 3, 4 as a second‐line treatment for advanced NSCLC patients. ICIs have been approved by the US Food and Drug Administration (FDA) to treat patients with 15 different cancer types.5 However, most tumors appear to lack T cell infiltration and active expression of immune genes. Only 20% of patients with advanced NSCLC benefit from the treatment, whereas up to 50% of patients experience treatment‐related adverse events (AEs). Considering the high cost of the drug, the limited population that benefits from it, and the potential for serious side effects, it is important to explore biomarkers for selecting patients with advanced NSCLC who might benefit from ICI treatment.
Recent studies have found that genetic changes in specific driver genes activate tumor cell proliferation, thereby supporting tumor growth. It has been shown that certain oncogenic pathways also affect the immune system's recognition of tumors, especially T cell‐mediated recognition. Smoking‐related KRAS mutations are the most common carcinogenic change in NSCLC.6, 7 Recent clinical evidence indicates that tumors classified as KRAS‐TP53 have an immunogenic phenotype and may be more sensitive to nivolumab.8
This study examined tumor mutation genes in the pathological tissues of 44 Chinese NSCLC patients treated with anti‐programmed death (PD)‐1 monoclonal antibodies (including pembrolizumab, nivolumab, and sintilimab) to identify genetic changes associated with the clinical benefit of immune checkpoint inhibitors (ICIs). The goal of the study is to accurately select the population that will benefit from immunotherapy.
Methods
Patients
A prospective analysis was conducted of patients with advanced NSCLC who visited the Peking Union Medical College Hospital from March 2018 to June 2019 and were instructed to use PD‐1/PD‐L1 inhibitors. According to the solid tumor response evaluation standard (Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1), there are four categories consisting of complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). Durable clinical benefit (DCB) is defined as CR, PR, or SD lasting more than six months. Patients who developed disease progression within six months were classified as having no durable benefit (NDB). Efficacy is determined every six to eight weeks after the start of the immunotherapy. In special cases, the time interval can be adjusted to suit the patients' needs. The enrollment deadline for patients was 30 June 2019, and the follow‐up deadline was 31 December 2019. The Ethics Committee of the Peking Union Medical College Hospital has approved this study, which is in line with the ethical principles of the Helsinki Declaration. All patients have signed informed consent.
Sample collection
Fresh tissue was sampled to detect gene mutation before immunotherapy, or a pathological white section of tumor tissue was used that was obtained within two years before treatment with PD‐1/PD‐L1 inhibitor. It is necessary to note the time of tumor tissue ex vivo; section requirements: tumor cells > 20%, area > 10 × 10 mm, thickness of 5–10 μm, and 15 slices or more.
Main experimental reagents and instruments
Tissue genomic DNA extraction kit DP304 (TIANGEN), KAPA HyperPlus Kits (Roche), HyperCap Bead Kit (Roche), SureSelect Target Enrichment Kit ILM Indexing Hyb Module Box 2 (Agilent), PlateLoc Thermal Microplate Sealer (Agilent), Herculase II Fusion DNA Polymerase Kit (Agilent), Sequencing and Library Building Platform (IIIumina USA) were used.
Experimental method
(i) Fresh tumor tissue was processed with quality control; (ii) DNA extraction of formalin‐fixed paraffin‐embedded (FFPE) samples was performed using the GeneRead DNA FFPE Tissue Kit; (iii) plasma and leukocytes were separated from peripheral blood samples; (iv) extraction of free DNA from peripheral blood: HiPure Circulating DNA kits were used to extract free DNA; (v) blood/cell/tissue genomic DNA extraction kit (DP304) was used to extract leukocyte DNA (germline DNA); (vi) a DNA library was established using KAPA Biosystems HyperPlus Kits to build the library; (vii) probe hybridization was performed for 642 gene panels(Appendix S1) with the Hyper Cap Target Enrichment Kit and SeqCap EZ Probes; (viii) full exon probe hybridization was performed using Agilent probes and related kits; (ix) to mix and dilute different libraries so that the DNA concentration in all libraries was 10 nM, and the total volume of the system is 20 μL; (x) online sequencing was performed using the Illumina HiSeq X Ten high‐throughput sequencing platform.
Statistical analysis
Descriptive analysis was used to summarize genetic characteristics. Fisher's exact test was used to study the association between mutations and DCB/NDB. DCB/NDB was used as the outcome variable, and specific gene mutations were used as independent variables for lasso regression, logistic regression, and machine learning random forest analysis to explore independent predictors related to the long‐term benefits of immunotherapy. The Kaplan‐Meier method was used to draw the progression‐free survival curve of NSCLC immunotherapy patients with or without specific gene mutations. A receiver operating characteristic (ROC) curve was drawn to evaluate the prediction ability.
Results
Tumor mutant gene characteristics
This prospective study included 63 patients with advanced NSCLC who received PD‐1 monoclonal antibody therapy, and 44 patients who could eventually provide sufficient tissue samples for the gene panel testing. Of these 44 patients, 66.15% were given pembrolizumab, 26.15% sindilizumab, and 7.69% nivolumab. The objective response rate (ORR) with PD‐1 was 43.18%, and the disease control rate (DCR) was 72.73%. The DCB group accounted for 43.18% of patients, and the NDB group accounted for 56.82%. The top 16 gene mutations were selected for statistical analysis. The mutation rates were TP53 38.64%, KRAS 31.82%, EGFR 20.45%, BRCA 20.45%, ERBB (excluding EGFR) 18.18%, PTEN 15.91%, CDK4/6 13.64%, POLE 11.36%, MET 11.36%, PIK3CA 9.10%, FGFR 9.10%, BRAF 9.10%, JAK 9.10%, ALK 6.82%, POLD1 4.55%, and BLM 4.55%.
Relationship between specific tumor gene mutations and possibility of DCB
Table 1 shows the correlation between DCB/NDB possibilities and molecular characteristics. Chi‐square test results showed a significant difference in the frequency of KRAS mutations (P < 0.001), KRAS + TP53 combination mutation (P = 0.005), TP53 + PTEN combination mutation (P = 0.036), PTEN mutation (P = 0.035), JAK1/2/3 mutation (P = 0.030), TP53 mutation (P = 0.042), EML4‐ALK mutation (P = 0.049), pan‐ErbB mutation (P = 0.039) between the DCB and NDB groups. The DCB group had higher rates of KRAS mutations, KRAS + TP53 combination mutations, TP53 + PTEN combination mutations, PTEN mutations, JAK1/2/3 mutations, TP53 mutations, and EML4‐ALK mutations, suggesting that patients with these genetic mutations may benefit from immunotherapy. The incidence of ERBB comutation in NDB was significantly higher than that in the DCB group, suggesting that the ERBB comutation may be a type that does not benefit immunotherapy. It is worth noting that the EML4‐ALK mutation, JAK1/2/3 mutation, KRAS + TP53 combination mutation, and the TP53 + PTEN combination mutation only occurred in DCB patients, suggesting that these four gene mutations may be potential genes for effective immunotherapy. BRCA mutations, CDK4/6 mutations, and BLM mutations had a higher incidence in the NDB group, but no statistical difference was shown due to the sample size, which indicates that if these gene mutations were used for immunotherapy, they would have poor curative effect, which requires further exploration.
Table 1.
Association between the possibility of DCB/NDB and tumor‐specific gene mutations
| Gene | NDB (n = 24) | DCB (n = 20) | P‐value |
|---|---|---|---|
| Pan‐ErbB | |||
| 1 | 12 | 4 | 0.039 |
| 0 | 12 | 16 | |
| KRAS | |||
| 1 | 1 | 13 | <0.001 |
| 0 | 23 | 7 | |
| BRCA | |||
| 1 | 7 | 2 | 0.117 |
| 0 | 17 | 18 | |
| EML4‐ALK | |||
| 1 | 0 | 3 | 0.049 |
| 0 | 24 | 17 | |
| PIk3CA | |||
| 1 | 2 | 2 | 1.000 |
| 0 | 22 | 18 | |
| MET | |||
| 1 | 3 | 2 | 1.000 |
| 0 | 21 | 18 | |
| TP53 | |||
| 1 | 6 | 11 | 0.042 |
| 0 | 18 | 9 | |
| FGFR | |||
| 1 | 2 | 2 | 1.000 |
| 0 | 22 | 18 | |
| BRAF | |||
| 1 | 2 | 2 | 1.000 |
| 0 | 22 | 18 | |
| CDK4/6 | |||
| 1 | 5 | 1 | 0.279 |
| 0 | 19 | 19 | |
| POLE | |||
| 1 | 3 | 2 | 1.000 |
| 0 | 21 | 18 | |
| POLD1 | |||
| 1 | 1 | 1 | 1.000 |
| 0 | 23 | 19 | |
| BLM | |||
| 1 | 2 | 0 | 0.493 |
| 0 | 22 | 20 | |
| JAK1/2/3 | |||
| 1 | 0 | 4 | 0.030 |
| 0 | 24 | 16 | |
| PTEN | |||
| 1 | 1 | 6 | 0.035 |
| 0 | 23 | 14 | |
| TP53 + PTEN | |||
| 1 | 0 | 4 | 0.036 |
| 0 | 24 | 16 | |
| KRAS + TP53 | |||
| 1 | 0 | 6 | 0.005 |
| 0 | 24 | 14 |
Bold font means P value <0.05, suggesting statistical significance.
Exploring independent predictors of outcomes for immunotherapy
Lasso regression was performed with the long‐term benefit as the outcome variable and the genetic mutation as the independent variable. The glmnet package in R software was used. When the penalty parameter lambda = lambda.1se = 0.125 773 1 was used, the error of the model was relatively small. At this time, the nonzero gene mutations (Table 2, Fig 1a–b) retained in the model were KRAS, EML4‐ALK, PTEN, and TP53 + PTEN. Among these, the ranking of KRAS > TP53 + PTEN > PTEN > EML4‐ALK was conducted according to importance.
Table 2.
Seventeen independent variable coefficients
| Gene | Coefficient |
|---|---|
| (Intercept) | −1.0209753 |
| Pan‐ErbB | |
| KRAS | 1.696 336 5 |
| BRCA | |
| EML4‐ALK | 0.126 027 6 |
| PIK3CA | |
| MET | |
| TP53 | |
| FGFR | |
| BRAF | |
| CDK4/6 | |
| POLE | |
| POLD1 | |
| BLM | |
| JAK1/2/3 | |
| PTEN | 0.588 657 5 |
| TP53 + PTEN | 0.816 064 3 |
| KRAS + TP53 |
Figure 1.

(a) Lasso coefficient curve for 17 independent variables; (b) log (Lambda) sequence coefficient distribution.
In the multivariate analysis, KRAS mutations and TP53 + PTEN mutations were included in logistic regression (due to sample size limitations), and the results showed that KRAS mutations were statistically significant (Table 3, P < 0.001). It is suggested that the mutation of the KRAS gene is an independent predictor of the long‐term benefit of immunotherapy. There was no significant statistical correlation between the TP53 + PTEN combination mutation and long‐term benefit outcomes.
Table 3.
Logistic regression analysis results
| Estimate | Std. | Error | P‐value |
|---|---|---|---|
| (Intercept) | −2.0369 | 0.6138 | <0.001 |
| KRAS | 3.8286 | 0.9799 | <0.001 |
| TP53 + PTEN | 20.6030 | 3261.3194 | 0.994 960 |
Unreliable conclusions are often obtained from logistic regression. Therefore, although logistic regression indicated that the KRAS gene mutation is an independent predictor of whether long‐term benefits are obtained, in order to form a closed loop of evidence, the random forest algorithm of machine learning was used to find important genes to compare with the results of the logistic regression analysis.
Predictive models were built based on whether the patient's long‐term benefit is the dependent variable and genetic mutation is the independent variable. The R window was used to draw a multidimensional scale (MDS), as shown in Fig 2, and 24 NDB samples (blue dots) and 20 DCB samples (red dots) were divided into two distinct categories. The RandomForest package in R software was used to perform random forest classification on the data. To prevent overfitting, a portion of the data was selected as the training set and another portion of the sample was selected as the test set. The purpose of this was to obtain the relationship between parameters and errors (Fig 3) and the relationship between the number of forests and errors (Fig 4).
Figure 2.
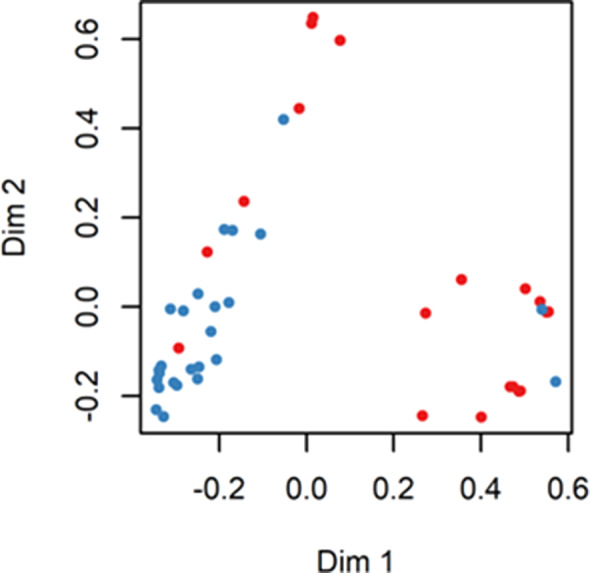
Multidimensional scale.
Figure 3.
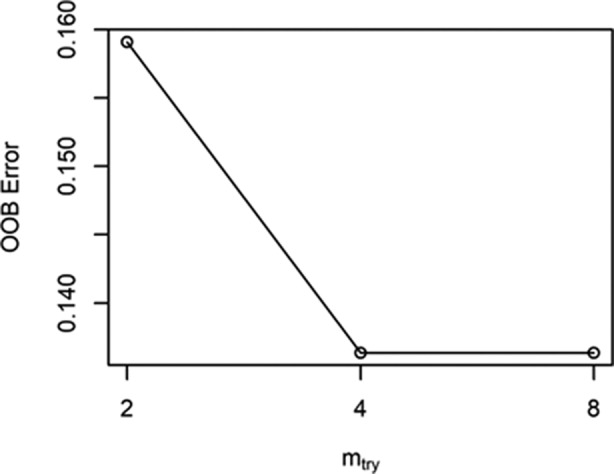
The relationship between parameters and errors.
Figure 4.
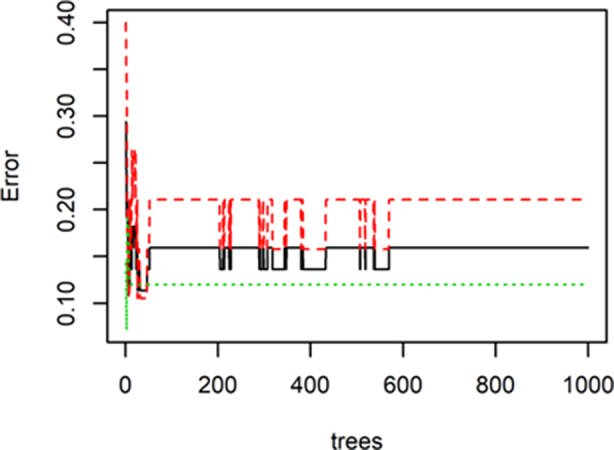
The relationship between the number of forests and errors.
It can be seen from the two figures that when the number of trees reaches more than 600, the error rate gradually stabilizes. The most optimal parameters are mtry = 4 and ntree = 1000.The out‐of‐bag (OOB) estimate of the error rate is 13.64%, which is equivalent to a cross‐validation classification accuracy rate of 86.36%, and the original sample back‐generation prediction accuracy rate is 100%. This indicates that there is a stable correlation between the predictors of this study and the long‐term benefits of immune checkpoint inhibitor treatment.
When the above parameters (mtry = 4, ntree = 1000) were fixed, the average minimum Gini index reduction (Mean Decrease Gini) and average accuracy decrease (Mean Decrease Accuracy) were calculated for each gene mutation. The purpose of this was to obtain a ranking chart (Fig 5) of the two accuracy indicators to evaluate the importance of the impact of different gene mutations on the outcome. The most important independent variable is the KRAS gene mutation, which ranks first. This shows that the KRAS gene is the most important gene in the classification algorithm.
Figure 5.
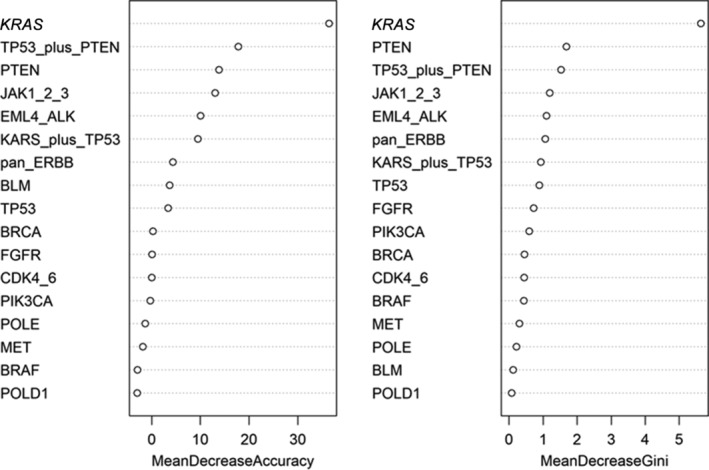
The ranking chart of the two accuracy indicators.
KRAS gene mutations are the most important variables in lasso regression, logistic regression, and machine learning random forest algorithms. Evidence of closed loops has accumulated, suggesting that KRAS gene mutations are independent predictors of whether immunotherapy can durably benefit patients.
Progression‐free survival curves with or without KRAS mutation
Fig 6 shows the Kaplan‐Meier method for mapping the association between mutations in KRAS genes and progression‐free survival in NSCLC patients who received immunotherapy. It can be seen that the difference in progression‐free survival times between the mutant group and the nonmutated group is statistically significant (PFS) (P = 0.00015). The KRAS mutation is therefore a favorable predictor of the long‐term benefit of immunotherapy in NSCLC patients.
Figure 6.
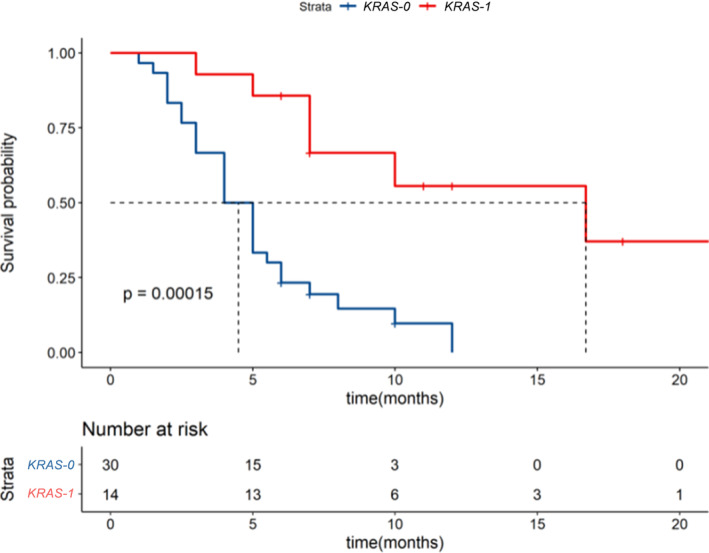
The Kaplan‐Meier method was used to draw a progression‐free survival curve for NSCLC patients with or without the KRAS gene mutation ( ) Strata, (
) Strata, ( ) KRAS = 0, (
) KRAS = 0, ( KRAS = 1).
KRAS = 1).
KRAS mutation predicts the long‐term benefits of immunotherapy
The ROC curve was drawn based on whether the long‐term benefit (two‐category outcome) is the dependent variable and whether the KRAS mutation is used as a predictor, as shown in Fig 7.
Figure 7.
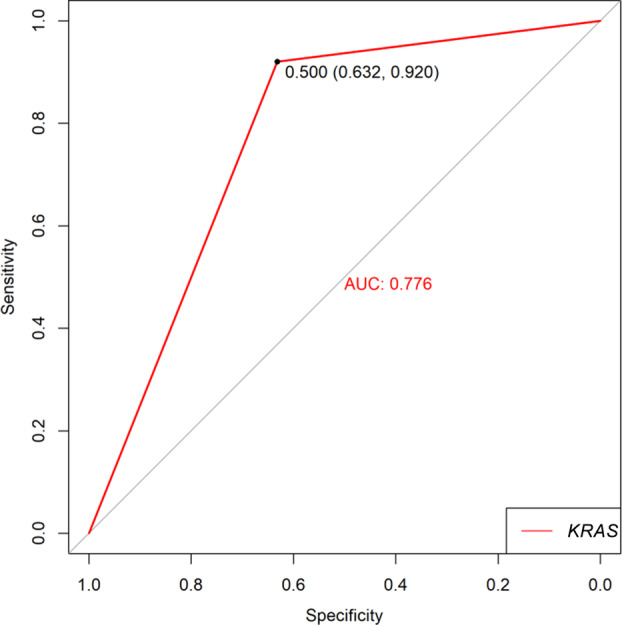
ROC curve of KRAS prediction outcome ( ) KRAS.
) KRAS.
The area under the curve is 0.776, and according to the principle of approximately equal exponential maximum, 0.5 is the most optimal cutoff value. At this time, the specificity is 0.632, and the sensitivity is 0.920. The probability of false negatives is 0.080, and the probability of false positives is 0.368, suggesting that the ability of the KRAS gene mutation to predict outcomes is moderately high.
Discussion
Genetic changes in specific driver genes activate tumor cell proliferation to support tumor growth. It has been shown that certain oncogenic pathways also affect the immune system's recognition of tumors, especially T cell‐mediated recognition. Identifying lung adenocarcinoma subtypes with carcinogenic drivers has revolutionized the treatment of NSCLC. KRAS mutations in solid tumors appear in 90% of pancreatic cancer cases, 10%–15% of lung cancer (mainly NSCLC) cases, and 30%–40% of colorectal cancer cases.
The KRAS mutation is the second most important oncogene‐driven mutation in lung adenocarcinoma, with KRAS missense mutations9 found in codons 12 and 13 appearing in more than 95% of cases. Unlike EGFR mutations, there is no gender difference with KRAS mutations, and they are more common in white populations than Asians, and in most patients who previously smoked or now smoke.10, 11 The biological and phenotypic heterogeneity of patients with KRAS mutations prevents the emergence of more effective treatment strategies for patients with KRAS mutations.12
Given that the activation of specific oncogenic pathways can have a broad effect on gene expression, the genetic makeup of cancer cells may have a significant impact on the immune tumor microenvironment (TME) by driving specific immune‐related pathways. This can be achieved by inducing immune checkpoints, secreting specific cytokines, or producing chemokines that recruit specific cell types.13 Recent studies have shown that KRAS‐mutant NSCLC expresses higher levels of PD‐L1 protein14, 15, 16 compared to corresponding wild‐type tumors. Therefore, it can be speculated that the most common smoking‐related mutation in lung adenocarcinoma, KRAS, can be used as an effective predictor of anti‐PD‐1/PD‐L1 immunotherapy.
Cinausero et al.17retrospectively analyzed 88 patients with locally advanced or metastatic nonsquamous NSCLC who received ICIs and found that patients with KRAS mutations had longer overall survival (OS) and PFS than patients with KRAS wild‐type, which was statistically significant. In addition, the presence of nonsynonymous KRAS mutations is associated with DCB. Dong et al.8 found that KRAS‐mutated tumors showed a significantly increased mutation load. KRAS mutations changed a group of genes involved in cell cycle regulation, DNA replication, and damage repair.
Public clinical trials and prospective observations of immunotherapy analysis have further confirmed that there is significant clinical benefit for patients with KRAS mutations to receive PD‐1 inhibitor treatment. In the current study, 44 patients were tested for tumor gene mutations, and multiple statistical analysis methods (lasso regression + logistic regression + machine learning random forest algorithm) were used to find that patients with KRAS mutations benefited from PD‐1 blockade. However, Jeanson et al.18 found that 282 patients with advanced NSCLC who received immunotherapy exhibited no significant KRAS mutations or any other mutations that made a difference in the ORR, PFS, or OS rates.
The underlying mechanism by which patients with KRAS‐activated mutations may benefit from PD‐1 blockade remains unclear. Most studies suggest that KRAS mutations can enhance PD‐L1 expression, promote T cell infiltration, and enhance tumor immunogenicity. This is attributed to the association between smoking and the presence of KRAS mutations.19 Snjezana et al.20 analyzed 3026 patients and found that KRAS mutations occurred in 34% of smokers and 6% of never‐smokers, and the most common G > T conversion mutation in smokers was KRAS G12C. In addition, it was found that any history of smoking significantly increased the possibility of finding KRAS mutations in lung cancer, regardless of the number of years of smoking.
The permanent damage to DNA caused by tobacco carcinogens obtained during smoking is the main source of most KRAS mutated lung adenocarcinomas. Therefore, the likelihood of KRAS mutations in lung cancer patients is determined by the number of years of smoking and does not significantly decrease over time after quitting. Dong et al.8 found a significant increase in the mutation load in KRAS‐mutant tumors, and also observed that KRAS mutations can disrupt DNA repair, especially in mismatch repair (MMR), which supports the idea that MMR deficiency can be a favorable factor for PD‐1 blockade. Therefore, the KRAS gene mutation may be a potential predictor of the success of immunotherapy involving the blockage of PD‐1.
In conclusion, the mutation of the KRAS gene in tumor tissues is an independent predictor of the long‐term benefit of immunotherapy, with strong predictive ability.
Disclosure
No authors report any conflict of interest.
Supporting information
Appendix S1: Supplementary Information
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Borghaei H, Paz‐Ares L, Horn L et al Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015; 373: 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Herbst RS, Baas P, Kim DW et al Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): A randomised controlled trial. Lancet 2016; 387: 1540–50. [DOI] [PubMed] [Google Scholar]
- 4. Rittmeyer A, Barlesi F, Waterkamp D et al Atezolizumab versus docetaxel in patients with previously treated non‐small‐cell lung cancer (OAK): A phase 3, open‐label, multicentre randomised controlled trial. Lancet 2017; 389: 255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018; 359: 1350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Del Re M, Rofi E, Restante G et al Implications of KRAS mutations in acquired resistance to treatment in NSCLC. Oncotarget 2018; 9: 6630–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Skoulidis F, Goldberg ME, Greenawalt DM et al STK11/LKB1 mutations and PD‐1 inhibitor resistance in KRAS‐mutant lung adenocarcinoma. Cancer Discov 2018; 8: 822–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dong ZY, Zhong WZ, Zhang XC et al Potential predictive value of TP53 and KRAS mutation status for response to PD‐1 blockade immunotherapy in lung adenocarcinoma. Clin Cancer Res 2017; 23: 3012–24. [DOI] [PubMed] [Google Scholar]
- 9. Forbes S, Clements J, Dawson E et al Cosmic 2005. Br J Cancer 2006; 94: 318–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buttitta F, Barassi F, Fresu G et al Mutational analysis of the HER2 gene in lung tumors from Caucasian patients: Mutations are mainly present in adenocarcinomas with bronchioloalveolar features. Int J Cancer 2006; 119: 2586–91. [DOI] [PubMed] [Google Scholar]
- 11. Suzuki M, Shigematsu H, Iizasa T et al Exclusive mutation in epidermal growth factor receptor gene, HER‐2, and KRAS, and synchronous methylation of nonsmall cell lung cancer. Cancer 2006; 106: 2200–7. [DOI] [PubMed] [Google Scholar]
- 12. Pao W, Girard N. New driver mutations in non‐small‐cell lung cancer. Lancet Oncol 2011; 12: 175–80. [DOI] [PubMed] [Google Scholar]
- 13. Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism‐driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer 2016; 16: 275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cortez MA, Ivan C, Valdecanas D et al PDL1 regulation by p53 via miR‐34. J Natl Cancer Inst 2016; 108: djv303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ji M, Liu Y, Li Q et al PD‐1/PD‐L1 expression in non‐small‐cell lung cancer and its correlation with EGFR/KRAS mutations. Cancer Biol Ther 2016; 17: 407–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cinausero M, Laprovitera N, De Maglio G et al KRAS and ERBB‐family genetic alterations affect response to PD‐1 inhibitors in metastatic nonsquamous NSCLC. Ther Adv Med Oncol 2019; 11: 1758835919885540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dong ZY, Zhong WZ, Zhang XC et al Potential predictive value of TP53 and KRAS mutation status for response to PD‐1 blockade immunotherapy in lung adenocarcinoma. Clin Cancer Res 2017; 23 (12): 3012–24. [DOI] [PubMed] [Google Scholar]
- 18. Jeanson A, Tomasini P, Souquet‐Bressand M et al Efficacy of immune checkpoint inhibitors in KRAS‐mutant non‐small cell lung cancer (NSCLC). J Thorac Oncol 2019; 14 (6): 1095–101. [DOI] [PubMed] [Google Scholar]
- 19. Rizvi NA, Hellmann MD, Snyder A et al Cancer immunology. Mutational landscape determines sensitivity to PD‐1 blockade in non‐small cell lung cancer. Science 2015; 348: 124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dogan S, Shen R, Ang DC et al Molecular epidemiology of EGFR and KRAS mutations in 3,026 lung adenocarcinomas: Higher susceptibility of women to smoking‐related KRAS‐mutant cancers. Clin Cancer Res 2012; 18 (22): 6169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supplementary Information


